Abstract
Background/Aims
Interleukin-28B (IL-28B) polymorphism is the strongest pretreatment predictor of viral clearance in the hepatitis C (HCV) population. Donor and recipient IL-28B genomic background may play an important role in post-transplant HCV recurrence. We sought to examine the role of IL-28B polymorphisms of donor and recipients in liver transplant patients with recurrent HCV and its impact on the response to interferon-based therapy.
Methods
The cohort study consisted of 135 adult liver transplant patients who received interferon-based therapy for recurrent HCV between 1996 and 2005 at the University of Florida. IL-28B single nucleotide polymorphism (rs. 12979860) was characterized using liver tissue from all donors and recipients.
Results
The CC genotype was observed in approximately 30% of donors and recipients. Sustained viral response (SVR) to HCV therapy was 100% if both recipient and donor were CC genotype, while the SVR was only 25% if neither donor nor recipient had a CC genotype. (Recipient, p=0.025, Donor, p<0.001). Recipients and donors with CC genotype had less fibrosis than recipients with genotypes CT and TT, but the difference was not statistically significant. IL-28B genotype did not seem to play a role in the overall survival in these patients.
Conclusion
In conclusion, recipient and donor CC genotype is associated with a better treatment response to interferon-based therapy after liver transplant. Our study suggests that using CC genotype donor livers for HCV patients may improve the overall clinical outcome after liver transplantation.
Keywords: sustained viral response, immunosuppression, fibrosis, survival, polymorphism
Hepatitis C (HCV) is the most common indication for liver transplant (LT) in the United States. HCV recurrence is universal following transplantation and leads to allograft failure in about ten percent of recipients by the fifth postoperative year.(1) In the LT setting, the factors associated with the more aggressive disease progression are complex and multi-factorial, including recipient, donor and viral components. It is likely that host immune responses, both innate and adaptive, play a pivotal role in the clearance of viral infections, ongoing liver damage, and response to antiviral therapy. Therefore, defining the mechanisms of host immune system interaction with HCV after LT is critical for understanding HCV-related allograft injury and response to antiviral treatment. This knowledge is also vital to guide immunosuppression strategies that minimize HCV disease progression and increase the overall survival of the graft.
Although multiple host factors have been implicated in HCV disease progression, interleukin-28B (IL-28B) single nucleotide polymorphism (SNP) on the chromosome 19, rs12979860, seems to be strongly associated with HCV clearance and treatment response.(2) So far, this SNP is the strongest predictor for sustained viral response (SVR) to interferon (IFN)-based therapy in the HCV population. The C allele is the most frequent allele in the white population associated with SVR. Patients with CC genotype have a two-fold chance of SVR compared to patients with T alleles.(3) In a recent study of IL-28B SNP in HCV LT recipients and donors, IL-28B genotypes predicted an SVR with 83% sensitivity and 82% specificity.(4) In addition, IL-28B recipient genotype was predictive of fibrosis, with the TT allele associated with more rapid fibrosis. Similar to interferon-based therapy in non-transplant patients, the CC genotype in either recipient or donor has been associated with increased rate of SVR.(5–7) Emerging evidence suggests that both donor and recipient IL-28B SNP background may play an important role in post-transplant HCV recurrence and its response to current therapy (8), but the available data is limited. Recently, others have reported that follow up liver biopsies may not be suitable for IL-28B determination.(9) Therefore, the objective of our study was to examine the role of IL-28B polymorphism of both donor and recipient livers in HCV disease progression after LT and its response to interferon-based therapy in a well characterized group of transplant patients.
PATIENTS and METHODS
Study Design & Study Population
We retrospectively reviewed and collected data on all LT performed at the University of Florida for HCV infection between 1996 and 2005. During the study period, 385 adult patients underwent LT for HCV infection. In our study group, we have selected 135 patients out of 173 patients who received post liver transplant treatment with interferon-based therapy during the study period and have IL-28B SNP determined by DNA sequencing. DNA was extracted from both the recipient liver tissue (explants) and the donor liver biopsy tissue (donor livers).
Human Subjects
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institutional review committee at the University of Florida. Also, no donor organs were obtained from executed prisoners or other institutionalized persons.
