Abstract
Decreasing the incidence of non-melanoma skin cancer (NMSC) is of great importance in regards to future healthcare services. Given the previously reported preventive effects of α-difluoromethylornithine (DFMO) in skin and colon cancer trials, we determined appropriate cause to update the clinical data on the subjects from the recently reported Randomized, Double-Blind, Placebo-Controlled Phase III Skin Cancer Prevention Study of DFMO. Our intention was to retrospectively assess the further incidence of skin cancer, other malignancies, and adverse events of patients accrued to our phase III skin cancer prevention study of DFMO. Clinical records of 209 UW Health subjects were reviewed, and 2092.7 person years of on study (884.3 person years) and post study (1208.4 person years) follow-up for these patients were assessed for new NMSC events and recurrence rates from the on study period, the post study period, and the two study periods combined. No evidence of increased significant diagnoses or serious adverse events was observed in the DFMO participants. The initially observed, marginally significant reduction (p=0.069) in NMSC rates for DFMO subjects relative to placebo continued without evidence of rebound. Event rates after discontinuation from study for total NMSCs (DFMO 0.236 NMSC/person/year, placebo 0.297, p=0.48) or the subtypes of BCCs (DFMO 0.179 BCC/person/year, placebo 0.190, p=0.77) and SCCs (DFMO 0.057 SCC/person/year, placebo 0.107, p=0.43) are listed. Follow-up data revealed a persistent but insignificant reduction in new NMSCs occurring in DFMO subjects without evidence of latent or cumulative toxicity relative to placebo subjects.
Introduction
Despite respectable intentions, education on the importance of limited or protected sun exposure has not been enough to halt the rising trend of the most commonly diagnosed malignancy in the United States, non-melanoma skin cancer (NMSC). For the year 2010, estimates expected greater than 2 million new cases of basal cell carcinoma (BCC) or squamouscell carcinoma (SCC) (1). Concerning trends note individual use of sunscreen tends to occur only with the intention to sunbathe, if at all, and limited prospective studies of sunscreens have not observed significant protection against BCC or malignant melanoma (2, 3). NMSC has many risk factors, namely ultraviolet (UV) radiation, and with continued depletion of the ozone layer, this under-recognized epidemic is projected to increase (4,5).
The increased incidence of NMSC especially impacts certain populations, an example being organ transplant recipients who are at increased risk (incidence ≥ 50%) with significant morbidity and increasing mortality (6–8). It is also alarming that women have a higher incidence of both BCC and SCC when compared to men and this is especially evident in women under 40 years of age where BCC incidence is increasing (9,10). The financial burden of this malignancy for US residents is quite substantial. One of the top five most costly cancers to Medicare, total yearly expenditures for NMSC care in the US have been estimated at $426 million and $650 million for the Medicare population and for the entire U.S. population respectively (11, 32). In regards to major implications for future healthcare services alone, decreasing the incidence of NMSC across all populationsis of great importance. Unfortunately, a successful and safe chemopreventive agent against NMSC does not exist (12–15).
Polyamines have been of research interest ever since their regulatory capabilities in normal cell signaling and growth were observed. Early work by O’Brien and Boutwell observed increased levels of polyamines in epithelial tumorigenesis (16). Epithelial carcinogenesis of skin, colon, and breast has specifically been linked to elevated levels of polyamines, spermidine and spermine (17). Preclinical research observing chemopreventive effects of polyamine depletion led to the development of α-difluoromethylornithine (DFMO), an analog of the amino acid ornithine, which irreversibly inhibits ornithine decarboxylase (ODC), the rate-limiting step of polyamine synthesis (18). While DFMO exhibited some positive therapeutic effects in clinical trials most of the recent focus has been toward cancer prevention effects (23, 24). This was recently highlighted by Meyskens et al. when they noted a significant reduction in colonic adenomas in participants taking DFMO + sulindac as compared to placebo (19).
The above data led to a single institution (UW) phase III double-blind, placebo-controlled skin cancer prevention study, of DFMO (500 mg/m2) for up to 5 years (22). There was a significant difference in new BCC of patients taking DFMO (163 cancers) versus placebo (243 cancers) as expressed as event rate of 0.28 BCC/person/year versus 0.40 BCC/person/year, (p = 0.03). The subjects showed exceptional compliance and the groups did not have a difference in toxicity/adverse events.
