Abstract
Microchimerism, the coexistence of genetically disparate populations of cells in a receptive host, is well described in both clinical and physiological settings, including transplantation and pregnancy. Microchimerism can also occur following allogeneic blood transfusion in traumatically injured patients, where donor cells have been observed decades after transfusion. To date, transfusion-associated microchimerism (TA-MC) appears confined to this clinical subset, most likely due to the immune perturbations that occur following severe trauma that allow foreign donor cells to survive. TA-MC appears to be unaffected by leukoreduction and has been documented following transfusion with an array of blood products. The only significant predictor of TA-MC to date is the age of red cells, with fresher units associated with higher risk. Thus far, no adverse clinical effect has been observed in limited studies of TA-MC. There are, however, hypothesized links to transfusion-associated graft vs. host disease (TA-GvHD) that may be unrecognized and consequently under-reported. Microchimerism in other settings has gained increasing attention due to a plausible link to autoimmune diseases, as well as its diagnostic and therapeutic potential vis-a-vis ante-natal testing and adoptive immunotherapy, respectively. Furthermore, microchimerism provides a tool to further our understanding of immune tolerance and regulation.
Keywords: Microchimerism, transfusion, chimerism, trauma, immunity, immune tolerance
INTRODUCTION
The word chimera is named for the mythological beast, originally referenced in Homer’s Iliad. This fire-breathing creature comprised the body of a lioness, the head of a goat and the tail of a transformed serpent. A sibling of both the Lernaean Hydra and Cerebrus, the multi-headed hound, a sighting of the chimera was said to be ominous of disaster.
The chimera in Science builds on the notion of a hybrid animal to represent an organism of mixed genetic origin. Specifically, chimerism refers to the enduring co-existence of genetically disparate populations of cells within a single host. Unlike mosaicism, where the discordant cell lines originate from the same zygote, the chimeric cell is truly foreign or allogeneic, having originated outside the host.
Although the term microchimerism (MC) is frequently used interchangeably with chimerism, it is generally accepted that the term MC should be reserved for circumstances in which the foreign populations account for less than 5% of host cellular content, or more specifically of nucleated cells in the relevant organ or blood. These allogeneic or non-self cells elude the host immune system and persist at low levels. The clinical significance of enduring chimerism includes both adverse effects (e.g., autoimmune diseases[1–3]) as well as potential therapeutic benefits (e.g., tolerance induction, tissue regeneration and repair[4, 5]).
Two key components define MC: (1) “quantity”, indicating that the population is of sufficient magnitude to be detectable using laboratory screening and (2) “longevity” indicating that the cells are durable over time. The latter is somewhat arbitrary, contingent on a persistence that is deemed excessive for the initiating event, e.g. persistence of allogeneic cells beyond 3–6 months following pregnancy or blood transfusion is indicative of long-term MC. Short-term MC (likely representing delayed clearance of allogeneic cells) is significantly more frequent than long-term MC and does not necessarily predict long-term durability. Several studies have reported survival of donor leukocytes after transfusion, yet limited follow-up precliudes a clear definition of durable MC. [6, 7] Should levels of chimerism start off below the limits of detection and increase over time, eventually long-term MC may occur in the absence of short-term MC; this may reflect expansion of a minute population, which is only detectable months to years following allogeneic exposure.
CLINICAL SETTINGS
MC may be iatrogenic following solid organ-, bone marrow transplantation or blood transfusion; it may also arise physiologically through pregnancy, twinning or intergenerational transfer (see below). [1–5, 8–12] Sexual transmission and needle sharing have also been suggested, as possible mechanisms for MC, yet have not been well documented.
Fetal-Maternal Microchimerism
Pregnancy is the most common setting in which MC is encountered, given bidirectional transfer of cells and cell-free DNA between mother and fetus. This may be necessary to establish immune tolerance between the mother and fetus[11], as evidenced by the detectable presence of both fetal cells and cell-free DNA in the maternal circulation in the majority of pregnancies. Chimeric cells increase from first appearance at 4–5 weeks up until parturition, when rapid and near-complete cellular clearance occurs[13]. [8], Long-lived MC is observed in some parous women, and has in some circumstances been documented decades following delivery.
Terminology surrounding pregnancy-associated chimerism may be confusing: fetal-maternal microchimerism (FM-MC) is a generic term to denote MC that occurs during or subsequent to pregnancy, irrespective of directionality. In contrast fetal-MC (F-MC) refers to the persistence of fetal cells in the mother while maternal-MC (M-MC) refers to the persistence of maternal cells in the fetus.[11, 14] F-MC can occur between members of a multiple pregnancy: indeed, the original research in MC was spurred by observations of pathology in freemartins (female twin cattle) arising through in-utero hormone exposure from their male siblings. Furthermore, the seminal work performed by geneticist Ray Owens documented blood group concordance among fraternal twin cattle.[15] Similarly in humans, up to 8% of twins and 21% of triplets have been shown to exhibit blood group chimerism.[16]
F-MC has been an unanticipated finding in a number of studies[17–19], including in control groups that have been selected using women that self-report as never having been pregnant. This underscores that pregnancy need not be recognized for MC to take place; up to 30% of pregnancies end in fetal loss with 14% going unnoticed.[20] Both “occult” pregnancy as well as “vanished” or resorbed twins are more common than is appreciated and may result in durable MC. In one report, F-MC was attributed to a vanished twin 40 years after the implicated pregnancy.[21] The transfer of cells from progeny to mother also raises the possibility that fetal cells, which have persisted in the mother following an antecedent pregnancy, can be exchanged with a sibling during a subsequent pregnancy (intergenerational transfer[9]).
Durable FM-MC is not unique to term gestation and has been reported following both spontaneous and elective abortions. Increased rates of MC are observed in the latter, which may be attributable to fetal-maternal hemorrhage with increased volume of cellular transmission.[22] Other factors that appear to impact FM-MC include fetal and placental abnormalities, fetal aneuploidy and the length of time lapsed between delivery and investigation.[22] In contrast, parity does not appear to have a significant impact on rates of FM-MC.[23]
Transplantation- and Transfusion-associated Microchimerism
Transplantation-associated MC can be coincidental or imposed for therapeutic benefit. In solid organ transplantation, there is opportunity for systemic migration of foreign cells beyond the graft, despite localization of the donor organ (e.g., kidney, liver (reviewed in [12]). In hematopoietic stem cell (HSC) transplantation, systemic chimerism is an intended outcome with a goal being either complete or partial replacement of the recipient’s HSC compartment.
Blood transfusion is the most frequently performed allogeneic transplantation. Interest in the kinetics of transfused donor cells dates back to the 1970s when Schechter et al. demonstrated karyotypic evidence of donor leukocyte proliferation within 7 days of blood transfusion.[24] Subsequent studies, however, were unable to detect evidence of donor leukocyte survival beyond 6 days in immunocompetent recipients.[25] Many of the earlier studies were, however, constrained by limited sensitivity of the technologies at time of evaluation. It was approximately twenty years later when an attempt to extend Schechter’s work lead to a serendipitous finding that reshaped our understanding of TA-MC. Lee et al. [26] conducted a case control study that evaluated subjects transfused during elective surgery (cases) and compared these with a control group comprising patients that had been transfused for traumatic injury. The kinetics observed in the case group replicated that of the earlier experience: rapid clearance of 99.9% donor leukocytes was followed by a transient expansion of donor cells observed between days 2 and 7 with complete clearance by 7 to 14 days after transfusion.[26] In contrast, the majority (7 out of 10) of the “control” group unexpectedly developed durable TA-MC beyond 7 days. Follow-up studies have supported the finding that transfusion in patients following severe traumatic injury results in high rates (20–40% [27] of patients affected) of enduring (up to 60 years) TA-MC. This pertains to both combat casualty and civilian trauma settings.[28, 29]
With rare exceptions, durable TA-MC has remained peculiar to patients transfused for severe traumatic injury. [29–31] The few studies that have sought to evaluate other populations at “risk” for TA-MC have yielded negative results. Some of these studies have investigated populations that share features with the trauma setting, thereby offering plausible risk of TA-MC. Examples include studies of TA-MC in the HIV-infected population, as a paradigm of an immunosuppressive state[32], elective surgery as a model of iatrogenic “trauma”[26], and sickle cell anemia and thalassemia[33] reflecting chronic transfusion risk. TA-MC was not significantly increased or durable in these patient populations.
A large cohort of adult and pediatric transfusion recipients were evaluated as part of the Transfusion Related Infections Study (TRIPS); 40 of the 207 (19%) adult and 44 of 202 (22%) of the pediatric transfusion recipients of leukoreduced and predominantly irradiated red cells and platelets, demonstrated transient TA-MC at 4 and/or 8-weeks post transfusion. However, on repeat testing only 12 (3%) showed TA-MC during the 2 months post-transfusion, none of which displayed durable chimerism.[34]
More recently, a study in South Africa, investigated whether TA-MC would occur in women transfused for peripartum hemorrhage. TA-MC was hypothesized to occur based on the overlap between the peripartum period and the trauma setting, given the acute blood loss, pain and similar immune perturbations that accompany parturition. However, despite a small sample size (n=22), the findings from this pilot study suggest that durable TA-MC does not occur in this population.[35]
Proposed reasons that place the trauma patient at greatest risk of durable TA-MC include the large number of unit exposures, which often include cellular components with relatively short periods of storage, as well as an altered immune response that occurs transiently following trauma.[36] This acute immunological imbalance is thought to predispose to a transient tolerance to allogeneic cells enabling low-level engraftment, thus manifesting as TA-MC. Both diminished innate and adaptive immune responses with transient T cell suppression and altered cytokine profiles are observed following injury. The latter is increasingly recognized as a complex, dynamic state in response to tissue damage, heightened stress, and the compounding contribution of exposure to microbial products from body site contamination. In addition predisposing genetic factors may account for variability of risk among transfusion recipients.[36]
The chimeric populations have been shown to comprise multi-lineage hematopoietic cells[37], supporting a hypothesis of stem cell engraftment; however, this has not been proven definitively.[26] Lee et al. evaluated chimeric cell phenotypes specifically in TA-MC using antibody-coated magnetic beads against CD4+ and CD8+ (T cells), CD15+ (myeloid cells), and CD19+ (B cells), and detected all 4 of these cell populations. Thus TA-MC is distinct from TA-GvHD, which involves excusive expansion of alloreactive donor lymphocytes targeting host histocompatibility antigens.