Histology
Postoperative protocol biopsies at seven days, four months, one year and annually were available as well as the final outcome of the subjects until the last clinic visit or death. Recurrent HCV disease was scored for inflammation [Hepatitis Activity Index, HAI (Score 0–18)] and fibrosis (Stage 0–6), using the modified Knodell scoring system of Ishak et al.(10) Combination therapy with IFN and ribavirin was initiated once patients developed significant fibrosis (Ishak fibrosis stage > 2) on liver biopsy. The duration of treatment was 48 weeks for genotype 1, and 24 weeks for genotype 2 and 3, if tolerated.
Immunosuppression
Standard immunosuppression consisted of cyclosporine in combination with prednisone + Azathioprine prior to 1997. After 1997, tacrolimus became the primary calcineurin inhibitor for immunosuppression. Typically, immunosuppression was tapered to monotherapy (tacrolimus or cyclosporine) within four to six months of LT as tolerated.
Interferon-based therapy
Combination therapy with interferon and ribavirin was utilized in those patients with significant fibrosis (Ishak fibrosis stage ≥ 2) on protocol or indication liver biopsy. Therapy was initiated at half dose [1.5 MU interferon alfa-2b SQ thrice weekly (tiw)/PEG interferon alfa-2a 135 μg weekly and ribavirin 400–600 mg daily] for 2 weeks, and if tolerated, the dose was increased to full dose (interferon alfa-2b 3 MU SC tiw/PEG interferon alfa-2a 180 μg weekly and ribavirin 800–1,200 mg daily). Ribavirin dosage was based on weight; patients < 75 kg received 800 mg and > 75 kg 1,000 mg. Therapy was discontinued in any patient who developed moderate to severe rejection, systemic bacterial infection, severe neuropsychiatric symptoms, or symptomatic anemia. The intended duration of treatment was 48 weeks for genotype 1 and 24 weeks for genotype 2 and 3.
IL-28B Single-nucleotide polymorphism Analysis
The IL-28B SNP (rs. 12979860) was tested. The selected formalin-fixed, paraffin-embedded tissue blocks were sectioned using a standard method. Two to four tissue sections of 10 μm were cut for this study. To eliminate tissue cross contamination, the microtome was thoroughly cleaned with 30% bleach solution before and after each usage, and a new blade was used for each tissue block. H&E stained sections were examined by a pathologist (CL). Hepatocytes in the lobules, not inflammatory cells in the portal tracts, were marked and macroscopically dissected for DNA extraction. The DNA was extracted using Trimgen WAXFREE™ FFPE DNA extraction kit (CAT # WF-100, 34 Loveton Circle, Suite 210 Sparks, Maryland 21152, USA). The extracted DNA was quantified by NanoDrop 8000 (Thermo Scientific NanoDrop ™ 8000 Spectrophotometer, Wilmington, Delaware, USA). All DNA solutions were diluted to the final concentration of 10 ng/μL using 1 x TE buffer.
The PCR assay was carried out using TaqMan real time PCR system (Applied Biosystems, Carlsbad, California, USA). The PCR reactions were setup according to the manufacture’s protocol using 10 ng genomic DNA in a final volume of 10 μL, and TaqMan® Genotyping Master Mix (Applied Biosystems, Carlsbad, California, USA, Cat# 4371355). The PCR program was: baseline data collection at 60 °C for 30 sec, 95°C for 12min, 40 cycles of 94 °C for 15 sec and ramp to 62 °C at 55% rate for one min, data collection was done at the end of each cycle. Automatic genomic allele calling was determined using Applied Biosystems StepOne ™ Real-Time PCR System Genotyping Software. All samples were tested in duplicate. The primers used for the assay are listed below:
AHLJH4R_F GCCTGTCGTGTACTGAACCA
AHLJH4R_R GCGCGGAGTGCAATTCAAC
AHLJH4R_V VIC TGGTTCGCGCCTTC
FAM CTGGTTCACGCCTTC
Statistics
For inferential purposes, we conducted four sets of analyses: IFN Response (Yes/No) as well as cirrhosis by five years where each analyzed jointly for the recipient (CC vs. not) and donor (CC vs. Not) by multiple logistic regression. For the analysis, the odds ratios were expressed as the ratio of the odds of IFN response for the non-CC to that of the CC and the ratio of the odds of cirrhosis for the non-CC to the CC. Values below the breakeven point of 1.00 suggest CC as having a higher IFN response or a higher cirrhosis rate, respectively. This analysis adjusts for donor CC vs. not when testing for a recipient association and vice-versa. Response for the 3 by 3 table, giving all three genotypes for donor by responder is also tabulated in Table 4. The semi-quantitative inflammation scores were averaged for five years using a missing at random presumption. These were compared for recipient CC vs. not and donor CC vs. not by two-sample t-tests, using a Satterthwaite correction for unequal population variances. This is more robust than a non-parametric test, which tests the narrow null hypothesis of identical distributions, whereas the t-test tests for equality of population means. Finally, we used a multiple Cox proportional hazards regression to compare survival. This analysis adjusts for donor CC vs. not when testing for a recipient association and vice-versa. The hazard ratio is expressed as instantaneous death probability ratio for Non CC to that of CC, and values above 1.00 favor donor CC. For descriptive purposes, we provide Kaplan-Meier survival curves for recipient (CC vs. not) and donor (CC vs. not). In addition, also for descriptive purposes, we provide non-actuarial death information for the 3 by 3 table (recipient genotype vs. donor genotype). All inferential analyses are two-sided and provide point estimates and 95% confidence limits. As will be seen by these confidence limits, non-significant results should be interpreted as inconclusive, rather than as proof of equality. All P-values are two-sided.
