Abstract
Problem
Expression patterns and regulation of cytosolic pattern recognition receptors (PRR) NOD-1, NOD-2, RIG-1, and MDA5 have not been elucidated in the human female reproductive tract (FRT).
Methods
Primary epithelial cells (EC) isolated from Fallopian tube (FT), endometrium (EM), cervix (Cx) and ectocervix (Ecx) were treated with estradiol, poly(I:C), Neisseria gonorrhea (GC), and HIV-1. PRR mRNA expressions were analyzed by Real-time RT-PCR. Conditioned media were analyzed for IL-8 by ELISA.
Results
EC from all FRT compartments constitutively expressed NOD1, NOD2, RIG-1, and MDA5 with highest levels expressed by FT. Stimulation with poly(I:C) resulted in upregulation of NOD2, RIG-1, and MDA5 in all FRT compartments and correlated with increased secretion of IL-8 whereas estradiol treatment had no effects. Exposure to GC and HIV-1 IIIB but not BaL resulted in selective upregulation of NOD2 and MDA5.
Conclusions
PRR are expressed throughout the FRT and differentially regulated by poly(I:C), GC and HIV-1.
Keywords: Estradiol, HIV, MDA5, NOD1, NOD2, Neisseria gonorrhea, Poly(I:C), RIG-1
INTRODUCTION
The human female reproductive tract (FRT) has to maintain the unique dual function of protecting the tract against invading pathogens while tolerating allogeneic sperm and a semi-allogeneic fetus. The FRT maintain a balance between immune activation and tolerance primarily through regulation by estradiol and progesterone 1, 2. The immune response to pathogens is initiated by recognition of pathogen associated molecular pattern (PAMP) as “non-self” through pattern recognition receptors (PRR). The most well characterized PRR are toll-like receptors (TLRs) which sense bacterial, viral and fungal PAMP at the surface of the cell or endosomal compartments 3, 4. More recently, intracellular cytosolic pathogen sensing receptors, NOD1, NOD2, RIG-1, and MDA5, have been described 5, 6 which can induce protective responses.
NOD-like receptors (NLR), nucleotide-binding domain-leucine-rich repeat-containing molecules act as cytosolic sensors to detect bacterial pathogens 5, although recently viral ligands have been described as well 7. Upon pathogen recognition, the NLR oligomerize and signal through NFkB and MAP kinase pathways to activate inflammatory caspases to stimulate the production of pro-inflammatory cytokines and the induction of apoptosis 8, 9. Retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) belong to the family of RIG-1 like receptor (RLR). The RLR sensors recognize viral RNA and induce a classical antiviral response of inflammatory cytokines and Type I Interferons (reviewed 10). RLR have also been demonstrated to recognize poly(I:C), a classic ligand for TLR3 6, 11–13.
Previously, we and others have shown that functional TLRs are expressed by epithelial cells of the FRT, the first line of defense against sexually transmitted pathogens 14–16. In contrast, there is scant information about NLR and RLR in the FRT. A study by King et al showed that NOD1 and NOD2 mRNA were constitutively expressed in the endometrium and the expression of NOD2 mRNA was altered by IL-1β as well as the menstrual cycle 17. In other studies using whole tissues from the FRT, Hart et al demonstrated that NOD1 and NOD2 are expressed in human Fallopian tube (FT), endometrium (EM), cervix (Cx), and ectocervix (Ecx) 18. However, the presence of RIG-1 and MDA5 in the human non-pregnant female reproductive tract has yet to be determined.
The TLR family recognizes sexually transmitted pathogens of the FRT 19, 20. Neisseria gonorrhea or gonococcus (GC) is the etiologic agent of gonorrhea, which, in women, infects the cervix and the Fallopian tubes. In women, infection is asymptomatic in approximately 50% of the time resulting in secondary complications such as pelvic inflammatory disease (leading to infertility) and/or systemic dissemination 21, 22. Although GC peptidoglycan has been shown to bind to TLR2, it is currently unknown whether GC can trigger NOD1, NOD2, RIG-1 or MDA5; GC has, however, been shown to activate NLRP3, a different NLR 23.
HIV-1 is a major sexually transmitted pathogen with the current world-wide epidemic being primarily heterosexual 24, 25. TLR 7 and 8 expressed by dendritic cells and macrophages have been shown to respond to HIV 26–28. It is unclear whether and how HIV is recognized by cytosolic PRR. One report has shown that HIV genomic RNA can be sensed by RIG-1 but not MDA5 29. Another study by Ogawa et al 30 reported no alterations in HIV replication by NOD1 and NOD2 agonists in Langerhans cells.
The human FRT is not an immunologically sterile but rather an immunologically active site 14, 31, 32. The upper (FT, EM) and lower (Cx, Ecx) tracts act in concert yet are distinct both anatomically and functionally. In this study, we mimicked in vitro physiological (constitutive and estradiol response) and pathological (poly(I:C), GC, HIV-1) conditions to demonstrate immune responses by primary FRT epithelial cells. To the best of our knowledge, no studies have comprehensively examined the expression patterns and regulation of NOD1, NOD2, RIG-1 and MDA5 throughout the FRT under these conditions. Our study demonstrates that epithelial cells (EC) in the upper and lower FRT express all four PRR with the highest constitutive expression in the FT. We also report that, whereas none of the receptors responded to estradiol treatment, poly(I:C) treatment upregulated NOD2, RIG-1 and MDA5 but not NOD1. In addition, we observed that sexually transmitted pathogens GC and HIV-1 IIIB selectively upregulated NOD2 and MDA5.
MATERIALS AND METHODS
Source of tissues
Human EM, FT, Cx and Ecx tissue were obtained from women undergoing hysterectomy at Dartmouth-Hitchcock Medical Center (Lebanon, NH). Tissues used in this study were collected from patients with benign conditions such as fibroids distal from the site of pathology. Sections were examined by a pathologist and identified to be free of pathological lesions. All human subject studies were carried out with the approval of the Dartmouth College Institutional Review Boards. Approval to use tissues was previously obtained from the Committee for the Protection of Human Subjects (CPHS).