PREDICTORS OF TA-MC
Multiple blood product and patient characteristics have been examined in an attempt to predict TA-MC. In a cohort of 45 transfused trauma patients, 53% of which had short-term TA-MC,[10] Utter et al. examined multiple factors including recipient age, sex, type of injury, injury severity, volume of transfusion, presence of hypotension, pre-injury comorbidities (e.g. cardiorespiratory disease, diabetes, substance abuse, or malignancy), and time interval between injury and transfusion, none of which were shown to have a significant association with TA-MC. TA-MC also does not appear to be influenced by the age of the recipient at time of transfusion; in one case TA-MC occurred following transfusion of a fetus, (donor cells were detected 25 years following intrauterine transfusion).[38] TA-MC is also relatively common in young men transfused following combat injury.
TA-MC occurs independent of leukoreduction[29, 39] with similar rates of TA-MC having been demonstrated following transfusion with both leukoreduced and non-leukoreduced products and hence is not WBC dose dependent. In a substudy of a double-blinded randomized-controlled trial evaluating the effect of leukoreduction on development of infection within 28 days of traumatic injury,[40] 9/32 (28.1%) patients in the non-leukoreduced arm had durable TA-MC while 13 of 35 (37.1%) in the leukoreduced arm developed TA-MC (p=0.43) on later follow-up.[31] This is not entirely unexpected; leukoreduction reduces white blood cells (WBCs) below 5×106 WBCs/component, but does not eliminate WBCs completely.
While donor recipient HLA mismatch has not been predictive of TA-MC in trauma patients in limited studies to date,[26] one retrospective study showed that transfusion of blood with at least one HLA haplotype shared between donor and recipient was more likely to result in TA-MC at 5–8 weeks of follow-up than receipt of mismatched blood (3/12 vs. 1/5, p=0.04).[6] However, the limited follow-up and transfusion shortly prior to renal transplantation limit the conclusion with respect to durable TA-MC.
Currently, many are advocating against use of older blood, citing concerns of heightened risk and reduced efficacy with prolonged storage. Reed et al. reported a significantly (p=0.024) different red cell storage time between non-TA-MC recipients (21±8.3 days) vs. TA-MC recipients (16.1±6.2 days).[28]Similarly the minimum storage time of packed red blood cells (pRBC) units was a median of 13 days in non-TA-MC recipients and 5 days [p=0.004] in TA-MC recipients.[28] Although this suggests that rates of TA-MC are inversely related to storage age, TA-MC has also been reported with older units (22 days following collection).[39]
The Immunology of Trauma, Transfusion and Microchimerism
Traumatic injury and transfusion are significant immunological stressors, triggering responses to the primary events themselves, as well as inducing major changes in the body’s response to associated immunological challenges posed by secondary pathogen exposure and surgical intervention. The immune modulation resulting from these events forges a unique environment that appears critical for the development of TA-MC.
The immune perturbations that accompany trauma may explain why patients that have been transfused following injury are uniquely susceptible to TA-MC. This was illustrated in a case-control study of 63 transfused trauma patients where the patients’ lymphocyte responses were compared with those of 10 non-transfused trauma patients and 10 healthy controls.[29] Lymphocyte responses were reduced in patients that had sustained traumatic injury, with even greater reduction in recipients who developed TA-MC. This trauma-induced immune hyporesponsiveness was not resolved at time of discharge. Furthermore, in another study, mixed lymphocyte reactions demonstrated a bidirectional hyporeactivity between donors and recipients that preceded transfusion.[41] Of note, recipients were shown to be least responsive to WBCs from the individual donors with whom they became chimeric. This strongly suggests a relative histocompatibility advantage of a particular donor in a particular recipient. In addition, TA-MC results from a single donor, despite potential exposure to multiple donors; this may be analogous to single donor stem cell engraftment observed in double cord blood transplantation.[29]
The immune response to trauma has been characterized through both evaluation of peripheral responses in humans as well as simulation of trauma using animal models. Following trauma, there are consistent increases in interleukin (IL)-6, IL-10, IL-1Ra, and IL-8; there may also be increases in tumor necrosis factor α and IL-4 yet the data in this regard are conflicted.[42–55] Other observed changes include increased blood levels of IgE and decreased IgM,[55–57] altered T cell effector function with increased regulatory T cell activity, decreased expression of HLA-DR and concomitant increases in IL-10 and IL-6 expression in monocytes.[58–65] A recent study demonstrated a genomic storm precipitated by trauma with massive changes in RNA expression profiles, suggesting a major shift in immune responsiveness.[66] Murine models, designed to investigate the response to traumatic stresses such as burns, fractures, hemorrhage, and/or laparotomy (surgical stress), both support the human study findings demonstrating elevated IL-6 and IL-10, as well as show altered ex vivo functions of T cells and dendritic cells, with increased regulatory T cell activity.[67–75]
There are multiple factors that can influence the host immune response to injury and thereby contribute to donor cell survival. These include –but are not limited to- the extent of tissue damage, exposure to microbial components, hemorrhage, stress/neuroendocrine activation, the patient’s age and sex.
Tissue damage can induce innate immune activation through several mechanisms. Alarmins, such as high-mobility group box 1 protein and heat-shock proteins, and damage-associated molecular patterns (DAMPS) such as mitochondrial DNA that are normally shielded in intracellular compartments can be released from necrotic cells and induce inflammation after injury.[76–78] Similarly, damage to the epithelium can allow for microbial entry, with recognition via innate immune receptors such as TLR4, while increased endothelial permeability can allow translocation of bacteria from the gastrointestinal tract into the blood stream.[79, 80]
Large volume hemorrhage may precipitate ischemia and later reperfusion injury compounding tissue damage and innate immune activation. In murine hemorrhage models where bleeding is induced either by depletion to a fixed volume or to a targeted blood pressure, short-term deficits in IL-2 production and T cell proliferative capacity have been observed.[81–83] Trauma also results in major endocrine and neurological changes that impact immunity. For example, the release of catecholamines has been shown to have an immunosuppressive effect leading to increased IL-10 production in vivo and ex vivo[84, 85] and inhibited responsiveness to lipopolysaccharide ex vivo.[86–88] Corticosteroids, released as part of the stress response, also lead to immunosuppression.[89–93]
The sex and age of the patient have a significant effect on the immune response to trauma: increased rates of death, sepsis, and multiple organ dysfunction syndrome have been demonstrated in men, with this sex difference modulated by patient age.[94–98] This difference appears to be regulated by sex hormones as manipulation in mice by oopherectomy, castration, or administration of androgens or estrogens, alters the response to trauma and septic challenges.[99, 100] Although significant differences in risk of TA-MC have not been reported between men and women or among different age groups, these factors may contribute in part to establishing an immunological environment that is favorable or unfavorable to development of TA-MC.
Allogeneic blood transfusion, itself, is a major immunological challenge (particularly when non-leukoreduced blood products are used). The immunosuppressive effect of allogeneic transfusion has long been recognized, and has been shown to confer both beneficial clinical effect, e.g. reduced graft failure after transplantation, [101–103] as well as adverse outcomes, e.g. increased cancer recurrence and susceptibility to infectious disease.[102, 104–109] This immunosuppression may also help to drive the development of microchimerism. In the non-trauma setting, transfusion of allogeneic blood can result in a proinflammatory allo-response, prompting both antibody and cellular responses against donor-specific antigens.[110–114] Ensuing alloimmunization may complicate future transfusions or solid organ transplantation.[112, 115, 116]
Historically, the initial response to severe injury was described as proinflammatory; referred to as the systemic inflammatory response syndrome (SIRS), this was thought to be beneficial both to contend with exposure to pathogens that accompany injury, as well as to promote wound healing. A compensatory anti-inflammatory response syndrome (CARS), which follows this initial phase, was deemed important to reduce tissue damage associated with the inflammatory phase. However both excessive immune suppression and activation introduce risk of exacerbating tissue damage and contribute to development of multiple organ failure (MOF). [117–119]
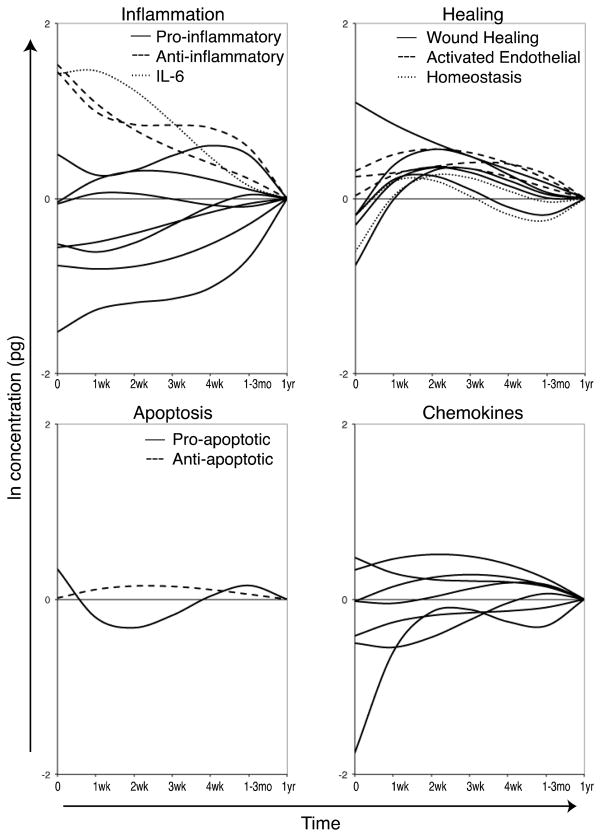
In contrast to the SIRS/CARS model, two recent studies demonstrate that the development of an immunosuppressive cytokine milieu after trauma occurs rapidly, and may already be established by the time the patient arrives in the hospital. This immunosuppression coincides with the timing of blood transfusion, suggesting that the host environment at the time of transfusion may determine the survival of donor cells. In one study, 56 trauma patients were evaluated prospectively from arrival in the emergency room up to one year after injury, using serial blood samples.[120] The levels of 41 different immunomodulatory proteins were tracked and analyzed with clinical data using a multivariable approach. This demonstrated a complex and evolving response to trauma, which begins with a mixed, but predominantly anti-inflammatory response (figure 2). The balance between pro- and anti-apoptotic factors was also shown to be altered by trauma, with an initial pro-apoptotic balance shifting towards being anti-apoptotic 1–4 weeks after injury (figure 2). A number of factors involved in tissue remodeling, lymphocyte homeostasis, and endothelial activation, also become upregulated in the blood during this same timeframe (1–4 weeks) as the system restores itself (figure 2). An early mixed response is consistent with another recent study examining RNA expression profiles post trauma. [66]
Figure 2. Kinetics of immune response to trauma.