Table 4.
Five year means (HAI) by Genotype Entries are Mean (SD)[N]
| Recipient\Donor | CC | Not CC |
|---|---|---|
| CC | 3.4(1.3)[9] | 3.7(1.8)[27] |
| CT | 3.4(1.1)[30] | 3.8(1.4)[66] |
HAI, Hepatitis Activity Index; SD, standard deviation; N, number
[Satterthwaite Corrected Student T-test: donor CC vs. Non-CC (P=0.12), recipient CC vs. Non-CC (P=0.97)]
RESULTS
Patient Characteristics
A total of 794 adult LT were performed at the University of Florida from 1996–2005. Among these patients, 385 patients were transplanted for HCV- cirrhosis. Our cohort consisted of 135 patients that received IFN therapy for HCV recurrence post-transplant. The mean age of the study group was 59.7 ± 7.7 years, 96 (71%) were male and 112 (83%) were Caucasian. Pre-transplant IFN therapy was administered to 54 (40%) patients independent of the time of transplant, only 19 (14%) received non-pegylated IFN based therapy. All of them were non-responder to treatment. We selected the treated patients as the study population. Table 1 describes the demographic and clinical features. As shown in the table, the vast majority of the patients (85%) were infected with genotype 1 HCV. Only 10/135 (7%) of the patients did not complete IFN-based therapy due to side effects or acute cellular rejection. At least 93/135 (69%) of the patients received 80% of the interferon and ribavirin dose.
Table 1.
General Characteristics of Study Group
| Study Years | 1996–2005 |
|
| |
| Total Adult Liver Transplants for HCV | 385 |
|
| |
| Patients characterized for IL-28B | 135 |
|
| |
| Patients treated with IFN therapy after LT | 135 |
|
| |
| Age (years ± SE) | 49 ± 7 |
|
| |
| Gender (F:M) | 39:96 |
|
| |
| Race (C/H/AA/A) | 112:11:9:3 |
|
| |
| HCV Genotype (%) | |
| 1 | 110 (85%) |
| 2 | 7 (5%) |
| 3 | 10 (8%) |
| 4 | 1 (1%) |
| 6 | 1 (1%) |
|
| |
| HCC at transplant | 21 (16%) |
|
| |
| Mean Donor Age (years ± SE) | 40.9 ± 1.7 |
HCV, hepatitis C infection; IL-28B, interleukin 28B; IFN, interferon; LT, liver transplant; SE, standard error; F, female; M, male; C, Caucasian; H, Hispanic; AA, African American; A, Asian; HCC, Hepatocellular carcinoma
Donor & Recipient’s IL-28B Genotype
Donor and recipient liver tissue was available for each patient treated for recurrent HCV post LT. Recipient tissue was obtained from the liver explants and donor tissue was from subsequent liver biopsies. IL28B polymorphism analysis was performed in all cases in our lab as described in the section of Materials and Methods. The predominant IL28B genotype was CT for donors and recipients, 63% and 53%, respectively. The CC genotype was found in about 30% in recipients and donors. We also compared the IL-28B SNP combination or chimerism of the donor liver and the recipient. The frequency of donor non-CC genotype with recipient non-CC genotype was 50% in our cohort, the highest of all the pair groups. Patients with CC genotype who also received CC genotype donor livers represented only 7% of the patients in our cohort (Table 2).
Table 2.