A key issue in the clinical viability of a chemoprevention agent after acute effectiveness and/or tolerance is the latent effectiveness and/or toxicity of the agent. Earlier chemoprevention research has observed positive and negative latent effects with chemopreventive agents. Namely, use of tamoxifen (5 years) for breast cancer prevention has observed even greater evidence of protection up to 5–10 years after stopping tamoxifen as compared to subjects on placebo (25). Contrary to this, early work with retinoids in oral cancer prevention has implied a rebound effect. When subjects discontinued the putative preventive agent, protective effects were not apparent due to increased development of second primary tumors (26). Also, the recent linkage between isotretinoin and inflammatory bowel disease is a concerning example of toxicity experienced years after use of a chronically administered agent (30).
Despite a wide spectrum of potential or ongoing clinical uses of DFMO, namely as a cancer preventive described above, as a treatment option in the management of human African trypanosomiasis (intravenous dose of 400 mg/kg per day for 14 days) (27, 28), or as an anti-Helicobactor pylori therapy (29), the sustainability of its effects or possible latent toxicities have not been assessed.
The continued interest in DFMO as a chemopreventive agent, along with other potential uses, provided cause to update the clinical data and overall health status of the available subjects from the phase III skin cancer prevention study of DFMO to understand the sustainability of the observed DFMO effects. After UW Health Sciences Institutional Review Board approval, we manually reviewed medical records of 243 subjects who received care at UW Health. The review focused on the skin cancer events by histology, other neoplasia (invasive and non-invasive), significant other diagnoses, and survival.
Materials and Methods
Subjects
Previously, two hundred and ninety-one participants (mean age, 61 years; 60% male) with a history of prior nonmelanoma skin cancer (NMSC; mean, 4.5 skin cancers) were randomized to 500 mg/m2/day oral DFMO (n=144) or placebo (n=147) for 4 to 5 years in the phase III skin cancer prevention study of DFMO, UWCCC Protocol CO9737. We pursued reviewing the clinical records of these original 291 subjects to establish what further incidence of malignancy (skin or otherwise) occurred after patients discontinued DFMO.
Study Design
Approval was received to review the 243 UW Health subjects from this study; 209 clinical records were subsequently used. The 34 records not included did not have post-study information; possible reasons for this included deaths of 7 patients (4 DFMO and 3 placebo) before the follow-up period began, lost affiliation with the UW Health, or not requiring medical assistance.
Specifically, the clinical records were reviewed by the authors (S.K., H.B.) to assess whether the patient was living or deceased, and date of death if applicable, the date of last contact with the patient, and the ICD9 Codes for relevant diagnoses. We documented the date (month/day/year) of diagnosis for relevant diseases of NMSC, other skin cancers, other cancers, cardiovascular or vascular disease, dementia, colonic polyps, hepatic and renal dysfunction. Data collection specifically for NMSC included lesion location, histology (basal or squamous), and total number of skin carcinomas.
Statistical Considerations
The primary objective was to determine whether the reductions in NMSC rate observed in subjects randomized to DFMO for up to 5 years were maintained, strengthened or reduced over the > 5 years since going off-study. As with the efficacy analysis in the manuscript for the original study, the primary endpoint used to address this was the rate of non-melanoma skin cancer recurrence; for this study we are interested in the interval from going off-study from CO9737 to the date of last contact for this follow-up study; the cut-off date for date of last contact was May 23, 2011. The rates of skin cancer recurrence were compared in the 209 subjects reviewed between the original randomization arms, DFMO vs. placebo, using a two-sample Student t-test. For greater precision, efficacy was evaluated using the exact probability value from the permutation test obtained from the randomization distribution. A similar analysis was performed for the original phase III DFMO manuscript. Estimates of the mean cumulative event rate over time were obtained by fitting a nonhomogenous Poisson process λ(t|z) = zλ0(t) using the nonparametric model of Huang et al, and are displayed in Figures 1(a–c). Analysing panel count data with informative observation times (33). In these models, the distributions of both the frailty variable z and observation times are considered as nuisance parameters. The way the data was collected, we expect observation times to be non-informative, although these models have been found to be insensitive to assumptions of informativeness of the observation times.
