Abstract
The objective of this study was to evaluate the relationship of amyloid burden, as assessed by florbetapir F 18 (18F-AV-45) amyloid PET, and cognition in healthy older control subjects (HC). Seventy-eight HC subjects were assessed with a brief cognitive test battery and PET imaging with florbetapir F 18. A standard uptake value ratio (SUVr) was computed for mean data from six cortical regions using a whole cerebellum reference region. Scans were also visually rated as amyloid positive (Aβ+) or amyloid negative (Aβ−) by three readers. Higher SUVr correlated with lower immediate memory (r=−0.33; p=0.003) and delayed recall scores (r=−0.25; p=0.027). Performance on immediate recall was also lower in the visually rated Aβ+ compared to Aβ− HC (p=0.04), with a similar trend observed in delayed recall (p=0.06). These findings support the hypothesis that higher amyloid burden is associated with lower memory performance among clinically normal older subjects. Longitudinal follow-up is ongoing to determine whether florbetapir F 18 may also predict subsequent cognitive decline.
Introduction
Converging evidence from autopsy, cerebrospinal fluid, and PET amyloid imaging studies suggests that a substantial proportion of clinically normal older individuals harbor amyloid-β pathology, one of the hallmark pathologic features of Alzheimer's disease (AD). This apparent dissociation between clinical and pathological status has sometimes been presented in refutation of the “amyloid cascade hypothesis” of AD. A number of recent studies, however, have reported that the presence of amyloid-β markers in clinically normal older individuals is associated with subtle functional and structural imaging alterations consistent with abnormalities seen in patients with AD dementia or mild cognitive impairment (MCI) (Becker, et al., 2011,Dickerson, et al., 2009,Hedden, et al., 2009,Mormino, et al., 2011,Sheline, et al., 2009,Sperling, et al., 2009). The relationship between amyloid-β burden and cognition among clinically normal older individuals remains to be fully elucidated, as the small number of studies published to date have yielded somewhat variable results (Mormino, et al., 2009,Pike, et al., 2007,Pontecorvo and MA, 2011,Rentz, et al., 2011,Rentz, et al., 2010,Resnick and Sojkova, 2011,Rodrigue, et al., 2012,Storandt, et al., 2009,Villemagne, et al., 2008).
Florbetapir (also known as 18F-AV-45) is an 18F labeled PET imaging tracer thought to bind to fibrillar forms of amyloid (Choi, et al., 2009). A recent autopsy study demonstrated high sensitivity and specificity of florbetapir in detection of amyloid pathology in comparison to autopsy verified histopathology (Clark, et al., 2011). In this study, we investigated the relationship of amyloid burden and cognition in a group of clinically normal, healthy older controls (HC) recruited for the multi-center Phase II study of florbetapir F18 PET imaging. As episodic memory impairment is one of the earliest and most salient symptoms reported in AD, we hypothesized that high amyloid burden would be associated with lower memory performance scores, even within the range of an older HC population.
Methods
The Phase II 18-F AV-45 PET study was conducted at 24 academic and private practice sites in order to recruit a large cohort of HC, MCI, and mild AD patients. HC subjects were recruited to be distributed approximately equally across age deciles (50–59, 60–69, 70–79 and ≥80 years of age) to better understand the interaction between age and probability of a positive scan. Subjects were recruited from existing normal cohorts at the sites or via advertisements. All subjects underwent a screening clinical assessment including a Clinical Dementia Rating Scale, MMSE, Geriatric Depression Scale, and a brief cognitive test battery, which included the Wechsler Logical Memory I and II Story A Immediate (WLM-I) and Delayed recall (WLM-D), Digit-Symbol substitution (DSS), verbal fluency (animals and vegetables), and the ADAS-Cog (11-item cognitive subscale). Eligible HC subjects were required to be > 50 years of age, and to have a reliable informant regarding their cognitive status. HC were further required to have a Clinical Dementia Rating Scale of 0 and an MMSE of 29–30, and not to meet criteria for MCI or AD dementia. Moreover the entry criteria for the study were such that if a subject had a subjective cognitive complaint that was verified by an informant (supporting a CDR of 0.5) they were placed in the MCI group, regardless of psychometric test scores. Subjects were also required to have an MRI performed within 6 months of enrollment or to undergo an MRI at screening to rule out any significant CNS lesions. Subjects with clinically significant cardiac or cerebrovascular disease (either by history or on MRI) were excluded. As these MRI scans were not performed with a standard protocol, the MRI data are not available for volumetric analyses.
Subjects underwent PET amyloid imaging during a 10-minute acquisition (acquired in 2×5 minutes frames), 50 minutes after intravenous injection of 10 mCi (370 MBq) of florbetapir F 18. The data was acquired from 22 different sites using ECAT HR+ (Siemens), Discovery LS PET/CT (General Electric), 16 slice Biograph PET/CT (Siemens) and Advance PET (GE) scanners. Data were reconstructed using an iterative reconstruction algorithm (4 iteration 16 subsets) with a post reconstruction Gaussian filter of 5mm. A semi-automated technique was used for image quantification and analysis as previously reported (Clark, et al., 2011,Fleisher, et al., 2011,Wong, et al., 2010). The two frames of each image were averaged and normalized to a florbetapir template in Talairach space using SPM-2. No partial volume correction was performed. Previously defined volumes of interest (VOI) representing grey matter regions known to be vulnerable to amyloid deposition in frontal, temporal, and parietal cortices, anterior cingulate, posterior cingulate, and precuneus were used to extract counts for each region. Standard Uptake Value ratios (SUVr) were calculated using whole cerebellum as the reference region. The average of the SUVr across the 6 cortical target regions was used for analysis.