IL28B Genotype frequency per donor or recipient status
| IL28B Genotype | Frequencies (%) |
|---|---|
|
| |
| (Recipient) | |
| CC | 37/135=27% |
| CT | 71/135=53% |
| TT | 27/135=20% |
|
| |
| (Donor) | |
| CC | 40/135=30% |
| CT | 85/135=63% |
| TT | 10/135=07% |
|
| |
| (Donor:Recipient) | |
| Donor CC: Recipient CC | 9/135=07% |
| Donor CC: Recipient non-CC | 31/135=23% |
| Donor non-CC: Recipient CC | 28/135=21% |
| Donor non-CC: Recipient non-CC | 67/135=50% |
IFN Treatment Response
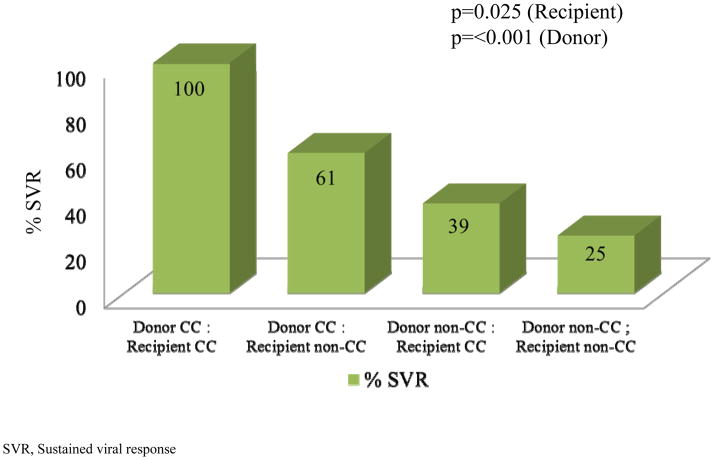
To assess the effect of IL-28B SNP on HCV treatment response after transplant, an analysis of SVR rates by IL-28B genotypes from donors and recipients was performed. The rate of SVR according to recipient and donor IL-28B genotype was cross tabulated as shown in Figure 1. The SVR was 100% if both recipients and donors were CC genotype, while the SVR was only 25% if neither donor nor recipient had a CC genotype. In the cases of donor and recipient mismatch, when the donor livers were CC genotype, the SVR was 61%. However, when the recipients were CC genotype, the SVR was lower at 39%. Recipients with non-CC genotype had a significantly lower rate of SVR than CC recipients [OR=0.38, (95% CI 0.17–0.89, p=0.025)] Also, donors with non-CC genotype had a significantly lower rate of SVR than CC donors [OR=0.16, (95% CI 0.068–0.366, p<0.001)]. An analysis was done using the data from genotype 1 only as this was the largest population of patients treated. We obtained similar results, with a notable exception. Recipients who were CC lost the significant predictive value for IFN response, but this predictive value was not lost for donors CC. The data suggests that the impact of the donor liver IL28B genotype appears to be most important.
Fig 1. SVR in Donors/Recipients by IL28B Genotype.
The SVR is 100% when both recipients and donors are CC genotype, 61% when donor is CC and recipient is non-CC, 39% when the recipient is CC and the donor non-CC and only 25% if neither donor nor recipient are CC genotype. Estimated odds ratio for Recipients, Non-CC: CC, adjusted for Donor IL28B genotype was 0.38, 95% CI 0.17–0.89, p=0.025. Estimated odds ratio for Donors, Not CC: CC, adjusted for Recipient IL-28 genotype was 0.16, 95% CI 0.068–0.366, p<0.001).
Histologic Progression
To determine if IL28B SNP plays a role in HCV disease progression after LT, we evaluated the association of recipient and donor IL-28B genotype with fibrosis progression and hepatitis inflammatory activity. A total of 135 donors with biopsies were available. A total of 120 recipient liver biopsies were available for analysis at 1 year post LT, 84 biopsies at 3-years post L, and 63 biopsies at 5-years post LT. At years one, three, and five, 31, 22 and 19 recipients had genotypes CC, respectively. Recipients and donors with CC genotype had less fibrosis than recipients with genotypes CT and TT, but the difference was not statistically significant. Our data showed that the time between liver transplant and the start of antiviral therapy for donor CC, CT and TT genotypes were 2.4 yrs., 2.3 yrs. and 2.4 yrs. respectively. For recipients CC, CT and TT genotypes were 2.3 yrs., 2.3 yrs. and 2.2 yrs. respectively. Therefore, no differences were found in this cohort.
