Abstract
The prevalence of the metabolic syndrome with attendant morbid obesity continues to increase nationwide. A concomitant increase in non-alcoholic steatohepatitis (NASH) and associated end-stage liver disease requiring transplantation is expected to parallel this trend. Between January 1, 1997 and December 31, 2008, our center performed 813 solitary adult deceased-donor liver transplants. Patients were divided into groups based on the World Health Organization International Classification of obesity. Patients within each obesity class were compared to normal weight recipients. Pre-operative demographics among all groups were similar. NASH was more common in higher BMI groups. Operative time, blood product usage, ICU length of stay, infectious complications, and biliary complications requiring intervention were all higher in obese recipients. Deep venous thrombosis occurred more commonly in patients with Class III obesity. Patients with Class II obesity had lower patient (HR 1.82, CI 1.09–3.01, p=0.02) and allograft survival (HR 1.62, CI 1.02–2.65, p=0.04). Obesity class did not reach statistical significance on multivariate analysis. Despite increased technical operative challenges and medical complexities associated with increasing recipient BMI, morbid obesity in and of itself should not be an absolute contraindication to liver transplantation as these patients have reasonable long-term outcomes.
Keywords: body-mass index, non-alcoholic steatohepatitis
INTRODUCTION
The prevalence of the metabolic syndrome with its attendant morbid obesity continues to increase nationwide. The National Health and Nutrition Education Survey series of studies have demonstrated a remarkable and steady increase in the number of Americans with all categories of obesity over the past 40 years.[1] A concomitant increase in non-alcoholic steatohepatitis (NASH) and associated end-stage liver disease requiring transplantation is expected to parallel this trend.[2] Increased morbidity and mortality have clearly been shown to be associated with surgical procedures performed in obese patients as compared to those performed in normal weight patients.[3–11] However, this has not been conclusively shown in the obese liver transplant recipient. Single-center studies have demonstrated the ability to achieve excellent outcomes in liver transplant recipients with a body-mass index (BMI)>30 kg/m2[12] or BMI>35 kg/m2.[13] Nonetheless, these outcomes have not been matched at all centers. A study from Denmark with a relatively small number patients with a BMI>30 kg/m2 (n=20) showed increased mortality and post-operative complications in this group as compared to patients with a BMI<30 kg/m2.[14] Earlier multicenter and database review studies have suggested that obesity should be a relative contraindication to liver transplantation. In a retrospective review of the United Network for Organ Sharing database compiled from 1988 to 1996, Nair et al. found lower patient survival associated with liver transplantation in patients with Class II and Class III obesity.[15] Contrary to this study, a recent review of the National Institute of Diabetes and Digestive and Kidney Diseases database by Leonard et al. demonstrated excellent outcomes in patients with Class II and Class III obesity when BMI was corrected for ascites.[16]
A comparison of these studies is made difficult by variable definitions of obesity, as well as a likely era effect between earlier and more recent works. Despite the potential for increased morbidity and mortality in patients with obesity, these patients have been shown to benefit from liver transplantation in all classes of obesity.[17] Nonetheless, there seems to be hesitancy to perform liver transplantation in this patient population, as these patients have increased waitlist times, as well as a higher frequency of rejected organ offers.[18] Studies to date have failed to address or account for any biases in patient wait listing relative to obesity.
Our patient demographic has permitted us to accumulate a large experience with liver transplantation in obese patients, and we have no restrictions on patient listing based on BMI. In total, we examined 306 obese liver transplant recipients. Forty-seven patients had a BMI>40 kg/m2, which is the largest single-center report to date.
PATIENTS AND METHODS
After Institutional Review Board approval was obtained, we performed a retrospective review of all adult, primary, deceased-donor, liver transplants (n=813) performed between January 1, 1997 and December 31, 2008 utilizing the University of Wisconsin prospectively-collected transplant database. BMI was calculated at the time of transplant by dividing the recipient weight in kilograms (kg) by their height in meters squared (m2). Patients were categorized according to the present World Health Organization International Classification of obesity: underweight (BMI <18 kg/m2, n=25), normal weight (BMI 18–25 kg/m2, n=216), overweight (BMI 25.1–30 kg/m2, n=266), Class I obese (BMI 30.1–35 kg/m2, n=176), Class II obese (BMI 35.1–40 kg/m2, n=83), and Class III obese (BMI>40 kg/m2, n=47).
The primary outcomes of interest were patient and graft survival, with secondary endpoints focused on intraoperative and post-operative complications. Diabetes was defined as permanent (>30 days) insulin use, and hypertension was defined as a systolic blood pressure requiring at least one permanent (>30 days) outpatient anti-hypertensive agent. These outcomes were compared between overweight and Class I–III obese recipients, and their normal weight counterparts (BMI 18–25 kg/m2).