Scans were also visually interpreted by 3 independent nuclear medicine physicians blinded to clinical information, and scored on a 5-point scale (0–4 rating), where 0 represented no appreciable cortical gray matter retention of compound, and 4 represented high specific cortical uptake ≥ white matter background, with multiple cortical regions strongly positive relative to cerebellum. Each scan was then assigned a qualitative rating of amyloid-β positive (Aβ+) or amyloid negative (Aβ−) where a score of 2 was defined as the minimum value for a positive scan. There was a significant inter-rater correlation (r=0.76 to 0.83, p<0.0001) between any 2 of the 3 raters. When simplified to a dichotomous Aβ+ or Aβ− rating, 2 of the 3 readers (reader 1 and reader 3) had excellent agreement with each other, with a kappa coefficient of 0.86. The third rater (reader 2) used consistently higher scores on the 0–4 scale, and thus had only moderate agreement with the other readers (kappa coefficient of 0.46 and 0.48). The median (majority) of the three readers' qualitative (Aβ+ or Aβ−) scores was used for this analysis. Overall (including all AD and MCI subjects enrolled in the Phase II trial), the median visual rating was statistically significantly correlated with the mean cortical SUVr (r=0.808, p<0.0001).
In order to assess the relationship between SUVr and cognitive performance and between SUVr and age, Pearson's correlation coefficient was calculated with a corresponding 2-sided significance test at the .05 significance level. To determine if there was a significant difference between the cognitive scores in the visually rated Aβ+ group compared to the Aβ− groups, a 2-sided t-test was performed comparing the mean of each group at the .05 significance level. Lastly, a stepwise regression was carried out to determine the best predictors of cognitive score. Each model used amyloid level (SUVr or blinded qualitative read), age, education level (in years) and APOE ε4 status as independent variables. In order to remain in the model, each variable needed to maintain a p-value < 0.15. We evaluated the appropriateness of using parametric statistics for these analyses with the Kolmogorov-Smirnov test by examining the normality of the residuals from linear regression analyses; p-values were all > 0.05 indicating no significant deviations from normality. The statistical analyses performed for this study were exploratory and the reported p-values were not adjusted for multiple comparisons. Nominal p-values < 0.05 were considered significant.
Results
Seventy-nine older subjects were enrolled as HC into the study. One HC subject received florbetapir but was not imaged due to a scanner failure, and thus is not included in the analyses below. The demographic variables are listed in Table 1. The mean age of the HC subjects was 69.4 ± 11.1 years old (Mean±SD, range 50–92), and the mean MMSE was 29.6 ± 0.5. The HC subjects had a mean of 15.2 ± 2.3 years of education; all HC subjects had at least a high school education, and 73.1% had attended at least some college or had an advanced degree. Seventy-two of the 78 HC underwent APOE genotyping, and 22.2% of those 72 possessed 1 or 2 ε4 alleles, consistent with the general population estimates (Roses and Saunders, 1997). The percentage of HC subjects with a positive family history of a first-degree relative diagnosed with dementia was only 17.9%, and this was similar in Aβ+ (18.2 %) and Aβ− (17.9%) groups.
Table 1.
Baseline Demographic Variables according to Aβ status on visual interpretation of Florbetapir images
| All HC (N=78)§ | Aβ+ (N=11)§ | Aβ− (N=67)§ | Aβ+ vs Aβ−* | |
|---|---|---|---|---|
| Age (yr) | 69.4 ±11.11 | 75.6 ±9.42 | 68.4 ±11.09 | p=0.0437 |
| APOE ε4 non-carrier APOE ε4 carrier** Not genotyped |
56 (71.8%) 16 (20.5%) 6 (7.7%) |
7 (63.6%) 3 (27.3%) 1 (9.1%) |
49 (73.1%) 13 (19.4%) 5 (7.5%) |
p=0.6821 |
| Education (yr) | 15.2 ±2.32 | 15.5 ±1.37 | 15.2 ±2.44 | p=0.6303 |
| MMSE | 29.6 ±0.50 | 29.5 ±0.52 | 29.6 ±0.50 | p=0.4928 |
| Race (Caucasian) | 71 (91.0%) | 10 (90.9%) | 61 (91.0%) | p=0.6708 |
| Gender (Female) | 44 (56.4%) | 5 (45.5%) | 39 (58.2%) | p=0.5190 |
Mean ±SD for continuous variables and count (percent) for categorical variables.
