Abstract
Inflammation of medium-sized, muscular arteries and coronary artery aneurysms are hallmarks of Kawasaki disease (KD), an acute, self-limited vasculitis of children. We previously reported that genetic variation in transforming growth factor (TGF)-β pathway genes influences both susceptibility to KD and coronary artery aneurysm (CAA) formation. TGF-β signaling has been implicated in the generation of myofibroblasts that influence collagen lattice contraction, antigen presentation, and recruitment of inflammatory cells as well as the generation of regulatory T-cells (Tregs). These processes could be involved in aneurysm formation and recovery in KD. Coronary artery tissues from 8 KD patient autopsies were stained to detect proteins in the TGF-β pathway, to characterize myofibroblasts, and to detect Tregs. Expression of proteins in the TGF-β pathway was noted in infiltrating mononuclear cells and spindle-shaped cells in the thickened intima and adventitia. Coronary arteries from an infant who died on Illness Day 12 showed α-smooth muscle actin (SMA)-positive, smoothelin-negative myofibroblasts in the thickened intima that co-expressed IL-17 and IL-6. CD8+ T-cells expressing HLA-DR+ (marker of activation and proliferation) were detected in the aneurismal arterial wall. Forkhead box P3 (FOXP3), whose expression is essential for Tregs, was also detected in the nucleus of infiltrating mononuclear cells, suggesting a role for Tregs in recovery from KD arteritis. TGF-β may contribute to aneurysm formation by promoting the generation of myofibroblasts that mediate damage to the arterial wall through recruitment of pro-inflammatory cells. This multi-functional growth factor may also be involved in the induction of Tregs in KD.
Keywords: endothelial-to-mesenchymal transition, myofibroblast, TGF-β, coronary artery aneurysm, Kawasaki disease
Introduction
Kawasaki disease (KD) is a self-limited vasculitis that is the most common cause of acquired heart disease in children [1]. Neither the trigger for the acute inflammation nor the mechanisms through which the immune activation is down-regulated are known. Based on echocardiographic data, 20–30% of KD patients will develop transient dilatation of the coronary arteries and 5–9% will develop coronary artery aneurysms despite timely therapy with intravenous immunoglobulin (IVIG) [2, 3].
The importance of transforming growth factor (TGF)-β in KD was initially highlighted by our finding that genetic variation in the TGF-β pathway influences both susceptibility and risk of coronary artery aneurysms (CAA) [4]. Single nucleotide polymorphisms (SNPs) in a ligand (TGFB2), a receptor (TGF-β receptor 2: TGFBR2), and a key signaling molecule (SMAD family member 3: SMAD3) in the pathway were associated with both KD susceptibility and formation of CAA. We also demonstrated that genes in the TGF-β signaling pathway were differentially expressed in whole blood during the acute and convalescent stages of KD. Based on these data, we predicted that TGF-β signaling would be important in the genesis of vasculitis. TGF-β signaling has been implicated in the generation of myofibroblasts that influence collagen lattice contraction, antigen presentation, and recruitment of inflammatory cells as well as the generation of regulatory T-cells (Tregs). Therefore we postulated that TGF-β induces transformation of cells of different lineages to myofibroblasts that mediate damage to the arterial wall. We further postulated a role for TGF-β in the genesis of Tregs and the down-regulation of inflammation based on our T-cell cloning studies using acute KD peripheral blood [5].
In this study, we investigated expression of proteins in the TGF-β pathway in the coronary arterial wall from KD autopsies. To test the hypothesis that myofibroblasts in the arterial wall participate in aneurysm formation, we performed immunohistochemical (IHC) staining for markers of smooth muscle cells (SMCs) (smoothelin), myofibroblasts (α-smooth muscle actin (SMA)), pro-inflammatory cytokines (Interleukin (IL)-17 and IL-6), mature T-cells (CD3+), cytotoxic T-cells (CD8+) and their activation/proliferation marker human leukocyte antigen (HLA)-DR. The potential anti-inflammatory effect of TGF-β was tested by IHC staining for nuclear FOXP3, a marker for Tregs.
Materials and Methods
Subjects
All subjects met American Heart Association (AHA) clinical criteria for KD during their acute illness [6]. CAA were defined according to Japanese Ministry of Health criteria as a coronary artery segment with an internal diameter 1.5 times that diameter of the adjacent segment [7]. All protocols were approved by the Institutional Review Board at University California, San Diego (UCSD) and Toho University, Japan, and written parental consent was obtained for the use of all tissues.
Tissues
We obtained formalin-fixed, paraffin embedded tissues from 8 KD patients (age 2m-5y2m) who expired 12 days-20m after onset of fever and from 2 age-similar control children who died from complications of Tetralogy of Fallot (TOF) and idiopathic acute encephalopathy.