All patients received dexamethasone or methylprednisolone at the time of implantation, and the majority of patients were maintained on either mycophenolate mofetil (CellCept, Roche) or mycophenolic acid (Myfortic, Novartis), steroids, and a calcineurin inhibitor (CNI, n=428). Of the remaining group, most were maintained on steroids and a CNI (n=337). Induction therapy with basiliximab (Simulect, Novartis, n=187), alemtuzumab (Campath-1H, ILEX, n=23), daclizumab (Zenapax, Roche, n= 6), or anti-thymocyte globulin (Thymoglobulin, Genzyme, n=5) was used at the discretion of the surgeon. During the study period, our group transitioned to a regimen that preferentially utilized tacrolimus (Prograf, Fujisawa, n=693) for a calcineurin inhibitor over cyclosporine (Neoral, Novartis, n=94). Steroids were tapered during the transplant hospitalization to prednisone 20 mg/day. This dose was tapered further over the first post-operative months to a baseline of 5–10 mg/day. Ultimately, steroids were tapered off in the majority of patients. Presently, patients receive basiliximab induction if they have pre-operative renal dysfunction, and are maintained on mycophenolic acid, tacrolimus, and low-dose prednisone post-operatively.
Cytomegalovirus (CMV) prophylaxis evolved over time as well. Initially, ganciclovir was utilized for CMV-negative recipients of CMV-positive donor organs. Since 2004, valganciclovir has been used on these high-risk recipients. Acyclovir was used for all other donor-recipient combinations for three months. In recent years, trimethoprim/sulfamethoxazole (160 mg/800 mg daily) was used for pneumocystis carinii prophylaxis for one year and oral nystatin or clotrimazole tablets were used for mucosal candidiasis prophylaxis for three months.
Unexplained elevations in liver function tests were initially evaluated with duplex ultrasonography of the liver allograft to assess vascular patency. If hepatic vascular flow was normal, percutaneous liver biopsy was performed and evaluated using hematoxylin and eosin staining.
Statistics
BMI was analyzed as a nominal categorical variable to reflect current clinical terminology, permit comparisons between obese BMI categories and normal weight recipients, and to avoid erroneous conclusions from lower BMI (underweight) patients who are known to have poorer outcomes following liver transplantation. Rates of infection, and patient and graft survival were estimated by Kaplan-Meier analysis. Group comparisons were performed by a log-rank test. Continuous data are presented as mean±standard deviation. Risk factors found to be significant on univariate analysis were used to construct a multivariate model. Statistical analysis was performed with SAS software. P-values <0.05 were considered significant.
RESULTS
Demographics
Pre-operative demographics among all groups were similar in regard to age, duration of illness, physiologic Model for End-stage Liver Disease (MELD), CMV status, warm and cold ischemic times, donor age, and donor gender. (Table 1) There was no statistically significant difference in the distribution of donation after cardiac death (DCD, n=70) and donation after brain death (DBD, n=743) donor organs between BMI groups (p=0.90). There was a tendency to use organs obtained from higher BMI donors in obese recipients, with mean donor BMI for Class I (27.5 kg/m2, p=0.007), Class II (28.2 kg/m2, p=0.003), and Class III (26.2 kg/m2, p=0.61) higher than that of normal weight recipients (25.7 kg/m2). Ascites, as judged at the time of transplant by the operating surgeon as none, slight, or significant, was not different between BMI groups (p=0.16). Pre-operative hypertension (p=0.0008) and diabetes mellitus (p=0.002) were more common in higher BMI recipient groups. Mean follow-up was 4.9±3.5 years. Six patients were lost to follow-up during the study period, and 35 patients transferred their care to another center.
Table 1.
Demographics. Pre-transplant DM and HTN were more common in all classes of obese recipients. Obese recipients were more likely to be female.
| BMI (kg/m2) |
Age (years) |
Male gender (%) |
Physiologic MELD |
Pre- transplant DM (%) |
Pre- transplant HTN (%) |
Donor age (years) |
Donor BMI (kg/m2) |
|---|---|---|---|---|---|---|---|
| 18–25 (n=216) | 52±11 | 60 | 19±9 | 17 | 27 | 39±17 | 26±5 |
| 25.1–30 (n=266) | 53±9 | 67 | 20±8 | 23 | 38 | 40±16 | 27±6 |
| 30.1–35 (n=176) | 54±9 | 68 | 20±9 | 28 | 40 | 42±16 | 27±7 |
| 35.1–40 (n=83) | 54±8 | 59 | 20±9 | 30 | 40 | 42±16 | 28±7 |
| >40 (n=47) | 50±9 | 43 | 21±11 | 43 | 53 | 41±16 | 26±6 |
| p-value | 0.27 | 0.002 | 0.56 | 0.002 | 0.0008 | 0.60 | 0.04 |
Waitlist time
Obese recipients had a greater length of time on the waiting list, with mean waitlist time for overweight (207 days, p=0.06), Class I (283 days, p=0.002), Class II (296 days, p=0.001), and Class III (232 days, p=0.13) recipients greater than the waitlist time for normal weight recipients (144 days). There were no differences in the MELD at the time of listing that would explain this correlation.
