Abstract
Persistent viral infection is often associated with dysfunctional immune responses against unrelated pathogens. Lymphocytic choriomeningitis virus (LCMV) can establish acute or chronic infections in mice and is widely used as a model for persistent virus infections in humans. Mice infected with LCMV develop a transient defect in antigen specific immunity against heterologous viral infection. Although it has been proposed that LCMV infection induces an immunosuppressed state within the host, our data show that infected mice successfully clear vaccinia virus through a mechanism that involves CD8+ T cell-derived IFNγ. This observation demonstrates that chronic LCMV infection does not impair protective immunity against heterologous viral challenge. Rather, a natural sterilizing immunity is induced following a primary infection that prevents a secondary infection. Our findings suggest a need to re-evaluate current thoughts about the immune suppression that might occur during a persistent infection.
Introduction
HIV and HCV-infected patients develop dysfunctional immune responses through mechanisms that remain unclear, but that may be related to the chronic nature of the diseases (1–8). Understanding how chronic viral infections disrupt immune system function is a major goal in the treatment of infectious diseases. Lymphocytic choriomeningitis virus (LCMV) is widely used to understand immune suppression and dysfunction during a persistent viral infection (9–18). Many studies have shown that chronic LCMV infection causes a transient defect in immune responses directed against subsequent infection replicating or non-replicating viruses (14, 19–22). As such, there has been intense interest in understanding the mechanisms of LCMV-induced immune suppression.
There are two LCMV clones that result in dramatically different infection outcomes in mice (12, 13). Whereas the Armstrong clone causes acute infection that resolves within 7 days, clone 13 causes a chronic infection in which virus replicates in some tissues for the life of the mouse. Comparison of the immune responses that develop against these LCMV clones has brought much insight to our understanding of immunological memory and immune dysfunction (12, 14, 23–26). Less well understood is how LCMV co-infections with other viruses affect the ability of the host to respond to either the primary or secondary viral challenge (14, 19, 21, 22). Most of the studies in this area have shown a decrease or lack of cell-mediated immunity against a second viral infection after a primary persistent LCMV infection. One report shows that neutralizing IFNα/β during Poly(I)(C) treatment neutralized the inhibition of a primary Vaccinia virus response (22). Alternatively, LCMV infection may dysregulate antigen presenting cell function to thwart immunity, against the second infection (19–21).
Given the central role of Type I and type II interferons in anti-viral immunity, LCMV infection has been widely used to study interferon responses in vivo (14, 27, 28). Type I interferon (IFN-I) include interferon beta and multiple subtypes of interferon alphas (29). These all bind to and signal through the same receptors (IFNAR1 and IFNAR2) (30). Studies with interferon receptor knockout mice demonstrate the importance of the interferon response following a virus infection in slowing virus replication and dissemination (31), and IFN has an important role in the activation of acquired immunity (reviewed in (32, 33)).
Like previous investigations, it was our goal to study the dynamics of co-infection between LCMV and vaccinia virus. We have found that persistent LCMV infection (clone 13) in mice transiently impairs an antigen specific immune response directed against a second pathogen. This transient immune suppression during LCMV also occurs in mice infected with the acute Armstrong strain of the virus. During this transient immune suppression the mice are surprisingly immune competent in being able to clear the second virus non-specifically. Our data clearly suggest a need to re-evaluate the natural in vivo antiviral state and what defines and drives immune suppression during a virus infection.
Materials and Methods
Mice and Infections
Female C57BL/6 from Charles River Laboratories (Wilmington, MA) and C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME) were used in these experiments. IFNAR mice were kindly provided by Mehrdad Matloubian. Charlie Kim provided the male perforin knockout mice. Mice were 6–8 weeks old at the time of primary infection. All mice were housed in specific-pathogen free conditions at the UCSF SFGH animal facility, and were used in accordance with university animal welfare guidelines (IACUC). LCMV Armstrong and LCMV Clone 13 were gifts from Dr. Rafi Ahmed and were propagated on BHK cells, aliquotted and stored at −80 C. Mice were infected with LCMV by tail vein injection of a volume of 200ul. Vaccinia Virus Western Reserve was a gift from Joshy Jacobs laboratory, Emory University. Vaccinia was propagated on Hela cells. Briefly, Helas cells were infected with Vaccinia at a low MOI, removed from flasks when they showed cytopathic effects, resuspended in PBS + 1 % FCS, freeze-thawed three times, centrifuged to remove debris, aliquotted and stored at −80 C. Mice were infected with Vaccinia by interperitoneal injection in a volume of 200 μl. Virus stocks were titered on Vero cells, as described below.
Viral Titers
Organs were harvested and frozen at −80 C in DMEM + 1% FCS. Organs were homogenized in a volume of 1 ml using a Polytron homogenizer (Kinematica). Samples were then serially diluted in DMEM/1% FCS, and 200 μl was added to confluent monolayers of Vero cells in 6-well plates. 60–90 minutes later the plates were overlaid with a 1:1 mixture of 2X Medium 199 (INVITROGEN) 1% Agarose (Lonza). Four days later the cells were overlaid with additional Medium 199/Agarose supplemented with Neutral Red dye. Plaques were read the next day. Vaccinia and LCMV plaques have clearly distinct morphology and were counted separately to calculate the plaque forming units per ovary. For quantitative PCR, viral DNA was isolated from individual ovaries using the Qiagen All-Prep kit. 10-fold dilutions of a plasmid standard containing the VACCINIA VIRUS-HA gene were used to generate a standard curve in each qPCR run. Invitrogen platinum SYBR green super mix was used. The qPCR primers for Vaccinia virus use are OPHA-F89 –GAT GAT GCA ACT CTA TCA TGT A and OPHA-R219 –GTA TAA TTA TCA AAA TAC CCG ACG TC, as described (34). Samples were run on an ABI Step One PCR machine.