P-values for continuous variables are from t-tests and from Fisher's exact test on categorical variables
APOE ε4 carrier with at least one e4 allele
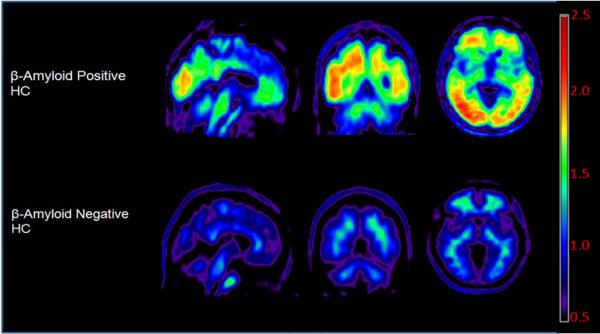
The mean florbetapir SUVr for the HC was 1.051 ± 0.16. Eighteen of the HC (23%) had SUVr values above 1.10, the pre-defined cutoff for negative florbetapir F18 scans as determined in a previous study with young subjects (Joshi et al., 2012). Eleven out of the 78 HC were also identified as Aβ+ on the qualitative blinded read, and 8/11 of these individuals were over the age of 70. With the exception of age, there were no significant differences between subjects visually rated as Aβ+ and those rated Aβ− (Table 1). Figure 1 shows representative images from Aβ− and Aβ+ HC subjects.
Figure 1.
Representative amyloid positive (Aβ+) and amyloid negative (Aβ−) clinically normal healthy controls (HC). Images have been fitted to Talairach space with each voxel normalized to the cerebellar reference region.
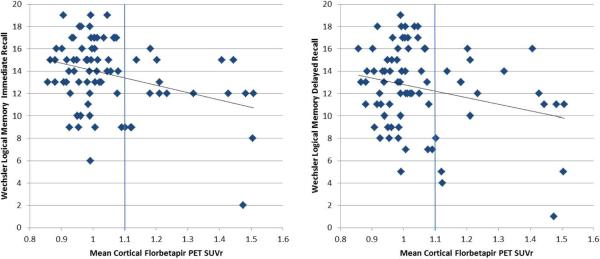
We first calculated the correlation between the mean cortical SUVr and the neuropsychological test performance variables across the entire HC group. There was a significant relationship between SUVr and performance on both memory measures: WLM-I (r=−.331; p=0.0032) and WLM-D (r=−.251; p=0.0270) (see Figure 2), as well as the ADAS (r=.288; p=0.0104), and DSS (r=−.24; p=0.0346), such that higher amyloid-β burden was associated with worse performance (Table 2).
Figure 2.
Wechsler Logical Memory Story A Immediate (left) and Delayed (right) recall scores as a function of mean cortical amyloid (SUVr), in the unadjusted regression analyses.
Table 2.
Correlation of Florbetapir PET SUVr with Cognitive Test Performance in Clinically Normal Healthy Controls
| ADAS | DSS | Verbal Fluency Vegetables | Verbal Fluency Animals | WLM-I | WLM-D | |
|---|---|---|---|---|---|---|
| Pearson's Pairwise Correlation | r = 0.288 p=0.0052 |
r = −0.240 p=0.0173 |
r = −0.002 p=0.4926 |
r = −0.017 p=0.4413 |
r = −0.331 p<0.0016 |
r = −0.251 p=0.0135 |
| Stepwise Regression: Parameter Estimate (p) | ||||||
| Intercept | −2.131 (0.3350) | 76.843 (<0.0001) | NA | NA | 20.381 (<0.0001) | 18.331 (<0.0001) |
| Age | 0.045 (0.0916) | −0.407 (<0.0001) | E | E | E | E |
| Education | E | E | E | E | E | E |
| APOE | E | E | E | E | E | E |
| SUVR | 3.488 (0.0600) | E | E | E | −6.340 (0.0060) | −5.542 (0.0470) |
E indicates that this variable was eliminated from the model as it did not meet the 0.15 p-value criterion for inclusion.
Across all HC subjects, the mean cortical SUVr was associated with age (r=.315; p=0.01), and the percentage of Aβ+ increased by decade, such that 25% of subjects above age 80 were Aβ+. There was also a trend for an overall difference in SUVr as a function of APOE genotype (APOE ε4 carriers vs ε3,3 vs APOE 2 carriers, p=0.0776). As age, education and APOE ε4 status are also known to have effects on cognitive performance, we then performed multiple stepwise regression analyses with SUVr, age, education and APOE ε4 status included as potential predictors in the model (Table 2). Both memory measures still showed a significant relationship with SUVr but not with any other predictor: Immediate recall: SUVr, p=0.006; Delayed recall: SUVr, p=0.0470. In contrast, DSS showed no significant relationship with SUVr (partial r=−.101; p=0. 383), but a strong relationship with age (p<0. 0001).