Fibrosis
The rate of cirrhosis by five years according to recipient and donor IL-28B genotype was cross tabulated as shown in Table 3. The estimated odds ratio for development of cirrhosis in non-CC recipients compared to CC recipients, adjusted for donor IL28B genotype, was 2.61, (95% CI 0.83–8.22, p=0.10). The estimated odds ratio for development of cirrhosis in non-cc donors compared to cc donors, adjusted for recipient IL-28B genotype was 2.28, (95% CI 0.79–6.59, p=0.13). There were too few events to analyze for cirrhosis at earlier time points after transplantation.
Table 3.
Cirrhosis in 5 years by IL28B Genotype of Donor and Recipient
| Recipient\Donor | CC | CT | TT | Total |
|---|---|---|---|---|
| CC | 0/9 (0%) | 4/25 (16%) | 0/3 (0%) | 4/37(11%) |
| CT | 4/25(16%) | 11/42 (42%) | 1/4 (25%) | 16/71 (23%) |
| TT | 1/6 (17%) | 5/18(28%) | 1/3(33%) | 7/27 (26%) |
| Total | 5/40(13%) | 20/85 (24%) | 2/10(20%) | 27/135 (20%) |
SVR, Sustained Viral Response
Odd ratio for development of cirrhosis in non-CC recipients vs. CC recipients, adjusted for donor IL28B genotype, was 2.61, (95% CI 0.83–8.22, p=0.10). Odd ratio for development of cirrhosis in non-cc donors vs. cc donors, adjusted for recipient IL-28B genotype was 2.28, (95% CI 0.79 -6.59, p=0.13).
Inflammation
The mean HAI over five years was also analyzed based on IL-28B genotypes. This index did not vary significantly between donor or recipients IL-28B genotypes [Satterthwaite Corrected Student T-test: donor CC vs. Non-CC (P=0.12), recipient CC vs. Non-CC (P=0.97)]. Details are in Table 4.
Patient and Graft Survival
No statistically significant difference in patient survival was identified according to recipient IL-28B polymorphism. The estimated hazard ratio for recipient Non-CC: CC, adjusted for donor IL28B genotype was 1.12 (95% CI 0.59–2.11), p=0.73. The estimated hazard ratio for donor Non-CC: CC, adjusted for recipient IL28B genotype, was 1.30 (95% CI 0.70–2.41), p=0.41. Figure 2 provides Kaplan-Meier curves for the recipient IL28B and donor IL28B genotypes respectively. Table 5 provides a descriptive report of the deaths by the combination of recipient and donor IL28B genotypes. Analysis for graft survival was not included according to recipient or donor IL-28B polymorphism because only 3 patients from this group had graft failure.
Fig 2. Patient survival according to Il-28B genotype.
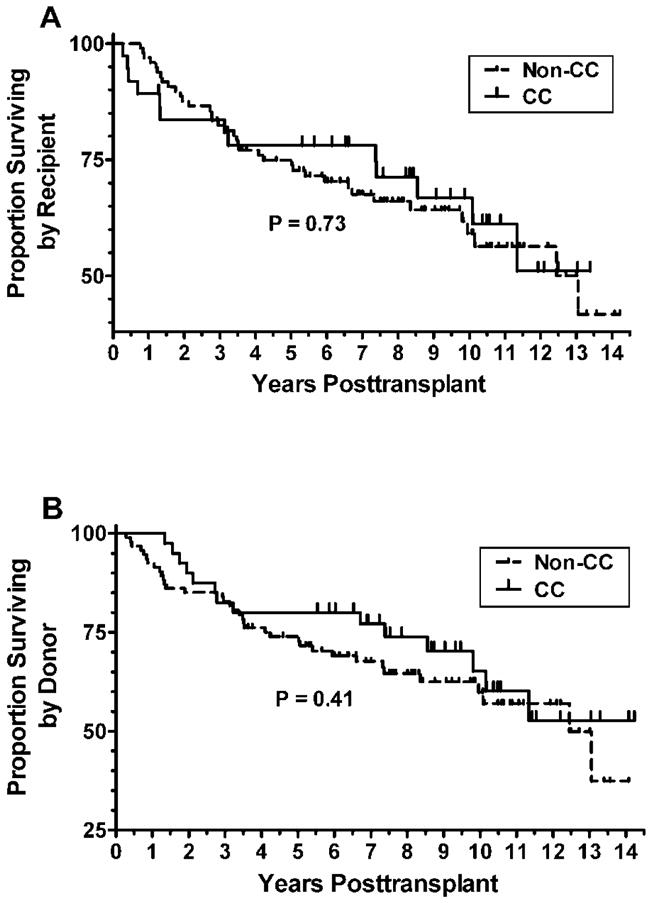
Panel A. Kaplan-Meier curves for the recipient IL28B genotypes. The estimated hazard ratio for recipient Non-CC: CC, adjusted for donor IL28B genotype was 1.12 (95% CI 0.59–2.11)), P=0.73. Panel B Kaplan-Meier curves for the donor IL28B genotypes. The estimated hazard ratio for donor Non-CC: CC, adjusted for recipient IL28B genotype was 1.30 (95% CI 0.70–2.41), P=0.41.