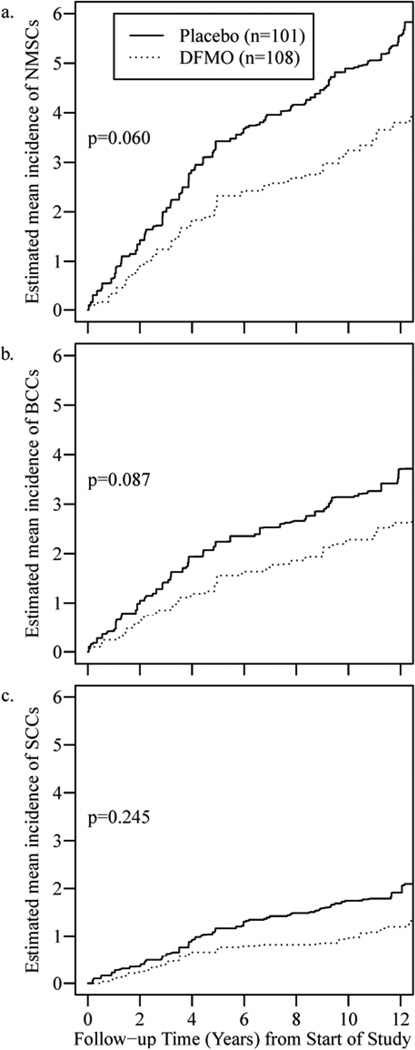
Figure 1.
(a, b, c). NMSC, BCC, SCC Incidence from Start of Study to Present.
For the secondary analyses, the rate of skin cancer recurrence from randomization onto CO9737 to the date of last contact for this follow-up study were compared between the original randomization arms, also with permutation test. Computations were performed and figures were created with R software (34).
Results
Baseline characteristics of the original study cohort and the post-study cohort are summarized in Tables 1. Height and weight was used to determine body surface area and the resulting mean and median values for randomized subjects were both 1.96 m2. The original study population had a total of 334 subjects enrolled over 2 years into the placebo run-in phase. After 28 days of the placebo run in, 291 subjects (87%) met the minimum compliance rate (≥80%) and were randomized to continue with blinded study treatment between September 1998 and July 2000. The mean age at enrollment was 60.9 years, with a median of 61.9 years. Among randomized subjects, 175 (60%) were male and 116 (40%) were female. Nearly all subjects (290, or 99.7%) were White, non-Hispanic with one Hispanic subject. Baseline variables across the two treatment groups seemed reasonably well balanced and consistent with randomization, with the possible exception of weight (Wilcoxon P = 0.060) and body surface area (Wilcoxon P = 0.063).
Table 1.
Randomized subject baseline characteristics.
| Population | Treatment Group | p-value | |
|---|---|---|---|
| Original Study | DFMO (n=144) | Placebo (n=147) | |
| Age(y) | 61.6 ± 10.7 | 60.2 ± 11.0 | |
| Gender | |||
| Female | 57 (39.6%) | 59 (40.1%) | |
| Male | 87 (60.4%) | 88 (59.9%) | |
| Race | |||
| White | 144 (99.3%) | 147 (100%) | |
| Hispanic | 1 (0.7%) | 0 (0%) | |
| Body Surface Area (m2) | 1.94 ± 0.23 | 1.99 ± 0.23 | |
| Prior NMSC | 4.2 ± 7.7 | 4.9 ± 5.7 | P=0.10 |
| Prior Tumor Rate | 2.3 ± 3.3 | 2.1 ± 3.4 | P=0.08 |
| Retrospective Study | DFMO (n=108) | Placebo (n=101) | |
| Age(y) | 61.4 ± 10.9 | 60.4 ± 11.0 | |
| Gender | |||
| Female | 43 (39.8%) | 46 (45.5%) | |
| Male | 65 (60.2%) | 55 (54.5%) | |
| Race | |||
| White | 107 (99.5%) | 101 (100%) | |
| Hispanic | 1 (0.5%) | 0 (0%) | |
| Body Surface Area (m2) | 1.94 ± 0.23 | 1.96 ± 0.23 | |
| Prior NMSC | 3.2 ± 3.0 | 5.5 ± 6.3 | P=0.01 |
| Prior Tumor Rate | 2.5 ± 3.5 | 2.4 ± 3.9 | P=0.57 |
Note: for continuous data, mean values ± SD are presented. For categorical data, N (%) are presented. Prior tumor rate is defined as the number of prior skin cancers divided by the time from initial diagnosis to randomization.
Baseline characteristics for the 209 prior study subjects that carried over into our retrospective study are summarized as the retrospective study population in Table 1. The mean age at enrollment was 60.9 years, with a median of 63.0 years. Among randomized subjects, 120 (57%) were male and 89 (43%) were female. Most subjects (208, or 99.5%) were White, non-Hispanic with one Hispanic subject. Baseline variables across the two treatment groups also seemed reasonably well balanced and consistent with randomization.