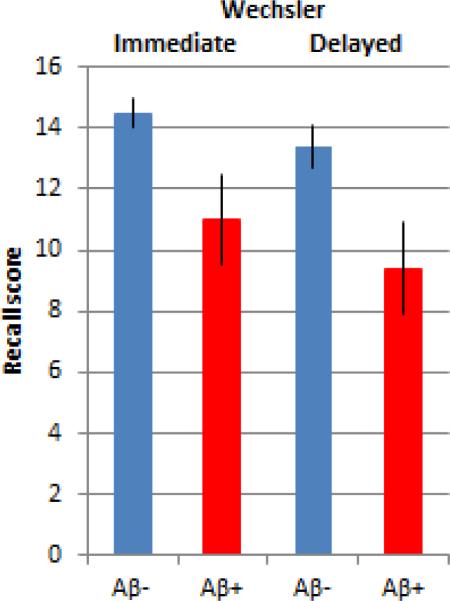
We also performed analyses with subjects dichotomously classified as amyloid-β positive (Aβ+) or negative (Aβ−). When subjects were classified on the basis of SUVr (Aβ− <1.10, Aβ+ ≥1.10) we found significant differences on the ADAS, DSS, WLM-I and WLM-D (Table 3). We also performed analyses based on the blinded qualitative read that classified subjects as Aβ+ or Aβ−. The 11 HC subjects classified as Aβ+ demonstrated lower performance on WLM-I and DSS with a trend towards lower performance on WLM-D (p=0.060), and ADAS-cog (p=0.052) (Table 3). As the Aβ+ group was older (75.6 +/− 9.4) than the Aβ− group (68.4 +/− 11.1), we repeated these analyses in the subset of HC who were ≥ 70 years of age (median age of all HC subjects). Although there were only 8 subjects ≥ 70 years of age who were rated qualitatively Aβ+, we still found differences between the Aβ+ and Aβ− groups, such that Aβ+ older subjects performed worse on the WLM-I (p=0.009) and WLM-D (p=0.015; Figure 3).
Table 3.
Cognitive Test Performance in Aβ+ and Aβ− Clinically Normal Healthy Controls
| ADAS | DSS | Verbal Fluency: Vegetables | Verbal Fluency: Animals | WLM-I | WLM-D | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUVR Classification | <1.1 (n=60) | >=1.1 (n=18) | <1.1 (n=60) | >=1.1 (n=18) | <1.1 (n=60) | >=1.1 (n=18) | <1.1 (n=60) | >=1.1 (n=18) | <1.1 (n=60) | >=1.1 (n=18) | <1.1 (n=60) | >=1.1 (n=18) |
| Mean±StdErr | 4.3±0.3 | 6.2±0.7 | 50.1±1.3 | 44.6±1.8 | 14.0±0.4 | 13.8±1.0 | 20.2±0.5 | 19.1±1.5 | 14.3±0.4 | 11.7±0.8 | 13.1±0.4 | 10.6±1.0 |
| P-value | 0.0026 | 0.0343 | 0.7962 | 0.4969 | 0.0013 | 0.0136 | ||||||
| ADAS | DSS | Verbal Fluency: Vegetables | Verbal Fluency: Animals | WLM-I | WLM-D | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual rating | Aβ − (n=67) | Aβ + (n=11) | Aβ − (n=67) | Aβ + (n=11) | Aβ − (n=67) | Aβ + (n=11) | Aβ − (n=67) | Aβ + (n=11) | Aβ − (n=67) | Aβ + (n=11) | Aβ − (n=67) | Aβ + (n=11) |
| Mean±StdErr | 4.5±0.3 | 6.0±0.8 | 49.9±1.2 | 42.3±2.2 | 13.9±0.4 | 14.2±1.4 | 19.9±0.5 | 20.1±2.2 | 14±0.4 | 11.9±1.2 | 12.8±0.4 | 10.5±1.3 |
| P-value | p=0.0520 | p=0.0160 | 0.8402 | 0.9325 | p=0.0401 | p=0.0611 | ||||||
ADAS = Alzheimer's Disease Assessment Scale; DSS= Digit Symbol Substitution; WLM-I= Wechsler Logical Memory –Immediate Recall; WLM-D = Wechsler Logical Memory –Delayed Recall
Figure 3.
Wechsler Logical Memory Story A performance compared between amyloid positive (n=8) and amyloid negative (n=32) clinically normal healthy controls ≥ 70 years old. Amyloid positivity was determined by visual rating. Amyloid positive older individuals performed significantly worse on both Immediate (p=0.009) and Delayed (p=0.015) recall measures.
As the number of Aβ+ subjects in our study was relatively small and the relationship between amyloid burden and cognition appeared to be driven partly by two subjects with very high amyloid burden and low logical memory scores (bottom right quadrant of figures in Figure 2), we conducted several post-hoc analyses without these two subjects. With these subjects removed, the statistical significance with the WLM was lost in the regression models, however, the correlation between the ADAS-COG and SUVr also continued to show a trend (r=0.223, p=0.054). Moreover, the dichotomized analyses based on SUVr still demonstrated worse cognition in the Aβ+ on the ADAS-COG (mean difference=1.67, p=0.0092) and WLM-I (mean difference=−1.83, p=0.02) compared to Aβ− subjects, even with the two high Aβ+ subjects removed.
Discussion
Our findings in subjects recruited as healthy controls in a multi-site Phase II PET amyloid imaging study suggest that the presence of occult amyloid-β deposition is associated with reduced episodic memory performance. These results remained significant in stepwise multiple regression analyses that included age and APOE. The HC subjects underwent a relatively brief neuropsychological battery that was geared towards detection of cognitive deficits in MCI and AD, and were determined to be “normal” by the site investigators. Nevertheless, amyloid-associated decrements in episodic memory performance were still evident in this apparently healthy control group. Although the relationship between amyloid and cognition is modest given the restricted range of neuropsychological scores in the healthy control group, our findings from this multi-site Phase II study add to the growing literature that suggests that high levels of amyloid accumulation in “normal” older individuals are associated with very subtle cognitive deficits.