Table 5.
Deaths by IL28B Genotype of Donor and Recipient
| Recipient\Donor | CC | CT | TT | Total |
|---|---|---|---|---|
| CC | 3/9 (33%) | 9/25 (36%) | 1/3 (33%) | 13/37 (35%) |
| CT | 8/25 (32%) | 19/42 (45%) | 1/4 (25%) | 28/71 (35%) |
| TT | 3/6 (50%) | 6/18 (33%) | 0/3 (0%) | 9/26 (35%) |
| Total | 14/40 (35%) | 34/85 (40%) | 2/10 (20%) | 50/135 (37%) |
DISCUSSION
Host factors and potential genetic components have long been suspected to play a role in HCV infection and treatment response. Recently, a single SNP (rs12979860) upstream of the IL28B gene has been associated with pegylated-IFN and ribavirin treatment response. This SNP has also been shown to be associated with spontaneous clearance of acute HCV.(11) Charlton et al. recently reported that donor and recipient IL-28B SNP is associated with HCV recurrence and treatment response after liver transplantation.(8) In our center’s report, we show data on the role of IL28B SNP in HCV disease progression and IFN-based treatment response after LT.
Using a well-documented patient cohort, we found that (1) the frequency of CT IL28B variant was predominant in both donor and recipients; (2) patients with CC genotype either at baseline or in the donor liver had significantly higher response rates to IFN therapy than the non-CC genotypes, while the donor liver CC genotype was more predictive for better outcome than the recipient’s; and (3) no difference in patient survival was present at any time point according to recipient IL-28B polymorphism.
A number of studies have now firmly established the association of IL-28B SNP and HCV infection outcome and treatment response. However, the underlying mechanisms of action remain elusive. The C or T allele of the IL-28B does not appear to affect the protein expression levels of the cytokine.(12) It is more likely that this SNP somehow changes the immune response, the virus, and the interferon signaling pathways. These changes will definitely affect the systemic immunity and the innate antiviral system of the HCV natural host, the liver. We believe that liver transplantation for HCV infection provides a unique model system to study the role of systemic immunity and liver innate immunity in the context of IL-28B polymorphism because the mismatched (chimeric) C or T allele in the recipients and liver donors will allow us to examine the impact of donor genotype and recipient genotype separately.
In our study, there was a large cohort of 135 patients. These patients had received IFN-based therapy after LT. Our standard biopsy protocol reduces the potential selection bias of indication only biopsy datasets. DNA was extracted from both the recipient liver tissue (explants) and the donor liver biopsy tissue (donor livers). We noticed the recent report by Coto-Llerena.(9) Clearly the liver biopsy tissue has both donor and recipient DNA. Therefore, it is critical to overcome this limitation for genotyping. In their report, they used direct PCR and DNA sequencing without macro dissection. To minimize effect of the recipient genomic DNA in the donor liver biopsy samples, we have histologically examined the liver biopsy tissue and macroscopically dissected the hepatocytes, not the inflammatory cells, for DNA extraction. Contamination of donor samples with recipient DNA cannot be excluded. This should not be a major problem, since the effect of the donor IL28B genotype becomes evident in those patients with donor/recipient mismatch, contamination will mainly reduce power but will probably not change the main findings. For the assay accuracy, we performed a validation assay. We identified two cell lines with genotype CC (LH86) and genotype TT (HCO2). We then mixed known percentage of each cell line and performed the allellic discrimination assay as described in the manuscript. The assay correctly called the predominant genotype in the presence of 20% of other genotype. This means that our assay accurately determined the correct genotype as long as the contamination (in this case the inflammatory cells in the liver biopsy) was below 20%. Even with a small fraction of recipient DNA from the inflammatory cells, it would not affect the final genotyping of the allele since the call of C or T is based on the predominant signal at the locus. IL-28B SNP was determined for the all the donor and recipients. Interestingly, patients with CC genotype represent only 27% of our patient population, which is more than 90% white. This is much less than the reported 51% of CC alleles among the white general population. This is indirect evidence that non-CC patients may have worse outcomes as they are over represented in the transplanted population with end-stage liver disease. Surprisingly, the CC allele is also lower than expected in the donor livers, which was 30% of the patient population. Comparing the donor and recipient IL-28B SNP, we found that majority of the patients are chimera CC and non-CC genotypes.