Intracellular Cytokine Staining
Single cell suspensions of splenocytes were prepared by mashing the spleen through a 70 um strainer and washing with RPMI. Red blood cells were lysed using ACK Buffer (Sigma-Aldrich, St Louis, MO). For intracellular cytokine staining assays, splenocytes were resuspended in RPMI supplemented with 10% FCS (Hyclone) and stimulated with 5 μg of indicated peptides for 5 to 6 hours at 37 C in the presence of GolgiPlug (BD Biosciences) and then stored at 4 C overnight. Cells were then stained for surface markers for 15–30 minutes at room temperature, washed twice with FACS buffer (PBS + 2% FCS) and fixed with 1% formaldehyde in PBS. Samples were then washed, permeabilized (FACS Buffer + 0.1% Saponin), stained for 15–30 minutes with antibodies for intracellular antigens, washed twice with Permeabilization buffer, and fixed in 1% formaldehyde. Monomers (B8R) were by synthesized by the Microchemical Facility Core (Emory University, Atlanta, GA) and conjugated into tetramers using Biotin APC (Invitrogen). Samples were read on an LSRII flow cytometer (BD Biosciences) and results were analyzed using FlowJo software (TreeStar, Ashland, OR). For FACS plots, CD8+CD4-CD19-cells are shown.
Antibodies and peptides
In vivo depleting antibodies for CD4 depletion (clone GK1.5), CD8 depletion (clone 2.43), NK depletion (clone PK136) and IFN gamma neutralization (clone XMG1.2) were purchased from the UCSF Monoclonal Antibody Core; 250 μg of each of these antibodies were administered interperitoneal at the indicated times. The IFNα/β receptor blocking antibody (clone MAR1-5A3) was from Leinco Technologies, St Louis, MO). 2.5 mg/mouse was administered interperitoneal in order to block IFN-I signaling (Sheehan, 2006). Antibodies used in flow cytometry analysis were CD8 Pacific Blue and CD4 Alexa-700 (Invitrogen), IFNγ FITC (eBiosciences), IL2 APC (ebioscience). Anaspec Corp (Fremont, CA) synthesized peptides for the immunodominant Vaccinia epitope B8R (TSYKFESV) and LCMV GP34 (AVYNFATC).
Type I IFN bioassay
Type I interferon bioassay was used to measure IFN-I as described (35). The murine IFN standard (Biomedical Laboratories PBL, 12100-1) at a final concentration of 100U ml-1 was used as a control.
IFNγ ELISA
The IFNγ ELISA was purchased from Invitrogen, mouse IFNγ DuoSet (DY485E). The assay was carried out according to manufactures instructions.
Results
The kinetics of LCMV and vaccinia virus CD8 T cell responses in co-infected mice
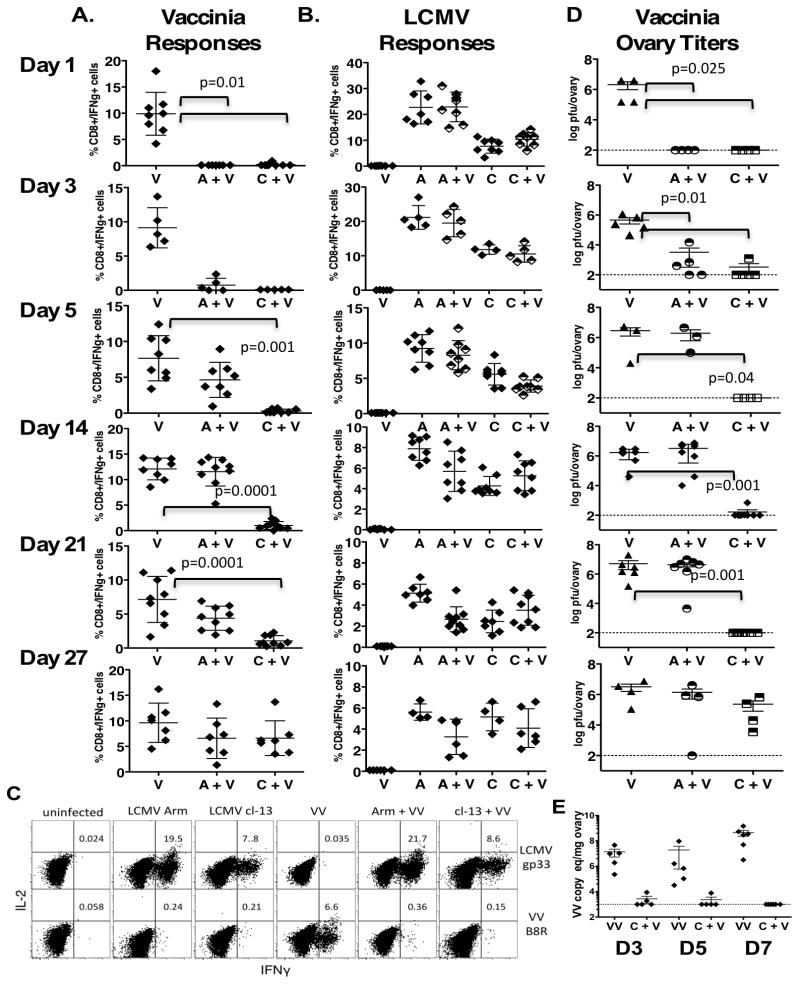
Mice infected intravenously with 2×106 pfu of either LCMV Armstrong (acute) or LCMV clone 13 (chronic) were co-infected at various times points with 1×106 pfu Vaccinia Virus (VV) intraperitoneally (i.p.). To study how the CD8+ T cell response to Vaccinia was altered at different stages of the underlying LCMV infections, mice were sacrificed at 6–7 days after secondary VV infection (Figure 1, experiment design). Splenocytes from the co-infected mice were harvested and cytokine production was measured after in vitro stimulation with an immunodominant CD8+ T cell epitope from Vaccinia, B8R (TSYKFESV) (Figure 2A) and LCMV gp33 peptide (Figure 2B). Co-infection with Vaccinia early after the primary LCMV infection (days 1 and 3) resulted in a total lack of T cell responses to VV, though mice had normal, robust anti-LCMV T cell responses (Figure 2A-B). Furthermore, mice infected with LCMV Clone 13 had undetectable or very low frequency VV CD8+ T cell responses during the first 21 days after LCMV infection. By 4 weeks, mice infected with LCMV Clone 13 could respond to the VV co-infection resulting in normal T cell responses to both viruses (Figure 2, representative dot plot Figure 2C).