Isolation of primary Fallopian tube, uterine, cervical and ectocervical epithelial cells
To isolate epithelial cells from hysterectomy tissues, tissues were rinsed with 1x HBSS with phenol red, containing 100 U/ml Penicillin, 100 μg/ml streptomycin (all Hyclone, Logan, UT), and 0.35 mg/ml NaCO3, and minced into 1–2 mm fragments prior to subjecting them to enzymatic digestion for 1 hr at 37°C on a rotating platform. The enzyme mixture contained 3.4 mg/ml pancreatin (Sigma, St Louis, MO), 0.1 mg/ml hyaluronidase (Sigma), 1.6 mg/ml collagenase-D (Roche, Indianapolis, IN), and 2 mg/ml D-glucose, in 1x HBSS. After digestion, cells were dispersed through a 250-mm nylon mesh screen (Small Parts, Miami Lakes, FL) and epithelial sheets were separated from stromal cells by filtration through a 20-mm screen. Epithelial cell sheets retained on the filter were recovered by back-flushing, then washed and resuspended in DMEM/F12 complete medium without phenol red, supplemented with 10 uM HEPES (both Invitrogen, Grand Island, NY), 100 ug/ml primocin (InvivoGen, San Diego, CA), 2 mM L-glutamine, 2.5% defined FBS (both Hyclone), and 2.5% NuSerum (BD Biosciences, Bedford, MA). Cell sheets were centrifuged at 500 × g for 10 min and analyzed for cell number and viability. By this procedure, we isolate purified epithelial cells that stain positive for the epithelial antigens Ber-EP4 and cytokeratin and negative for CD4, CD45, and vimentin 33.
Cell culture and treatments with Estradiol, poly(I:C) and LPS
To establish a cell culture system of polarized human EM, FT, and Cx epithelial cells with both apical and basolateral compartments, primary cells were cultured on matrix coated Falcon cell culture inserts in 24-well culture plates (Fisher Scientific, Pittsburgh, PA) in complete medium (DMEM/F12 without phenol red, supplemented with 10 uM HEPES (both Invitrogen, Grand Island, NY), 100 ug/ml primocin (InvivoGen, San Diego, CA), 2 mM L-glutamine, 2.5% defined FBS (both Hyclone), and 2.5% NuSerum (BD Biosciences, Bedford, MA). For these experiments, apical and basolateral compartments contained 300 and 500μl of complete medium, respectively. In order to keep the culture conditions similar, the same procedure was followed and same media was used for culturing squamous Ecx epithelial cells (EC), which do not polarize. Media were changed every 2 days. The cells were treated apically with 25μg/ml TLR3 agonist poly(I:C) (Invivogen) or 10ng/ml Escherichia coli ultra-pure LPS (Invivogen) for 24 hrs in triplicate. Apical and basolateral conditioned media were collected and centrifuged for 5 min at 10,000xg and stored at −80°C until used. For estradiol studies, cells were grown to confluence and allowed to polarize (for upper FRT EC) and then switched to media with 10% stripped FBS (Hyclone) to minimize the presence of endogenous hormones present in FBS. Estradiol was prepared as described previously 34. Epithelial cells were treated with estradiol (5×10−8M) both apically and basolaterally. After 24 hr incubation, apical and basolateral conditioned media were collected, centrifuged for 5 min at 10,000xg and stored at −80°C until assayed as described below.
Measurement of transepithelial resistance (TER)
Tight junction formation of cultured epithelial cell monolayers was assessed by periodically measuring transepithelial resistance (TER) using an EVOM electrode and Voltohmmeter (World Precision Instruments, Sarasota, FL), as described previously 33. TER is a functional measurement of the integrity of tight junctions in an epithelial cell monolayer. Since the presence of non-epithelial cells in the culture interferes with the formation of tight junctions and therefore prevents an increase in TER, TER is also an indicator for the purity of the epithelial monolayer.
Measurement of IL-8 protein secretion
Concentrations of IL-8 in the apical and basolateral conditioned media from epithelial cells were determined using an ELISA Duoset kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol. Triplicate wells were assayed for each treatment. Amounts of IL-8 were measured based on a standard curve after OD measurements at 450 nm on an ELISA reader (Dynex, Chantilly, VA).
Taqman real-time polymerase chain reaction
Real-time RT-PCR was done with a two-step protocol as described previously 35. Total RNA was isolated from cells using TRIzol Reagent according to the manufacturer’s recommendations (Invitrogen Life Technologies) and purified with RNAeasy columns (Qiagen, Valencia, CA). In some cases RNA was isolated using RLT buffer (RNAeasy Kit, Qiagen), purified using Qiashredder columns (Qiagen) prior to loading them on RNAeasy columns for further purification. Coincident with RNA purification was on-column DNase digestion using the RNase-Free DNase set (Qiagen). For each specimen, 400ng of total RNA was reverse-transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s recommendations in a 20μl volume. Relative mRNA expression levels of NOD1, NOD2, RIG-1, and MDA5 were measured using the 5′ fluorogenic nuclease assay in real-time quantitative PCR using TaqMan chemistry on the ABI 7300 Prism real-time PCR instrument (Applied Biosystems, Foster City, CA). NOD1, NOD2, RIG-1, MDA5 and β-actin primer/MGB probe sets were obtained from Applied Biosystems assays-on-demand (ID nos. Hs00196075_m1, Hs00223394_m1, Hs01058986_m1, Hs00223420_m1 and 4333762T, respectively). β-actin was selected as house-keeping gene for these studies as preliminary optimization experiments did not show significant differences in the expression of β-actin between untreated Control groups and treatment groups. PCR was conducted using the following cycle parameters: 95°C, 12 min for 1 cycle (95°C, 20 s; 60°C, 1 min), for 40 cycles. Analysis was conducted using the sequence detection software supplied with the ABI 7300. The software calculates the threshold cycle (Ct) for each reaction which was used to quantify the amount of starting template in the reaction. Ct values for each set of duplicate reactions were averaged for all subsequent calculations. A difference in Ct values (ΔCt) was calculated for each gene by taking the mean Ct of each gene of interest and subtracting the mean Ct for the housekeeping gene β-actin for each cDNA sample. Assuming that each reaction functions at 100% PCR efficiency, a difference of one Ct represents a 2-fold difference. Relative expression levels were reported as a fold-increase in mRNA expression where the untreated control was normalized to 1 and calculated using the formula 2−ΔΔCt. Total mRNA copies normalized to β-actin were calculated using the formula 2−ΔCt × 105 36.