Similar to the results of Charlton et al (8), our data showed that patients with CC genotype had generally better response to IFN therapy, especially if the donor liver is also CC genotype (100% response rate). In contrast, their study showed only 65 patients on treatment post-LT with no patients with TT genotypes. In our study, we have a larger cohort of 135 patients, treated with IFN based regimen similar to the German study by Eurich et al using rs8099917.(7) Charlton study combined the group of CC/r : nonCC/d with CC/d:non-CC/r for a 50% SVR. We separated those groups and showed a higher SVR rate in those who were CC/d: nonCC/r of 61% vs. 39% SVR on the CC/r: nonCC/d. On the contrary, patients with non-CC genotype who also received a donor liver with non-CC genotype have much lower chance of response to therapy (25%). This data suggests that patient’s own IL-28B genotype is a predictor for SVR, implying that systemic immune responses may play a role in determining the response to therapy. In addition, patients with non-CC genotype who received a CC donor liver had better chance to achieve SVR than those patients who received a non-CC donor liver, 39% vs. 25%. The data suggests that liver innate antiviral response may play a more important role in the outcome of the treatment.
We then examined the impact of IL-28B on histopathology of the liver biopsies. The result showed that patients with CC genotypes had lower HAI score and less fibrosis than the non-CC genotypes. The favorable histology with CC genotype is consistent with other studies. This data further supports the notion than IL-28B SNP affects HCV outcome through the host immune system. We also analyzed the impact of IL-28B SNP on the graft overall survival. The data showed no difference between the CC genotype and the non-CC genotype regarding the overall survival of the liver graft. One potential explanation for this result is the fact that the numbers of patients with CC genotype are rather small. The impact of IL28B genotypes on fibrosis progression could be explained by the effect on treatment outcome, but a specific effect on fibrosis progression remains speculative. The suggestion that host and donor IL-28B have a relationship to fibrosis progression and thus when we initiated HCV therapy is a very interesting topic. There is extensive literature on this topic, but not very clear. In a recent paper by Duarte-Rojo et al(13), recipient IL28B CC genotype was associated with lower viral load at recurrence and a lower frequency of F ≥2 on liver biopsy at 1 year after LT, when compared with the non-CC genotype. Therefore, you may think that the time between transplant and initiation of therapy could be influenced by the recipient genotype. Other studies have shown in multiple logistic regression analysis contribution of the IL28B genotype to the risk of severe HCV recurrence independently of other associated factors.(14) The allele T in the donor had an opposite effect than that in the recipient, but this study was underpowered to demonstrate any effect. Our data showed that the time between liver transplant and the start of antiviral therapy for donor have no differences In our group, it will be hard to compare given the different strategies used over the years to treat patients with recurrent HCV post-LT. More research is needed to further clarify this issue.
In summary, our study clearly demonstrated that both recipient and donor CC genotype is a favorable biomarker for liver histopathology and treatment response to IFN therapy after liver transplantation. Our data highlights an opportunity to consider targeted donor distribution based on genotype of IL28B SNP; if the CC donors are allocated to HCV-infected recipients, the clinical outcome after liver transplantation may dramatically improve.
Acknowledgments
Financial Support: This work was partially supported by grants 1UL1RR029890 and U54RR025208 from the National Institute of Research Resources, National Institutes of Health, and R01 AI061158.
We thank Douglas Theriaque for assistance with statistical graphs.
List of Abbreviations
- HCV
Hepatitis C infection
- LT
Liver transplant
- IL-28B
Interleukin 28B
- SNP
Single-nucleotide polymorphism
- SVR
Sustained viral response
- IFN
Interferon
- HAI
Hepatitis activity index
Contributor Information
Roberto J. Firpi, Email: Roberto.Firpi@medicine.ufl.edu.
Huijia Dong, Email: donghj@pathology.ufl.edu.
Virginia C. Clark, Email: Virginia.Clark@medicine.ufl.edu.
Consuelo Soldevila-Pico, Email: Consuelo.Soldevila@medicine.ufl.edu.