Immunohistochemical staining
Tissue sections were deparaffinized and rehydrated. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide in methanol. Antigen retrieval was performed either in citrate buffer (unmasking solution, Vector Laboratories, Cat. H-3300) or in ethylenediaminetetraacetic acid (EDTA) (pH 9.0 for CD8) buffer in a microwave oven for 10 minutes. Slides were incubated with 1.5% normal goat or horse serum blocking solution at room temperature for 30 min (Vector Laboratories) then incubated in PBS with 1.5% goat or horse serum overnight at 4°C or for 60 minutes at room temperature with the following antibodies: TGF-β2 (sc-90), TGF-βR2 (sc-400), pSMAD2/3 (sc-11769), Smoothelin (sc-28562), IL-17 (sc-7927) from Santa Cruz Biotechnology; Connective tissue growth factor (CTGF) (ab5097), IL-6 (ab6672), Forkhead box P3 (FOXP3) (ab10901), HLA-DR (ab92511) from Abcam; α-SMA (M0851), CD3 (N1580), CD8 (IS623) from DAKO (detailed information in Supplemental Table 1). Antibodies were detected using biotin-avidin, ABC (Vectastain Rabbit: PK-6101, Mouse: PK-6102) followed by visualization with the AEC substrate kit (Invitrogen 00-2007) or biotin-streptavidin, LSAB2 System-HRP (DAKO K0675) followed by coloring with AEC or PowerVision Poly-AP IHC Detection Systems (Leica Biosystems, Cat. PV6133) and coloring with permanent red (DAKO, K0640) according to the manufacturer’s instructions (Supplemental Table 1). Negative staining controls were performed using either normal rabbit immunoglobulin G (IgG) (Dako, Cat. X0936) or mouse universal negative control (Dako, Cat. N1698).
For immunofluorescent double-staining, tissue sections were treated as described previously except without quenching endogenous peroxidase activity and with blocking buffer containing 5% normal donkey serum. Tissue sections were incubated with antibodies for α-SMA, smoothelin, IL-17, IL-6, HLA-DR, and CD8 overnight at 4°C. Binding of antibodies was detected following incubation with donkey anti-mouse Dyelight 488 (Jackson Immunoresearch laboratory, Cat. 715-485-150) (1:100 to 200) for α-SMA and donkey anti-rabbit Dyelight 594 (Jackson Immunoresearch laboratory, Cat. 711-515-152) (1:200) for the other antibodies. Negative control primary antibodies were normal rabbit IgG (Dako, Cat. X0936) and mouse universal negative control (Dako, Cat. N1698). Background fluorescent intensities were subtracted from staining with each specific antibody.
Image capture
Digital images were captured using Motic Images (Advanced v. 3.2) and on a Motic microscope for light microscopic observation. Fluorescent images were captured using Softwarx (v.4.1.0) on a DeltaVision RT Deconvolution Images inverted microscope. Filters used to detect fluorescence were as follows: DeltaVision RT Deconvolution Images, Ex 480–500nm and Em 508–548nm for DyeLight 488 conjugated donkey anti-mouse IgG, Ex 540–570nm and Em 580–654nm for DyeLight 594 conjugated donkey anti-rabbit IgG and Ex 340–380nm and Em 427–577nm for DAPI. Images were acquired sequentially to ensure that individual channels were devoid of fluorescence from other emission sources.