Treatment of epithelial cells with Neisseria gonorrhea
N. gonorrhea 31426 (American Type Culture Collection, Manassas, VA) was passaged daily on chocolate agar plates (Remel, Lenexa, KS) and incubated in a 5% CO2 humidified incubator. A single N. gonorrhea colony was inoculated into GC broth (15 g proteose peptone no. 3, 4 g K2HPO4, 1 g KH2PO4, 5 g NaCl, 1 g corn starch, and 1% GCHI enrichment supplement per liter) and incubated for 18–20 hrs in a 5% CO2 humidified incubator. For the preparation of heat-killed lysates, overnight cultures of GC were incubated for 45 minutes in a 65°C water bath. Integrity of the bacteria following heat killing was verified via light microscopy. For the preparation of GC lysates, overnight cultures were passed through a 25-gauge needle 20 times. Shearing of intact bacteria was verified via light microscopy.
Heat-killed or sheared GC were diluted 1:5 in GC broth and applied apically to confluent polarized monolayers of epithelial cells isolated from FT, and Cx. Each treatment was done in triplicate. After 6 hrs of exposure, apical and basolateral supernatants were harvested, centrifuged at 10,000xg for 5 min to eliminate cell debris, and frozen at −80°C until needed. RNA was isolated as described under “Taqman Real-time RT-PCR” section and expression of NOD1, NOD2, RIG-1 and MDA5 was determined.
Exposure of epithelial cells to HIV-1
EM epithelial cell monolayers were grown to confluence and high TER as described in a previous section. HIV-1 IIIB (CXCR4 tropic) and BaL (CCR5 tropic) were diluted in DMEM/F12 complete medium without phenol red, supplemented with 20mM HEPES, 2mM L-glutamine (all from Invitrogen Life Technologies), 50μg/ml primocin (Invivogen, San Diego, CA), and 10% defined FBS (Hyclone, Logan, UT) to 1×106 infectious units (i.u.). Virus was added to the apical side of polarized EC and cells were exposed for 24 hrs prior to collecting apical and basolateral supernatants and isolating RNA (as described in a previous section). Expression of NOD1, NOD2, RIG-1, and MDA5 were analyzed by Taqman realtime RT-PCR.
Statistics
A two-tailed paired t test or a one-way ANOVA with Bonferonni’s post-test was performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). A p value of <0.05 was taken as indicative of statistical significance.
RESULTS
Human FRT epithelial cells constitutively express NOD1, NOD2, RIG-1 and MDA5
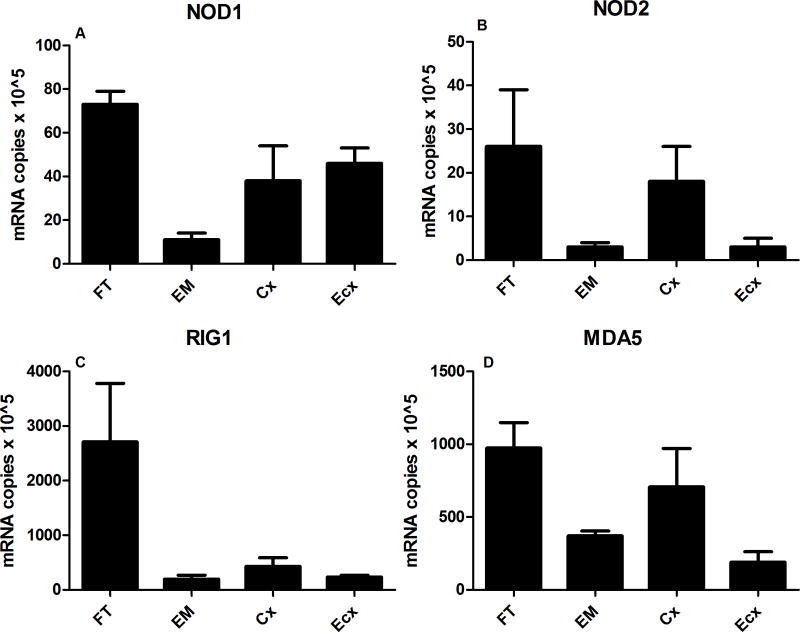
Epithelial cells isolated from human Fallopian tube (FT), endometrium (EM), cervix (Cx), and ectocervix (Ecx) were grown on cell inserts until confluent and polarized (except Ecx). Expression of NOD1, NOD2, RIG-1 and MDA5 was determined by Taqman real-time RT-PCR. When tissues from FT (n=5), EM (n=3), Cx (n=5), and Ecx (n=5) were averaged, mRNA expression of all four genes was highest in the FT, followed by the Cx with lowest levels found either in EM and/or Ecx epithelial cells (Figure 1A–D). Of the four genes analyzed, RIG-1 showed the highest constitutive expression followed by MDA5; both NOD1 and NOD2 showed low mRNA expression with NOD2 being the lowest of the four genes (Figure 1 A–D). When all four tissues could be obtained and analyzed from an individual patient (a total of 3 individuals with four tissues each were analyzed), we observed the same pattern i.e. the expression of all four genes was highest in the FT when compared to EM, Cx, and Ecx (data not shown).
Figure 1. Constitutive mRNA expression of NOD1, NOD2, RIG-1 and MDA5 by epithelial cells of the female reproductive tract in multiple patients.
Real-time RT-PCR was used to determine the mRNA expression levels of NOD1, NOD2, RIG-1, and MDA5 in primary FT, EM, Cx, and Ecx epithelial cells. Values from each sample were normalized to housekeeping gene β-actin and expressed as copies of mRNA × 105. An average of 3–5 different patient tissues were used from each compartment. Expression of NOD1 in FT, EM, Cx, and Ecx is shown in (A), NOD2 in (B), RIG-1 in (C) and MDA5 in (D).