These HC subjects were recruited from a variety of academic and clinical sites, and were required to have a near-perfect score on MMSE (29 or 30) and a normal CDR. Subjects with any evidence of corroborated cognitive complaint were placed in the MCI cohort. Thus, it is very unlikely that there was intermingling of the HC group in this study with subjects that might be classified as MCI or AD in other studies and we believe these HC subjects would have been considered cognitively normal in standard clinical practice. Similarly, there was the expected normal population frequency of the APOE ε4 allele in this group and percentage of subjects with a positive family history of dementia consistent with the general population estimates (Huang, et al., 2004,Roses and Saunders, 1997). Our results demonstrate that, although all of these HC subjects were still considered clinically normal, amyloid-β accumulation was associated with lower memory performance. Our finding is consistent with two prior autopsy studies demonstrating relationships between lower episodic memory performance and post-mortem AD pathology, specifically amyloid plaque burden, among community-dwelling older adults free of MCI or dementia prior to death (Bennett, et al., 2006,Price, et al., 2009). It is important to acknowledge, however, that the relationship between amyloid and cognition among normal controls has been variable in the PET studies published to date, and that the effects may be driven by a relatively small number of subjects who have very elevated Aβ levels.
The overall percentage of amyloid positive HC in this study (14%) is lower than in some previous reports with 11-C PiB (Jack, et al., 2010,Jack, et al., 2008,Mintun, et al., 2006,Pike, et al., 2007,Rowe, et al., 2010,Vlassenko, et al., 2011), which may in part reflect somewhat different populations, particularly the inclusion of younger subject groups in the present study. Consistent with other reports, 25% of the HC over age 80 were Aβ+, but it is also possible that the stringent MMSE inclusion criteria screened out a few more Aβ+ older subjects who would still be classified as normal in other studies. This multi-center study also did not recruit healthy controls on the basis of family history, and had a lower proportion of APOE e4 carriers than several other cohorts that may have a relative over-representation of individuals with strong family histories of AD (Rowe, et al., 2010,Vlassenko, et al., 2011). It is also theoretically possible that F18 PET amyloid imaging agents are less sensitive to low levels of amyloid deposition; however, until direct comparison between the C11 PiB and F18 PET amyloid imaging is performed in large numbers of healthy controls, hypotheses related to the differential sensitivity for detection of low levels of amyloid deposition cannot be adequately tested. Despite the relative small number of HC subjects with clearly positive amyloid scans in the present study, we still found a significant relationship between amyloid burden and episodic memory performance.
Our findings from this multi-center study should be put in the context of a somewhat variable literature on the relationship of amyloid burden and neuropsychological performance in clinically normal older individuals. Whereas some studies have reported no relationship between β-amyloid deposition as estimated by PET imaging and cognitive performance (Aizenstein, et al., 2008,Jack, et al., 2008,Mintun, et al., 2006,Rowe, et al., 2010,Storandt, et al., 2009), several others have reported significant inverse correlations between amyloid burden and baseline memory performance, as well as longitudinal decline in cognition (Chetelat, et al., 2011,Kantarci, et al., 2012,Knopman, et al., 2012,Pike, et al., 2007,Rentz, et al., 2010,Resnick, et al., 2010,Roe, et al., 2008,Rosenberg, et al., In press,Villemagne, et al., 2011). A recent cross-sectional amyloid imaging study across a wide age range reported correlations between amyloid levels and processing speed, working memory, and reasoning, but not episodic memory (Rodrigue, et al., 2012). Another PET study found a relationship between amyloid and memory performance only in the setting of hippocampal atrophy (Mormino, et al., 2008). Differences in subject selection and the neuropsychological batteries utilized may partially explain the divergent results across studies. Two studies have demonstrated that cognitive reserve can partially mask the effects of amyloid deposition on cognitive performance in clinically normal older individuals (Rentz, et al., 2010,Roe, et al., 2008). One recent study found a relationship between amyloid burden and cognition only in women (Pike, et al., 2011), and another large single-site study reported a relationship only among APOE ε4 carriers (Kantarci, et al., 2012). Additionally, for all of these studies, the potential relationship between amyloid burden on the PET scan and cognitive performance is limited by the entry criteria for “normal”. Aβ+ subjects who show even subtle cognitive impairment may tend to be placed into the MCI category, particularly in centers that specialize in early diagnosis, and in this study, participants with any corroborated subjective cognitive complaints were classified as MCI. As a result, potential effect size for cognitive performance within the normal group is constrained and, as in the present study, is necessarily relatively modest. Consistent with this notion, scatter plots of PET binding vs. cognition in the present study (Figure 2), and in some previous studies (Pike, et al., 2007,Rentz, et al., 2010), are notable for the absence of high performers in the Aβ+ groups, rather than a preponderance of really poor performers.