Results
Cells in the arterial wall express proteins in the TGF-β pathway
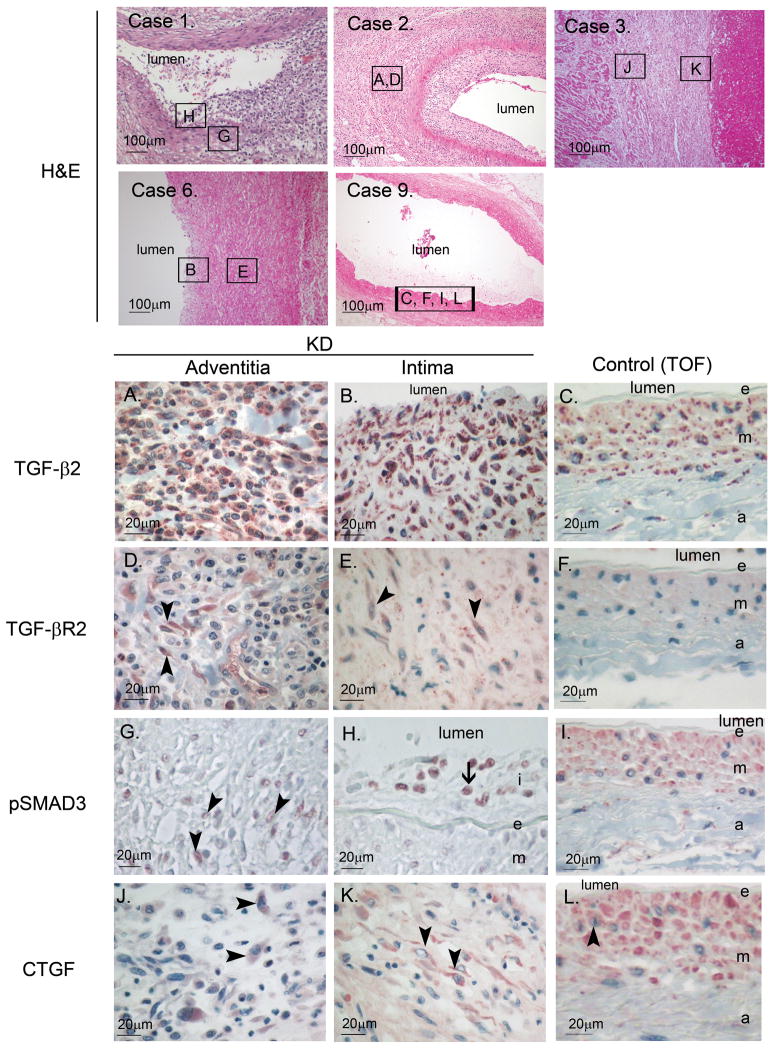
All coronary arteries from KD subjects demonstrated thickening of the intima with or without inflammatory infiltrates or obliteration of the intima associated with thrombosis by H&E staining (Supplemental Figure 1). Positive staining for the receptor, TGF-βR2 (Figure 1, D–F, Figure 2), was observed in spindle-shaped cells in the thickened intima and adventitia of arteries from KD autopsies (Figure 1D and 1E arrow heads) but not control tissues (Figure 1F). In KD Case 1, for whom we had both proximal as well as distal, intramuscular coronary artery segments, only the proximal segment expressed TGF-βR2 in the thickened intima (Figure 2). The ligand, TGF-β2 (Figure 1, A–C, Figure 2), was expressed by cells in all layers of the coronary artery as well as by inflammatory cells infiltrating the arterial wall of KD subjects (Figure 1A and 1B), but was expressed mainly by smooth muscle cells in media of controls (Figure 1C). Staining to detect nuclear localization of activated SMAD3 (Figure 1G and 1H, arrow heads) and the downstream target protein, CTGF (Figure 1J and 1K, arrow heads), yielded variable results in KD subjects with some positive staining in mononuclear cells and spindle-shaped cells. In control tissues, nuclear-localized pSMAD3 staining was not detected (Figure 1I) and CTGF staining was positive only in SMCs in the media and endothelial cells but not in spindle-shaped cells in the adventitia or intima (Figure 1L). Coronary arteries from subjects who died later after the onset of KD (3–20 mos) tended to have fewer cells expressing TGF-βR2, pSMAD3, and CTGF (Figure 2).
Figure 1. TGF-β2, TGF-βR2, pSMAD3 and CTGF expression in coronary arterial wall in KD and control autopsy tissues.
Case 1, 2, 3, 6 (KD), and 9 (Tetrology of Fallot (TOF) negative control) (×100): H&E staining of coronary arteries. Boxed area indicates location of section used for IHC shown in A-L. TGF-β2 (A–C): Positive staining of mononuclear and spindle-shaped cells in KD arteries (A and B). Control shows rare positively stained spindle-shaped cell in adventitia (C). TGF-βR2 (D–F): Positive staining of spindle-shaped cells (arrow head) only in KD arteries (D and E) but not control tissues (F). pSMAD3 (G–I): Positive nuclear staining of spindle-shaped cells (arrow head) in adventitia and mononuclear cells (arrow) in intima from KD artery (G and H) but not control (I). CTGF (J–L): Positive staining of spindle-shaped cells (arrow head) in adventitia and intima of KD artery (J and K) and media of control artery (L). TGF-β2: transforming growth factor-β2, TGF-βR2: TGF-β receptor 2, pSMAD3: phosphorylated SMAD3, CTGF: connective tissue growth factor.
Figure 2.
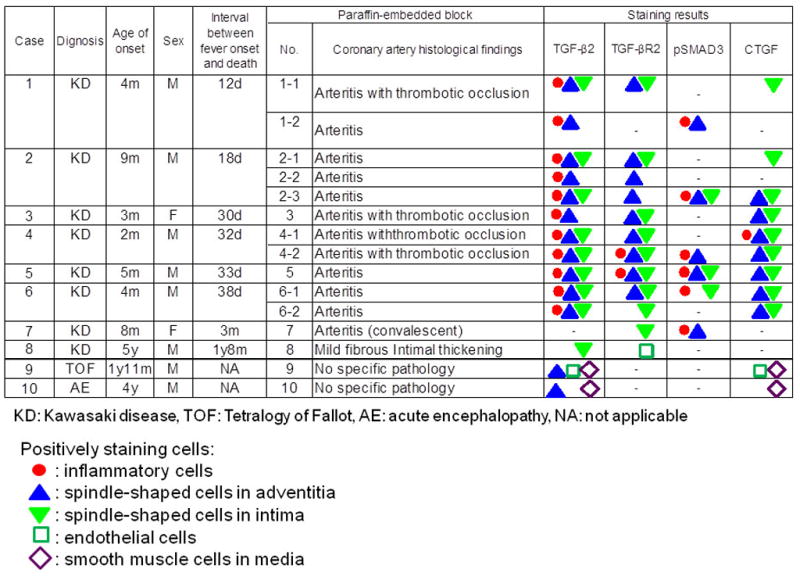
Expression of TGF-β2, TGF-βR2, pSMAD3 and CTGF in KD and control tissues.