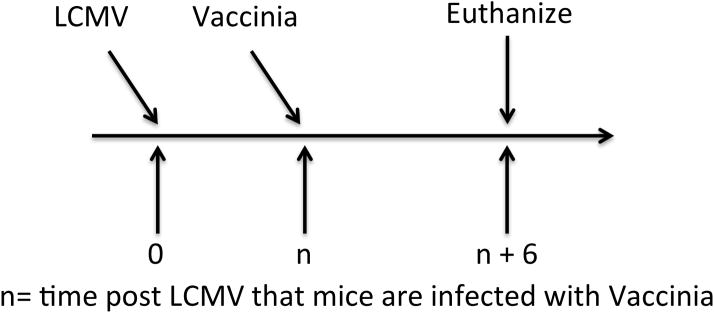
Figure 1. Experimental Design.
Naïve mice are infected i.v. with LCMV clone 13 or clone Armstrong (2×106 pfu). Mice are then are co-infected i.p. with Vaccinia virus (1×106 pfu) at different times (n). Mice are sacrificed 6 days following the secondary Vaccinia infection for analysis.
Figure 2. The kinetics of the non-specific antiviral state during LCMV infection in mice.
Mice are infected i.v. with LCMV clone 13 (C) or clone Armstrong (A) (2×106 pfu) at various time-points following i.p. infection with Vaccinia virus (V)(1×106). Virus-specific responses were evaluated 6 days following Vaccinia infection. In vitro peptide stimulation was used to determine (22)the percentage of CD8+ IFN-g+ cells in response to the Vaccinia B8R epitope A. and the LCMV gp34 epitope B C. A representative dot plot of cytokine production. D. A standard plaque assay or E. quantitative real time PCR measured vaccinia virus titers in the ovaries. The numbers (D3, D5 and D7) indicate the time following LCMV that mice were co-infected with Vaccinia virus. A total of 3 independent experiments with similar results were done. Error bars represent mean + SD. All statistical comparisons were made using the two tailed students t-Test.
Clearance of vaccinia virus occurs in LCMV infected mice that lack an antigen specific vaccinia immune response
With a lack of vaccinia specific T cell response during the first few weeks following the LCMV infection we measured VV replication in the co-infected mice. LCMV infected mice that were co-infected with vaccinia during the period of 1– 22 days post clone 13 infection clear the VV infection (Figure 2D). At 5 days, Armstrong infected mice become susceptible to VV co-infection (Figure 2D) and this correlates with a return in their ability to respond to the second infection immunologically (Figure 2A). By 4 weeks, clone 13 infected mice make immune responses to VV similar to VV infected control mice (Figure 2A) and harbor high levels of VV titers similar to control infected mice (Figure 2D). Immune responses against the primary LCMV infection were for the most part unaltered in the co-infected mice (Figure 2B). We did detect low levels of VV in the co-infected mice by PCR (Figure 2E) though all mice at these time points following VV co-infection were negative for VV replication as measured by a standard plaque assay (Figure 2D).
LCMV infected Type I Interferon Receptor Knockout Mice are resistant to Vaccinia Virus co-infection after 72 hours
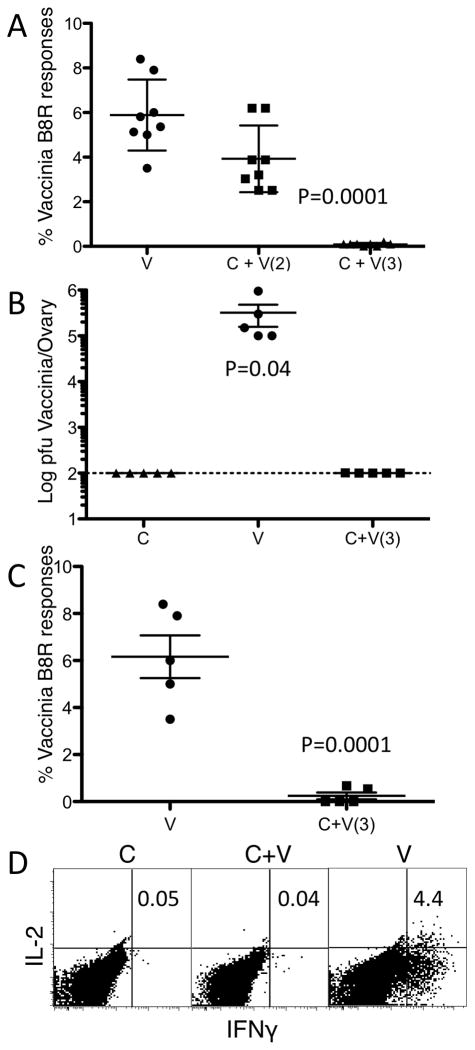
We hypothesized that IFN-1 was responsible for the resistance to VV co-infection during LCMV since type I IFN is known as an innate antiviral cytokine and VV is sensitive to IFN-1 (36, 37). To test this we infected IFN-1 receptor knockout mice (IFNAR) with clone 13 and co-infected them with VV. Importantly, IFNAR mice infected with 1×106 pfu of VV alone succumb to the infection. Therefore, the infectious dose of VV was reduced to 1×104 in order that the IFNAR mice would survive. We found that LCMV infected IFNAR mice were susceptible to VV during the first 2 days following a primary LCMV infection (Figure 3A). Surprisingly, when the clone 13 infected IFNAR mice are co-infected on day 3, the mice are able to clear the VV infection (Fig 3B) without mounting an anti-VV immune response (Fig 3A and 3D). Therefore, by 72 hours following a primary LCMV systemic virus infection IFN-1 signaling was not needed to prevent the secondary VV infection.
Figure 3. IFNAR mice can clear a secondary VACCINIA VIRUS infection non-specifically by 72 hours following a primary LCMV infection.