Indication for transplant
The majority of patients were transplanted for alcoholic liver disease (31%, n=255) or hepatitis C-induced cirrhosis (23%, n=189). Other common indications included primary sclerosing cholangitis (9.4%, n=76), biliary cirrhosis (5.2%, n=42), alpha-one anti-trypsin deficiency (3.6%, n=29) and autoimmune hepatitis (3.2%, n=26). (Figure 1) Steatohepatitis was a progressively more common indication for transplantation in patients with increasing BMIs. NASH was the primary indication for transplant in 4.1% (n=11) of overweight, 8.5% (n=15) of Class I, 18% (n=15) of Class II, and 28% (n=13) of Class III recipients, versus only 0.93% (n=2) of normal weight recipients (p<0.0001). When NASH was included as one of three listed indications for transplantation in addition to the primary indication, the prevalence increased. NASH was present in 5.3% (n=14) of overweight, 10% (n=18) of Class I, 18% (n=15) of Class II, and 45% (n=21) of Class III recipients versus only 1.9% (n=4) of normal weight recipients (p<0.0001).
Figure 1. Indication for transplantation.
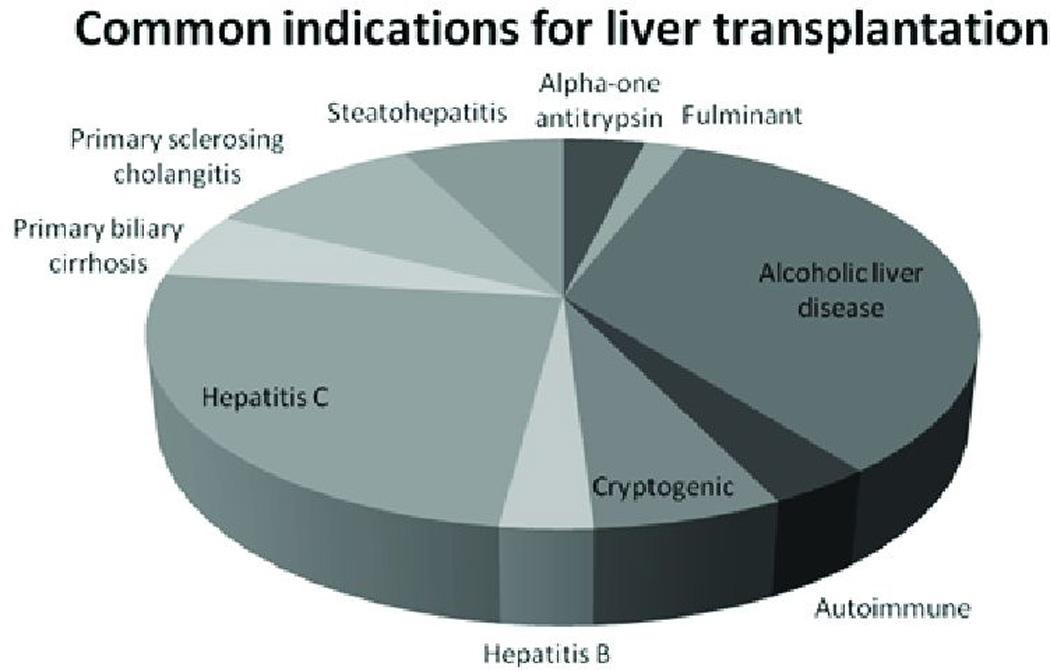
The most common indications for transplantation included alcoholic liver disease and hepatitis C, which accounted for more than 50% of cases. Other common indications included primary sclerosing cholangitis (9%), steatohepatitis (7%), cryptogenic cirrhosis (6%), and primary biliary cirrhosis (5%).
Patient and graft survival
There were no significant patient or graft survival differences between overweight, Class I, or Class III obese recipients as compared to normal weight recipients. However, patients with Class II obesity had lower patient (HR 1.82, CI 1.09–3.01, p=0.02) and allograft survival (HR 1.62, CI 1.02–2.65, p=0.04). Patient survival at 1, 3, and 5 years was 91%, 78%, and 78% for patients with Class II obesity, versus 94%, 86%, and 83% for normal weight recipients. (Figure 2) Graft survival at 1, 3, and 5 years in patients with Class II obesity was 87%, 76%, and 74% versus 91%, 84%, and 80% in normal weight recipients. (Figure 3) On multivariate analysis, obesity did not prove to be an independent risk factor relative to patient or allograft survival. As shown in Table 2A, significant independent risk factors for patient survival included receipt of a DCD liver, recipient diabetes, and donor age. Table 2B shows a similar analysis for independent factors for graft survival. Independent risk factors for graft survival were receipt of a DCD liver, recipient hypertension, MELD at the time of transplant, and donor age.
Figure 2. Patient survival.