Giuseppe Morelli, Email: Giuseppe.Morelli@medicine.ufl.edu.
Roniel Cabrera, Email: Roniel.Cabrera@medicine.ufl.edu.
Oxana Norkina, Email: Oxana.Norkina@medicine.ufl.edu.
Jonathan J. Shuster, Email: Jshuster@biostat.ufl.edu.
David R. Nelson, Email: David.Nelson@medicine.ufl.edu.
Chen Liu, Email: Liu@pathology.ufl.edu.
References
- 1.Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996 Mar;334(13):815–820. doi: 10.1056/NEJM199603283341302. [DOI] [PubMed] [Google Scholar]
- 2.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009 Sep 17;461(7262):399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 3.Balagopal A, Thomas DL, Thio CL. IL28B and the control of hepatitis C virus infection. Gastroenterology. 2010 Dec;139(6):1865–1876. doi: 10.1053/j.gastro.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuhara T, Taketomi A, Motomura T, Okano S, Ninomiya A, Abe T, et al. Variants in IL28B in liver recipients and donors correlate with response to peg-interferon and ribavirin therapy for recurrent hepatitis C. Gastroenterology. 2010 Nov;139(5):1577–85. 1585. doi: 10.1053/j.gastro.2010.07.058. [DOI] [PubMed] [Google Scholar]
- 5.Lange CM, Moradpour D, Doehring A, Lehr HA, Mullhaupt B, Bibert S, et al. Impact of donor and recipient IL28B rs12979860 genotypes on hepatitis C virus liver graft reinfection. J Hepatol. 2011 Aug;55(2):322–327. doi: 10.1016/j.jhep.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 6.Coto-Llerena M, Perez-Del-Pulgar S, Crespo G, Carrion JA, Martinez SM, Sanchez-Tapias JM, et al. Donor and recipient IL28B polymorphisms in HCV-infected patients undergoing antiviral therapy before and after liver transplantation. Am J Transplant. 2011 May;11(5):1051–1057. doi: 10.1111/j.1600-6143.2011.03491.x. [DOI] [PubMed] [Google Scholar]
- 7.Eurich D, Boas-Knoop S, Ruehl M, Schulz M, Carrillo ED, Berg T, et al. Relationship between the interleukin-28b gene polymorphism and the histological severity of hepatitis C virus-induced graft inflammation and the response to antiviral therapy after liver transplantation. Liver Transpl. 2011 Mar;17(3):289–298. doi: 10.1002/lt.22235. [DOI] [PubMed] [Google Scholar]
- 8.Charlton MR, Thompson A, Veldt BJ, Watt K, Tillmann H, Poterucha JJ, et al. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology. 2011 Jan;53(1):317–324. doi: 10.1002/hep.24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coto-Llerena M, Crespo G, Gonzalez P, Koutsoudakis G, Miquel R, Navasa M, et al. Determination of IL28B polymorphisms in liver biopsies obtained after liver transplantation. J Hepatol. 2012 Feb;56(2):355–358. doi: 10.1016/j.jhep.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1991 Oct;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 11.Tillmann HL, Thompson AJ, Patel K, Wiese M, Tenckhoff H, Nischalke HD, et al. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology. 2010 Nov;139(5):1586–92. 1592. doi: 10.1053/j.gastro.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Cyr DD, Lucas JE, Thompson JW, Patel K, Clark PJ, Thompson A, et al. Characterization of serum proteins associated with IL28B genotype among patients with chronic hepatitis C. PLoS One. 2011;6(7):e21854. doi: 10.1371/journal.pone.0021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duarte-Rojo A, Veldt BJ, Goldstein DD, Tillman HL, Watt KD, Heimbach JK, et al. The Course of Posttransplant Hepatitis C Infection: Comparative Impact of Donor and Recipient Source of the Favorable IL28B Genotype and Other Variables. Transplantation. 2012 Jul 27;94(2):197–203. doi: 10.1097/TP.0b013e3182547551. [DOI] [PubMed] [Google Scholar]
- 14.Cisneros E, Banos I, Citores MJ, Duca A, Salas C, Noblejas A, et al. Increased Risk of Severe Hepatitis C Virus Recurrence After Liver Transplantation in Patients With a T Allele of IL28B rs12979860. Transplantation. 2012 Aug 15;94(3):275–280. doi: 10.1097/TP.0b013e31825668f6. [DOI] [PubMed] [Google Scholar]