Type I IFN receptor knockout mice (IFNAR) were infected with LCMV clone 13 (c) and co-infected either on day 2 or 3 (the day is indicated in parentheses) with 1×104 pfu of Vaccinia virus (v). Vaccinia specific B8R responses were measured in the spleen 30 days following co-infection. A. % IFNγ+ CD8 cells. IFNAR mice were infected with LCMV clone 13 and infected with Vaccinia virus 3 days later. Mice were sacrificed 6 days following VACCINIA VIRUS infection and, B. VACCINIA VIRUS titers in the ovaries and VACCINIA VIRUS specific B8R responses were measured in the spleen, C. % IFNγ+ CD8 cells. D. Representative dot plot of B8R specific IFNγ producing CD8 cells. Data are representative of four to five mice per group and of two to three individual experiments with similar results. Error bars represent mean + SD. Statistics were analyzed by student two-tailed t-Test.
The interferon response during an LCMV infection
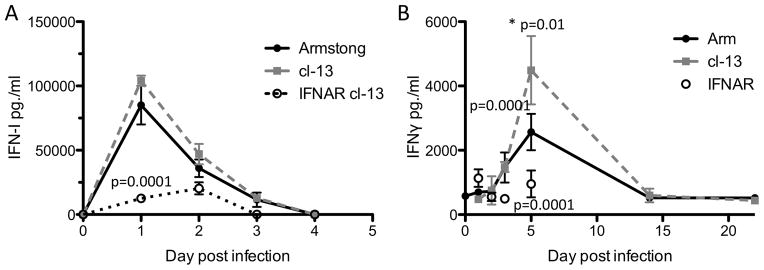
Since within 72 hours of an LCMV infection, IFNAR mice are capable of clearing VV nonspecifically (Figure 3C-D), we postulated that IFNγ might have a role. We measured the type I IFN and IFNγ responses during LCMV. Similar to published data (14, 38), we found that LCMV infected mice had a robust type I IFN response beginning on the first day following infection that slowly wanes over time (Figure 4A). The type I IFN response in IFNAR mice was significantly reduced on day 1 following an LCMV clone 13 infection (p=0.0001, student two tailed T-test and Figure 4A) and is not detected by 3 days of infection. Therefore, IFNAR mice can produce type I IFN but not respond to it. We could detect IFNγ responses also as early as day 3 (Figure 4B). By day 5 following infection there was a significant increase in IFNγ levels in mice infected with LCMV clone 13 as compared to LCMV Armstrong (Figure 4B, p=0.01 two tailed student t-Test). The levels of IFNγ were sustained in LCMV infected mice even in Armstrong infected mice that become susceptible to VV co-infection at 5 days following the primary LCVM infection (Figure 1). LCMV clone 13 infected IFNAR mice have a significant reduction in IFNγ production as compared to clone 13 infected WT mice on days 3 and 5 following infection (Figure 4B, p<0.0001 at both time points, IFNAR cl-13 infected mice as compared to WT cl-13 infected mice by non parametric two tailed student t-Test).
Figure 4. Type I and type II interferon production following LCMV infection.
WT mice are infected with LCMV clone 13 or Armstrong at 2×106 pfu i.v. n=5–10/ group. IFNAR mice were infected with LCMV clone 13 at 2×106 pfu i.v. n=5–10/group. WT mice were bled on 1, 2, 3, 5, 14 and 22 days following infection. IFNAR mice were bled out to 5 days post infection. A. Type I IFN responses were measured using a standard bioassay. B. IFNγ responses were measured by a standard ELISA. Day 1 IFN-I levels are significantly decreased in IFNAR mice as compared to all other groups. Day 5 WT LCMV clone 13 IFNγ levels are significantly increased as compared to day 5 Armstrong levels. Day 5 clone 13 infected IFNAR IFNγ levels are significantly decreased as compared to day 5 WT clone 13 IFNγ levels. Error bars represent mean + SD. Statistics performed using the two tailed students t-Test.
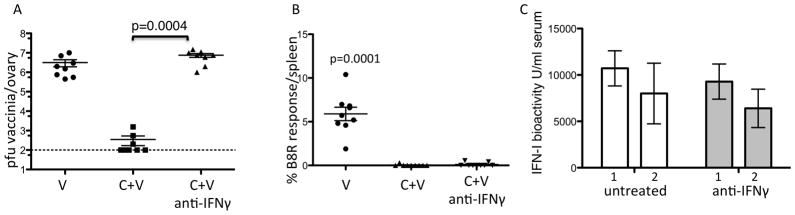
The nonspecific clearance of Vaccinia is dependent on IFNγ
Since LCMV infected IFNAR mice are susceptible to a VV co-infection during the first 2 days but resistant by day 3 and since we can detect IFNγ in the serum of LCMV infected mice on day 3 (Figure 4B) we postulated that IFNγ might have a role. In wild type mice, we administered an IFNγ neutralizing antibody at days 1 and 3 after LCMV, and infected with VV on day 3. Surprisingly, these mice were highly susceptible to Vaccinia infection, harboring very high titers of VV (Figure 5A, p=0.0004 two tailed student t-Test). These mice also do not have detectable Vaccinia-specific CD8+ T cells (Figure 5B, p=0.0001 two tailed student t-Test) suggesting that the transient immune suppression is independent of the in vivo antiviral state. The type I IFN response in these WT mice was intact (Figure 5C). In addition, if we bock IFNγ in LCMV infected IFNAR mice, the mice are susceptible to a day 3 VV co-infection (data not shown). Therefore, by 72 hours of an LCMV infection IFN-I was insufficient to render mice resistant to a secondary infection without IFNγ.
Figure 5. The nonspecific clearance of Vaccinia is dependent on IFNγ.
Wild type mice were infected with LCMV clone 13 at 2×106 pfu i.v. Mice were co-infected with Vaccinia on day 3. Six days after co-infection mice were sacrificed. A. Levels of Vaccinia in ovaries by standard plaque assay. B. % Vaccinia B8R IFNγ producing cells in the spleen. C. Day 1 and 2 post clone 13 infection IFN-I bioactivity as measured by the standard IFN-I bioassay white bars are untreated and grey bars are anti-IFNγ treated. Error bars represent mean + SD. Two independent experiments combined n=8–10/group. Statistics performed using the two tailed students t-Test.