TLR3 agonist poly(I:C) treatment results in upregulation of NOD1, NOD2, RIG-1, and MDA5
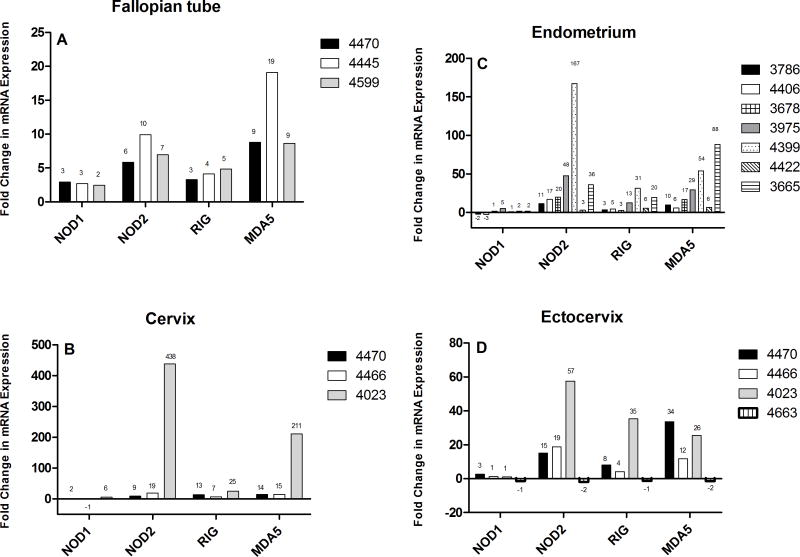
Previously we and others have shown that epithelial cells from both the upper and lower FRT express toll-like receptors (TLRs) and respond to treatment by TLR ligands 14, 18, 32. To determine whether NOD1, NOD2, RIG-1, and MDA5 expression by epithelial cells throughout the FRT is regulated by TLR agonists, epithelial cells were grown to confluence and high TER (when applicable) and treated with TLR agonists. As seen in Figure 2, treatment of FT, EM, Cx, Ecx epithelial cells with TLR3 agonist poly(I:C), a synthetic mimic for viral double-stranded RNA, induced mRNA expression of NOD2, RIG-1, MDA5 and to a lesser extent, NOD1, relative to untreated controls. Time course experiments indicated that all four genes were upregulated at 6 hrs and was measurable at 24 hrs (data not shown). At 24 hrs of poly(I:C) treatment (Figure 2 AD), all four genes were upregulated greater than 2-fold in the FT in 3/3 patient samples. In the EM, NOD2, RIG-1, and MDA5 were upregulated in 7/7 samples. In contrast, NOD1 was upregulated in only 3/7 samples. In Cx and Ecx, NOD2, RIG-1, and MDA5 were upregulated in 3/3 samples while NOD1 was upregulated in 2/3 of Cx and 1/4 of Ecx.
Figure 2. Induction of NOD1, NOD2, RIG-1, and MDA5 mRNA by epithelial cells from EM, FT, Cx, and Ecx, upon treatment with Poly(I:C).
Primary FRT epithelial cells were treated with poly(I:C) for 24 hr. Real-time RT-PCR was used to determine mRNA expression levels of NOD1, NOD2, RIG-1, and MDA5. After normalization to endogenous control β-actin, each patient sample was further calibrated to its own untreated control and expressed as relative fold change. Data from (A) three distinct FT, (B) seven EM, (C) three Cx, and (D) four Ecx are shown. A 2-fold or greater change was considered to be significant.
In contrast to poly(I:C), TLR2 and 4 agonist LPS showed modest stimulatory effects on all four genes in 50% or less of the samples tested (data not shown). In addition, upon treatment of EM EC with IL-1β, we found that IL-1β had no effect on NOD1, NOD2, RIG-1 or MDA5 (data not shown). The effect of poly(I:C), GC, and HIV were therefore selective as no upregulation of the PRRs were observed upon exposure to TLR 2/4 ligand LPS and proinflammatory stimulant IL-1β, which stimulates other genes in the FRT 37, 38.
TLR3 agonist poly(I:C) treatment results in increased secretion of IL-8
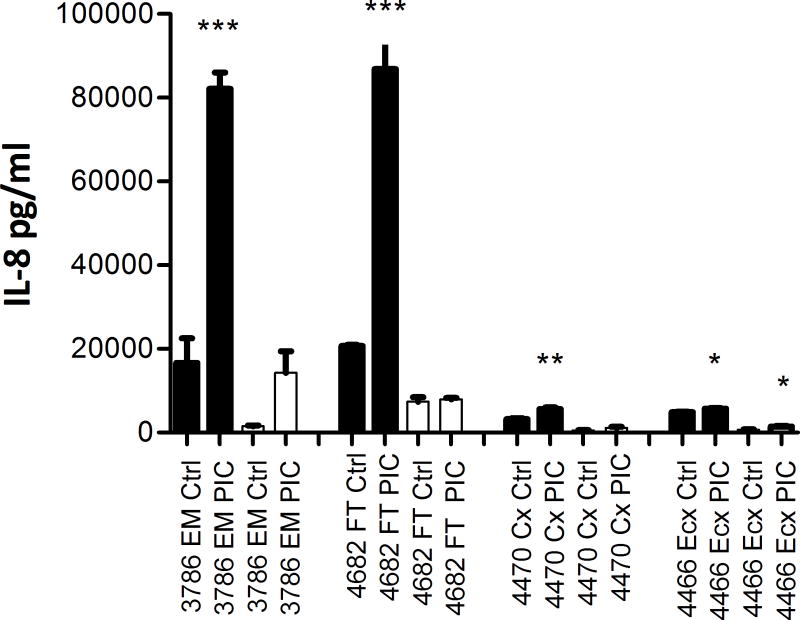
To determine if increase in expression of intracellular sensors correlated with the induction of epithelial immune responsiveness, we measured the secretion of IL-8 in both apical and basolateral supernatants collected from epithelial cells (FT, EM, Cx, Ecx) grown on inserts. As seen in Figure 3, following a 24 hrs treatment with poly(I:C), IL-8 secretion was significantly enhanced in apical secretions in all FRT compartments. Basolateral secretion of IL-8 was also induced but did not reach significance except in the Ecx.
Figure 3. Enhanced secretion of IL-8 by primary FRT epithelial cells following stimulation with TLR3 agonist poly(I:C).
Primary epithelial cells from FT, EM, Cx, and Ecx were treated with poly(I:C) for 24 hr. Apical and basolateral conditioned media were collected and assayed for IL-8 secretion by ELISA. Data from one representative patient sample from each compartment is shown. Apical secretions are shown in black and basolateral secretions are shown in white. The p values were calculated by comparing triplicate wells for Control and Treatment, using a two-tailed paired t test. Significance denoted by *; *, p<0.05; **, p<0.01; ***, p<0.001.