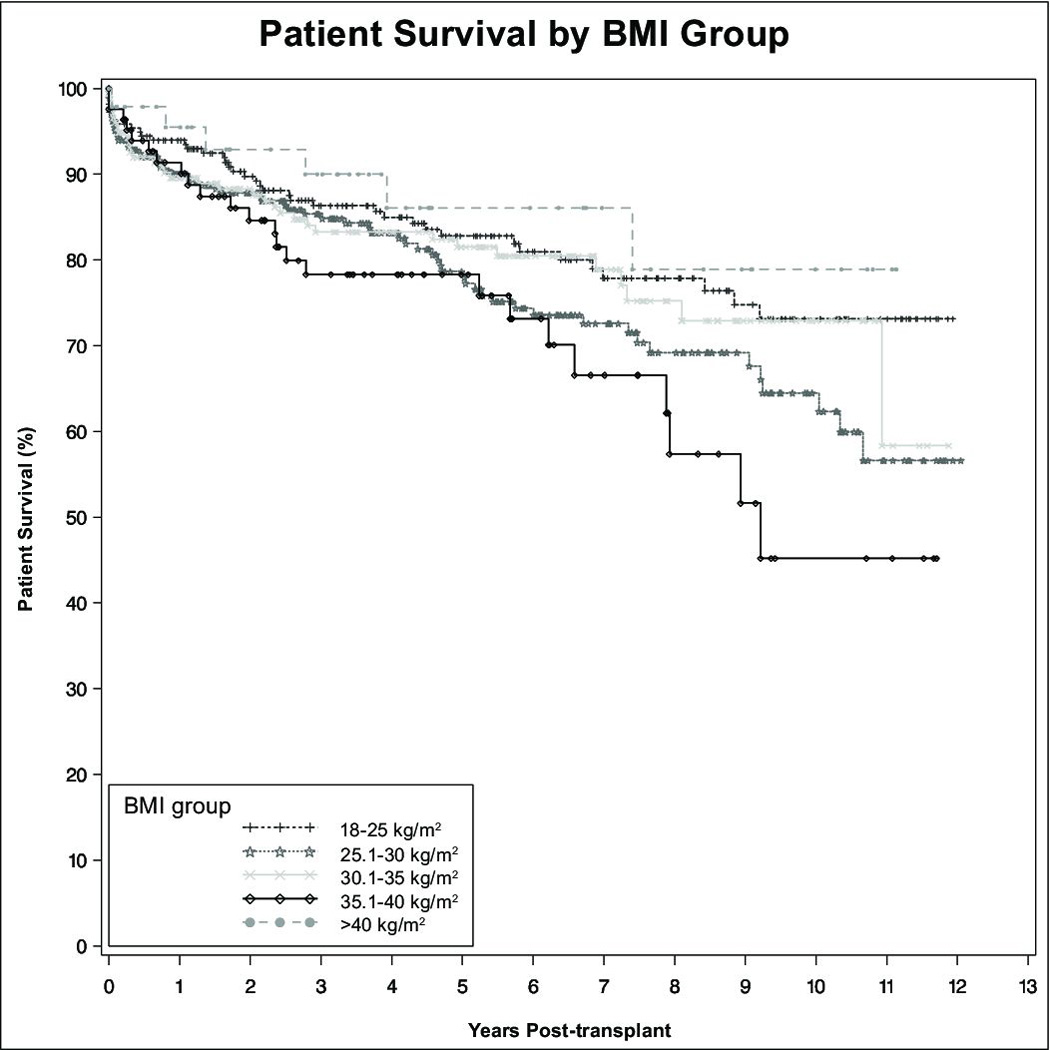
Patient survival was similar between all groups except patients with Class II obesity, who had statistically-significant lower patient survival following liver transplantation.
Figure 3. Graft survival.
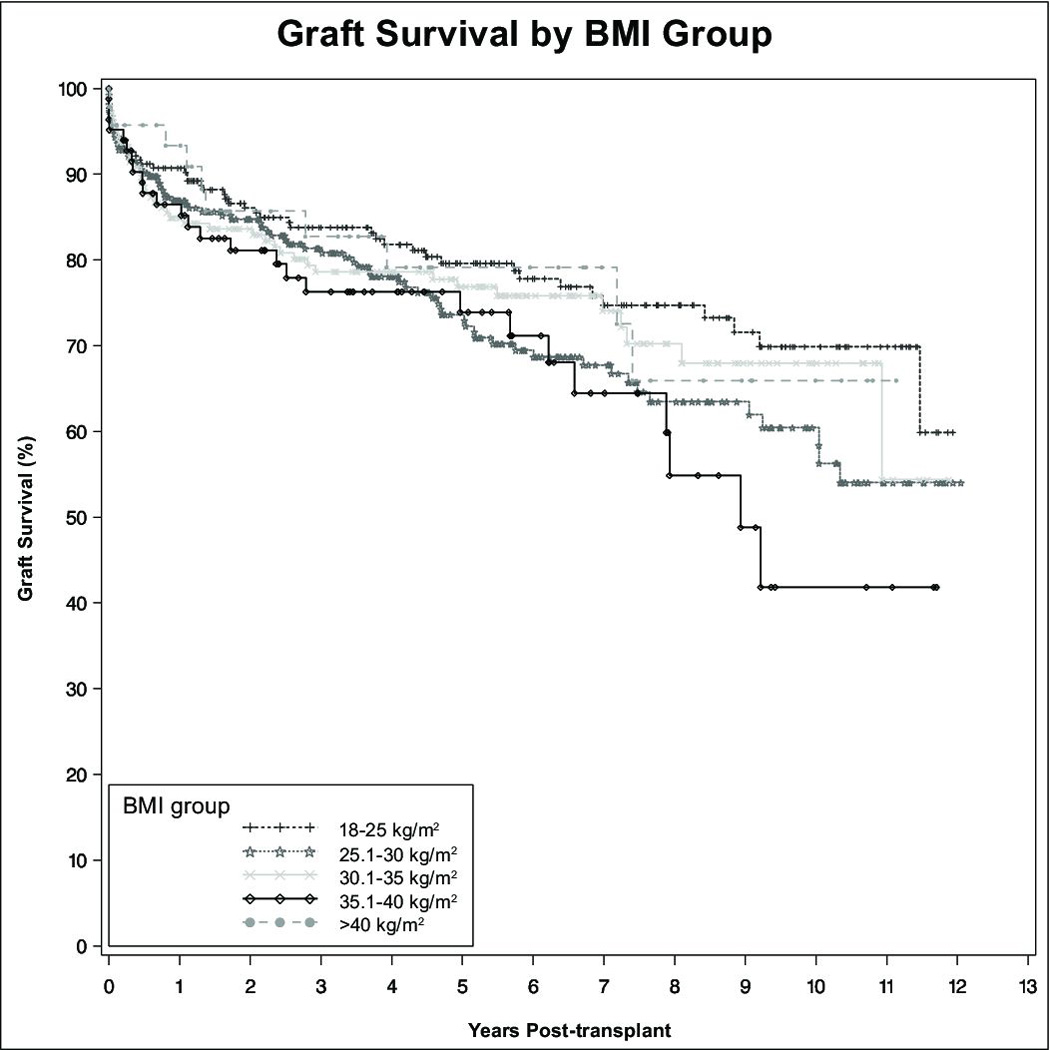
Graft survival was similar between all groups except patients with Class II obesity, who had statistically-significant lower graft survival following liver transplantation.