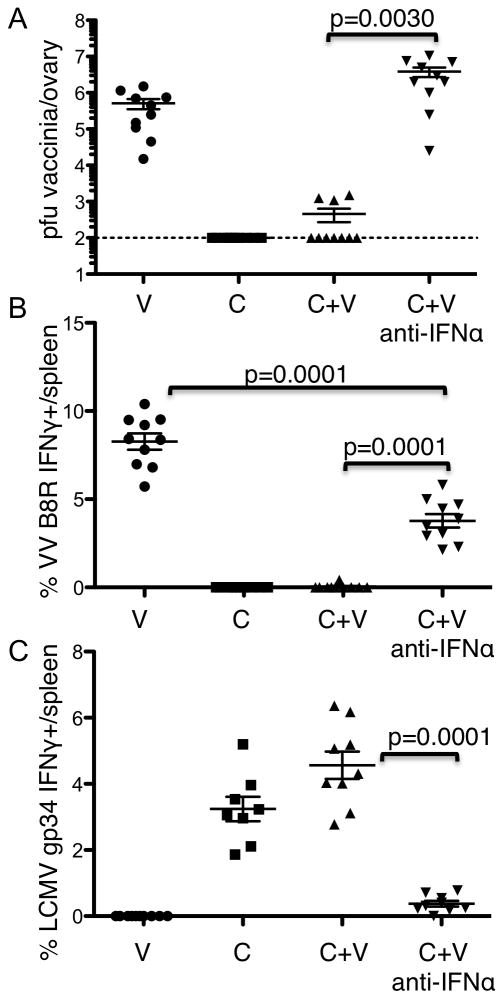
Type I IFN signaling is required for an IFNγ response capable of clearing high doses of VV in LCMV infected mice
Because the resistance of VV in IFNAR mice was with a lower dose of the virus we wanted to test infection with the high dose of VV by blocking the IFN signaling in WT mice. We blocked IFN-I signaling by administering 2.5mg of an IFN receptor-blocking antibody 1 day before infection of WT mice with LCMV clone 13. WT LCMV infected mice were then injected with Vaccinia at 1×106 pfu 3 days later, and sacrificed 6 days following VV. These results are consistent with our original hypothesis that IFN-I was required for the in vivo resistance to Vaccinia; these mice had high Vaccinia titers and normal frequency Vaccinia-specific CD8+ T cell responses at the time of sacrifice (Fig 6A and 6B respectively). However, it is known that type I IFN signaling is necessary for a robust CD8+ T cell response (39) and these anti-IFNAR treated mice have a significant reduction in their LCMV specific CD8 T cell responses (Figure 6C, p<0.0001 t-test). Therefore, we hypothesize that low levels of IFNγ produced in the absence of IFN-1 signaling can resist low dose VV infection (Figure 2). Whereas, IFN-1 signaling is required to generate an IFNγ response necessary to resist high dose VV (Figure 6) and this dose response effect has been suggested previously (21).
Figure 6. Type I IFN signaling is required for an IFNγ response capable of clearing high dose VACCINIA VIRUS infection.
WT mice were treated with 2.5mg of an anti-IFNAR antibody. One day later the mice were infected with LCMV clone 13 at 2×106 pfu i.v. Three days after the LCMV infection mice were co-infected with VACCINIA VIRUS. Six days following Vaccinia infection mice were analyzed. A. Vaccinia titers in the ovary. Splenocytes were harvested and antigen specific responses to B. Vaccinia B8R and. C. LCMV gp34 were measured. Two independent experiments were combined n=8–10/group. Error bars represent mean + SD. Statistics performed using the two tailed students t-Test.
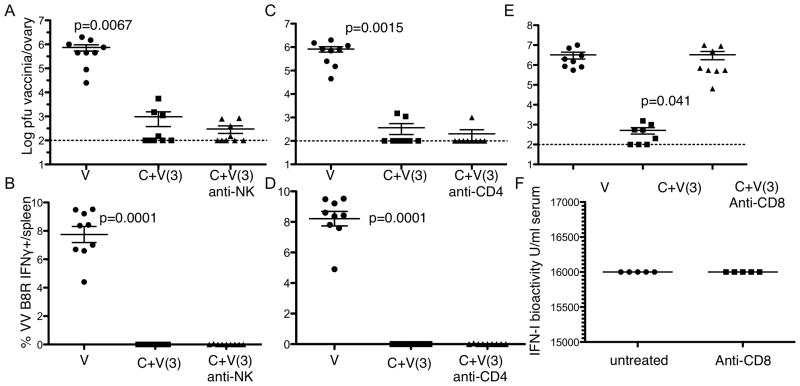
The in vivo antiviral state is dependent on CD8+ cells
Having observed that IFNγ is required for LCMV-induced resistance to Vaccinia infection, we next determined what particular subsets of lymphocytes mediated this resistance. Subsets of cells that produce IFNγ include NK, CD4+ and CD8+ T cells (40). NK cells are activated and produce IFNγ very early after LCMV infection in an IFN-I-dependent mechanism (41), so we expected these cells to be crucial for Vaccinia resistance. However, depletion of NK cells at the time of LCMV infection did not affect whether animals were susceptible to Vaccinia infection 3 days later (Figure 7A and B). Similar to intact mice, co-infected NK1.1-depleted mice did not have detectable Vaccinia-specific CD8+ T cell responses (Figure 7A) and had very low Vaccinia titers (Figure 7B). Likewise, depletion of CD4+ cells had no effect on susceptibility to the second infection. CD4-depleted animals had negligible CD8+ T cell responses to Vaccinia (Figure 7C), though were still able to clear VV from the ovaries by day 6 after Vaccinia (Fig. 7D). In contrast, depletion of CD8+ cells showed a dramatic effect. Mice depleted of CD8s at the time of LCMV infection were susceptible to Vaccinia, having high titers at the time of sacrifice (Fig. 7E). In these anti-IFNγ treated mice, there was no significant change in the levels of type I IFN (Figure 7F).
Figure 7. The in vivo antiviral state is dependent on CD8+ T cells.