Original study results of the 291 participants randomized to oral DFMO (500 mg/m(2)/day) or placebo for 4 to 5 years revealed a marginally statistically significant (p=0.069) decrease in total NMSCs (DFMO, 259 cancers; placebo, 363 cancers) in participants randomized to DFMO. Analysis by specific NMSC type revealed a statistically significant difference in new BCCs (DFMO, 162 cancers; placebo, 245 cancers; expressed as event rate of 0.28 BCC/person/year versus 0.40 BCC/person/year, p = 0.03). Table 2(a) displays results while on study for the 209 subjects, who are the focus of this retrospective review. Post study data of 209 study subjects displayed in Table 2(b) did not show a significant difference between groups in total NMSCs or individual cancer types (SCC or BCC). The BCC post-study event rate of DFMO subjects was similar to the placebo subjects (DFMO 0.179 BCC/person/year, placebo 0.190, p=0.765) (Table 2(b)). Interestingly, the post study period rate of SCCs decreased when compared to placebo (DFMO 0.057 SCC/person/year, placebo 0.107, p=0.426. SCCs: DFMO 40, placebo 64).
Table 2.
| (a). Cancers During Study. | ||||
|---|---|---|---|---|
| Treatment Group | DFMO (n=108) |
Placebo (n=101) |
Overall (n=209) |
p-value |
| Time under obs(years)/avg | 463.6 / 4.29 | 420.7 / 4.17 | 884.3 / 4.23 | |
| Subjects with new NSMC | 70 | 69 | 139 | |
| Total New NMSCs | 207 | 308 | 515 | |
| New NMSCs/year (SE) | 0.444 (0.063) | 0.701 (0.095) | 0.568 (0.057) | 0.012 |
| Subjects with new BCC | 57 | 55 | 112 | |
| Total New BCCs | 133 | 201 | 334 | |
| New BCCs/year (SE) | 0.294 (0.049) | 0.466 (0.067) | 0.377 (0.041) | 0.014 |
| Subjects with new SCC | 32 | 43 | 75 | |
| Total New SCCs | 74 | 107 | 181 | |
| New SCCs/year (SE) | 0.150 (0.040) | 0.236 (0.056) | 0.191 (0.034) | 0.223 |
| (b). Post Study Cancers. | ||||
|---|---|---|---|---|
| Treatment Group | DFMO (n=108) |
Placebo (n=101) |
Overall (n=209) |
p-value |
| Time under obs(years)/avg | 627.2 / 5.81 | 581.2 / 5.75 | 1208.4 / 5.78 | |
| Subjects with new NSMC | 49 | 52 | 101 | |
| Total New NMSCs | 146 | 170 | 316 | |
| New NMSCs/year (SE) | 0.236 (0.039) | 0.297 (0.081) | 0.266 (0.044) | 0.484 |
| Subjects with new BCC | 44 | 42 | 86 | |
| Total New BCCs | 106 | 106 | 212 | |
| New BCCs/year (SE) | 0.179 (0.035) | 0.190 (0.042) | 0.185 (0.027) | 0.765 |
| Subjects with new SCC | 28 | 22 | 50 | |
| Total New SCCs | 40 | 64 | 104 | |
| New SCCs/year (SE) | 0.057 (0.011) | 0.107 (0.054) | 0.081 (0.027) | 0.426 |
| (c). All Cancers Combined. | ||||
|---|---|---|---|---|
| Treatment Group | DFMO (n=108) |
Placebo (n=101) |
Overall (n=209) |
p-value |
| Time under obs(years)/avg | 1090.8 / 10.10 | 1002.0 / 9.92 | 2092.7 / 10.01 | |
| Subjects with new NSMC | 76 | 77 | 153 | |
| Total New NMSCs | 353 | 478 | 831 | |
| New NMSCs/year (SE) | 0.336 (0.041) | 0.509 (0.085) | 0.420 (0.046) | 0.060 |
| Subjects with new BCC | 70 | 68 | 138 | |
| Total New BCCs | 239 | 307 | 546 | |
| New BCCs/year (SE) | 0.231 (0.034) | 0.334 (0.053) | 0.281 (0.031) | 0.087 |
| Subjects with new SCC | 42 | 47 | 89 | |
| Total New SCCs | 114 | 171 | 285 | |
| New SCCs/year (SE) | 0.106 (0.022) | 0.175 (0.053) | 0.139 (0.028) | 0.245 |
Table 2(c) displays the combined data of the 209 subjects from study initiation to end of the current retrospective period with a follow-up from 2.3 years to 12.7 years.