Table 2.
| A. Multivariate analysis of patient survival. Recipient diabetes, receipt of a DCD donor liver, and donor age were significant independent risk factors for patient survival. | ||
|---|---|---|
| Hazard ratio | p-value | |
| Overweight | 1.38 | 0.35 |
| Class I obesity | 0.99 | 0.98 |
| Class II obesity | 0.86 | 0.75 |
| Class III obesity | 0.51 | 0.31 |
| Recipient age | 1.01 | 0.31 |
| Male gender | 1.34 | 0.30 |
| MELD at transplant | 1.03 | 0.17 |
| Hepatitis B | 0.97 | 0.96 |
| Hepatitis C | 1.06 | 0.85 |
| Hepatocellular carcinoma | 0.54 | 0.10 |
| Recipient hypertension | 1.65 | 0.06 |
| Recipient diabetes | 1.92 | 0.02 |
| DCD donor | 2.72 | 0.006 |
| Donor age | 1.03 | <0.001 |
| Donor BMI | 0.35 | 0.98 |
| B. Multivariate analysis of graft survival. MELD at transplant, recipient hypertension, receipt of a DCD donor liver, and donor age were significant independent risk factors for graft survival. | ||
|---|---|---|
| Hazard ratio | p-value | |
| Overweight | 1.49 | 0.18 |
| Class I obesity | 0.92 | 0.81 |
| Class II obesity | 0.80 | 0.60 |
| Class III obesity | 0.75 | 0.57 |
| Recipient age | 1.00 | 0.80 |
| Male gender | 1.00 | 0.99 |
| MELD at transplant | 1.04 | 0.02 |
| Hepatitis B | 1.14 | 0.80 |
| Hepatitis C | 1.24 | 0.39 |
| Hepatocellular carcinoma | 0.53 | 0.06 |
| Recipient hypertension | 1.72 | 0.02 |
| Recipient diabetes | 0.57 | 0.06 |
| DCD donor | 4.25 | <0.0001 |
| Donor age | 1.04 | <0.0001 |
| Donor BMI | 0.99 | 0.40 |
Intraoperative complications
Case duration (defined as skin incision to skin closure) was longer in obese recipients, with mean operative time in Class I (7.7 hours, p=0.009), Class II (7.9 hours, p=0.008), and Class III (8.2 hours, p=0.003) recipients greater than that for normal weight recipients (7.2 hours). Obese recipients also required more packed red blood cell transfusions intraoperatively and within the first 48 hours post-operatively. Class I (15 units, p=0.005), Class II (16 units, p=0.002), and Class III (15 units, p=0.08) recipients each required more than normal weight recipients (11 units).
Post-operative complications
The intensive care unit (ICU) length of stay was different only for Class II recipients, who required a mean ICU length of stay of 4.1 days versus 2.6 days for normal weight recipients (p=0.04). The overall length of stay was significantly greater in patients with Class III obesity, with a mean length of stay of 30 days versus 17 days in normal weight recipients (p=0.002). There were no significant differences among the other BMI groups.
Infections
Infectious complications were more common in obese recipients. Wound infections which were clinically diagnosed and treated with antibiotics within the first 60 days post-operatively occurred more often in patients with Class II (n=15, HR 4.03, CI 1.85–8.79, p=0.0005) and Class III (n=13, HR 6.27, CI 2.81–14.01, p<0.0001) obesity. Patients with Class III obesity were also more likely to develop intraabdominal infections which were diagnosed radiographically or intraoperatively and treated with antibiotics and/or percutaneous drainage within the first 30 days following transplantation (n=4, HR 7.21, CI 1.60–32.45, p=0.01). No significant differences were noted in either Class I or Class II obesity in regard to intraabdominal infections.