Wild type mice were infected with LCMV clone 13 (c). Three days later some mice were co-infected with vaccinia virus (c+v). Some mice were treated with the NK depleting antibody (PK136) on day’s 0 and 3 post LCMV infection. A, Vaccinia virus titers in the ovaries and B, % CD8+ T cell response to the dominant vaccinia epitope B8R responses. Some infected mice were treated at the time of LCMV infection with anti-CD4 antibody (GK1.5) and C. Vaccinia virus titers in the ovaries and D, CD8+ T cell response to the vaccinia epitope B8R responses. Some mice were treated with anti-CD8 antibody (2.43) on day’s 0 and 3 post LCMV infection and E, Vaccinia virus titers in the ovary measured. F. Type I IFN bioactivity was measured day 2 post clone 13 infection. Plots represent the combined data from at least 2 independent experiments. Error bars represent mean + SD. Population distributions were compared by nonparametric two-tailed student t-Test.
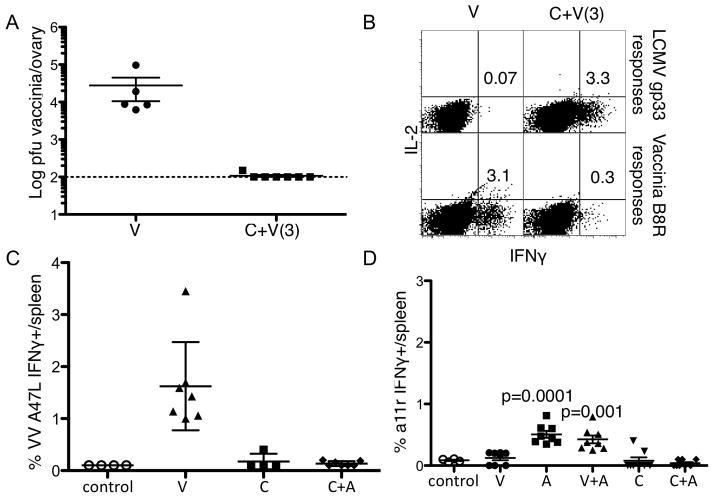
The IFNγ antiviral state is independent of perforin, sub-dominant, or cross-reactive T cell responses
CD8 T cytotoxic T cells have a variety of effector functions. To determine whether other CD8 functions are required for this non-specific antiviral state we infected perforin knockout mice with LCMV clone 13 and co-infected the mice with Vaccinia three days later. We found that perforin knockout mice are also resistant to the Vaccinia co-infection (Figure 8A) and do not mount as high an antigen specific immune response to the secondary Vaccinia infection (Figure 8B). We also measured sub-dominant VV specific responses to the A47L epitope (Figure 8C) and to published cross-reactive CD8 T cell epitopes in the co-infected mice (42). Some of these cells were detected 21 days following an Armstrong infection but not at any time during the LCMV clone 13 infection (Figure 8D and data not shown). Therefore, perforin, subdominant Vaccinia specific response or published cross-reactive T cells do not have a role in the CD8 mediated IFNγ non-specific antiviral state.
Figure 8. The IFNγ antiviral state is independent of perforin, sub-dominant, or cross-reactive T cell responses.
Perforin knockout mice were infected with LCMV clone 13 (c) and some were co-infected with Vaccinia (c+v) three days later. A, Vaccinia virus titers in the ovaries and B, % CD8+ T cell response to the dominant vaccinia epitope B8R responses. WT mice were infected with LCMV clone 13 (C) and co-infected with VACCINIA VIRUS on day 8 post primary LCMV infection. Six days after VACCINIA VIRUS co-infection mice were sacrificed and C, VACCINIA VIRUS specific A47L responses were measured. WT mice infected with either LCMV Arm (A) or clone 13 (C) and were co-infected with VACCINIA VIRUS 21 days later. E, % IFNγ produced by cross reactive a11r CD8 T cell responses were measured in uninfected controls, singly or co-infected mice. Vaccinia virus titers represent the data from one of at least 2 independent experiments with similar results. Error bars represent mean + SD. Population distributions were compared by nonparametric two-tailed student t-Test.
Discussion
Here we describe the susceptibility and T cell responses to a secondary viral infection following infection of mice with either acute or chronic LCMV. We found that mice infected with either strain of LCMV were transiently resistant to subsequent infection with VV for different periods of time. This resistance required IFN-I signaling but rapidly becomes dependent on IFNγ and CD8+ cells. We do not believe that the resistance observed here is due to cross-recognition of VV epitopes by LCMV-specific CD8+ T cells or by the activation of subdominant epitopes that were not detected in the co-infected mice. Rather, the mechanism of preventing a secondary virus infection during a primary LCMV systemic viral infection appears to be the non-specific establishment of a potent antiviral state. This state results in sterilizing immunity against the second VV infection since neither VV virus replication nor VV specific immunity is detected up to 27 days post VV co-infection in IFNAR mice (data not shown and Figure 3A).
Several labs have reported diminished cell mediated immune responses towards a second infection in LCMV infected mice (14, 19–22). These studies concluded that the inability to respond to the second infection is due to the immune suppressive qualities of the strain of LCMV used. However, even mice that are infected with an acute strain of LCMV do not mount an antigen specific response to the second VV infection. This suggests this transient suppression is independent of disease outcome. Additionally, mice infected with either acute or persistent strains of LCMV are immune competent and clear the secondary VV infection non-specifically. Further, we found that in vivo this non-specific clearance is rapidly dependent on IFNγ production by the acquired immune response.
We found that, when IFNγ is blocked, the LCMV infected mice become susceptible to the VV co-infection. However, in these susceptible mice, an immune response to the second VV infection is still prevented. Treating mice with anti-IFNγ followed by infection with VV alone has been shown to have no effect on the VV immune response (43). This suggests that the IFNγ non-specific clearance of the secondary VV infection and the inability to mount a specific immune response to the secondary VV infection are independent. We therefore propose that the inability of the LCMV infected mice to mount an antigen specific response to the VV secondary infection in a partial immune suppression. The purpose of the partial immune suppression may reflect defects in antigen presenting cell function and this has been studied extensively (44). We conclude that the partial immune suppression occurs during both acute and persistent LCMV infections as soon as 24 hours following infection, and that it is independent of the final outcome of the infection, clearance or persistence.