Estimates of the mean cumulative event rate over time from start of the prior study to end of the retrospective study (approximately 12 years) for NMSC, BCC, and SCC are displayed in Figures 1(a)–1(c).
In the prior study, gastrointestinal adverse events were the most commonly observed toxicity, and nausea or diarrhea were often attributed as possibly related to study drug. Twelve study subjects died during study participation or follow-up, seven on the DFMO arm (ages 69–78 years) and five on the placebo arm (ages 62–78 years). Although no deaths were felt to be possibly or probably related to the study drug, four deaths on the DFMO arm were previously described: congestive heart failure, a ruptured spleen and congestive heart failure, a cerebrovascular accident, and acute renal failure. We examined the medical records for diagnoses of or evidence for malignancies and noninvasive neoplasms, cardiac, vascular, endocrine, neurological, gastrointestinal, renal, hepatic and ophthalmologic events. Table 3 compares the DFMO and placebo groups relative to general system events that occurred post-study. We compiled cardiac conditions as chronic heart failure, valve disorders, coronary artery disease, abnormal ECG, endocarditis, abnormal heart rate and cardiomegaly, which occurred in 23 DFMO participants vs. 22 placebo participants. The renal conditions (chronic renal failure, abnormal creatinine, kidney cyst and calculi) were noted in 4 of the DFMO group as compared to 2 in Placebo. Hepatic disorders, including hepatitis, cholecystitis, hepatic and pancreatic cysts, abnormal liver function tests (transaminases, total bilirubin, alkaline phosphatase) , ascites, microalbumin and common bile duct obstruction, were observed for 9 DFMO participants and 3 placebo patients.
Table 3.
Number (%) of Patients with Each Condition Post-Study.
| N (%) of Patients | |||||||
|---|---|---|---|---|---|---|---|
| DFMO (N=108) | Placebo (N=108) | All (N=209) | p-valuea | ||||
| Hematological Malig. | 4 | (3.7) | 2 | (2.0) | 6 | (2.9) | 0.684 |
| Prostate Cancer | 5 | (4.6) | 2 | (2.0) | 7 | (3.3) | 0.447 |
| Noninvasive Neoplasm | 23 | (21.3) | 28 | (27.7) | 51 | (24.4) | 0.334 |
| Cardiacb | 23 | (21.3) | 22 | (21.8) | 45 | (21.5) | 1.000 |
| Vascular Disease | 22 | (20.4) | 20 | (19.8) | 42 | (20.1) | 1.000 |
| Diabetes Mellitus Type 2 | 8 | (7.4) | 5 | (5.0) | 13 | (6.2) | 0.572 |
| Neurological Disorders | 22 | (20.4) | 17 | (16.8) | 39 | (18.7) | 0.595 |
| Colonic Polyps | 16 | (14.8) | 11 | (10.9) | 27 | (12.9) | 0.418 |
| Renal Diseasec | 4 | (3.7) | 2 | (2.0) | 6 | (2.9) | 0.684 |
| Hepatic Disordersd | 9 | (8.3) | 3 | (3.0) | 12 | (5.7) | 0.137 |
| Eye Disorders | 7 | (6.5) | 7 | (6.9) | 14 | (6.7) | 1.000 |
| Death | 10 | (9.3) | 6 | (5.9) | 16 | (7.7) | 0.441 |
Fisher’s exact test
Chronic Heart Failure, valve disorders, CAD, abnormal ECG, endocarditis, abnormal heart rate, cardiomegaly.
Chronic Renal Failure, abnormal creatinine, kidney cyst, calculus
Hepatitis, cholecystitis, hepatic cyst, abnormal LFTs, ascites, microalbumin, common bile duct obstruction.
Discussion
Our results after updating the clinical data on subjects from the Randomized, Double-Blind, Placebo-Controlled Phase 3 Skin Cancer Prevention Study of α-Difluoromethylornithine imply that up to 5 years of DFMO did not result in latent toxicity and the initial observed insignificant reduction in NMSC and significant reduction in BCCs did not result in a “rebound” increase in rate of BCCs or SCCs after stopping DFMO. Further evaluation of rates of BCC’s and SCC’s in Figures 1(a)–1(c), which depict the cumulative mean incidence of events over time (rate) suggest the following: The initial diverging rates of BCCs during the study (consistent with the observed significant reduction in BCC rate) is followed by parallel rates from years 5 to 12 implying initial protection followed by no protection or a return to baseline risk; the initial lack of any divergence in rates of SCCs between DFMO and placebo during the study imply minimal to no protection but in the initial years post-study (years 5–10) there is divergence of the rates suggestive of DFMO protection; the combined results of BCCs and SCCs (NMSCs) show a persistent, but not significant (p=0.060), divergence in rates from year 1–10 consistent with the above results for BCCs and SCCs. These data strongly imply DFMO at 500 mg/m2/day for up to 5 years provides a small to moderate reduction in the risk of NMSCs for up to 5 to 10 years. There is clearly is no evidence of any increase in incidence of NMSCs upon discontinuation of DFMO.