Technical complications
Intraoperative
An increased rate of intraoperative technical problems as recorded in the operative note occurred in patients with Class II obesity (n=11, HR 3.30, CI 1.37–4.00, p=0.008). Problems included hepatic arterial injury or malposition (n=6), inferior vena cava injury (n=3), air embolism (n=1), and uncontrolled bleeding (n=1). Although there was no significant difference in the rate of return to the OR within the first 7 days (p=0.18), patients with Class II obesity had a higher rate of complications requiring eventual operative revision (n=14, HR 3.78, CI 1.68–8.51, p=0.001). Operations included repair or revision of the hepatic arterial anastomosis (n=8), repair or revision of the caval anastomosis (n=5), and revision of the portal vein anastomosis (n=1).
Short term (within 180 days post-operative)
There were no significant differences in the rate of portal venous thrombosis (p=0.31), hepatic artery thrombosis (p=0.75), or primary non-function (p=0.32) among different BMI groups. There was no significant difference in the rate of complications requiring surgical intervention for bile leak (n=32) or revision of the biliary anastomosis (n=18) between BMI groups (p=0.82). (Table 3) However, an increased rate of biliary complications requiring endoscopic or percutaneous intervention was noted in patients with obesity at all levels. This reached statistical significance in overweight patients (n=81, HR 1.81, CI 1.25–2.63, p=0.002) and patients with Class II obesity (n=28, HR 2.04, CI 1.27–3.30, p=0.003), and approached statistical significance in patients with Class I (p=0.09) and Class III (p=0.06) obesity. The most common intervention was endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy (n=141), followed by ERCP with stenting (n=33) and biliary balloon dilation (n=22). There was no correlation between donor BMI and either operative or non-operative biliary complications.
Table 3.
Technical complications following transplantation. Non-operative biliary complications were higher in all classes of obesity.
| BMI (kg/m2) |
Portal vein thrombosis n (%) |
Hepatic artery thrombosis n (%) |
Non-operative biliary complications n (%) |
Operative biliary complications n (%) |
Primary non- function n (%) |
Hernia n (%) |
|---|---|---|---|---|---|---|
| 18–25 (n=216) | 10 (4.6) | 7 (3.2) | 42 (19) | 12 (5.6) | 0 (0.0) | 21 (10) |
| 25.1–30 (n=266) | 10 (3.7) | 15 (5.6) | 81 (30) | 17 (6.4) | 5 (1.9) | 32 (12) |
| 30.1–35 (n=176) | 11 (6.3) | 11 (6.3) | 45 (26) | 12 (6.8) | 2 (1.1) | 20 (11) |
| 35.1–40 (n=83) | 8 (9.6) | 6 (7.2) | 28 (34) | 7 (8.4) | 0 (0.0) | 9 (11) |
| >40 (n=47) | 3 (6.4) | 2 (4.3) | 14 (30) | 2 (4.3) | 1 (2.1) | 11(23) |
| p-value | 0.31 | 0.75 | 0.0006 | 0.82 | 0.32 | 0.74 |
Wound complications requiring operative revision were more common in higher BMI groups, with more patients with Class I (n=3), Class II (n=4), and Class III (n=5) obesity requiring treatment for wound dehiscence. No normal weight recipients experienced this complication.
Long term (within 2 years post-operative)
The rate of hernia varied from 10 to 12% in the first 2 years following transplantation in normal weight, overweight, and Class I–II obese recipients (p=0.74). Although the rate of hernia in Class III recipients was 23%, this did not reach statistical significance when compared to the normal weight group (p=0.57).
Medical complications
Thromboembolism
There was a significantly higher rate of deep venous thrombosis (DVT) seen in patients with Class III obesity (n=8, HR 4.71, CI 1.82–12.22, p=0.001), but no difference in the rate of pulmonary embolism (p=0.51). There was no significant difference in the rate of cerebrovascular accident (p=0.92), myocardial infarction (p=0.98), or renal failure (p=0.49) between groups. There was no significant difference in the rate of readmission between BMI groups (p=0.44).
Pulmonary
Post-operative pneumonia occurred more commonly in overweight (n=14, HR 3.80, CI 1.10–13.23, p=0.04) and Class I obese patients (n=10, HR 3.98, CI 1.10–14.48, p=0.04). There were no differences in the rate of post-operative reintubation and/or tracheostomy (p=0.93) between groups. Mean post-operative ventilatory time was similar between groups (overall mean 43.8 hours, p=0.82).
Cause of allograft failure
There were no significant differences noted among BMI groups related to the cause of allograft loss. At the time of most recent follow-up, 74% of patients (n=603) were alive with a functioning primary liver allograft. The most common cause of allograft failure in all categories was death with a functioning graft (n=108). Other common causes of graft loss were recurrence of original disease (n=25), hepatic arterial thrombosis (n=21), biliary complications (n=12), and primary non-function (n=8). Recurrent steatohepatitis accounted for only one graft loss.
Cause of patient death
There were no statistically significant differences noted among BMI groups related to cause of patient death. At the time of most recent follow-up, 79% of patients (n=638) were alive. In patients with a known cause of death, the most common causes included infection (n=43), malignancy (n=29), and liver allograft failure (n=21). Less common causes included cardiac (n=14) and cerebrovascular events (n=11).