A remaining question is whether fibrillar forms of amyloid-β are responsible for the cognitive deficits, as laboratory data suggest that oligomeric forms may be more directly toxic to synaptic function (Shankar, et al., 2008,Walsh, et al., 2002). Although it is possible that fibrillization of amyloid-β and plaque formation represent a protective sequestration phenomemona, it is likely that there are oligomeric forms of amyloid-β in equilibrium, perhaps in so-called “toxic halos” surrounding plaques (Koffie, et al., 2009,Lesne, et al., 2008). Thus, although all PET tracers for amyloid detection bind more avidly to β pleated sheet structures (Villemagne, et al., 2008), they may serve as good proxies for the presence of other more soluble forms of amyloid-β. A related question is whether evidence of high fibrillar amyloid burden is also a marker that these individuals harbor “downstream” AD pathology, such as neurofibrillary tangles or neuronal loss. The previous report that the range of memory impairment among Aβ+ clinically normal individuals is mediated through hippocampal volume (Mormino, et al., 2008), supports the hypothesis that amyloid accumulation is necessary but may not be sufficient to result in memory impairment in the absence of neurodegeneration (Jack, et al., 2010,Sperling, et al., 2011a).
There are several limitations to our study. The analyses with specific cognitive measures were exploratory and the reported p-values were unadjusted for multiple comparisons. These results therefore require replication in other studies. Nevertheless, our key finding that amyloid-β burden was associated with lower memory performance in normal elderly individuals was observed consistently whether we analyzed the data using an SUVr cutpoint, visual reads, correlational analyses, or stepwise regressions with other covariates, and across multiple neuropsychological measures of memory. The sample size, and particularly the number of amyloid positive clinically normal cognitively healthy controls was relatively small, and potentially vulnerable to effects of outliers; nevertheless, even after removing two subjects who had very high florbetapir uptake and low Logical Memory scores, we still found evidence that Aβ+ subjects performed worse on some cognitive measures. From this data set, it is difficult to determine whether there is truly a continuous relationship between amyloid burden and cognition in clinically normal subjects (e.g., Table 2) or whether the observed correlations are driven by a dichotomous relationship (e.g., Table 3). The brief neuropsychological battery included in this study did not measure all domains of cognition. We did find evidence of an effect of amyloid-β burden on a measure of attention/speed of processing; however, this was not significant in some analyses that included age as a covariate. Future studies with more sensitive measures of executive function may reveal additional deficits in this and other domains of cognition. We do not have high resolution volumetric MRI scans with a uniform acquisition protocol available on these HC subjects to determine if they had evidence of cortical thinning or hippocampal volume loss in addition to amyloid-β burden. Finally, although it is likely that these subjects in a multi-center study recruited from a variety of settings are somewhat more representative of the older population than some previous reports, they still represent a “convenience” sample of subjects who are relatively highly educated. For all of the above reasons, larger studies in community-based samples across a range of socio-economic status are needed to fully elucidate the relationship between amyloid burden and cognition in the general elderly population. In this vein, it is particularly important to note a recently published large community-based study using PIB PET imaging that also found significant correlations of PiB amyloid load with both memory and global cognition among normal older adults, particularly those individuals carrying one or more APOE ε4 alleles (Kantarci, et al., 2012).
The question as to whether the relationship between amyloid burden and cognitive performance in clinically normal older individuals represents a continuum or evidence of a “state” or “threshold” effect remains to be disambiguated. The range of amyloid burden in our sample is likely truncated by the rigorous requirements for being a “healthy normal control”, and it is clear there is a tail end of the distribution that partially drives the observed relationship in our study, as well as in majority of the published studies. It is likely that large longitudinal studies will be required to determine if there is a clear threshold above which all normal older subjects will eventually manifest cognitive impairment. Nevertheless, our findings from this multi-center study suggest that very subtle cognitive impairments may exist in some Aβ+ subjects that might otherwise be considered cognitively normal on routine clinical evaluation. A recent study from the Mayo Clinic found that Aβ+ HC with evidence of neurodegenerative markers and lower cognitive performance were indeed more likely to progress to MCI over a one-year period (Knopman, et al., 2012). Identification of such individuals could be a potentially welcome advance, particularly if treatment interventions were available that could prevent further cognitive decline (Sperling, et al., 2011b).
In summary, we found preliminary evidence of a relationship between episodic memory performance and amyloid-β deposition as estimated by florbetapir F18 among a group of clinically normal healthy controls. Our findings, the first from a multi-center study, add to the growing evidence that amyloid-β accumulation may not be benign in older individuals. Recent longitudinal studies, both retrospective and prospective, of normal older individuals imaged with 11C-PiB have suggested an association between higher amyloid burden and more rapid deterioration on cognitive tests (Resnick, et al., 2010,Storandt, et al., 2009,Villemagne, et al., 2008) or an increase in the risk of change in cognitive status from normal to MCI (Knopman, et al., 2012) or early dementia (Morris, et al., 2009). Similarly, studies with cerebrospinal fluid measurement of amyloid-β in clinically normal older individuals have reported that low CSF A-β1–42 (also thought to represent evidence of cerebral A-β accumulation) confers increased risk of cognitive decline (Fagan, et al., 2007,Li, et al., 2007). Longitudinal follow-up of the present and other cohorts imaged with florbetapir F18 are ongoing to determine if these Aβ+ clinically normal individuals are indeed in the preclinical stages of AD (Sperling, et al., 2011a), and will demonstrate cognitive decline towards MCI and AD dementia.