Myofibroblasts in the arterial wall
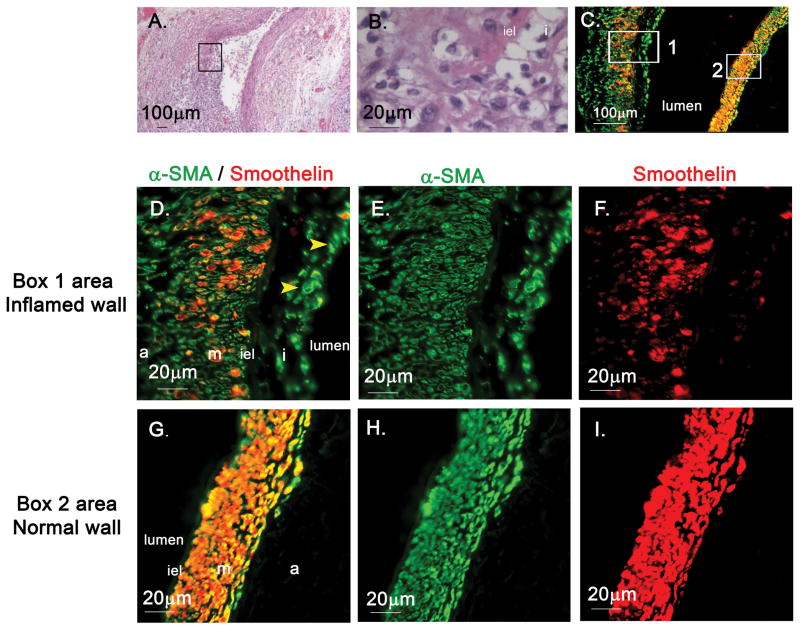
To study the mechanism by which TGF-β signaling may contribute to the development of arteritis, we sought myofibroblasts in the coronary arteries from Case 1, a 3 month-old male who died without IVIG treatment from a myocardial infarction on the 12th day after fever onset. To detect myofibroblasts in the arterial wall, we performed double staining for α-SMA and smoothelin. Cells expressing both markers were considered to be mature SMC, while cells expressing α-SMA but not smoothelin were considered to be myofibroblasts [8–10]. A longitudinal section through the coronary artery included one wall of the dilated artery that was unaffected and one wall that was highly inflamed (Figure 3 A, B). α-SMA alone was expressed by cells in the edematous, thickened intima and adventitia from the inflamed arterial wall suggesting a myofibroblast phenotype (Figure 3 C–I). SMC in the media of both the normal and abnormal vessel wall segments expressed both markers. (Supplemental Figure 2: co-localization analysis). α-SMA+/smoothelin–cells (myofibroblast phenotype) were also seen in the thickened intima and adventitia of the inflamed right coronary and mesenteric arteries (data not shown) of Case 1. To identify myofibroblasts in the thickened intima from other KD CA sections, we stained the sections from Cases 2–8 for expression of α-SMA. Expression was detected in the thickened intima and media of the coronary arteries or occluded coronary artery aneurysms (Supplemental figure 3).
Figure 3. α-SMA and smoothelin staining of coronary artery from Case 1 (Illness Day 12).
A–B, Coronary artery aneurysm from Case 1 (H&E, A: ×40, B: ×400), A: One side of the coronary arterial wall is well-preserved with normal architecture. Opposite wall shows disruption of arterial wall structures, thickened initma, and focal breaks in internal elastic lamina (iel) and infiltration of inflammatory cells (boxed area magnified in B). C–I: α-SMA(green) and smoothelin (red) staining of coronary artery(boxed area 1 magnified in D–F, boxed area 2 magnified in G–I), C, D and G: merged fluorescence α-SMA+/smoothelin-cells (myofibroblasts) in thickened intima(arrowhead in D). Double-staining smooth muscle cells (orange) in media(D and G). α-SMA: α-smooth muscle actin.
Secretion of cytokines by myofibroblasts in the arterial wall
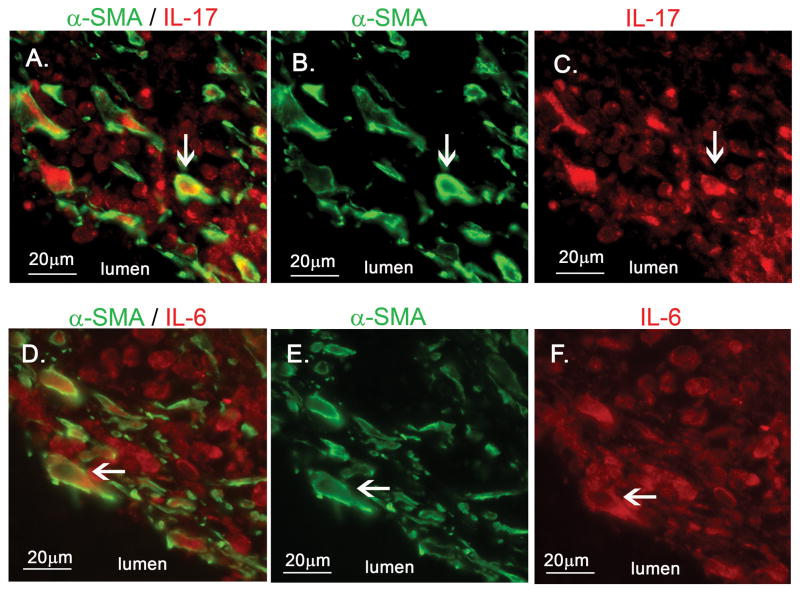
To understand how myofibroblasts might mediate damage in the arterial wall, we studied expression of cytokines by myofibroblasts. Using double staining, α-SMA+ myofibroblasts in the intima expressed IL-17 and IL-6 (Figure 4). IL-17-positive spindle-shaped cells were observed in both the coronary and mesenteric arteries from Case 1 who died on Illness Day 12 (Supplemental figure 4A, B). Weak staining of IL-17 was observed in spindle-shaped cells in the thickened intima of the coronary artery from Case 2 who died on Illness Day 18 (Supplemental figure 4C). Because IL-17 induces inflammation by recruiting neutrophils and T-cells, we stained the thickened coronary artery intima from Case 2 for CD3, a marker for mature T-cells, and infiltrating CD3+ T-cells were identified (Supplemental figure 4E).