From these data it is possible to hypothesize DFMO more strongly inhibits later stages of basal cell carcinogenesis with less effect on early carcinogenic processes, as evidenced by a near immediate decrease in BCCs during 5 years of DFMO followed by a rapid return to a rate of BCCs similar to placebo subjects (no evidence of latent protection). Contrary to BCCs, DFMO’s effect on SCCs appears to be more strongly impactful on early carcinogenesis rather than later given the observed minimal if any reduction in incidence/rate during DFMO administration, but observed trend toward a reduced rate of SCCs in the 5 years after DFMO administration. As we discussed in our initial report (22), it should not be surprising if DFMO or any potential skin cancer prevention agent had differing effects against squamous or basal cell carcinogenesis given the known differences in critical oncogenic pathways between the two. While the relatively small size of our studies limits the ability to establish significant small to moderate changes, the consistent decreased numbers of NMSCs in participants having received DFMO is noteworthy.
As discussed prior, key considerations toward a potential chemopreventive agent besides acute and latent protection from cancer are safety and compliance. The initial study results (22) observed high compliance (>95%), even when DFMO was a liquid form rather than tablet, and minimal toxicity other than a significant (p<0.05) increase in uniformly transient audiometric (but not clinically detectable) hearing loss in participants on DFMO. Thecombined toxicity data from our prospective and follow-up studies continue to imply an acceptable immediate and long-term safety profile of daily DFMO. However, as with all prevention agents, larger and/or longer prospective, randomized trials that could discern increased rates of uncommon events are still needed to more definitively establish long-term safety.
The limitations of our follow-up study includethe relatively small size of our study (noted above), the inability to review the full 291 patients from the original study (48 patient records were not affiliated with UW Health and 34 subjects from UW Health were lost to various reasons) and the retrospective nature (follow-up guidelines from the prior study were not in place and subjects may have been more or less closely followed than previously). Events may have been missed if recorded by other providers as subjects may have sought care outside the UW Health system. This was a manual process, and errors in recording may have occurred, but data were cross-checked by study statistician and compared by paper records and electronic health records. Based on the above experience, we have started incorporating prospective, long-term followup plans into our phase 3 chemoprevention trial proposals with the hopes of improving our ability to detect beneficial or detrimental latent effects.
While it could be debated whether the observed differences in NMSC between participants who took DFMO daily for up to 5 years as compared to those who took placebo are large enough to justify its continued development as a “solo” agent for cancer prevention, these results show a “biological” signal is there. Namely, clinically safe administration of DFMO produces an alteration in skin cancer development. At a minimum these data support the exploration of DFMO in combination with other agents, especially given the data of Meyskens et al. and Elmets et al. in colon cancer and skin cancer prevention, respectively (19, 31).
Acknowledgements
We thank the University of Wisconsin Carbone Cancer Center and the University of Wisconsin School of Medicine and Public Health for their support of this research.
Grant Support
This work was supported in part by Core grant NIH P30 CA14520 through the University of Wisconsin Carbone Cancer Center, and by the Herman and Gwendolyn Shapiro Foundation at the University of Wisconsin School of Medicine and Public Health.
Footnotes
Financial Support – Source of Funding for participation in this project:
This work was supported in part by
1Core grant NIH P30 CA14520 through the University of Wisconsin Carbone Cancer Center, and by the
2Herman and Gwendolyn Shapiro Foundation at the University of Wisconsin School of Medicine and Public Health.