Estradiol treatment of FRT epithelial cells did not induce expression of NOD1, NOD2, RIG-1, and MDA5
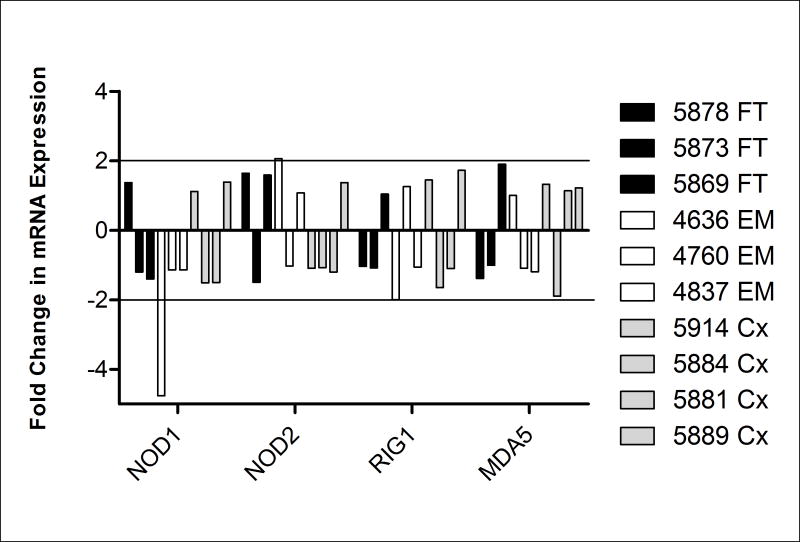
We and others have previously shown that estradiol affects multiple aspects of the FRT immune system during the menstrual cycle, pregnancy and aging (Reviewed 39). To determine whether the presence of estradiol influences the expression of intracellular PRR, FT, EM and Cx epithelial cells were treated with estradiol both apically and basolaterally at a concentration of 5×10−8M for 24 hrs. Real-time RT-PCR was used to check for expression of the four intracellular cytosolic sensors. As seen in Figure 4, in 3/3 EM, 3/3 FT, and 4/4 Cx samples, estradiol had no consistent direct effect on the expression of NOD1, NOD2, RIG-1, and MDA5.
Figure 4. No change in mRNA expression of NOD1, NOD2, RIG-1, and MDA5 by primary FRT epithelial cells upon estradiol treatment.
Primary epithelial cells from FT, EM, and Cx, were treated apically and basolaterally with 5×10−8M E2 for 24 hr and real-time RT-PCR was used to determine expression of NOD1, NOD2, RIG-1, and MDA5. After normalization to endogenous control β-actin, each sample was further calibrated to its own untreated control and expressed as relative fold change. Data from three distinct FT (black), three EM (white), and four Cx (grey) are shown. A ≥2-fold change was considered to be significant.
Neisseria gonorrhea, an intracellular, sexually transmitted bacterial pathogen, selectively induced NOD2 and MDA5 in the Cx but not in the FT
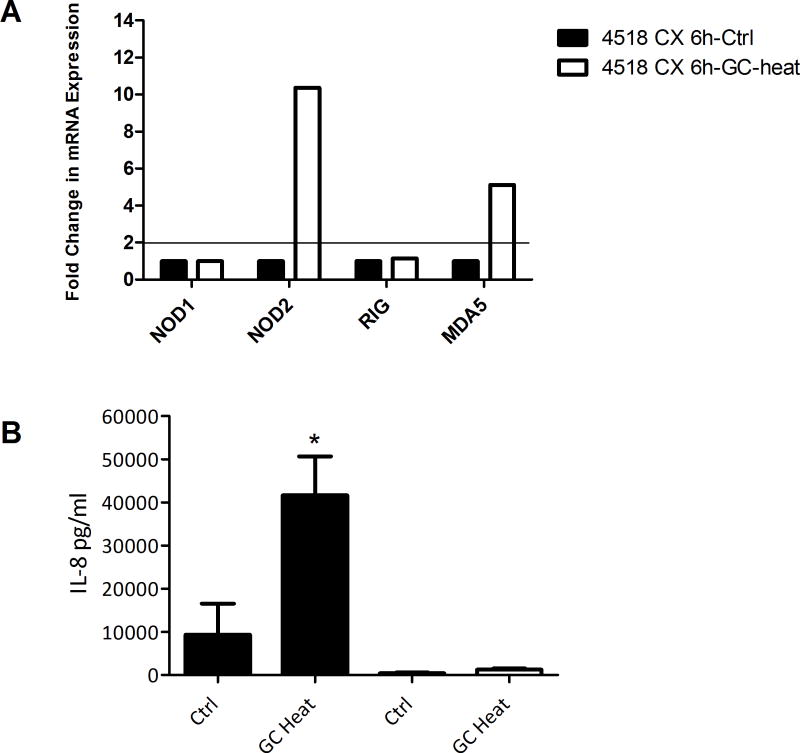
Since NOD1, NOD2, RIG-1, and MDA5 are intracellular pathogen sensors we tested the ability of an intracellular bacterial pathogen, GC, to induce NLR expression. FT and Cx were selected for these experiments as these sites are clinically affected upon GC infection. Interestingly, none of the PRR were induced in GC-treated FT epithelial cells in 3/3 patients tested (data not shown). However, when polarized epithelial cells from Cx were treated with heat-killed or sheared preparations of GC apically for 6 hrs, we found significant upregulation of the expressions of NOD2 and MDA5 but not NOD1 and RIG-1 in 2/3 samples (Fig 5A and data not shown). Figure 5A shows one of three representative cervical samples. We also observed that cervical epithelial cell upregulation of NOD2 and MDA5 in response to GC was accompanied by increased secretion of IL-8. Figure 5B shows upregulation of IL-8 secretion from the same samples analyzed in 5A. In contrast, we observed that FT epithelial cells that did not increase expression of NOD1, NOD2, RIG-1, and MDA5 upon GC treatment failed to show any upregulation of IL-8 secretion (data not shown). These findings indicate selective response of cervical but not FT epithelial cells to heat-killed GC.
Figure 5. Selective upregulation of NOD2 and MDA5 by primary cervical epithelial cells upon exposure to heat-killed Neisseria gonorrhea.
Primary epithelial cells from Cx, were treated with heat-killed GC for 6 hrs. (A) shows induction of NOD2 and MDA5 mRNA by Cx epithelial cells (Control, black; heat-killed, white). (B) shows significantly higher secretion of IL-8 by Cx epithelial cells upon GC treatment (apical conditioned media, black; basolateral, white). The p values were calculated by comparing triplicate wells for Control and Treatment, using a two-tailed paired t test; *, p<0.05.