Blood samples were collected from trauma patients beginning with arrival to the emergency room and up to 1 year after injury. Multiplexing techniques were used to measure the levels of 41 immunomodulatory proteins in the plasma. Multivariable generalized estimating equations models were generated using the natural log of the concentration of each protein as the dependent variable and time since trauma, injury severity score, injury type, size of transfusion, age, sex, and microchimerism as the independent variables. Overlays of the models’ prediction of the influence of time since trauma controlling for the other covariates are plotted by protein type. Predicted values at 1 year after trauma are set as the baseline (0) for each cytokine to show elevation or depression relative to this value. The inflammation plot includes the pro-inflammatory cytokines interleukin (IL)-1α, IL-5, IL-9, IL-17, tumor necrosis factor α, tumor necrosis factor β, and macrophage migration inhibitory factor, the anti-inflammatory cytokines IL-1 Receptor a and IL-10, and IL-6, which has both pro- and anti-inflammatory properties. The healing plot includes the wound healing proteins epidermal growth factor, fibroblast growth factor-2, vascular endothelial growth factor, matrix metallopeptidase 9, and total plasminogen activator inhibitor-1, the activated endothelial markers soluble E-Selectin, soluble inter-cellular adhesion molecule-1, and soluble vascular cell adhesion molecule-1, and the homeostasis cytokines IL-7 and IL-15. The apoptosis plot includes the pro-apoptotic soluble FasL and the anti-apoptotic soluble Fas. The chemokine plot includes interferon gamma-induced protein-10, IL-8, macrophage inflammatory protein 1 α, monocyte chemotactic protein-1, eotaxin, fractalkine, and macrophage derived chemokine. (Adapted from Jackman, R. P., et al. 2012, Transfusion. doi: 10.1111/j.1537-2995.2012.03618.x, with permission)
[120]
TECHNIQUES FOR DETECTION OF MC
Detection of MC has inherent challenges: the target cellular population is minute and can represent as little as 1 chimeric cell per 106 WBC, which may be overwhelmed by the host’s cellular burden. Therefore, detection of chimerism is rarely based on cell recognition such as in microscopy or by fluorescent in-situ hybridization (FISH),[121, 122] but on molecular signals from whole blood or enriched subpopulations of cells. Therefore, primers or target probes are needed that adhere uniquely to the chimeric minor population (See Table 1).
Table 1.
Techniques for detection of microchimerism
| Technique | Brief Description | Advantages | Disadvantages |
|---|---|---|---|
| Identification of minor population using the Y-chromosome |
|
|
|
| FISH* | In-situ hybridization using a labeled probe e.g. against Y-chromosome | Visual representation |
|
| PCR | Amplification of minor population e.g. using a Y-chromosome based probe | Use in both FM-MC and TA-MC |
|
| Real Time-PCR using HLA-DR | Quantitative amplification of HLA-DR loci | Used for both TA-MC and FM-MC | Can under-represent MC |
| Real Time-PCR using both HLA-DR and InDel probes | Quantitative amplification of multiple loci (HLA and non-HLA targets) |
|
May under-represent MC |
Abbreviations
MACS: magnetic-activated cell sorting
FACS: fluorescence-activated cell sorting
FISH: Fluorescent In-Situ Hybridization
Early techniques largely employed gender discordance, through targeting the Y-chromosome, for evaluation of MC. This has inherent problems both for FM-MC as well as TA-MC. In FM-MC, a Y-chromosome probe restricts evaluation to a single male chimeric population in the maternal host, excluding female progeny. It also introduces both unexpected findings following occult pregnancy or abortion, as well as imprecision with respect to origin of the chimeric population in parous women. Similarly, in the setting of TA-MC, use of sex-based probes excludes detection of female blood donors or the study of male recipients (who are the majority of trauma patients). Furthermore, the majority of transfusion recipients receive more than one blood product, again, imparting a significant limitation in discrimination of chimeric populations.
Enhanced Methods for Detection of MC
The availability of PCR has dramatically improved sensitivity of MC detection, and was applied early to the study of TA-MC, initially through the use of semi-quantitative PCR assays. These assays employed radioactive isotope-labeled probes for detection of the PCR product with subsequent semi-quantification.[123] Although sensitive, these assays were both labor intensive and prone to PCR product contamination. In addition, the dynamic range of semi-quantification PCR systems was limited to approximately 2–3 Logs.
Development of real-time PCR-based assays vastly improved both the sensitivity and specificity of the detection and quantification of MC in humans[13, 26, 33, 124–126] and mice.[127–132] In real time PCR amplification, when a minor population of cells is spiked into a dominant host background (major population), the major host allele appears at an earlier amplification cycle number from that of the spiked minor allele. The difference in amplification cycles between the major and minor populations can be used to estimate frequency of the minor relative to the major alleles. With a limit of detection as low as one chimeric cell in a background of 106 host cells, real time PCR has proven effective in detecting MC in both transfused trauma patients and FM-MC. Given the greater extent of automation, these assays are less prone to PCR product contamination and much less labor intensive than the antecedent semi-quantitative assays. In addition, real-time PCR assays are quantitative with a broad dynamic range of 6–7 Logs.
Unlike the initial assays that targeted the Y-chromosome, these newer assays employ panels that combine HLA- as well as insertion/deletion (InDel) polymorphisms. These assays employ sequence-specific primers that exploit 3–4 base pair differences in the target sequences and dye intercalation to detect specific amplicons. In doing so, they achieve equivalent sensitivity and specificity as probe based systems.[133–135] This has been validated both through sequencing of reaction products as well as spiking serial dilutions of target DNA into negative DNA for the targeted polymorphism.[41] These real-time quantitative PCR assay systems are also amenable to high throughput, and high DNA input for detection of MC.
The HLA panel targets donor-recipient differences in class II genes (see figure 1). One group uses HLA-DR for this purpose, as it is highly polymorphic and has been well characterized. Although sensitive, there are limitations to using an HLA-DR panel exclusively for the detection of MC. Firstly, HLA-based techniques rely on donor-recipient mismatch and would therefore fail to detect TA-MC if the targeted HLA DR alleles are shared. Second, given the breadth of HLA-DR types, it is not feasible to incorporate the complete spectrum of HLA-DR types in the panel, and thus certain cases of TA-MC will go undetected, thereby under-representing the true frequency of TA-MC. Finally, genetic polymorphisms within the HLA class II genes may, in part, be responsible for tolerance and therefore contribute to development of MC. These concerns, therefore, motivated for introduction of a second, complementary, testing array: the InDel panel.
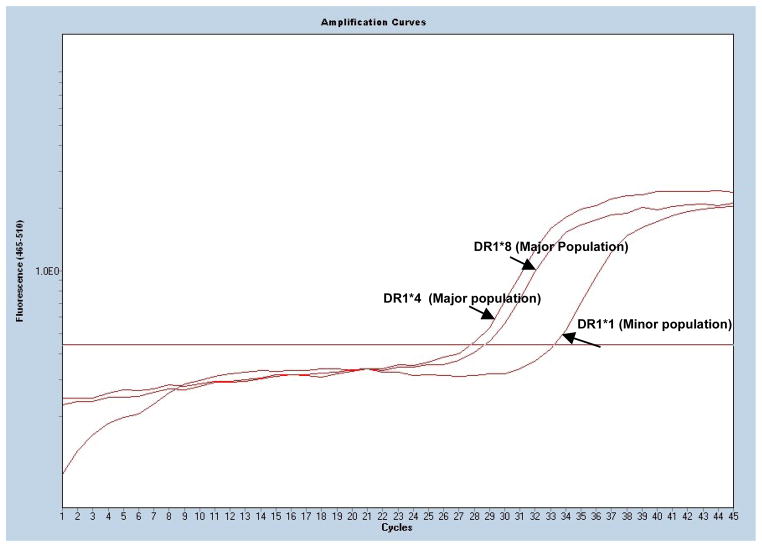
Figure 1. Real time PCR and Detection of Microchimerism.
Fluorescence intensity is plotted on the y-axis while cycles are plotted on the x-axis. Cycle threshold (CT) is defined as the cycle where the fluorescence intensity of amplified DNA crosses a background threshold. Lower cycle thresholds indicate presence of more DNA compared to samples with higher CT s. The patient pre-transfusion sample was typed as HLA DR1*4 and HLA DR1*8, with cycle thresholds for the major types of 27.8 and 28.6 cycles, respectively. Following identification of the subject type, the post-transfusion sample was probed for microchimerism by amplifying for HLA-DR1*1, 3, 4, 7, 8, 9, 10, 11, 12,13, 15 and 16. The cycle threshold for the post-transfusion minor population of DR1*1 is 33.3 cycles.
The InDel panel was adapted for MC analysis from earlier work conducted by Alizadeh, who focused on detection of low level engraftment in hematopoietic stem cell transplant recipients.[136] Unlike HLA-DR, InDel polymorphisms are bi-allelic loci that are unrelated to the immune response genes and distributed across somatic chromosomes, thereby eliminating linkage bias associated with HLA-based ascertainment of TA-MC. The HLA-DR and InDel assays are complementary for both the identification and quantification of MC, as evidenced by validation experiments. The combination of HLA-DR and InDel panels enables identification of an informative polymorphism in 99.5% of donor-recipient pairings.[137] The lower limit of detection for the combined HLA-DR and InDel panels is approximately 1 in 105 to 1 in 106 cells, which is largely impacted by the DNA input in the sample or the number of recipient aliquots evaluated.