Disclosure of Potential Conflicts of Interest
No authors had any potential conflicts of interest to disclose.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. “Cancer Statistics, 2010,” CA: A Cancer Journal for Clinicians. 2010 Sep-Oct;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Thieden E, Philipsen PA, Sandby-Møller J, Wulf HC. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005 Aug;141(8):967–973. doi: 10.1001/archderm.141.8.967. [DOI] [PubMed] [Google Scholar]
- 3.Green A, Williams G, Neale R, Hart V, Leslie D, Parsons P, et al. Daily sunscreen application and beta carotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomized controlled trial. Lancet. 1999;354:723–729. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- 4.Norval M, Lucas RM, Cullen AP, de Gruijl FR, Longstreth J, Takizawa Y, et al. The human health effects of ozone depletion and interactions with climate change. PhotochemPhotobiol Sci. 2011 Feb;10(2):199–225. doi: 10.1039/c0pp90044c. [DOI] [PubMed] [Google Scholar]
- 5.Ulrich C, Kanitakis J, Stockfleth E, Euvrard S. Skin cancer in organ transplant recipients: where do we stand today? Am J Transplant. 2008 Nov;8(11):2192–2198. doi: 10.1111/j.1600-6143.2008.02386.x. [DOI] [PubMed] [Google Scholar]
- 6.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010 Mar;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 7.Ramsay HM, Reece SM, Fryer AA, Smith AG, Harden PN. Seven-year prospective study of nonmelanoma skin cancer incidence in U.K. renal transplant recipients. Transplantation. 2007;84:437–439. doi: 10.1097/01.tp.0000269707.06060.dc. [DOI] [PubMed] [Google Scholar]
- 8.Bath-Hextall F, Leonardi-Bee J, Somchand N, Webster A, Delitt J, Perkins W. Interventions for the prevention of non-melanoma skin cancers in high-risk groups. (Protocol) Cochrane Database of Systematic Reviews. 2007 Oct;17(4):CD005414. doi: 10.1002/14651858.CD005414.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birch-Johansen F, Jensen A, Mortensen L, Olesen AB, Kjær SK. Trends in the incidence of nonmelanoma skin cancer in Denmark 1978–2007: Rapid incidence increase among young Danish women. International journal of cancer. 2010 Nov 1;127(9):2190–2198. doi: 10.1002/ijc.25411. [DOI] [PubMed] [Google Scholar]
- 10.Christenson LJ, Borrowman TA, Vachon CM, Tollefson MM, Otley CC, Weaver AL, et al. Incidence of Basal Cell and Squamous Cell Carcinomas in a Population Younger Than 40 Years. JAMA. 2005 Aug 10;294(6):681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, G, Fleischer AB, Jr, Smith ED, Kancler C, Goldman ND, Williford PM, et al. Cost of nonmelanoma skin cancer treatment in the United States. Dermatol Surg. 2001 Dec;27(12):1035–1038. doi: 10.1046/j.1524-4725.2001.01004.x. [DOI] [PubMed] [Google Scholar]
- 12.Wright TI, Spencer JM, Flowers FP. Chemoprevention of nonmelanoma skin cancer. Journal of the American Academy of Dermatology. 2006 Jun;54(6):933–946. doi: 10.1016/j.jaad.2005.08.062. quiz 947-50. [DOI] [PubMed] [Google Scholar]
- 13.Levine N, Moon TE, Cartmel B, Bangert JL, Rodney S, Dong Q, et al. Trial of Retinol and Isotretinoin In Skin Cancer Prevention: A Randomized, Double-Blind, Controlled Trial. Cancer Epi BiomPrev. 1997;6:957–961. [PubMed] [Google Scholar]
- 14.Frieling UM, Schaumberg DA, Kupper TS, Muntwyler J, Hennekens CH. A randomized, 12-Year Primary-Prevention Trial of Beta Carotene Supplementation for Nonmelanoma Skin Cancer in the Physicians’ Health Study. Arch Dermatol. 2000;136:179–184. doi: 10.1001/archderm.136.2.179. [DOI] [PubMed] [Google Scholar]
- 15.Duffield-Lillico AJ, Slate EH, Reid ME, Turnbull BW, Wilkins PA, Combs GF, et al. Selenium Supplementation and Secondary Prevention of Nonmelanoma Skin Cancer in a Randomized Trial. JNCI. 2003;95:1477–1481. doi: 10.1093/jnci/djg061. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien TG, Simsiman RC, Boutwell RK. Induction of the polyamine biosynthetic enzymes in mouse epidermis by tumor-promoting agents. Cancer Res. 1975;35:1662–1670. [PubMed] [Google Scholar]