Exposure to HIV-1 upregulated NOD2 and MDA5 in EM
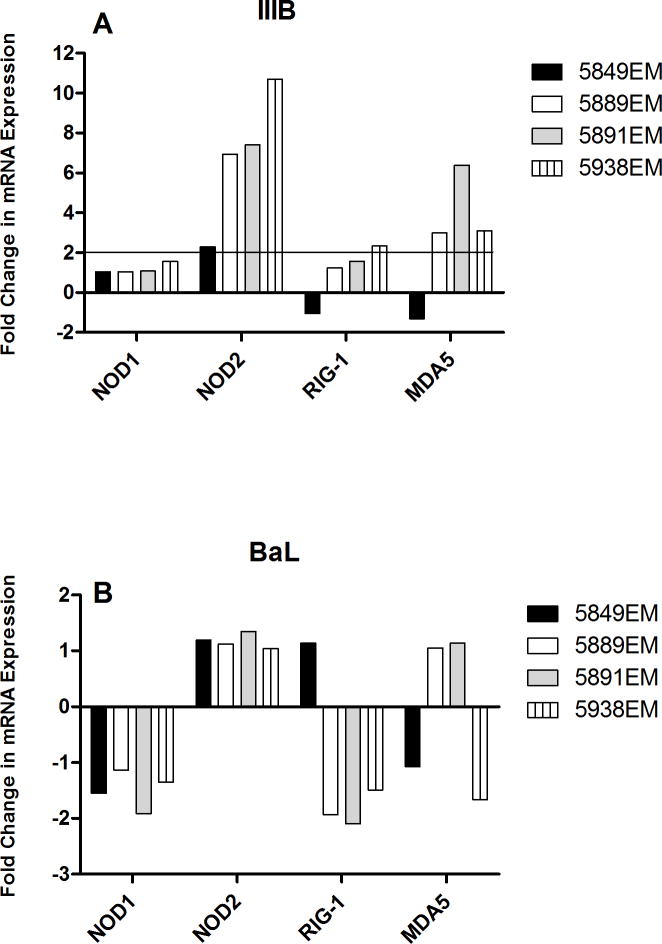
The primary route of HIV infection in women is through the epithelial barrier of the FRT. It is inconclusive whether HIV can productively infect FRT EC; however it is clear that response to HIV alters immune parameters in the FRT. We exposed EM EC apically to 106 infectious units of HIV IIIB and BaL for 24 hrs. RNA was then isolated from infected cells and tested for expression of NOD1, NOD2, RIG1, and MDA5 by realtime RT-PCR. We found that when exposed to IIIB, EC significantly upregulated (>2-fold), NOD2 (4/4) and MDA5 in (3/4) patients tested (Figure 6A). RIG-1 was upregulated in 1/4 and NOD1 in 0/4 patients. However, in case of exposure to BaL (Figure 6B), no upregulation of any of the PRRs were observed. Unlike GC, HIV-1 treatment of EC did not show a corresponding upregulation in IL-8 secretion (data not shown). Our data indicate that specific strains of HIV might be able to differentially upregulate selective intracellular PRRs.
Figure 6. Selective upregulation of NOD2 and MDA5 by primary endometrial epithelial cells upon exposure to HIV-1.
Primary epithelial cells from EM were grown to confluence and high TER prior to exposing them apically to 106 infectious units of HIV-1 for 24 hrs. RNA was extracted from cells and the expression of NOD1, NOD2, RIG-1, and MDA5 was determined by real-time RT-PCR. (A) shows induction of NOD2 (4/4 samples) and MDA5 (3/4 samples) upon treatment with HIV IIIB whereas no such induction was observed in (B) when cells were similarly treated with HIV BaL.
DISCUSSION
The present study demonstrates that epithelial cells from the upper and lower human FRT express intracellular cytosolic receptors NOD1, NOD2, RIG-1, and MDA5 with the Fallopian tube expressing the highest amounts constitutively. Poly(I:C) treatment upregulated mRNA for all sensors except NOD1, whereas exposure to GC selectively upregulated NOD2 and MDA5 mRNA. With HIV, exposure to the CXCR4-tropic IIIB strain but not CCR5-tropic BaL resulted in an upregulation of NOD2 and MDA5 by EM EC. In contrast, estradiol had no effect on the expression of NOD1, NOD2, RIG-1 and MDA5 in epithelial cells from either the upper or the lower tract. To our knowledge, this is the first comprehensive demonstration of intracellular cytosolic receptors in epithelial cells throughout the FRT. This study further demonstrates the differential regulation of selective receptors by pathogens and pathogenic mimics.
Previously believed to be sterile, the upper FRT is now recognized as being continuously exposed to pathogens 40. It has been shown that pathogens can reach upper FRT by binding to sperm shortly after ejaculation 40. Given that sexually transmitted pathogens have access to the upper FRT, we studied both the upper and lower tract in terms of expression of intracellular pathogenic sensors in epithelial cells that line the lumen and serve as the first line of defense against potential pathogens. Our findings indicate that NOD1, NOD2, RIG-1 and MDA5 are constitutively expressed and expression levels vary considerably throughout the FRT when compared in the same patient or between multiple patients. Interestingly, FT showed the highest expression of the four genes analyzed whereas EM and/or Ecx typically showed the lowest expression relative to the other sites. The high constitutive expression of pathogenic sensors in the FT might indicate a mechanism where, if a pathogen reaches the FT, it could be rapidly eliminated. Physiologically, the FT might require a high degree of vigilance as it opens into the peritoneal cavity. Failure to protect at this level would lead to infection in the peritoneal cavity, followed by entry into the bloodstream leading to systemic infection. Previously we observed high constitutive expression in epithelial cells from the FT of MIP-3α and Trappin-2/Elafin, endogenous antimicrobials that also have anti-HIV functions 41, 42. The present study extends our earlier findings of antimicrobial expression by demonstrating that FT epithelial cell expression of intracellular PRR is higher than all other FRT epithelial cells examined in this study.
Others have demonstrated that NOD1 and NOD2 are expressed in the FRT and are responsive to iE-DAP and MDP 17, 18. We have confirmed and extended these findings in our demonstration of NOD1, NOD2, as well as RIG-1 and MDA5 in the FRT. To the best of our knowledge, the present study is the first to demonstrate the presence of RIG-1 and MDA5 in the FRT. In the current study, poly(I:C) induced NOD2, RIG-1, and MDA5 and, to a much lesser extent, NOD1 in all four compartments of the FRT. We also observed significantly higher secretion of IL-8 from the FRT epithelial cells following poly(I:C) treatment. Since we and others have previously shown that poly(I:C) induces signaling through TLR3 pathway in the FRT 14, 31, 32, we cannot definitively determine whether the IL-8 upregulation was a result of signaling through TLR, NLR, or RLR pathways. Further studies are needed to define the relative contributions of these receptors to viral pathogens.