Figure 4.
Fluorescent double staining for α-SMA with either IL-17 or IL-6 of coronary artery from Case 1 (Illness Day 12). A–C, α-SMA (green) with IL-17 (red). D–F, α-SMA (green) with IL-6 (red). α-SMA positive myofibroblasts expressing IL-17 (A) and IL-6 (D).
CD8+/HLA-DR+ mononuclear cells in the coronary arterial wall
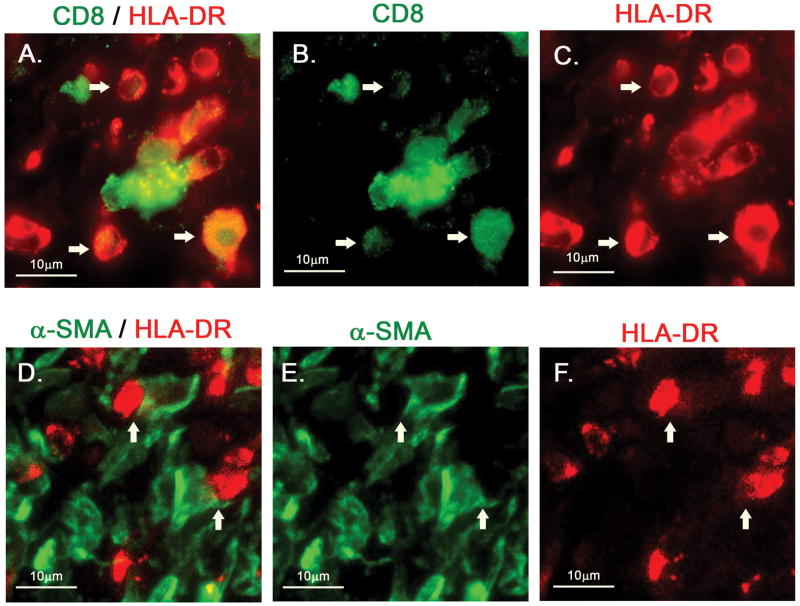
Double staining for CD8 and HLA-DR in the thickened intima of the coronary arterial wall from Case 1 showed that many CD8+ T-cells express HLA-DR, suggesting an activated phenotype (Figure 5. A–C, white arrows). Myofibroblasts were seen in close proximity to these activated T-cells (Figure 5, D–F, white arrows).
Figure 5. HLA-DR+/CD8+ mononuclear cells in arterial wall of a KD aneurysm.
Thickened intima of coronary arterial wall from Case 1 was stained. A–C, double staining for CD8 (green) with HLA-DR (red). Many of the HLA-DR+ round, mononuclear cells are also CD8+ (white arrows). D–F, double staining for α-SMA (green) with HLA-DR (red). α-SMA+ myofibroblasts adjacent to HLA-DR+ mononuclear cells (white arrows).
Regulatory T-cells in the coronary arterial wall
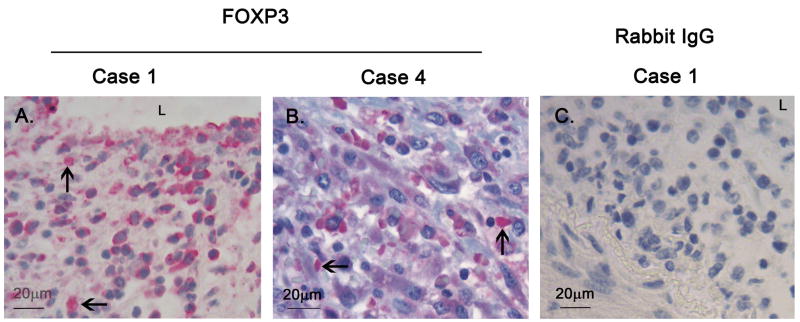
Because the TGF-β pathway may also participate in the differentiation of naive and Th17+T-cells toward a regulatory phenotype, we stained the thickened intima of the coronary arterial wall from Case 1 for expression of the transcription factor FOXP3, which is a hallmark of regulatory T-cells (Tregs). Nuclear-localized FOXP3 was observed in mononuclear cells infiltrating the arterial wall (Figure 6A and B).