In prospective TA-MC studies, pre-transfusion samples are collected first to identify the major alleles of transfusion recipients by typing the recipient using the combined InDel and HLA-DR panels. Any additional alleles that are subsequently detected in the post-transfusion sample are thereby assumed to be of donor origin and consequently chimeric alleles. In the absence of a recipient pre-transfusion samples, or in cross-sectional studies, the post-transfusion sample is serially diluted and amplified to identify the major host alleles through disproportionate dilution of the chimeric population, rendering the latter undetectable. Once the major allele is known, the sample is amplified again using probes that target non-host alleles.
Finally, although the real-time PCR assays are quantitative, the signals of MC are extremely low and most samples require a large DNA input in order to obtain a signal sufficiently strong for quantification. For example, if the true MC concentration is 1 in 105 cells, a minimum DNA input equivalent of approximately 106 cells is required to generate a signal that is both detectable and quantifiable.
Quantification of MC
There are different strategies for reporting MC. Some report MC as a dichotomous outcome (positive vs. negative) while others extrapolate results e.g. report the MC concentration at a given cell number e.g. 100 chimeric cells per 106 cells.
The knowledge that 10-fold dilutions in the sample each PCR cycle will correlate with a doubling in DNA (3.3 cycle difference), enables quantification. One can compare the amplification curve of the sample with that of a standard that has similarly been serially diluted. This comparison enables quantification to a given percentage; an early cycle threshold corresponds to a high input percentage while a later cycle threshold corresponds to a lower input percentage.
In order to establish the proportion of chimeric cells, one needs first to establish the total genomic DNA input (or amplifiable genomic DNA) for the sample (denominator). In our studies this is achieved by amplifying HLA-DQ∝, a conserved region in the major histocompatibility complex II locus, and comparing the output to that of a reporting standard, i.e. samples of known genomic DNA input e.g. 103, 104, 105 cells/mL. Amplification of HLA-DQ∝ also provides for a common method of MC quantification, which is to divide the copy number of the chimeric allele e.g. HLA-DR4 as established first through one of the above detection methods, and to divide this by total genomic DNA input as determined by the level of HLA-DQ∝. The chimeric population is therefore reported as a percentage of the total input. Although relatively simply to calculate, this method neglects a variable efficiency of detection with respect to the different PCR targets, thereby potentially introducing inconsistency in the results.
Once the total DNA is known, a second amplification is conducted against alleles specific to the chimeric population, which is similarly compared to a reporting standard, i.e., samples of known DNA input, e.g. 1, 10, 100 cells/mL. The reporting standard used for the chimeric population is different from that used to gauge total DNA and contains the same specific genetic sequence (HLA-DR and InDel) targeted on the chimeric population. In TA-MC, the actual donor standard is unknown and an external standard is instead employed.
MC can also be reported as genomic equivalents (GEq) although this has not been widely used in reporting of TA-MC. The use of GEq is based on a correlation between DNA input and a given cell number i.e. 1μg DNA is equal to approximately 1.5×105 cells. The total DNA input is therefore critical to the sensitivity of the assay. With this reporting method, if the total DNA input is low, e.g. a total DNA input equivalent to 104 cells, one should not report the chimeric population per 106 cells as this could overstate the sensitivity of the assay.
CLINICAL SIGNIFICANCE OF TA-MC
The clinical ramifications of TA-MC are still unknown. TA-MC has not been extensively investigated and the studies to date have primarily focused on trying to establish the settings in which TA-MC occurs and the immunology of trauma that uniquely place transfusion recipients at “risk”. The clinical effects therefore remain speculative, based on study of MC in other settings, specifically FM-MC.[2, 22] FM-MC has been associated with a wide array of both immune and non-immune diseases, including scleroderma, multiple sclerosis[8, 138], systemic lupus erythematosis (SLE), polymorphic eruption of pregnancy[139], autoimmune thyroiditis[140], biliary atresia[141, 142] and preeclampsia. Furthermore, the observation of histopathological overlap between scleroderma, an autoimmune disease of uncertain etiology, with GvHD, a predictable effect of MC in the setting of transplantation, spurred a novel hypothesis that links FM-MC to autoimmune disease.[143, 144]
In a large study of TA-MC by Utter et al, 163 transfused combat veterans (World War II, the Korean War, and the Vietnam War) were compared with age- and gender-matched blood donor controls.[10] 9.8% of veterans vs. 0.7% of controls (RR 14.7; 95% CI, 2.0–110) demonstrated MC 46±12 years following transfusion. Subjects were questioned regarding autoimmune disease (such as Lupus, Scleroderma, Sjögrens syndrome, Reynaud’s Syndrome, multiple sclerosis), coronary artery disease and any cancer. Extending the hypothesized link between F-MC and autoimmune disease, subjects were also questioned regarding symptoms or signs that might allude to undiagnosed autoimmune disease. Similarly, subjects were questioned about symptoms that might suggest chronic GvHD, another speculated manifestation of TA-MC. No significant difference was detected between the TA-MC vs. non-TA-MC groups in any of these outcomes. Although limited by survivor bias and self-reporting, this suggests that TA-MC does not confer adverse effect in the majority of recipients. However, the numbers of subjects were very small (16 veterans with TA-MC in the Utter study), which is limiting when investigating rare outcomes such as autoimmune disease.
All TA-MC studies to-date have been performed on whole blood, given both the suitability in evaluating MC as well as the ease of availability. As a caveat, although evaluation of MC using whole blood is logistically convenient, it may under-represent the extent of systemic involvement. Tissue is necessary if one is to understand the true distribution, physiology and downstream clinical ramifications of MC.
Transfusion Associated Graft versus Host disease
One extreme clinical manifestation of TA-MC is transfusion-associated Graft vs. Host disease (TA-GvHD), a rare complication of allogeneic blood transfusion. This results from transfusion with donor cells that are homozygous for a haplotype shared by the recipient. The chimeric donor cells go unrecognized by the host, allowing them to engraft and attack the haploidentical recipient. Unlike GvHD in hematopoietic stem cell transplant, where the marrow is donor-derived and therefore spared, TA-GvHD incites global damage and is uniformly fatal with only rare, anecdotal exceptions.[145] TA-GvHD is the principle reason for irradiation of blood products collected from family members or those to be transfused to immunosuppressed patients. While rare in the United States, the incidence can be high in populations with high ethnic homogeneity such as Japan.[146] Risk of TA-GvHD has led to routine irradiation of all blood products in Japan. Although typically a dramatic clinical presentation, there remains the possibility that TA-GvHD represents a clinical spectrum that includes more subtle changes that could go unnoticed or be ascribed to comorbid disease. For example, patients transfused for trauma may develop complications such as multiorgan failure that are readily attributable to the trauma, so that TA-MC driven TA-GvHD might not be considered. Further work is in progress to test this hypothesis.
Alloimmunization
Blood transfusion and pregnancy are both established risk factors for alloimmunization, resulting in antibody formation against red cell, platelet or granulocyte antigens in the transfusion recipient or parous female respectively. The role of TA-MC in development of alloimmunization is not yet known, but is plausible. Alloimmunization adversely affects both the eligibility for transplantation as well as the ability to procure compatible blood for transfusion, particularly in chronically transfused patients. This association is also under study both with respect to WBC and RBC alloimmunization.
Consideration for Patient Typing
TA-MC may hinder resolution of complex immunohematology work-ups of the patients that have been multiply transfused or have a positive direct antiglobulin test.[147] Conventional techniques used to establish red cell phenotyping, e.g. reticulocyte separation, are complex, time-intensive and occasionally still fail to provide resolution. Patient red cell genotyping is an emerging technology to help predict antibodies that the patient could make through generation of a genotype-informed phenotype. TA-MC, whether transient or durable, can confound results both through the laboratory’s unintended serological or geno-typing of a minor population.
POSSIBLE BENEFICIAL EFFECTS OF MC
A “repair hypothesis” proposes that chimeric cells with pluripotent progenitor function can develop into parenchymal cells in response to tissue injury.[148] Fetal progenitor cells have the ability for multi-lineage differentiation with subsequent recruitment and rejuvenation following tissue injury.[4, 22] Fetal chimeric cells have been shown to be significantly more common in organs of women with certain disease processes (such as SLE) with related injury, compared to both healthy controls (p<0.001) and to the organs from women with SLE without injury (p=0.036).[148] A problem shared with the autoimmune-MC hypothesis, however, is the inability to establish causality (the chicken / egg paradigm). Although chimeric cells may serve in a reparative capacity, an alternative hypothesis is that of global cellular recruitment in the setting of inflammation in affected organs that might include non-selective recruitment of resident chimeric cells.[149]
Finally, the search for improved methods of antenatal diagnosis provided the impetus that chanced upon MC. Prenatal genetic diagnosis has relied on invasive sampling e.g. chorionic villus biopsy and amniocentesis, which incurs risk of fetal loss. If chimeric fetal cells or free DNA were reliably detectable in the peripheral maternal circulation, this would bypass the need for high-risk sampling. [14, 150] Bianchi et al. evaluated the ability to detect MC and aneuploidy in women carrying singleton male fetuses using a blinded FISH study. They found at least one cell with an X and Y signal in 41.4% of cases. The detection rate of at least one aneuploid cell in cases of fetal aneuploidy was 74.4% with a false-positive rate estimated to be between 0.6% and 4.1%.[150] Although, comparable to single marker prenatal serum screening, further work is needed if this is to replace or compliment existing strategies of antenatal diagnosis.[121, 150]
FUTURE STUDIES ON TA-MC
Research on TA-MC has been constrained by challenges inherent to tracing large numbers of transfused subjects for prolonged periods, the high costs of laboratory evaluation and the lack of expertise and funding given a relatively esoteric field. Ongoing areas of study include populations at risk, primarily focused on patients that share overlap with the trauma population, clinical outcomes and mechanisms.