Acknowledgments
Drs. Sperling and Johnson's efforts were supported by P01AG036694– the Harvard Aging Brain Study. The authors wish to acknowledge the clinical research staffs at all of the A05 study centers, and the dedication of research participants in this study. This paper is dedicated to Dr. Chris Clark, who sadly passed away during the preparation of this manuscript, but contributed greatly to this work and the field of Alzheimer's disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Sperling has served as a site investigator for Avid, Bristol-Myers-Squibb, Elan, Janssen, Pfizer, and Wyeth, a consultant to Bayer, Bristol-Myers-Squibb, Elan, Eisai, Janssen, Pfizer, and Wyeth, and an unpaid consultant to Avid. She has received speaking honoraria from Pfizer, Janssen, Eli Lilly, and Bayer. Dr. Johnson has served as a site investigator for Avid, Pfizer, Janssen, Bristol-Myers-Squibb, and as a consultant to Bristol-Myers-Squibb and Bayer. Dr. Doraiswamy has served as a paid advisor/speaker and/or received research grants from Avid/Lilly and several pharmaceutical companies. He owns stock in Sonexa and Clarimedix. Dr. Reiman has served as a paid consultant to Eli Lilly for its amyloid-modifying drug development programs, but has not served as a paid consultant to Eli Lilly or Avid for the development of florbetapir F18, and has served as a site investigator for Eli Lilly and Avid. Dr. Fleisher is a consultant for Lilly SAB and has received grant funding from Avid. Dr Sabbagh has served as a site investigator for Avid, BMS, Elan, Janssen, Pfizer, Wyeth, Baxter, Bayer, GE, Lilly, Genentech, Eisai, Ceregene, and Celgene, and a consultant to BMS, Bayer, Lilly, Avid, Amerisciences, Eisai, and Takeda. Dr. Sadowsky is on the speaker's bureau for Novartis, Forest and Axona, and is a site investigator for Avid. Dr. Davis performed statistical analysis under a consulting contract from Avid. Dr. Grundman is a paid consultant to Avid, Eli Lilly and other pharmaceutical companies. Drs. Carpenter, Davis, Flitter, Joshi, Mintun, Skovronsky and Pontecorvo are all employees of Avid Radiopharmaceuticals.
References
- 1.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65(11):1509–17. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, Fischl B, Greve DN, Marshall GA, Salloway S, Marks D, Buckner RL, Sperling RA, Johnson KA. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69(6):1032–42. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett D, Schneider J, Arvanitakis Z, Kelly J, Aggarwal N, Shah R, Wilson R. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 4.Chetelat G, Villemagne VL, Pike KE, Ellis KA, Bourgeat P, Jones G, O'Keefe GJ, Salvado O, Szoeke C, Martins RN, Ames D, Masters CL, Rowe CC. Independent contribution of temporal beta-amyloid deposition to memory decline in the pre-dementia phase of Alzheimer's disease. Brain. 2011;134(Pt 3):798–807. doi: 10.1093/brain/awq383. [DOI] [PubMed] [Google Scholar]
- 5.Choi S, Golding G, Zhuang Z, Zhang W, Lim N, Hefti F, Benedum T, Kilbourn M, Skovronsky D, Kung H. Preclinical properties of 18 F-AV-45: A PET agent for AB plaques in the brain. J Nucl Med. 2009;50:1887–994. doi: 10.2967/jnumed.109.065284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, Pontecorvo MJ, Hefti F, Carpenter AP, Flitter ML, Krautkamer MJ, Kung HF, Coleman RE, Doraiswamy PM, Fleisher A, Sabbagh M, Sadowsky C, Reiman EM, Zehntner SP, Skovronsky D, Group A-AS. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–83. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64(3):343–9. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 9.Fleisher AS, Chen K, Liu X, Roontiva A, Thiyyagura P, Ayutyanont N, Joshi AD, Clark CM, Mintun MA, Pontecorvo MJ, Doraiswamy PM, Johnson KA, Skovronsky DM, Reiman EM. Using Positron Emission Tomography and Florbetapir F 18 to Image Cortical Amyloid in Patients With Mild Cognitive Impairment or Dementia Due to Alzheimer Disease. Arch Neurol. 2011 doi: 10.1001/archneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- 10.Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29(40):12686–94. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang W, Qiu C, von Strauss E, Winblad B, Fratiglioni L. APOE genotype, family history of dementia, and Alzheimer disease risk: a 6-year follow-up study. Arch Neurol. 2004;61(12):1930–4. doi: 10.1001/archneur.61.12.1930. [DOI] [PubMed] [Google Scholar]
- 12.Jack CR, Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR, Jr., Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Knopman DS, Boeve BF, Klunk WE, Mathis CA, Petersen RC. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131(Pt 3):665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, Adler LP, Kovnat KD, Seibyl JP, Arora A, Saha K, Burns JD, Lowrey MJ, Mintun MA, Skovronsky DM. Florbetapir F 18 Study Investigators. Performance characteristics of amyloid PET with florbetapir F 18 in patients with alzheimer's disease and cognitively normal subjects. J Nucl Med. 2012 Mar;53(3):378–84. doi: 10.2967/jnumed.111.090340. [DOI] [PubMed] [Google Scholar]