In our model, the exact role of the type I IFN response, which is potent during the first few days of the LCMV infection, is not clear. VV is both sensitive and resistant to type I IFN and IFNγ (reviewed (36, 45)). Treating mice with exogenous type I IFN can prevent VV infection in mice (37). In out studies, we found that type I IFN signaling without IFNγ signaling was not sufficient to prevent VV. Type I IFN has recently been shown to stimulate direct IFNγ production by NK cells (28), though we found that depletion of NK cells had no effect on the IFNγ clearance of VV. In addition type I IFN signaling is important to activate dendritic cells and T cells (46–53) and can promote IFNγ production by T cells (54). In addition, neutralizing IFNα/β during Poly(I)(C) treatment in mice neutralized the inhibition of a primary VV immune response (22). We found that wild type mice given an anti-IFNAR blocking antibody prior to the LCMV infection were susceptible to the VV co-infection. These mice had a significant reduction in their LCMV specific responses. Our findings suggest that type I IFN is required during the first few days of infection but following 72 hours supports IFNγ production through direct or indirect activation of CD8 T cells that is required for the extended in vivo antiviral state.
The down modulation of the IFNγ antiviral state occurred five days following an LCMV Armstrong infection, although high levels of IFNγ are still detected in the serum of the mice. In addition, the in vivo antiviral state is still in place in mice 15 days following infection with LCMV clone 13, though there is no detection of IFNγ in the serum at this time. Therefore, the presence of IFNγ alone is not sufficient for the in vivo antiviral state. Other factors must be important in maintaining the in vivo antiviral state such as the expression of interferon receptors, the translation of interferon response genes, or a threshold of virus present. The in vivo bioactive levels of IFNγ may also be below the level of detection by our assays.
The antiviral properties of type I IFN and IFNγ are well characterized and similar to many virus infections, VV is sensitive to interferon. Type I IFN and IFNγ have been shown to be key to clear a poxvirus infection independently (37, 55, 56). Using the LCMV system we, we have shown that there is a close interplay between the innate and acquired immune response during the antiviral state. The innate response very early seems to pass this role onto the acquired immune response, which may reflect the evolution of IFNγ produced by activated CD8 T cells as a mechanism to sustain the antiviral state following more complex infections. Our results do not support a model in which animals experiencing an LCMV chronic viral infection are immune compromised with regard to secondary infection. Rather, this result highlights the effectiveness of the non-specific antiviral response at protecting against infection, even in diseased hosts. Additionally, we found our work here demonstrates that the in vivo antiviral state is ineffective without IFNγ signaling. Further analysis will be required to carefully understand this early interplay between the innate and adaptive immune response and whether the host in response to the LCMV infection initiates the partial immune suppression.
Acknowledgments
Support
This work was supported by the Harvey V. Berneking Living Trust, by the generous support of the Hellman Fellows Fund,” established by Warren and Chris Hellman and in part by US National Institutes of Health R00 AI076346-01. Laura Valentines was supported in part by the NIH grant T32AI007334. Rashaun Potts was supported by NIH (NIGMS) MBRS-RISE grant R25-GM059298.
We would like to thank Mehrdad Matloubian (UCSF) for providing the type I IFN receptor knockout mice and Charlie Kim (UCSF) for providing the perforin knockout mice used in these studies. We would like to thank members of the Department of Experimental Medicine at UCSF for their comments and suggestions on the experiments done in this study and for their review of the manuscript.
References
- 1.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R, Pape GR, Klenerman P. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–5558. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 4.van Grevenynghe J, Cubas RA, Dafonseca S, Metcalf T, Tremblay CL, Trautmann L, Sekaly RP, Schatzle J, Haddad EK. Foxo3a: An integrator of immune dysfunction during HIV infection. Cytokine Growth Factor Rev. 2012;23:215–221. doi: 10.1016/j.cytogfr.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV. Current opinion in HIV and AIDS. 2010;5:498–503. doi: 10.1097/COH.0b013e32833ed6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epeldegui M, Vendrame E, Martinez-Maza O. HIV-associated immune dysfunction and viral infection: role in the pathogenesis of AIDS-related lymphoma. Immunol Res. 2010;48:72–83. doi: 10.1007/s12026-010-8168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kottilil S, Shin K, Jackson JO, Reitano KN, O’Shea MA, Yang J, Hallahan CW, Lempicki R, Arthos J, Fauci AS. Innate immune dysfunction in HIV infection: effect of HIV envelope-NK cell interactions. J Immunol. 2006;176:1107–1114. doi: 10.4049/jimmunol.176.2.1107. [DOI] [PubMed] [Google Scholar]
- 8.Westby M, Marriott JB, Guckian M, Cookson S, Hay P, Dalgleish AG. Abnormal intracellular IL-2 and interferon-gamma (IFN-gamma) production as HIV-1-assocated markers of immune dysfunction. Clinical and experimental immunology. 1998;111:257–263. doi: 10.1046/j.1365-2249.1998.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 12.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuniga EI, Liou LY, Mack L, Mendoza M, Oldstone MB. Persistent virus infection inhibits type I interferon production by plasmacytoid dendritic cells to facilitate opportunistic infections. Cell Host Microbe. 2008;4:374–386. doi: 10.1016/j.chom.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MB. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest. 2004;113:737–745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naniche D, Oldstone MB. Generalized immunosuppression: how viruses undermine the immune response. Cellular and molecular life sciences: CMLS. 2000;57:1399–1407. doi: 10.1007/PL00000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tishon A, Lewicki H, Rall G, Von Herrath M, Oldstone MB. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology. 1995;212:244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- 19.Althage A, Odermatt B, Moskophidis D, Kundig T, Hoffman-Rohrer U, Hengartner H, Zinkernagel RM. Immunosuppression by lymphocytic choriomeningitis virus infection: competent effector T and B cells but impaired antigen presentation. Eur J Immunol. 1992;22:1803–1812. doi: 10.1002/eji.1830220720. [DOI] [PubMed] [Google Scholar]