The clinical significance and potential utility of MC remains a major interest. While unrelated to TA-MC, ongoing studies are investigating the role of FM-MC in autoimmune disease. Transfusion medicine experts can play an active role in MC research given the ability to access population and pregnancy data, necessary for these studies. For example, through linkage of blood donors with known repositories (e.g. Recipient Epidemiology and Donor Evaluation Studies), one can determine rates of MC in women with different autoimmune diseases such as SLE, RA and MS and compare these with healthy female donors matched for parity and maternal-fetal HLA compatibility. Germane to transfusion is the relationship between FM-MC and alloimmunization, and whether MC influences the development and persistence of RBC and platelet alloantibodies among parous female donors. This can be extended to similar allo-responsiveness in transfusion recipients with an evaluation of TA-MC.
The mechanisms driving TA-MC have yet to be fully elucidated. While the immune changes following trauma and blood transfusion may enable MC to occur, only a subset of transfused trauma patients develop durable TA-MC, suggesting that trauma and transfusion are necessary, but not sufficient for the development of TA-MC. Further work is needed to determine whether other factors such as extent of donor/recipient HLA matching, injury severity and variability of transfused products contribute to TA-MC, and specifically, whether they influence the type of immune response.
The mechanisms enabling transfused donor cells to evade the host immune response are still unknown. Short-term term TA-MC may be explained by the transient immune disarray following trauma; however, it is less clear how the chimeric cells continue to survive (years to decades), well after the host immune system has been restored. One explanation is that of long-term peripheral tolerance to the chimeric population, either through deletion of reactive host cells, or by induction of anergy. Given the long-term persistence, the demonstration of multiple cell lineages and the increase in cell numbers in selected patients over time, the more widely accepted explanation is that of donor stem cell engraftment, with integration into the “self” repertoire, thereby imparting central tolerance to the donor. [26, 29, 34] However, persistence of tolerance-inducing donor cells could also result from long-term survival of differentiated cells.
Finally, the functionality of chimeric cells -specifically whether they are able to participate in the normal host immune response and tissue repair- is unknown. While chimeric cells do not appear to inflict damage, further work is necessary to determine whether subtle adverse or beneficial effects are being missed. Alternatively, the chimeric cells may be anergic, rendering them incapable of any functional effect.
CONCLUSION
Chimerism research still remains at a foundational level, and further work is needed to elevate this beyond scientific curiosity. FM-MC has gained some traction with compelling hypotheses linked to autoimmune disease and repair, and clinical application to antenatal diagnostics. The mechanisms that enable FM-MC to occur also hold wide-ranging implications for immunology and tolerance. In contrast, TA-MC has received comparatively less interest with studies largely confined to delineating the clinical settings in which it occurs, and the factors that predict TA-MC. To date, durable, high-level TA-MC appears confined to patients transfused following severe traumatic injury, explained in part by the characteristic immune milieu potentiated by injury. The limited clinical consequences of TA-MC observed to date should not detract from the potential importance of TA-MC, given unexplored yet plausible links to GvHD and alloimmunization. Sighting of the mythological chimera was once considered ominous of disaster; only through improved understanding of this phenomenon can we ensure that this does not resonate with reality.
Acknowledgments
The authors wish to thank Ms. Leilani Montalvo of Blood Systems Research Institute for her kind assistance with figure 1. This work was supported by NIH RO1 HL-083388-01A1.
Footnotes
DISCLOSURE: The authors have no conflicts of interest or other financial involvement to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelson JL. Microchimerism in human health and disease. Autoimmunity. 2003;36:5–9. doi: 10.1080/0891693031000067304. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi DW. Fetal cells in the mother: from genetic diagnosis to diseases associated with fetal cell microchimerism. Eur J Obstet Gynecol Reprod Biol. 2000;92:103–8. doi: 10.1016/s0301-2115(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi DW. Fetomaternal cell trafficking: a new cause of disease? Am J Med Genet. 2000;91:22–8. doi: 10.1002/(sici)1096-8628(20000306)91:1<22::aid-ajmg4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. Jama. 2004;292:75–80. doi: 10.1001/jama.292.1.75. [DOI] [PubMed] [Google Scholar]
- 5.Johnson KL, Samura O, Nelson JL, McDonnell MdWM, Bianchi DW. Significant fetal cell microchimerism in a nontransfused woman with hepatitis C: Evidence of long-term survival and expansion. Hepatology. 2002;36:1295–7. doi: 10.1053/jhep.2002.35622. [DOI] [PubMed] [Google Scholar]
- 6.Vervoordeldonk SF, Doumaid K, Remmerswaal EB, ten Berge IJ, Wilmink JM, de Waal LP, et al. Long-term detection of microchimaerism in peripheral blood after pretransplantation blood transfusion. Br J Haematol. 1998;102:1004–9. doi: 10.1046/j.1365-2141.1998.00862.x. [DOI] [PubMed] [Google Scholar]
- 7.Lapierre V, Auperin A, Robinet E, Ferrand C, Oubouzar N, Tramalloni D, et al. Immune modulation and microchimerism after unmodified versus leukoreduced allogeneic red blood cell transfusion in cancer patients: results of a randomized study. Transfusion. 2007;47:1691–9. doi: 10.1111/j.1537-2995.2007.01344.x. [DOI] [PubMed] [Google Scholar]
- 8.Willer CJ, Sadovnick AD, Ebers GC. Microchimerism in autoimmunity and transplantation: potential relevance to multiple sclerosis. J Neuroimmunol. 2002;126:126–33. doi: 10.1016/s0165-5728(02)00048-6. [DOI] [PubMed] [Google Scholar]
- 9.Rust DW, Bianchi DW. Microchimerism in endocrine pathology. Endocr Pathol. 2009;20:11–6. doi: 10.1007/s12022-009-9064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Utter GH, Lee TH, Rivers RM, Montalvo L, Wen L, Chafets DM, et al. Microchimerism decades after transfusion among combat-injured US veterans from the Vietnam, Korean, and World War II conflicts. Transfusion. 2008;48:1609–15. doi: 10.1111/j.1537-2995.2008.01758.x. [DOI] [PubMed] [Google Scholar]
- 11.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–5. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Demetris AJ, Murase N, Trucco M, Thomson AW, Rao AS, et al. Chimerism after organ transplantation. Curr Opin Nephrol Hypertens. 1997;6:292–8. doi: 10.1097/00041552-199705000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ariga H, Ohto H, Busch MP, Imamura S, Watson R, Reed W, et al. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: implications for noninvasive prenatal diagnosis. Transfusion. 2001;41:1524–30. doi: 10.1046/j.1537-2995.2001.41121524.x. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi DW, Robert E. Gross Lecture Fetomaternal cell trafficking: a story that begins with prenatal diagnosis and may end with stem cell therapy. J Pediatr Surg. 2007;42:12–8. doi: 10.1016/j.jpedsurg.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 15.Owen R. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;28:400–1. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 16.van Dijk BA, Boomsma DI, de Man AJ. Blood group chimerism in human multiple births is not rare. Am J Med Genet. 1996;61:264–8. doi: 10.1002/(SICI)1096-8628(19960122)61:3<264::AID-AJMG11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Yan Z, Lambert NC, Guthrie KA, Porter AJ, Loubiere LS, Madeleine MM, et al. Male microchimerism in women without sons: quantitative assessment and correlation with pregnancy history. Am J Med. 2005;118:899–906. doi: 10.1016/j.amjmed.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 18.Bloch EM, Reed WF, Lee TH, Montalvo L, Shiboski S, Custer B, et al. Male microchimerism in peripheral blood leukocytes from women with multiple sclerosis. Chimerism; 2:6–10. doi: 10.4161/chim.2.1.15151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert NC, Pang JM, Yan Z, Erickson TD, Stevens AM, Furst DE, et al. Male microchimerism in women with systemic sclerosis and healthy women who have never given birth to a son. Ann Rheum Dis. 2005;64:845–8. doi: 10.1136/ard.2004.029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Chen C, Wang L, Chen D, Guang W, French J. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577–84. doi: 10.1016/s0015-0282(02)04694-0. [DOI] [PubMed] [Google Scholar]
- 21.de Bellefon LM, Heiman P, Kanaan SB, Azzouz DF, Rak JM, Martin M, et al. Cells from a vanished twin as a source of microchimerism 40 years later. Chimerism; 1:56–60. doi: 10.4161/chim.1.2.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khosrotehrani K, Bianchi DW. Multi-lineage potential of fetal cells in maternal tissue: a legacy in reverse. J Cell Sci. 2005;118:1559–63. doi: 10.1242/jcs.02332. [DOI] [PubMed] [Google Scholar]
- 23.Gammill HS, Guthrie KA, Aydelotte TM, Waldorf KM, Nelson JL. Effect of parity on fetal and maternal microchimerism: interaction of grafts within a host? Blood. 116:2706–12. doi: 10.1182/blood-2010-02-270942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schechter GP, Whang-Peng J, McFarland W. Circulation of donor lymphocytes after blood transfusion in man. Blood. 1977;49:651–6. [PubMed] [Google Scholar]
- 25.Adams PT, Davenport RD, Reardon DA, Roth MS. Detection of circulating donor white blood cells in patients receiving multiple transfusions. Blood. 1992;80:551–5. [PubMed] [Google Scholar]