Since estradiol is a major regulator of immune functions in the FRT, we were surprised when we observed no effect of estradiol on any of the four PRR. Interestingly we observed previously that estradiol had no direct effect on the expression of TLR (Schaefer, unpublished observations) or interferon-induced antiviral factors MxA, OAS, PKR (Patel et al, in press). It is well known that estrogens have diverse regulatory functions that can be both pro-inflammatory and anti-inflammatory 34. Numerous studies have shown that the epithelial cells of the FRT have intracellular estrogen receptors capable of binding to the estrogen response elements in the promoter regions of various genes, thereby affecting their transcription 34, 43. It is possible that the effects of hormones on NOD1, NOD2, RIG-1 and MDA5 could be indirect. Estradiol has been shown to act indirectly on the FRT EC by stimulating growth factor production by the underlying stromal cells 2, 44, 45. It is also possible that innate immune protection is so critical to the FRT that the receptors which recognize potential pathogens, specifically the TLRs, NOD-and RIG-like-receptors, remain constant throughout the menstrual cycle.
Unlike several bacterial and viral pathogens have been studied in terms of their recognition by NLR and RLR, little has been published in regards to the sexually transmitted pathogen Neisseria gonorrhea. We observed that treatment of FRT EC with heat-killed GC resulted in modest upregulation of NOD2 and MDA5 by Cx EC. This response correlated with a significant upregulation of IL-8, thereby demonstrating a distinct functional response to GC. To our knowledge, this is the first demonstration of GC recognition by intracellular receptors of the FRT. Further studies are required to determine which intermediates in the GC lifecycle promote recognition by these PRR. In our study, whereas GC enhanced NOD2 and MDA5 expression by Cx EC, no effect was observed in the FT, a site affected by GC pathology. One explanation is that FT cells must be infected by viable GC in order to be responsive. Alternatively, since we observed very high constitutive levels of NOD1, NOD2, RIG-1, and MDA5 by FT EC, it is possible that no further induction by GC was possible. Additionally, we decided upon a 6 hrs GC treatment as that is the time-point when we observed upregulation of the intracellular receptors upon poly(I:C) treatment. Since the time course might be dependent upon the pathogen tested, the timing of GC response can be very different. We plan to address optimizing GC response in FRT EC in future studies.
A recent study by Solis et al reported that in macrophages, RIG-1 but not MDA5 can bind HIV RNA 29. In our study, however, we found that FRT epithelial cells when exposed to HIV selectively upregulate NOD2 and MDA5. The absence of RIG-1 upregulation in our system might indicate the fundamental differences in host immune responses depending on location. Genital mucosa is a unique location where HIV will first “see” the epithelial cells during sexual transmission. Under these circumstances the virus might be recognized by a set of very different innate immune sensors. Although NOD2 is classically a bacterial sensor, recent studies have implicated it in viral recognition 7. However, to our knowledge, this is the first demonstration of both NOD2 and MDA5 upregulation as a result of HIV exposure. Another interesting aspect of our data is that the X4-tropic virus IIIB seemed to be “recognized” more readily by the innate immune sensors. Typically in sexual transmission of HIV, R5-tropic viruses are the ones selectively transmitted although the exact mechanism for this remains controversial 46, 47. Several studies have indicated that the R5 viruses are able to transcytose through the epithelial barrier whereas the X4 might be able to replicate in the epithelial cells and thereby is not able to pass through unnoticed by the immune system 48, 49. Our data extend these previous findings by demonstrating that the X4 not the R5 virus can upregulate both NOD2 and MDA5 thereby inducing an immune response which might lead to its sequestration or destruction. This is an area of future investigations with larger sample size and including EC from all compartments of the FRT.
Overall, the present study demonstrates that epithelial cells from both the upper and lower female reproductive tract differentially express cytosolic receptors NOD1, NOD2, RIG-1, MDA5. Receptor mRNA expression is selectively stimulated by poly(I:C) as well as Neisseria gonorrhea and HIV-1 but not by estradiol. From a physiological perspective, our findings add yet another layer to the complexity of immune responses in the FRT. Each compartment in the FRT has different functions and therefore can respond differentially to pathogens. The balance between immune tolerance required for implantation and immune activation required to protect against pathogenic invasions is intricately regulated by multiple parameters that can only be fully defined upon further extensive investigations. Our findings suggest that NOD- and RIG-like-receptors throughout the FRT contribute to a level of previously unrecognized protection against pathogens.
Acknowledgments
FUNDING
This work was supported by the National Institute of Health [AI51877 and AI071761 C.W.].
The authors thank Richard Rossoll, M.S., Yan Song, M.Sc., and Danica Hickey, Ph.D. at Dartmouth Medical School for excellent technical assistance in the preparation of samples, cells and useful edits and comments.
Additionally, the authors would like to thank the staff in the Department of Pathology at Dartmouth-Hitchcock Medical Center, and the patients who participated in our study.
References
- 1.Hickey DK, Patel MV, Fahey JV, Wira CR. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. J Reprod Immunol. 2011;88:185–194. doi: 10.1016/j.jri.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 2010;63:544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostuni R, Zanoni I, Granucci F. Deciphering the complexity of Toll-like receptor signaling. Cell Mol Life Sci. 2010;67:4109–4134. doi: 10.1007/s00018-010-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 5.Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3:371–382. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- 6.Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, Gale M, Jr, Akira S, Yonehara S, Kato A, Fujita T. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 7.Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. Activation of innate immune antiviral responses by Nod2. Nature immunology. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 9.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 10:688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fensterl V, Sen GC. Interferons and viral infections. Biofactors. 2009;35:14–20. doi: 10.1002/biof.6. [DOI] [PubMed] [Google Scholar]
- 11.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv Drug Deliv Rev. 2008;60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 15.Aflatoonian R, Fazeli A. Toll-like receptors in female reproductive tract and their menstrual cycle dependent expression. J Reprod Immunol. 2008;77:7–13. doi: 10.1016/j.jri.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Abrahams VM, Mor G. Toll-like receptors and their role in the trophoblast. Placenta. 2005;26:540–547. doi: 10.1016/j.placenta.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 17.King AE, Horne AW, Hombach-Klonisch S, Mason JI, Critchley HO. Differential expression and regulation of nuclear oligomerization domain proteins NOD1 and NOD2 in human endometrium: a potential role in innate immune protection and menstruation. Mol Hum Reprod. 2009;15:311–319. doi: 10.1093/molehr/gap020. [DOI] [PubMed] [Google Scholar]
- 18.Hart KM, Murphy AJ, Barrett KT, Wira CR, Guyre PM, Pioli PA. Functional expression of pattern recognition receptors in tissues of the human female reproductive tract. J Reprod Immunol. 2009;80:33–40. doi: 10.1016/j.jri.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaushic C, Ferreira VH, Kafka JK, Nazli A. HIV infection in the female genital tract: discrete influence of the local mucosal microenvironment. Am J Reprod Immunol. 2010;63:566–575. doi: 10.1111/j.1600-0897.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- 20.Sonnex C. Toll-like receptors and genital tract infection. Int J STD AIDS. 2010;21:153–157. doi: 10.1258/ijsa.2009.009525. [DOI] [PubMed] [Google Scholar]
- 21.CDC. 2009 http://www.cdc.gov/std/stats09/gonorrhea.htm.