Figure 6. Immunohistochemical staining of mononuclear cells with nuclear FOXP3 in KD arterial wall.
A and B: Thickened intima of coronary arteries from Case 1 (A) and Case 4 (B) showing mononuclear cells expressing FOXP3 in the nucleus (arrows). C: Rabbit IgG negative antibody control. FOXP3: Forkhead box P3, L: lumen.
Discussion
We previously reported that genetic variation in TGF-β pathway genes influences susceptibility to KD and CAA formation [4]. Here we demonstrate expression of TGF-β pathway gene products in cells in the damaged coronary arterial wall from KD autopsies. We further identified cells with a myofibroblast phenotype that can result from mesenchymal transition mediated by TGF-β. These myofibroblasts in the arterial wall expressed IL-17 and IL-6 in addition.
Myofibroblasts are reported to secrete many inflammatory cytokines and chemokines and to present antigen through MHC Class I and II [11–13]. This is the first report to show IL-17-expressing myofibroblasts in the damaged coronary arterial wall from autopsies of children after KD. IL-17 induces a strong pro-inflammatory response by stimulating secretion of IL-6, several growth factors including granulocyte macrophage colony-stimulating factor (GMCSF) and vascular endothelial growth factor (VEGF), and chemokines (CXCL8 (IL-8), CXCL1/3/5/6) that recruit neutrophils and T-cells [14]. Both neutrophils and T-cells (predominantly CD8+) have been documented in the intima and media in KD autopsy tissues [15, 16]. Therefore, recruitment of activated CD8+ T-cells to the arterial wall may contribute to aneurysm formation. Infiltration of HLA-DR+ mononuclear cells into the arterial wall has been previously reported in KD arteritis [17]. Cells with a phenotype consistent with HLA-DR+ mature myeloid dendritic cells have been reported adjacent to CD3+ cells in the arterial wall suggesting that in situ activation of the T-cells may contribute to the pro-inflammatory state in the arterial wall [18]. Here we present data suggesting that myofibroblasts may also have a role in recruiting and activating T-cells in the arterial wall.
Epithelial or endothelial to mesenchymal transition (EMT) is a process that generates myofibroblasts during normal development and also in disease conditions such as cancer, transplant rejection, and transplant vasculopathy [19]. Through this process, myofibroblasts can be generated from endothelial cells, resident fibroblasts in the adventitia, SMCs, and bone marrow-derived, circulating fibrocytes [19]. During EMT, cells transitioning to myofibroblasts up-regulate α-SMA expression and down-regulate expression of other markers associated with their original phenotype such as smoothelin in the case of SMCs. Although myofibroblasts were originally described based on their morphology by transmission electron microcroscopy, it is now accepted that myofibroblasts can be recognized by their IHC phenotype with expression of α-SMA in the absence of smoothelin [20]. Cells expressing α-actin and TGFB1were reported previously in thickened intima from KD autopsies but TGFB1 was not expressed in age-matched control coronary arteries [21]. Taken together, our data coupled with these reports suggest the TGF-β contributes to the pathogenesis of KD.
Genetic variation in multiple gene pathways appears to play a role in myofibroblast function. Recently we reported the association of susceptibility to KD and aneurysm formation with genetic variation in SMAD3 and ATP-binding cassette, sub-family C, member 4 (ABCC4) [4, 22]. Myofibroblasts from smad3 knockout mice show a distinct phenotype with increased proliferation, decreased extracellular matrix production, and impaired contraction of collagen lattice [23]. ABCC4 stimulates proliferation of myofibroblasts in thickened intima following arterial injury [24]. Thus, variation in these genes may also influence myofibroblast proliferation and function and enhance the arteritis in KD.
We also found nuclear localized FOXP3-expressing Tregs in KD arteries. We previously described circulating, peripherally-induced Tregs in KD patients during the acute phase [5]. In humans it has been reported that retinoic acid, together with TGF-β, induces the differentiation of naïve T-cells into Tregs with potent and stable immunosuppressive capability [25]. Cooperative activation of nuclear factor of activated T-cells (nfat) and smad3 leads to the induction of Foxp3 and the generation of Tregs in mice, although data are lacking in humans [26, 27]. Our genetic studies have also implicated the calcineurin-NFAT pathway in influencing KD susceptibility and coronary artery aneurysm formation [28]. Thus, the intersection of the TGF-β and calcineurin-NFAT pathways may influence both induction and regulation of inflammation.
Reducing TGF-β signaling in KD with angiotensin receptor blocking agents [29] might reduce transformation of different cell types to myofibroblasts, but could have an undesirable effect on regulation of inflammation and elastin degradation. In a mouse model of coronary arteritis, TGF-β blockade resulted in increased elastin degradation associated with increased matrix metalloproteinase (MMP)-9 activity that resulted from TGF-β-mediated decrease in plasminogen activator inhibitor-1, an inhibitor of plasmin that activates MMP-9 [30]. Thus, caution must be excercised in drawing conclusions about the desirability of inhibiting TGF-β signaling in this patient population.