- 26.Lee TH, Paglieroni T, Ohto H, Holland PV, Busch MP. Survival of donor leukocyte subpopulations in immunocompetent transfusion recipients: frequent long-term microchimerism in severe trauma patients. Blood. 1999;93:3127–39. [PubMed] [Google Scholar]
- 27.Dunne JR, Lee TH, Burns C, Cardo LJ, Curry K, Busch MP. Transfusion-associated microchimerism in combat casualties. J Trauma. 2008;64:S92–7. doi: 10.1097/TA.0b013e318160a590. discussion S7–8. [DOI] [PubMed] [Google Scholar]
- 28.Reed W, Lee TH, Norris PJ, Utter GH, Busch MP. Transfusion-associated microchimerism: a new complication of blood transfusions in severely injured patients. Semin Hematol. 2007;44:24–31. doi: 10.1053/j.seminhematol.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Utter GH, Owings JT, Lee TH, Paglieroni TG, Reed WF, Gosselin RC, et al. Microchimerism in transfused trauma patients is associated with diminished donor-specific lymphocyte response. J Trauma. 2005;58:925–31. doi: 10.1097/01.ta.0000162142.72817.5c. discussion 31–2. [DOI] [PubMed] [Google Scholar]
- 30.Utter GH, Owings JT, Lee TH, Paglieroni TG, Reed WF, Gosselin RC, et al. Blood transfusion is associated with donor leukocyte microchimerism in trauma patients. J Trauma. 2004;57:702–7. doi: 10.1097/01.ta.0000140666.15972.37. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 31.Utter GH, Nathens AB, Lee TH, Reed W, Owings JT, Nester T, et al. Leukoreduction of blood transfusions does not diminish transfusion-associated microchimerism in transfused trauma patients. Transfusion. 2006;46:1863–9. doi: 10.1111/j.1537-2995.2006.00991.x. [DOI] [PubMed] [Google Scholar]
- 32.Kruskall MS, Lee TH, Assmann SF, Laycock M, Kalish LA, Lederman MM, et al. Survival of transfused donor white blood cells in HIV-infected recipients. Blood. 2001;98:272–9. doi: 10.1182/blood.v98.2.272. [DOI] [PubMed] [Google Scholar]
- 33.Reed W, Lee TH, Vichinsky EP, Lubin BH, Busch MP. Sample suitability for the detection of minor white cell populations (microchimerism) by polymerase chain reaction. Transfusion. 1998;38:1041–5. doi: 10.1046/j.1537-2995.1998.38111299056314.x. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez R, Lee TH, Wen L, Montalvo L, Schechterly C, Colvin C, et al. Absence of transfusion-associated microchimerism in pediatric and adult recipients of leukoreduced and gamma-irradiated blood components. Transfusion. 2011;52:936–45. doi: 10.1111/j.1537-2995.2011.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bloch EM, Busch MP, Lee T-H, Montalvo L, Bird A, Mathews Y, et al. Microchimerism in the Transfused Obstetric Population (Abstract). 32nd International Congress of the ISBT 2012; [DOI] [PubMed] [Google Scholar]
- 36.Gill RM, Lee TH, Utter GH, Reed WF, Wen L, Chafets D, et al. The TNF (-308A) polymorphism is associated with microchimerism in transfused trauma patients. Blood. 2008;111:3880–3. doi: 10.1182/blood-2007-08-107144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens AM, McDonnell WM, Mullarkey ME, Pang JM, Leisenring W, Nelson JL. Liver biopsies from human females contain male hepatocytes in the absence of transplantation. Lab Invest. 2004;84:1603–9. doi: 10.1038/labinvest.3700193. [DOI] [PubMed] [Google Scholar]
- 38.Vietor HE, Hallensleben E, van Bree SP, van der Meer EM, Kaal SE, Bennebroek-Gravenhorst J, et al. Survival of donor cells 25 years after intrauterine transfusion. Blood. 2000;95:2709–14. [PubMed] [Google Scholar]
- 39.Flesland O, Ip LS, Storlien AS, Spurkland A, Larsen J, Solheim BG. Microchimerism in immune competent patients related to the leukocyte content of transfused red blood cell concentrates. Transfus Apher Sci. 2004;31:173–80. doi: 10.1016/j.transci.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Nathens AB, Nester TA, Rubenfeld GD, Nirula R, Gernsheimer TB. The effects of leukoreduced blood transfusion on infection risk following injury: a randomized controlled trial. Shock. 2006;26:342–7. doi: 10.1097/01.shk.0000228171.32587.a1. [DOI] [PubMed] [Google Scholar]
- 41.Lee TH, Paglieroni T, Utter GH, Chafets D, Gosselin RC, Reed W, et al. High-level long-term white blood cell microchimerism after transfusion of leukoreduced blood components to patients resuscitated after severe traumatic injury. Transfusion. 2005;45:1280–90. doi: 10.1111/j.1537-2995.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 42.Foex BA, Lamb WR, Roberts TE, Brear SG, Macartney I, Hammer M, et al. Early cytokine response to multiple injury. Injury. 1993;24:373–6. doi: 10.1016/0020-1383(93)90098-q. [DOI] [PubMed] [Google Scholar]
- 43.Mimasaka S, Funayama M, Hashiyada M, Nata M, Tsunenari S. Significance of levels of IL-6 and IL-8 after trauma: a study of 11 cytokines post-mortem using multiplex immunoassay. Injury. 2007;38:1047–51. doi: 10.1016/j.injury.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 44.Partrick DA, Moore FA, Moore EE, Biffl WL, Sauaia A, Barnett CC, Jr, Jack A. Barney Resident Research Award winner. The inflammatory profile of interleukin-6, interleukin-8, and soluble intercellular adhesion molecule-1 in postinjury multiple organ failure. American journal of surgery. 1996;172:425–9. doi: 10.1016/s0002-9610(96)00252-8. discussed 9–31. [DOI] [PubMed] [Google Scholar]
- 45.Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25:1813–9. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 46.Endo S, Inada K, Yamada Y, Takakuwa T, Kasai T, Nakae H, et al. Plasma endotoxin and cytokine concentrations in patients with hemorrhagic shock. Crit Care Med. 1994;22:949–55. doi: 10.1097/00003246-199406000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Ferguson KL, Taheri P, Rodriguez J, Tonapi V, Cardellio A, Dechert R. Tumor necrosis factor activity increases in the early response to trauma. Acad Emerg Med. 1997;4:1035–40. doi: 10.1111/j.1553-2712.1997.tb03676.x. [DOI] [PubMed] [Google Scholar]
- 48.Hoch RC, Rodriguez R, Manning T, Bishop M, Mead P, Shoemaker WC, et al. Effects of accidental trauma on cytokine and endotoxin production. Crit Care Med. 1993;21:839–45. doi: 10.1097/00003246-199306000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647–64. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giannoudis PV, Smith RM, Perry SL, Windsor AJ, Dickson RA, Bellamy MC. Immediate IL-10 expression following major orthopaedic trauma: relationship to anti-inflammatory response and subsequent development of sepsis. Intensive Care Med. 2000;26:1076–81. doi: 10.1007/s001340051320. [DOI] [PubMed] [Google Scholar]
- 51.Rabinovici R, John R, Esser KM, Vernick J, Feuerstein G. Serum tumor necrosis factor-alpha profile in trauma patients. J Trauma. 1993;35:698–702. doi: 10.1097/00005373-199311000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Roumen RM, Hendriks T, van der Ven-Jongekrijg J, Nieuwenhuijzen GA, Sauerwein RW, van der Meer JW, et al. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993;218:769–76. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seekamp A, Jochum M, Ziegler M, van Griensven M, Martin M, Regel G. Cytokines and adhesion molecules in elective and accidental trauma-related ischemia/reperfusion. J Trauma. 1998;44:874–82. doi: 10.1097/00005373-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 54.Sherry RM, Cue JI, Goddard JK, Parramore JB, DiPiro JT. Interleukin-10 is associated with the development of sepsis in trauma patients. J Trauma. 1996;40:613–6. doi: 10.1097/00005373-199604000-00016. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 55.DiPiro JT, Howdieshell TR, Goddard JK, Callaway DB, Hamilton RG, Mansberger AR., Jr Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130:1159–62. doi: 10.1001/archsurg.1995.01430110017004. discussion 62–3. [DOI] [PubMed] [Google Scholar]
- 56.DiPiro JT, Hamilton RG, Howdieshell TR, Adkinson NF, Jr, Mansberger AR., Jr Total IgE in plasma is elevated after traumatic injury and is associated with sepsis syndrome. Ann Surg. 1992;215:460–5. doi: 10.1097/00000658-199205000-00008. discussion 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bjornson AB, Altemeier WA, Bjornson HS, Tang T, Iserson ML. Host defense against opportunist microorganisms following trauma. I. Studies to determine the association between changes in humoral components of host defense and septicemia in burned patients. Ann Surg. 1978;188:93–101. doi: 10.1097/00000658-197807000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faist E, Kupper TS, Baker CC, Chaudry IH, Dwyer J, Baue AE. Depression of cellular immunity after major injury. Its association with posttraumatic complications and its reversal with immunomodulation. Arch Surg. 1986;121:1000–5. doi: 10.1001/archsurg.1986.01400090026004. [DOI] [PubMed] [Google Scholar]
- 59.O’Mahony JB, Palder SB, Wood JJ, McIrvine A, Rodrick ML, Demling RH, et al. Depression of cellular immunity after multiple trauma in the absence of sepsis. J Trauma. 1984;24:869–75. doi: 10.1097/00005373-198410000-00001. [DOI] [PubMed] [Google Scholar]
- 60.Keane RM, Birmingham W, Shatney CM, Winchurch RA, Munster AM. Prediction of sepsis in the multitraumatic patient by assays of lymphocyte responsiveness. Surgery, gynecology & obstetrics. 1983;156:163–7. [PubMed] [Google Scholar]
- 61.Livingston DH, Appel SH, Wellhausen SR, Sonnenfeld G, Polk HC., Jr Depressed interferon gamma production and monocyte HLA-DR expression after severe injury. Arch Surg. 1988;123:1309–12. doi: 10.1001/archsurg.1988.01400350023002. [DOI] [PubMed] [Google Scholar]
- 62.Szabo G, Kodys K, Miller-Graziano CL. Elevated monocyte interleukin-6 (IL-6) production in immunosppressed trauma patients. II. Downregulation by IL-4. J Clin Immunol. 1991;11:336–44. doi: 10.1007/BF00918799. [DOI] [PubMed] [Google Scholar]