- 22.Fichorova RN, Desai PJ, Gibson FC, 3rd, Genco CA. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun. 2001;69:5840–5848. doi: 10.1128/IAI.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan JA, Gao X, Huang MT, O’Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182:6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UNAIDS. UNAIDS Publication; 2011. http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/20111130_UA_Report_en.pdf). In. [Google Scholar]
- 25.HIVAIDS. HIVAIDS; 2009. http://www.kff.org/hivaids/upload/6092-09.pdf. [Google Scholar]
- 26.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 27.Meier A, Alter G, Frahm N, Sidhu H, Li B, Bagchi A, Teigen N, Streeck H, Stellbrink HJ, Hellman J, van Lunzen J, Altfeld M. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. Journal of virology. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gringhuis SI, van der Vlist M, van den Berg LM, den Dunnen J, Litjens M, Geijtenbeek TB. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nature immunology. 2010;11:419–426. doi: 10.1038/ni.1858. [DOI] [PubMed] [Google Scholar]
- 29.Solis M, Nakhaei P, Jalalirad M, Lacoste J, Douville R, Arguello M, Zhao T, Laughrea M, Wainberg MA, Hiscott J. RIG-I-mediated antiviral signaling is inhibited in HIV-1 infection by a protease-mediated sequestration of RIG-I. Journal of virology. 2011;85:1224–1236. doi: 10.1128/JVI.01635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa Y, Kawamura T, Kimura T, Ito M, Blauvelt A, Shimada S. Gram-positive bacteria enhance HIV-1 susceptibility in Langerhans cells, but not in dendritic cells, via Toll-like receptor activation. Blood. 2009;113:5157–5166. doi: 10.1182/blood-2008-10-185728. [DOI] [PubMed] [Google Scholar]
- 31.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–1446. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh M, Schaefer TM, Fahey JV, Wright JA, Wira CR. Antiviral responses of human Fallopian tube epithelial cells to toll-like receptor 3 agonist poly(I:C) Fertil Steril. 2008;89:1497–1506. doi: 10.1016/j.fertnstert.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meter RA, Wira CR, Fahey JV. Secretion of monocyte chemotactic protein-1 by human uterine epithelium directs monocyte migration in culture. Fertil Steril. 2005;84:191–201. doi: 10.1016/j.fertnstert.2005.01.104. [DOI] [PubMed] [Google Scholar]
- 34.Fahey JV, Wright JA, Shen L, Smith JM, Ghosh M, Rossoll RM, Wira CR. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol. 2008;1:317–325. doi: 10.1038/mi.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godfrey TE, Kim SH, Chavira M, Ruff DW, Warren RS, Gray JW, Jensen RH. Quantitative mRNA expression analysis from formalin-fixed, paraffin-embedded tissues using 5′ nuclease quantitative reverse transcription-polymerase chain reaction. J Mol Diagn. 2000;2:84–91. doi: 10.1016/S1525-1578(10)60621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia D, Sanders A, Shah M, Bickerstaff A, Orosz C. Real-time polymerase chain reaction analysis reveals an evolution of cytokine mRNA production in allograft acceptor mice. Transplantation. 2001;72:907–914. doi: 10.1097/00007890-200109150-00028. [DOI] [PubMed] [Google Scholar]
- 37.Pioli PA, Weaver LK, Schaefer TM, Wright JA, Wira CR, Guyre PM. Lipopolysaccharide-induced IL-1 beta production by human uterine macrophages up-regulates uterine epithelial cell expression of human beta-defensin 2. J Immunol. 2006;176:6647–6655. doi: 10.4049/jimmunol.176.11.6647. [DOI] [PubMed] [Google Scholar]
- 38.Schaefer TM, Wright JA, Pioli PA, Wira CR. IL-1beta-mediated proinflammatory responses are inhibited by estradiol via down-regulation of IL-1 receptor type I in uterine epithelial cells. J Immunol. 2005;175:6509–6516. doi: 10.4049/jimmunol.175.10.6509. [DOI] [PubMed] [Google Scholar]
- 39.Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65:196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piomboni P, Baccetti B. Spermatozoon as a vehicle for HIV-1 and other viruses: a review. Mol Reprod Dev. 2000;56:238–242. doi: 10.1002/(SICI)1098-2795(200006)56:2+<238::AID-MRD5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. Trappin-2/Elafin: a novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology. 129:207–219. doi: 10.1111/j.1365-2567.2009.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR. CCL20/MIP3alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am J Reprod Immunol. 2009;62:60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 44.Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coleman KD, Wright JA, Ghosh M, Wira CR, Fahey JV. Estradiol modulation of hepatocyte growth factor by stromal fibroblasts in the female reproductive tract. Fertil Steril. 2009;92:1107–1109. doi: 10.1016/j.fertnstert.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee B, Doranz BJ, Ratajczak MZ, Doms RW. An intricate Web: chemokine receptors, HIV-1 and hematopoiesis. Stem Cells. 1998;16:79–88. doi: 10.1002/stem.160079. [DOI] [PubMed] [Google Scholar]
- 47.Margolis L, Shattock R. Selective transmission of CCR5-utilizing HIV-1: the ‘gatekeeper’ problem resolved? Nature reviews Microbiology. 2006;4:312–317. doi: 10.1038/nrmicro1387. [DOI] [PubMed] [Google Scholar]
- 48.Asin SN, Fanger MW, Wildt-Perinic D, Ware PL, Wira CR, Howell AL. Transmission of HIV-1 by primary human uterine epithelial cells and stromal fibroblasts. The Journal of infectious diseases. 2004;190:236–245. doi: 10.1086/421910. [DOI] [PubMed] [Google Scholar]
- 49.Saidi H, Magri G, Nasreddine N, Requena M, Belec L. R5- and X4-HIV-1 use differentially the endometrial epithelial cells HEC-1A to ensure their own spread: implication for mechanisms of sexual transmission. Virology. 2007;358:55–68. doi: 10.1016/j.virol.2006.07.029. [DOI] [PubMed] [Google Scholar]