- 20.Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roost H, Charan S, Gobet R, Ruedi E, Hengartner H, Althage A, Zinkernagel RM. An acquired immune suppression in mice caused by infection with lymphocytic choriomeningitis virus. Eur J Immunol. 1988;18:511–518. doi: 10.1002/eji.1830180404. [DOI] [PubMed] [Google Scholar]
- 22.Brenan M, Zinkernagel RM. Influence of one virus infection on a second concurrent primary in vivo antiviral cytotoxic T-cell response. Infect Immun. 1983;41:470–475. doi: 10.1128/iai.41.2.470-475.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackburn SD, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends in microbiology. 2007;15:143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Oldstone MB. A suspenseful game of ‘hide and seek’ between virus and host. Nat Immunol. 2007;8:325–327. doi: 10.1038/ni0407-325. [DOI] [PubMed] [Google Scholar]
- 27.Lee LN, Burke S, Montoya M, Borrow P. Multiple mechanisms contribute to impairment of type 1 interferon production during chronic lymphocytic choriomeningitis virus infection of mice. J Immunol. 2009;182:7178–7189. doi: 10.4049/jimmunol.0802526. [DOI] [PubMed] [Google Scholar]
- 28.Mack EA, Kallal LE, Demers DA, Biron CA. Type 1 Interferon Induction of Natural Killer Cell Gamma Interferon Production for Defense during Lymphocytic Choriomeningitis Virus Infection. MBio. 2011:2. doi: 10.1128/mBio.00169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagata S, Mantei N, Weissmann C. The structure of one of the eight or more distinct chromosomal genes for human interferon-alpha. Nature. 1980;287:401–408. doi: 10.1038/287401a0. [DOI] [PubMed] [Google Scholar]
- 30.Mogensen KE, Uze G, Eid P. The cellular receptor of the alpha-beta interferons. Experientia. 1989;45:500–508. doi: 10.1007/BF01990498. [DOI] [PubMed] [Google Scholar]
- 31.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welsh RM, Bahl K, Marshall HD, Urban SL. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathog. 2012;8:e1002352. doi: 10.1371/journal.ppat.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulesh DA, Baker RO, Loveless BM, Norwood D, Zwiers SH, Mucker E, Hartmann C, Herrera R, Miller D, Christensen D, Wasieloski LP, Jr, Huggins J, Jahrling PB. Smallpox and pan-orthopox virus detection by real-time 3′-minor groove binder TaqMan assays on the roche LightCycler and the Cepheid smart Cycler platforms. J Clin Microbiol. 2004;42:601–609. doi: 10.1128/JCM.42.2.601-609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cembrzynska-Nowak M. Different antiviral activity and cell specificity of interferon preparations produced by mouse peritoneal cells at 37 degrees C and at 26 degrees C. Arch Immunol Ther Exp (Warsz) 1989;37:499–502. [PubMed] [Google Scholar]
- 36.Esteban M, Benavente J, Paez E. Mode of sensitivity and resistance of vaccinia virus replication to interferon. J Gen Virol. 1986;67(Pt 4):801–808. doi: 10.1099/0022-1317-67-4-801. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez JR, Rodriguez D, Esteban M. Interferon treatment inhibits early events in vaccinia virus gene expression in infected mice. Virology. 1991;185:929–933. doi: 10.1016/0042-6822(91)90575-v. [DOI] [PubMed] [Google Scholar]
- 38.Stamm A, Valentine L, Potts R, Premenko-Lanier M. An intermediate dose of LCMV clone 13 causes prolonged morbidity that is maintained by CD4+ T cells. Virology. 2012 doi: 10.1016/j.virol.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biron CA. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin Immunol. 1998;10:383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- 40.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 41.Mack EA, Kallal LE, Demers DA, Biron CA. Type 1 interferon induction of natural killer cell gamma interferon production for defense during lymphocytic choriomeningitis virus infection. MBio. :2. doi: 10.1128/mBio.00169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen ZT, Brehm MA, Daniels KA, Sigalov AB, Selin LK, Welsh RM, Stern LJ. Bi-specific MHC heterodimers for characterization of cross-reactive T cells. J Biol Chem. 2010;285:33144–33153. doi: 10.1074/jbc.M110.141051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leist TP, Eppler M, Zinkernagel RM. Enhanced virus replication and inhibition of lymphocytic choriomeningitis virus disease in anti-gamma interferon-treated mice. J Virol. 1989;63:2813–2819. doi: 10.1128/jvi.63.6.2813-2819.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan BM, Emonet SF, Welch MJ, Lee AM, Campbell KP, de la Torre JC, Oldstone MB. Point mutation in the glycoprotein of lymphocytic choriomeningitis virus is necessary for receptor binding, dendritic cell infection, and long-term persistence. Proc Natl Acad Sci U S A. 2011;108:2969–2974. doi: 10.1073/pnas.1019304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perdiguero B, Esteban M. The interferon system and vaccinia virus evasion mechanisms. J Interferon Cytokine Res. 2009;29:581–598. doi: 10.1089/jir.2009.0073. [DOI] [PubMed] [Google Scholar]
- 46.Bogdan C, Mattner J, Schleicher U. The role of type I interferons in non-viral infections. Immunol Rev. 2004;202:33–48. doi: 10.1111/j.0105-2896.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 47.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 48.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–2969. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 49.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 50.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 51.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 52.Trinchieri G, Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978;147:1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I. Direct effects of type I interferons on cells of the immune system. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:2619–2627. doi: 10.1158/1078-0432.CCR-10-1114. [DOI] [PubMed] [Google Scholar]
- 54.Cousens LP, Peterson R, Hsu S, Dorner A, Altman JD, Ahmed R, Biron CA. Two roads diverged: interferon alpha/beta-and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J Exp Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruby J, Ramshaw I. The antiviral activity of immune CD8+ T cells is dependent on interferon-gamma. Lymphokine and cytokine research. 1991;10:353–358. [PubMed] [Google Scholar]
- 56.Atrasheuskaya AV, Bukin EK, Fredeking TM, Ignatyev GM. Protective effect of exogenous recombinant mouse interferon-gamma and tumour necrosis factor-alpha on ectromelia virus infection in susceptible BALB/c mice. Clinical and experimental immunology. 2004;136:207–214. doi: 10.1111/j.1365-2249.2004.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]