- 63.Faist E, Schinkel C, Zimmer S, Kremer JP, Von Donnersmarck GH, Schildberg FW. Inadequate interleukin-2 synthesis and interleukin-2 messenger expression following thermal and mechanical trauma in humans is caused by defective transmembrane signalling. J Trauma. 1993;34:846–53. doi: 10.1097/00005373-199306000-00016. discussion 53–4. [DOI] [PubMed] [Google Scholar]
- 64.Lyons A, Kelly JL, Rodrick ML, Mannick JA, Lederer JA. Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection. Ann Surg. 1997;226:450–8. doi: 10.1097/00000658-199710000-00006. discussion 8–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacConmara MP, Maung AA, Fujimi S, McKenna AM, Delisle A, Lapchak PH, et al. Increased CD4+ CD25+ T regulatory cell activity in trauma patients depresses protective Th1 immunity. Ann Surg. 2006;244:514–23. doi: 10.1097/01.sla.0000239031.06906.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–90. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murphy TJ, Ni Choileain N, Zang Y, Mannick JA, Lederer JA. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol. 2005;174:2957–63. doi: 10.4049/jimmunol.174.5.2957. [DOI] [PubMed] [Google Scholar]
- 68.Kelly JL, Lyons A, Soberg CC, Mannick JA, Lederer JA. Anti-interleukin-10 antibody restores burn-induced defects in T-cell function. Surgery. 1997;122:146–52. doi: 10.1016/s0039-6060(97)90003-9. [DOI] [PubMed] [Google Scholar]
- 69.Ni Choileain N, MacConmara M, Zang Y, Murphy TJ, Mannick JA, Lederer JA. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol. 2006;176:225–36. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- 70.Hsieh CH, Frink M, Hsieh YC, Kan WH, Hsu JT, Schwacha MG, et al. The role of MIP-1 alpha in the development of systemic inflammatory response and organ injury following trauma hemorrhage. J Immunol. 2008;181:2806–12. doi: 10.4049/jimmunol.181.4.2806. [DOI] [PubMed] [Google Scholar]
- 71.Kawasaki T, Fujimi S, Lederer JA, Hubbard WJ, Choudhry MA, Schwacha MG, et al. Trauma-hemorrhage induces depressed splenic dendritic cell functions in mice. J Immunol. 2006;177:4514–20. doi: 10.4049/jimmunol.177.7.4514. [DOI] [PubMed] [Google Scholar]
- 72.Kobbe P, Vodovotz Y, Kaczorowski DJ, Mollen KP, Billiar TR, Pape HC. Patterns of cytokine release and evolution of remote organ dysfunction after bilateral femur fracture. Shock. 2008;30:43–7. doi: 10.1097/SHK.0b013e31815d190b. [DOI] [PubMed] [Google Scholar]
- 73.Mack VE, McCarter MD, Naama HA, Calvano SE, Daly JM. Dominance of T-helper 2-type cytokines after severe injury. Arch Surg. 1996;131:1303–8. doi: 10.1001/archsurg.1996.01430240057007. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 74.Kang SC, Matsutani T, Choudhry MA, Schwacha MG, Rue LW, Bland KI, et al. Are the immune responses different in middle-aged and young mice following bone fracture, tissue trauma and hemorrhage? Cytokine. 2004;26:223–30. doi: 10.1016/j.cyto.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 75.Wichmann MW, Ayala A, Chaudry IH. Severe depression of host immune functions following closed-bone fracture, soft-tissue trauma, and hemorrhagic shock. Crit Care Med. 1998;26:1372–8. doi: 10.1097/00003246-199808000-00024. [DOI] [PubMed] [Google Scholar]
- 76.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. International immunology. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 77.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO reports. 2004;5:825–30. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roumen RM, Hendriks T, Wevers RA, Goris JA. Intestinal permeability after severe trauma and hemorrhagic shock is increased without relation to septic complications. Arch Surg. 1993;128:453–7. doi: 10.1001/archsurg.1993.01420160095016. [DOI] [PubMed] [Google Scholar]
- 80.Rush BF, Jr, Sori AJ, Murphy TF, Smith S, Flanagan JJ, Jr, Machiedo GW. Endotoxemia and bacteremia during hemorrhagic shock. The link between trauma and sepsis? Ann Surg. 1988;207:549–54. doi: 10.1097/00000658-198805000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abraham E, Chang YH. Cellular and humoral bases of hemorrhage-induced depression of lymphocyte function. Crit Care Med. 1986;14:81–6. doi: 10.1097/00003246-198602000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Abraham E, Freitas AA. Hemorrhage produces abnormalities in lymphocyte function and lymphokine generation. J Immunol. 1989;142:899–906. [PubMed] [Google Scholar]
- 83.Stephan RN, Kupper TS, Geha AS, Baue AE, Chaudry IH. Hemorrhage without tissue trauma produces immunosuppression and enhances susceptibility to sepsis. Arch Surg. 1987;122:62–8. doi: 10.1001/archsurg.1987.01400130068010. [DOI] [PubMed] [Google Scholar]
- 84.van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Invest. 1996;97:713–9. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woiciechowsky C, Asadullah K, Nestler D, Eberhardt B, Platzer C, Schoning B, et al. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat Med. 1998;4:808–13. doi: 10.1038/nm0798-808. [DOI] [PubMed] [Google Scholar]
- 86.Hasko G, Szabo C, Nemeth ZH, Deitch EA. Dopamine suppresses IL-12 p40 production by lipopolysaccharide-stimulated macrophages via a beta-adrenoceptor-mediated mechanism. J Neuroimmunol. 2002;122:34–9. doi: 10.1016/s0165-5728(01)00459-3. [DOI] [PubMed] [Google Scholar]
- 87.Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annual review of pharmacology and toxicology. 1995;35:417–48. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- 88.Severn A, Rapson NT, Hunter CA, Liew FY. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J Immunol. 1992;148:3441–5. [PubMed] [Google Scholar]
- 89.Barton RN, Cocks RA, Doyle MO, Chambers H. Time course of the early pituitary-adrenal and metabolic responses to accidental injury. J Trauma. 1995;39:888–94. doi: 10.1097/00005373-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 90.Barton RN, Stoner HB, Watson SM. Relationships among plasma cortisol, adrenocorticotrophin, and severity of injury in recently injured patients. J Trauma. 1987;27:384–92. doi: 10.1097/00005373-198704000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Schrader KA. Stress and immunity after traumatic injury: the mind-body link. AACN clinical issues. 1996;7:351–8. doi: 10.1097/00044067-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 92.Stoner HB, Frayn KN, Barton RN, Threlfall CJ, Little RA. The relationships between plasma substrates and hormones and the severity of injury in 277 recently injured patients. Clin Sci (Lond) 1979;56:563–73. doi: 10.1042/cs0560563. [DOI] [PubMed] [Google Scholar]
- 93.Woolf PD. Hormonal responses to trauma. Crit Care Med. 1992;20:216–26. doi: 10.1097/00003246-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 94.Morris JA, Jr, MacKenzie EJ, Damiano AM, Bass SM. Mortality in trauma patients: the interaction between host factors and severity. J Trauma. 1990;30:1476–82. [PubMed] [Google Scholar]
- 95.Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000;48:932–7. doi: 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 96.Schroder J, Kahlke V, Staubach KH, Zabel P, Stuber F. Gender differences in human sepsis. Arch Surg. 1998;133:1200–5. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 97.Wichmann MW, Inthorn D, Andress HJ, Schildberg FW. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med. 2000;26:167–72. doi: 10.1007/s001340050041. [DOI] [PubMed] [Google Scholar]
- 98.Wohltmann CD, Franklin GA, Boaz PW, Luchette FA, Kearney PA, Richardson JD, et al. A multicenter evaluation of whether gender dimorphism affects survival after trauma. American journal of surgery. 2001;181:297–300. doi: 10.1016/s0002-9610(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 99.Choudhry MA, Bland KI, Chaudry IH. Trauma and immune response--effect of gender differences. Injury. 2007;38:1382–91. doi: 10.1016/j.injury.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yokoyama Y, Schwacha MG, Samy TS, Bland KI, Chaudry IH. Gender dimorphism in immune responses following trauma and hemorrhage. Immunol Res. 2002;26:63–76. doi: 10.1385/ir:26:1-3:063. [DOI] [PubMed] [Google Scholar]
- 101.Opelz G, Vanrenterghem Y, Kirste G, Gray DW, Horsburgh T, Lachance JG, et al. Prospective evaluation of pretransplant blood transfusions in cadaver kidney recipients. Transplantation. 1997;63:964–7. doi: 10.1097/00007890-199704150-00010. [DOI] [PubMed] [Google Scholar]
- 102.Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. 1973;5:253–9. [PubMed] [Google Scholar]
- 103.Opelz G, Terasaki PI. Prolongation effect of blood transfusions on kidney graft survival. Transplantation. 1976;22:380–3. doi: 10.1097/00007890-197610000-00010. [DOI] [PubMed] [Google Scholar]
- 104.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–48. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 105.Okuno K, Ozaki M, Shigeoka H, Nakajima I, Nakamura K, Hirohata T, et al. Effect of packed red cell and whole blood transfusion on liver-associated immune function. American journal of surgery. 1994;168:340–4. doi: 10.1016/s0002-9610(05)80161-8. [DOI] [PubMed] [Google Scholar]
- 106.Pirenne J, Kitade H, Kawai M, Koshiba T, Van Damme B, Mathieu C, et al. Regulatory cells, TH1/TH2 unbalance, and antibody-induced chronic rejection in operational tolerance induced by donor-specific blood transfusion. Transplantation. 2005;79:S25–7. doi: 10.1097/01.tp.0000153295.51565.f1. [DOI] [PubMed] [Google Scholar]
- 107.Salvatierra O, Jr, Vincenti F, Amend W, Potter D, Iwaki Y, Opelz G, et al. Deliberate donor-specific blood transfusions prior to living related renal transplantation. A new approach. Ann Surg. 1980;192:543–52. doi: 10.1097/00000658-198010000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singal DP, Joseph S. Role of blood transfusions on the induction of antibodies against recognition sites on T lymphocytes in renal transplant patients. Hum Immunol. 1982;4:93–108. doi: 10.1016/0198-8859(82)90010-6. [DOI] [PubMed] [Google Scholar]