We recognize several strengths and limitations to our study. Autopsy tissue from the acute stage of KD is thankfully rare. Currently, the mortality rate for KD is reported as 0.01% [7], but, this includes late mortality as a consequence of myocardial infarction long after disease onset. We were fortunate to have access to tissues from children who tragically died early in the course of their illness. Using this extremely scarce resource, we were able to demonstrate myofibroblasts secreting pro-inflammatory mediators that likely contribute to the vasculitis in this disease. Limitations inherent to all IHC studies include potential bias due to limited sampling and the inability to study the evolution of vascular lesions over time. Animal models of coronary arteritis might be appropriate settings in which to further dissect the time course of the vascular changes and cell populations in the arterial wall [31, 32]. Another limitation is that we have not explored the complex non-canonical TGF-β signaling pathways that use SMAD3-independent pathways such as mitogen-activated protein kinase (MAPK), Jun N-terminal kinase (JNK), p38, extracellular signal-regulated kinases (Erk), Rho and phosphatidylinositol 3-kinase (PI3K)/Akt. Although all of these pathways are reported to regulate EMT in different ways [33], in this study we have only tested the SMAD canonical pathway. The non-canonical TGF-β signaling pathway has proved critical to an understanding of aortic wall destruction in Marfan’s syndrome and a detailed analysis in KD arteritis may be helpful to further our understanding of aneurysm formation [34].
In summary, we propose a mechanism of acute arterial wall damage initiated by TGF-β signaling that results in transformation of cells to a myofibroblast phenotype, secretion of IL-17 leading to recruitment of neutrophils and CD8+ T-cells, and activation of these CD8+ T-cells in the tissues (Figure 7). Histologic studies of KD animal models of coronary arteritis may help to clarify the time course of these events. TGF-β may also play a critical role in the generation of Tregs that may be instrumental in down regulating the inflammatory state. Therefore, TGF-β may be the proverbial double edged-sword and therapeutic intervention to reduce TGF-β signaling should be approached with caution.
Figure 7. Proposed mechanism of aneurysm formation mediated by TGF-β.
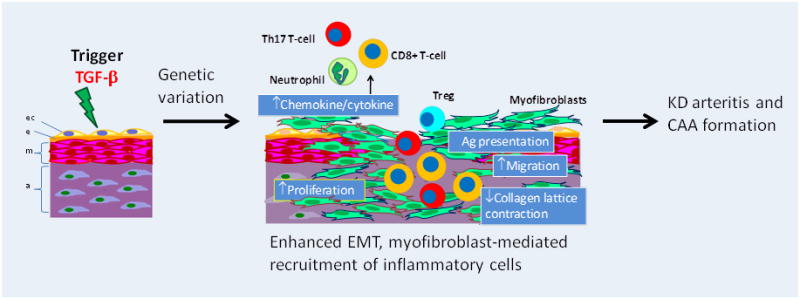
Local secretion of TGF-β generates myofibroblasts from endothelial cells, fibroblasts in adventitia, smooth muscle cells in media, and fibrocytes in the peripheral circulation. Myofibroblasts secrete cytokines (IL-17, IL-6) and chemokines that recruit inflammatory cells. Myofibroblasts also present antigen leading to activation and proliferation of T-cells. Cessation of inflammation is mediated by T-cell regulation (FOXP3 + cells). Genetic variation in TGF-β pathway genes may modulate this cascade either up (more damage) or down (less damage). ec: endothelial cell; e: internal elastic lamina; m: media; a: adventitia. EMT: endothelial/epithelial to mesenchymal transition, Treg: regulatory T-cell.
Supplementary Material
Acknowledgments
We thank Nissi Varki, M.D. and MaryAnn Lawrence at UCSD Histopathology Resources Cancer and Mouse Histopathology and James S. Hagood M.D., Dept. of Pediatrics, UCSD, for helpful discussion. We thank Jennifer Meerloo for imaging support at UCSD Neuroscience Microscopy Shared Facility and DeeAnna Scherrer for technical assistance. We thank Seema Aceves, M.D. PhD. for kind gift of pSMAD antibody. Sherry Huang, M.D. for kind gift of positive control tissue for pSMAD. We thank the parents who donated their children’s tissues for these studies.