- 15.Kantarci K, Lowe V, Przybelski SA, Weigand SD, Senjem ML, Ivnik RJ, Preboske GM, Roberts R, Geda YE, Boeve BF, Knopman DS, Petersen RC, Jack CR. APOE modifies the association between Abeta load and cognition in cognitively normal older adults. Neurology. 2012;78(4):232–40. doi: 10.1212/WNL.0b013e31824365ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knopman DS, Jack CR, Jr., Wiste HJ, Weigand SD, Vemuri P, Lowe V, Kantarci K, Gunter JL, Senjem ML, Ivnik RJ, Roberts RO, Boeve BF, Petersen RC. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78(20):1576–82. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009;106(10):4012–7. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lesne S, Kotilinek L, Ashe KH. Plaque-bearing mice with reduced levels of oligomeric amyloid-beta assemblies have intact memory function. Neuroscience. 2008;151(3):745–9. doi: 10.1016/j.neuroscience.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Kaye JA, Raskind MA, Zhang J, Peskind ER, Montine TJ. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69(7):631–9. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 20.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–52. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 21.Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ. Episodic memory loss is related to hippocampal-mediated {beta}-amyloid deposition in elderly subjects. Brain. 2008 doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(Pt 5):1310–23. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mormino EC, Smiljic A, Hayenga AO, Onami SH, Greicius MD, Rabinovici GD, Janabi M, Baker SL, Yen IV, Madison CM, Miller BL, Jagust WJ. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex. 2011;21(10):2399–407. doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA. Pittsburgh Compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66(12):1469–75. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pike KE, Ellis KA, Villemagne VL, Good N, Chetelat G, Ames D, Szoeke C, Laws SM, Verdile G, Martins RN, Masters CL, Rowe CC. Cognition and beta-amyloid in preclinical Alzheimer's disease: Data from the AIBL study. Neuropsychologia. 2011;49(9):2384–90. doi: 10.1016/j.neuropsychologia.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007;130(Pt 11):2837–44. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 27.Pontecorvo M, MA M. PET amyloid imaging as a tool for early diagnosis and identifying patients at risk for progression to Alzheimer's disease. Alzheimer's Research and Therapy. 2011;3:11. doi: 10.1186/alzrt70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price JL, McKeel DW, Jr., Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30(7):1026–36. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rentz DM, Amariglio RE, Becker JA, Frey M, Olson LE, Frishe K, Carmasin J, Maye JE, Johnson KA, Sperling RA. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia. 2011;49(9):2776–83. doi: 10.1016/j.neuropsychologia.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Sperling RA, Johnson KA. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67(3):353–64. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resnick SM, Sojkova J. Amyloid imaging and memory change for prediction of cognitive impairment. Alzheimers Res Ther. 2011;3(1):3. doi: 10.1186/alzrt62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, Dannals RF, Mathis CA, Klunk WE, Ferrucci L, Kraut MA, Wong DF. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74(10):807–15. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigue KM, Kennedy KM, Devous MD, Sr., Rieck JR, Hebrank AC, Diaz-Arrastia R, Mathews D, Park DC. β-Amyloid burden in healthy aging: Regional distribution and cognitive consequences. Neurology. 2012;78(6):387–95. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roe CM, Mintun MA, D'Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol. 2008;65(11):1467–71. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg PB, Wong DF, Edell S, et al. Cognition and amyloid load in Alzheimer's imaged with florbetapir F18 (AV-45) PET. Am J Geriatr Psychiatry. doi: 10.1016/j.jagp.2012.11.016. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roses AD, Saunders AM. Apolipoprotein E genotyping as a diagnostic adjunct for Alzheimer's disease. Int Psychogeriatr. 1997;9(Suppl 1):277–88. doi: 10.1017/s1041610297005012. discussion 317–21. [DOI] [PubMed] [Google Scholar]
- 37.Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O'Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–83. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2009;67(6):584–7. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr., Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011a;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sperling RA, Jack CR, Jr., Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011b;3(111):111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–88. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66(12):1476–81. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, Ackermann U, Jones G, Szoeke C, Salvado O, Martins R, O'Keefe G, Mathis CA, Klunk WE, Ames D, Masters CL, Rowe CC. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69(1):181–92. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villemagne VL, Pike KE, Darby D, Maruff P, Savage G, Ng S, Ackermann U, Cowie TF, Currie J, Chan SG, Jones G, Tochon-Danguy H, O'Keefe G, Masters CL, Rowe CC. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer's disease. Neuropsychologia. 2008;46(6):1688–97. doi: 10.1016/j.neuropsychologia.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Vlassenko AG, Mintun MA, Xiong C, Sheline YI, Goate AM, Benzinger TL, Morris JC. Amyloid-beta plaque growth in cognitively normal adults: longitudinal [11C]Pittsburgh compound B data. Ann Neurol. 2011;70(5):857–61. doi: 10.1002/ana.22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 48.Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, Dannals RF, Nandi A, Brasic JR, Ye W, Hilton J, Lyketsos C, Kung HF, Joshi AD, Skovronsky DM, Pontecorvo MJ. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18) J Nucl Med. 2010;51(6):913–20. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]