- 109.Burrows L, Tartter P, Aufses A. Increased recurrence rates in perioperatively transfused colorectal malignancy patients. Cancer Detect Prev. 1987;10:361–9. [PubMed] [Google Scholar]
- 110.Dutcher JP, Schiffer CA, Aisner J, Wiernik PH. Alloimmunization following platelet transfusion: the absence of a dose-response relationship. Blood. 1981;57:395–8. [PubMed] [Google Scholar]
- 111.Fauchet R, Genetet B, Gueguen M, Leguerrier A, Rioux C, Logeais Y. Transfusion therapy and HLA antibody response in patients undergoing open heart surgery. Transfusion. 1982;22:320–2. doi: 10.1046/j.1537-2995.1982.22482251219.x. [DOI] [PubMed] [Google Scholar]
- 112.Howard JE, Perkins HA. The natural history of alloimmunization to platelets. Transfusion. 1978;18:496–503. doi: 10.1046/j.1537-2995.1978.18478251250.x. [DOI] [PubMed] [Google Scholar]
- 113.Heddle NM, Soutar RL, O’Hoski PL, Singer J, McBride JA, Ali MA, et al. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol. 1995;91:1000–5. doi: 10.1111/j.1365-2141.1995.tb05425.x. [DOI] [PubMed] [Google Scholar]
- 114.Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The Trial to Reduce Alloimmunization to Platelets Study Group. N Engl J Med. 1997;337:1861–9. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 115.Desmarets M, Cadwell CM, Peterson KR, Neades R, Zimring JC. Minor histocompatibility antigens on transfused leukoreduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood. 2009;114:2315–22. doi: 10.1182/blood-2009-04-214387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kissmeyer-Nielsen F, Olsen S, Petersen VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2:662–5. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 117.Giannoudis PV. Current concepts of the inflammatory response after major trauma: an update. Injury. 2003;34:397–404. doi: 10.1016/s0020-1383(02)00416-3. [DOI] [PubMed] [Google Scholar]
- 118.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. Journal of the American College of Surgeons. 2001;193:237–44. doi: 10.1016/s1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 119.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–10. doi: 10.1097/00005373-199604000-00001. discussion 10–2. [DOI] [PubMed] [Google Scholar]
- 120.Jackman RP, Utter GH, Muench MO, Heitman JW, Munz MM, Jackman RW, et al. Distinct roles of trauma and transfusion in induction of immune modulation after injury. Transfusion. 2012 doi: 10.1111/j.1537-2995.2012.03618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klintschar M, Immel UD, Kehlen A, Schwaiger P, Mustafa T, Mannweiler S, et al. Fetal microchimerism in Hashimoto’s thyroiditis: a quantitative approach. Eur J Endocrinol. 2006;154:237–41. doi: 10.1530/eje.1.02080. [DOI] [PubMed] [Google Scholar]
- 122.Bianchi DW. From Michael to microarrays: 30 years of studying fetal cells and nucleic acids in maternal blood. Prenat Diagn. 2010;30:622–3. doi: 10.1002/pd.2512. [DOI] [PubMed] [Google Scholar]
- 123.Lee TH, Donegan E, Slichter S, Busch MP. Transient increase in circulating donor leukocytes after allogeneic transfusions in immunocompetent recipients compatible with donor cell proliferation. Blood. 1995;85:1207–14. [PubMed] [Google Scholar]
- 124.Lee T-H, Donegan E, Slichter S, Busch M. Transient increase in circulating donor leukocytes after allogeneic transfusion in immunocompetent recipients compatible with donor cell proliferation. Blood. 1995;85:1207–14. [PubMed] [Google Scholar]
- 125.Reed WF, Lee TL, Trachtenberg E, Vinson M, Busch MP. Detection of microchimerism by PCR is a function of amplification strategy. Transfusion. 2001;41:39–44. doi: 10.1046/j.1537-2995.2001.41010039.x. [DOI] [PubMed] [Google Scholar]
- 126.Reed W, Kong DZ, Lee TH, Cowan MJ, Busch MP, Baxter-Lowe LA. Non-invasive determination of the paternal HLA haplotype of a fetus using kinetic PCR to detect fetal microchimerism in maternal plasma. Bone Marrow Transplant. 2002;29:527–9. doi: 10.1038/sj.bmt.1703411. [DOI] [PubMed] [Google Scholar]
- 127.Lee TH, Reed W, Mangawang-Montalvo L, Watson J, Busch MP. Donor WBCs can persist and transiently mediate immunologic function in a murine transfusion model: effects of irradiation, storage, and histocompatibility. Transfusion. 2001;41:637–42. doi: 10.1046/j.1537-2995.2001.41050637.x. [DOI] [PubMed] [Google Scholar]
- 128.Carrier E, Lee TH, Busch MP, Cowan MJ. Induction of tolerance in nondefective mice after in utero transplantation of major histocompatibility complex-mismatched fetal hematopoietic stem cells. Blood. 1995;86:4681–90. [PubMed] [Google Scholar]
- 129.Carrier E, Lee TH, Busch MP, Cowan MJ. Recruitment of engrafted donor cells postnatally into the blood with cytokines after in utero transplantation in mice. Transplantation. 1997;64:627–33. doi: 10.1097/00007890-199708270-00014. [DOI] [PubMed] [Google Scholar]
- 130.Carrier E, Gilpin E, Lee TH, Busch MP, Zanetti M. Microchimerism does not induce tolerance after in utero transplantation and may lead to the development of alloreactivity. J Lab Clin Med. 2000;136:224–35. doi: 10.1067/mlc.2000.108942. [DOI] [PubMed] [Google Scholar]
- 131.Chou SH, Chawla A, Lee TH, Zhou Y, Busch MP, Balassanian R, et al. Increased engraftment and GVHD after in utero transplantation of MHC-mismatched bone marrow cells and CD80low, CD86(-) dendritic cells in a fetal mouse model. Transplantation. 2001;72:1768–76. doi: 10.1097/00007890-200112150-00010. [DOI] [PubMed] [Google Scholar]
- 132.Donahue J, Gilpin E, Lee TH, Busch MP, Croft M, Carrier E. Microchimerism does not induce tolerance and sustains immunity after in utero transplantation. Transplantation. 2001;71:359–68. doi: 10.1097/00007890-200102150-00004. [DOI] [PubMed] [Google Scholar]
- 133.Malinen E, Kassinen A, Rinttila T, Palva A. Comparison of real-time PCR with SYBR Green I or 5′-nuclease assays and dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantification of selected faecal bacteria. Microbiology. 2003;149:269–77. doi: 10.1099/mic.0.25975-0. [DOI] [PubMed] [Google Scholar]
- 134.Maeda H, Fujimoto C, Haruki Y, Maeda T, Kokeguchi S, Petelin M, et al. Quantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS Immunol Med Microbiol. 2003;39:81–6. doi: 10.1016/S0928-8244(03)00224-4. [DOI] [PubMed] [Google Scholar]
- 135.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- 136.Alizadeh M, Bernard M, Danic B, Dauriac C, Birebent B, Lapart C, et al. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood. 2002;99:4618–25. doi: 10.1182/blood.v99.12.4618. [DOI] [PubMed] [Google Scholar]
- 137.Lee T-H, Chafets D, Reed W, Wen L, Yang Y, Chen J, et al. Enhanced ascertainment of microchimerism with real-time quantitative PCR amplification of insertion/deletion polymorphisms. Transfusion. 2006;46:1870–8. doi: 10.1111/j.1537-2995.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- 138.Willer CJ, Herrera BM, Morrison KM, Sadovnick AD, Ebers GC. Association between microchimerism and multiple sclerosis in Canadian twins. J Neuroimmunol. 2006 doi: 10.1016/j.jneuroim.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 139.Aractingi S, Berkane N, Bertheau P, Le Goue C, Dausset J, Uzan S, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898–901. doi: 10.1016/S0140-6736(98)05121-6. [DOI] [PubMed] [Google Scholar]
- 140.Klintschar M, Schwaiger P, Mannweiler S, Regauer S, Kleiber M. Evidence of fetal microchimerism in Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2001;86:2494–8. doi: 10.1210/jcem.86.6.7540. [DOI] [PubMed] [Google Scholar]
- 141.Suskind DL, Rosenthal P, Heyman MB, Kong D, Magrane G, Baxter-Lowe LA, et al. Maternal microchimerism in the livers of patients with biliary atresia. BMC Gastroenterol. 2004;4:14. doi: 10.1186/1471-230X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hayashida M, Nishimoto Y, Matsuura T, Takahashi Y, Kohashi K, Souzaki R, et al. The evidence of maternal microchimerism in biliary atresia using fluorescent in situ hybridization. J Pediatr Surg. 2007;42:2097–101. doi: 10.1016/j.jpedsurg.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 143.Nelson JL. Maternal-fetal immunology and autoimmune disease: is some autoimmune disease auto-alloimmune or allo-autoimmune? Arthritis Rheum. 1996;39:191–4. doi: 10.1002/art.1780390203. [DOI] [PubMed] [Google Scholar]
- 144.Nelson JL. Microchimerism and autoimmune disease. N Engl J Med. 1998;338:1224–5. doi: 10.1056/NEJM199804233381711. [DOI] [PubMed] [Google Scholar]
- 145.Dwyre DM, Holland PV. Transfusion-associated graft-versus-host disease. Vox Sang. 2008;95:85–93. doi: 10.1111/j.1423-0410.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- 146.Sakurai M, Moizumi Y, Uchida S, Imai Y, Tabayashi K. Transfusion-associated graft-versus-host disease in immunocompetent patient: early diagnosis and therapy. Am J Hematol. 1998;58:84–6. doi: 10.1002/(sici)1096-8652(199805)58:1<84::aid-ajh17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 147.Reid ME, Rios M, Powell VI, Charles-Pierre D, Malavade V. DNA from blood samples can be used to genotype patients who have recently received a transfusion. Transfusion. 2000;40:48–53. doi: 10.1046/j.1537-2995.2000.40010048.x. [DOI] [PubMed] [Google Scholar]
- 148.Kremer Hovinga IC, Koopmans M, Baelde HJ, de Heer E, Bruijn JA, Bajema IM. Tissue chimerism in systemic lupus erythematosus is related to injury. Ann Rheum Dis. 2007;66:1568–73. doi: 10.1136/ard.2007.070516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kremer Hovinga IC, Koopmans M, de Heer E, Bruijn JA, Bajema IM. Chimerism in systemic lupus erythematosus--three hypotheses. Rheumatology (Oxford) 2007;46:200–8. doi: 10.1093/rheumatology/kel379. [DOI] [PubMed] [Google Scholar]
- 150.Bianchi DW, Simpson JL, Jackson LG, Elias S, Holzgreve W, Evans MI, et al. Fetal gender and aneuploidy detection using fetal cells in maternal blood: analysis of NIFTY I data. National Institute of Child Health and Development Fetal Cell Isolation Study. Prenat Diagn. 2002;22:609–15. doi: 10.1002/pd.347. [DOI] [PubMed] [Google Scholar]