Grant support
This work supported in part by grants from the National Institutes of Health, National Heart, Lung, Blood Institute HL69413 awarded to JCB and HL103536 awarded to JCB and AF and the iDASH grant U54HL108460. We also acknowledge NIH support through the UCSD Neuroscience Microscopy Shared Facility Grant P30 NS047101.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364:533–544. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 2.Tremoulet AH, Best BM, Song S, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153:117–121. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newburger JW, Takahashi M, Beiser AS, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu C, Jain S, Davila S, et al. Transforming growth factor-beta signaling pathway in patients with Kawasaki disease. Circ Cardiovasc Genet. 2011;4:16–25. doi: 10.1161/CIRCGENETICS.110.940858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco A, Shimizu C, Tremoulet AH, Burns JC. Memory T-cells and characterization of peripheral T-cell clones in acute Kawasaki disease. Autoimmunity. 2010;43:317–324. doi: 10.3109/08916930903405891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 7.Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2008)--digest version. Circulation journal : official journal of the Japanese Circulation Society. 2010;74:1989–2020. doi: 10.1253/circj.cj-10-74-0903. [DOI] [PubMed] [Google Scholar]
- 8.Christen T, Verin V, Bochaton-Piallat M, et al. Mechanisms of neointima formation and remodeling in the porcine coronary artery. Circulation. 2001;103:882–888. doi: 10.1161/01.cir.103.6.882. [DOI] [PubMed] [Google Scholar]
- 9.Skalli O, Schurch W, Seemayer T, et al. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989;60:275–285. [PubMed] [Google Scholar]
- 10.van der Loop FT, Gabbiani G, Kohnen G, Ramaekers FC, van Eys GJ. Differentiation of smooth muscle cells in human blood vessels as defined by smoothelin, a novel marker for the contractile phenotype. Arterioscler Thromb Vac Biol. 1997;17:665–671. doi: 10.1161/01.atv.17.4.665. [DOI] [PubMed] [Google Scholar]
- 11.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 12.Roberts AI, Nadler SC, Ebert EC. Mesenchymal cells stimulate human intestinal intraepithelial lymphocytes. Gastroenterology. 1997;113:144–150. doi: 10.1016/s0016-5085(97)70089-1. [DOI] [PubMed] [Google Scholar]
- 13.Saada JI, Pinchuk IV, Barrera CA, et al. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J Immunol. 2006;177:5968–5979. doi: 10.4049/jimmunol.177.9.5968. [DOI] [PubMed] [Google Scholar]
- 14.Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C. IL-17 and IL-22: siblings, not twins. Trends Immunol. 2010;31:354–361. doi: 10.1016/j.it.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Brown TJ, Crawford SE, Cornwall ML, Garcia F, Shulman ST, Rowley AH. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis. 2001;184:940–943. doi: 10.1086/323155. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K, Oharaseki T, Naoe S, Wakayama M, Yokouchi Y. Neutrophilic involvement in the damage to coronary arteries in acute stage of Kawasaki disease. Pediatr Int. 2005;47:305–310. doi: 10.1111/j.1442-200x.2005.02049.x. [DOI] [PubMed] [Google Scholar]
- 17.Terai M, Kohno Y, Namba M, et al. Class II major histocompatibility antigen expression on coronary arterial endothelium in a patient with Kawasaki disease. Hum Pathol. 1990;21:231–234. doi: 10.1016/0046-8177(90)90135-r. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz A, Rowley A, Schulte DJ, et al. Activated myeloid dendritic cells accumulate and co-localize with CD3+ T cells in coronary artery lesions in patients with Kawasaki disease. Exp Mol Pathol. 2007;83:93–103. doi: 10.1016/j.yexmp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki A, Miyagawa-Tomita S, Komatsu K, et al. Immunohistochemical study of apparently intact coronary artery in a child after Kawasaki disease. Pediatr Int. 2004;46:590–596. doi: 10.1111/j.1442-200x.2004.01943.x. [DOI] [PubMed] [Google Scholar]
- 22.Khor CC, Davila S, Shimizu C, et al. Genome-wide linkage and association mapping identify susceptibility alleles in ABCC4 for Kawasaki disease. J Med Genet. 2011;48:467–472. doi: 10.1136/jmg.2010.086611. [DOI] [PubMed] [Google Scholar]
- 23.Dobaczewski M, Bujak M, Li N, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sassi Y, Lipskaia L, Vandecasteele G, et al. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J Clin Invest. 2008;118:2747–2757. doi: 10.1172/JCI35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Huizinga TW, Toes RE. De novo generation and enhanced suppression of human CD4+CD25+ regulatory T cells by retinoic acid. J Immunol. 2009;183:4119–4126. doi: 10.4049/jimmunol.0901065. [DOI] [PubMed] [Google Scholar]
- 26.Maitra U, Davis S, Reilly CM, Li L. Differential regulation of Foxp3 and IL-17 expression in CD4 T helper cells by IRAK-1. J Immunol. 2009;182:5763–5769. doi: 10.4049/jimmunol.0900124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Onouchi Y, Gunji T, Burns JC, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40:35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC., 3rd Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med. 2008;358:2787–2795. doi: 10.1056/NEJMoa0706585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvira CM, Guignabert C, Kim YM, et al. Inhibition of transforming growth factor beta worsens elastin degradation in a murine model of Kawasaki disease. Am J Pathol. 2011;178:1210–1220. doi: 10.1016/j.ajpath.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duong TT, Silverman ED, Bissessar MV, Yeung RS. Superantigenic activity is responsible for induction of coronary arteritis in mice: an animal model of Kawasaki disease. Int Immunol. 2003;15:79–89. doi: 10.1093/intimm/dxg007. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Oharaseki T, Wakayama M, Yokouchi Y, Naoe S, Murata H. Histopathological features of murine systemic vasculitis caused by Candida albicans extract--an animal model of Kawasaki disease. Inflamm Res. 2004;53:72–77. doi: 10.1007/s00011-003-1225-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holm TM, Habashi JP, Doyle JJ, et al. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.