- 17.Nowotarski SL, Origanti S, Shantz LM. Posttranscriptional regulation of ornithine decarboxylase. Methods Mol Biol. 2011;720:279–292. doi: 10.1007/978-1-61779-034-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haegele KD, Alken RG, Grove J, Schechter PJ, Koch-Weser J. Kinetics of alpha-difluoromethylornithine: an irreversible inhibitor of ornithine decarboxylase. ClinPharmacolTher. 1981;30:210–217. doi: 10.1038/clpt.1981.150. [DOI] [PubMed] [Google Scholar]
- 19.Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008 Jun;1(1):9–11. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pegg AE. Regulation of ornithine decarboxylase. J Biol Chem. 2006;281:14529–14532. doi: 10.1074/jbc.R500031200. [DOI] [PubMed] [Google Scholar]
- 21.Hughes DJ, Hlavatá I, Soucek P, Pardini B, Naccarati A, Vodickova L, et al. Ornithine Decarboxylase G316A Genotype and Colorectal Cancer Risk. Colorectal Dis. 2011 Aug;13(8):860–864. doi: 10.1111/j.1463-1318.2010.02300.x. [DOI] [PubMed] [Google Scholar]
- 22.Bailey HH, Kim K, Verma AK, Sielaff K , Larson PO, Snow S, et al. A Randomized, Double-Blind, Placebo-Controlled Phase 3 Skin Cancer Prevention Study of α-Difluoromethylornithine in Subjects with Previous History of Skin Cancer. Cancer Prev Res (Phila) 2010 Jan;3(1):8–11. doi: 10.1158/1940-6207.CAPR-09-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simoneau AR, Gerner EW, Nagle R, Ziogas A, Fujikawa-Brooks S, Yerushalmi H, et al. The effect of difluoromethylornithine on decreasing prostate size and polyamines in men: results of a year-long phase IIb randomized placebo-controlled chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2008 Feb;17(2):292–299. doi: 10.1158/1055-9965.EPI-07-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izbicka E, Streeper RT, Yeh IT, Pressley O, Grant M, Andrews JV, et al. Effects of alpha-difluoromethylornithine on markers of proliferation, invasion, and apoptosis in breast cancer. Anticancer Res. 2010 Jun;30(6):2263–2269. [PubMed] [Google Scholar]
- 25.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010 Jun;3(6):696–706. doi: 10.1158/1940-6207.CAPR-10-0076. Epub 2010 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klaassen I, Braakhuis BJ. Anticancer activity and mechanism of action of retinoids in oral and pharyngeal cancer. Oral oncology. 2002 Sep;38(6):532–542. doi: 10.1016/s1368-8375(01)00118-x. [DOI] [PubMed] [Google Scholar]
- 27.Oguri H, Hiruma T, Yamagishi Y, Oikawa H, Ishiyama A, Otoguro K, et al. Generation of Anti-trypanosomal Agents through Concise Synthesis and Structural Diversification of Sesquiterpene Analogues. Journal of the American Chemical Society. 2011;133(18):7096–7105. doi: 10.1021/ja200374q. [DOI] [PubMed] [Google Scholar]
- 28.Pépin J, Khonde N, Maiso F, Doua F, Jaffar S, Ngampo S, et al. Short-course eflornithine in Gambian trypanosomiasis: a multicentre randomized controlled trial. Bull WHO. 2000;78:1284–1295. [PMC free article] [PubMed] [Google Scholar]
- 29.Barry DP, Asim M, Leiman DA, deSablet T, Singh K, Casero RA, Jr, et al. Difluoromethylornithine is a novel inhibitor of Helicobacter pylori growth, CagA translocation, and interleukin-8 induction. PLoS One. 2011 Feb 28;6(2):e17510. doi: 10.1371/journal.pone.0017510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sewell JL, Mahadevan U. Of Blemishes and Bowels: Isotretinoin and Inflammatory Bowel Disease. Gastroenterology. 2010 Jan;138(1):392–394. doi: 10.1053/j.gastro.2009.11.029. Epub 2009 Nov 20. [DOI] [PubMed] [Google Scholar]
- 31.Elmets CA, Athar M. Targeting ornithine decarboxylase for the prevention of nonmelanoma skin cancer in humans. Cancer Prevention Research. 2010 Jan;3(1):8–11. doi: 10.1158/1940-6207.CAPR-09-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Housman TS, Feldman SR, Williford PM, Fleischer AB, Jr, Goldman ND, Acostamadiedo JM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am AcadDermatol. 2003 Mar;48(3):425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 33.Huang C, Wang M, Zhang Y. Analysing panel count data with informative observation times. Biometrika. 2006;93(4):763–776. doi: 10.1093/biomet/93.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R. Core Development Team. R Foundation for Statistical Computing, Vienna; Austria: 2011. R: A language and environment for statistical computing. ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]