DISCUSSION
The transplant community is progressively being challenged with obese donors and recipients. Furthermore, the prevalence of NASH as an indication for transplantation is increasing nationwide.[19] Insight into the complexities in the management of these patients is critical to ensure adequate patient and allograft survival. This cohort of patients represents the largest single-center report of liver transplantation in patients with a BMI≥40 kg/m2. There seems to be a general hesitancy to offer liver transplantation to these patients, as suggested by the longer waitlist time for obese patients in both our series and national database analysis.[18] Patients with obesity tended to receive livers from donors with higher BMIs, and this effort to match donor and recipient BMI might at least partially be responsible for the higher waitlist time. Whether this represents an institutional bias to avoid transplantation of livers from high BMI donors into low BMI donors, or to match high BMI donors to high BMI recipients is unclear.
If there is, in fact, a slight hesitancy in offering transplantation to patients in higher BMI groups, there may be listing bias that would make conclusions regarding waitlist mortality difficult. Although the 2008 review of the Scientific Registry of Transplant Recipients database did not show a difference in waitlist mortality between BMI groups,[17] this may be because the patients of higher BMI groups had additional comorbidities which precluded listing for transplant. Our center has no absolute limitation on BMI for potential liver transplant recipients. The increased pre-operative morbidities common in higher BMI recipients may prolong the pre-operative evaluation. Although patients with obesity undergo similar pre-operative evaluations to their normal weight counterparts, this pre-operative selection bias is difficult to quantify. All patients undergo preoperative cardiac testing including either stress echocardiogram or echocardiogram and nuclear imaging studies. Subsequent cardiac catheterization is performed at the discretion of the transplant team. Patients with all classes of obesity were more likely to undergo preoperative cardiac catheterization then normal weight recipients. This suggests that these patients undergo more rigorous preoperative screening, which could ultimately lead to fewer patients being placed on the transplant waiting list. This could explain why no difference in waitlist mortality is noted on large database studies, as patients with elevated BMIs would be expected to have a higher waitlist mortality given the associated comorbidities. Nonetheless, with appropriate patient selection, patients with obesity can safely undergo liver transplantation with excellent patient and allograft survival.
The transplant operation is subjectively more technically challenging in obese recipients, and this is reflected by an increased operative time and an increased perioperative transfusion requirement in obese patients. The rate of intraoperative complications was significantly greater in patients with Class II obesity, and this patient population had more complications that ultimately required operative revision. This was not seen in patients with Class III obesity, which may be secondary to a smaller sample size in this group, or may be reflective of selection bias in this group. Furthermore, the rate of biliary complications requiring non-operative intervention was greater in obese patients. This is similar to our finding in a recent analysis of DCD liver transplantation.[20] While the pathophysiology of biliary injury is unclear, one possible theory is that an increase in small vessel disease results in decreased blood supply to the recipient biliary tree. This potentially decreased blood supply could render the biliary tree of the donor liver more susceptible to ischemia-reperfusion injury from the transplant procedure.
Patients with obesity had increased rates of complications associated with the metabolic syndrome, and patients with obesity were more likely to suffer from hypertension and diabetes prior to transplantation. It is perhaps not unexpected that these comorbidities, in conjunction with the technical difficulties associated with the operation, could at least partially explain the slightly lower allograft survival noted in patients with Class II obesity. Differential results of the effect of BMI on patient and allograft survival noted in prior studies likely represent selection bias. It is intuitive, but difficult to demonstrate, that more liberal policies for patient selection will tend towards poorer outcomes. Prior studies demonstrating survival benefits for patients undergoing liver transplantation in all BMI categories[17] may justify inclusion of patients with an elevated BMI, especially in light of the excellent results that can be achieved. A significant number of our recipients expired with a functioning graft, and, in fact, the causes of death in obese recipients were similar to normal weight recipients. On multivariate analysis, BMI was not an independent factor for either patient death or graft loss. This suggests that although transplantation should not be contraindicated in these patients, meticulous attention to detail in their pre- and post-operative management, with particular attention to the cardiovascular system, may be needed to achieve equivalent outcomes to normal weight recipients.
The increased likelihood of wound infection, wound dehiscence, intraabdominal infection, and pneumonia seen in morbidly obese patients is similar to that noted in outcomes studies in other surgical subspecialities. Although the frequency of incisional hernia was the highest in Class III obese recipients, there was no statistically significant difference between groups. These collective complications mandate particular attention to modifiable intraoperative and post-operative factors such as hyperglycemia, hypothermia, and hypotension, as well as pre- and intra-operative antibiotic administration. Morbid obesity with resultant decreased perioperative mobility can explain the increased rate of DVTs and pneumonia seen in patients with obesity, and we are now particularly attentive to pharmacologic and mechanical DVT prophylaxis, as well as early mobilization, in post-operative patients.
A limitation of the retrospective nature of this study is that patients who were evaluated and deemed not candidates for liver transplantation were not captured in the analysis. Although other studies have quantified the degree of ascites, and corrected the BMI for this value, we did not feel that this would be particularly helpful in the assessment of a given recipient’s suitability for liver transplantation. These studies used the degree of ascites at transplant to correct the BMI, and clearly this is not helpful in pre-operative patient assessment. Pre-operatively determined estimates of ascites based on imaging or paracentesis will, by necessity, be highly variable. Furthermore, the fluid balance of a given patient will have significant day-to-day variability. In the largest study to address this issue, only 4 of 37 patients were down-staged into the Class II group from the Class III group after correcting for ascites.[16]
Liver transplantation in the morbidly obese is not a trivial undertaking, and medical complications leading to increased length of stay can be expected. Nonetheless, with appropriate patient selection, excellent patient and allograft survival can be achieved in patients with morbid obesity. Morbid obesity in and of itself should not be a contraindication to liver transplantation.
ACKNOWLEDGEMENTS
The authors wish to thank Barbara Voss and Glen Leverson for assistance in the analysis of data and the preparation of this manuscript.
Footnotes
AUTHOR CONTRIBUTIONS
Designed study: JCL/DPF/JDM
Collected data: JCL
Analyzed data: JCL/DPF/JDM
Prepared manuscript: JCL/JDM
Edited manuscript: JCL/DPF/LAF/JDP/AIM/AMD/JDM
REFERENCES
- 1.Flegal KM. Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav. 2005;86(5):599–602. doi: 10.1016/j.physbeh.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 2.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2(12):1048–1058. doi: 10.1016/s1542-3565(04)00440-9. [DOI] [PubMed] [Google Scholar]
- 3.Postlethwait RW, Johnson WD. Complications following surgery for duodenal ulcer in obese patients. Arch Surg. 1972;105(3):438–440. doi: 10.1001/archsurg.1972.04180090043011. [DOI] [PubMed] [Google Scholar]
- 4.Pemberton LB, Manax WG. Relationship of obesity to postoperative complications after cholecystectomy. Am J Surg. 1971;121(1):87–90. doi: 10.1016/0002-9610(71)90081-x. [DOI] [PubMed] [Google Scholar]
- 5.Benoist S, et al. Impact of obesity on surgical outcomes after colorectal resection. Am J Surg. 2000;179(4):275–281. doi: 10.1016/s0002-9610(00)00337-8. [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 7.Pasulka PS, et al. The risks of surgery in obese patients. Ann Intern Med. 1986;104(4):540–546. doi: 10.7326/0003-4819-104-4-540. [DOI] [PubMed] [Google Scholar]
- 8.Choban PS, et al. Increased incidence of nosocomial infections in obese surgical patients. Am Surg. 1995;61(11):1001–1005. [PubMed] [Google Scholar]
- 9.Canturk Z, et al. Nosocomial infections and obesity in surgical patients. Obes Res. 2003;11(6):769–775. doi: 10.1038/oby.2003.107. [DOI] [PubMed] [Google Scholar]
- 10.Choban PS, Flancbaum L. The impact of obesity on surgical outcomes: a review. J Am Coll Surg. 1997;185(6):593–603. doi: 10.1016/s1072-7515(97)00109-9. [DOI] [PubMed] [Google Scholar]
- 11.Flancbaum L, Choban PS. Surgical implications of obesity. Annu Rev Med. 1998;49:215–234. doi: 10.1146/annurev.med.49.1.215. [DOI] [PubMed] [Google Scholar]
- 12.Braunfeld MY, et al. Liver transplantation in the morbidly obese. J Clin Anesth. 1996;8(7):585–590. doi: 10.1016/s0952-8180(96)00142-0. [DOI] [PubMed] [Google Scholar]
- 13.Sawyer RG, Pelletier SJ, Pruett TL. Increased early morbidity and mortality with acceptable long-term function in severely obese patients undergoing liver transplantation. Clin Transplant. 1999;13(1 Pt 2):126–130. doi: 10.1034/j.1399-0012.1999.130111.x. [DOI] [PubMed] [Google Scholar]
- 14.Hillingso JG, et al. Obesity increases mortality in liver transplantation--the Danish experience. Transpl Int. 2005;18(11):1231–1235. doi: 10.1111/j.1432-2277.2005.00206.x. [DOI] [PubMed] [Google Scholar]
- 15.Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35(1):105–109. doi: 10.1053/jhep.2002.30318. [DOI] [PubMed] [Google Scholar]
- 16.Leonard J, et al. The impact of obesity on long-term outcomes in liver transplant recipients-results of the NIDDK liver transplant database. Am J Transplant. 2008;8(3):667–672. doi: 10.1111/j.1600-6143.2007.02100.x. [DOI] [PubMed] [Google Scholar]
- 17.Pelletier SJ, et al. Effect of body mass index on the survival benefit of liver transplantation. Liver Transpl. 2007;13(12):1678–1683. doi: 10.1002/lt.21183. [DOI] [PubMed] [Google Scholar]
- 18.Segev DL, et al. Prolonged waiting times for liver transplantation in obese patients. Ann Surg. 2008;248(5):863–870. doi: 10.1097/SLA.0b013e31818a01ef. [DOI] [PubMed] [Google Scholar]
- 19.Angulo P. Nonalcoholic fatty liver disease and liver transplantation. Liver Transpl. 2006;12(4):523–534. doi: 10.1002/lt.20738. [DOI] [PubMed] [Google Scholar]
- 20.Foley DP, et al. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253(4):817–825. doi: 10.1097/SLA.0b013e3182104784. [DOI] [PMC free article] [PubMed] [Google Scholar]


