Abstract
Circulating Foxp3+ regulatory T cells (Treg) may arise in the thymus (natural Treg, nTreg) or through the adaptive upregulation of Foxp3 after T cell activation (induced Treg, iTreg). In this brief review, we explore evidence for the formation and function of iTreg during pathologic conditions. Determining the ontogeny and function of Treg populations has relied on the use of manipulated systems in which either iTreg or nTreg are absent, or lineage tracing of T cell clones through repertoire analyses. iTreg appear particularly important at mucosal interfaces. iTreg can also ameliorate tissue-specific autoimmunity and are a prominent source of tumor-infiltrating Treg in some models. However, under many conditions, including in CNS autoimmunity, diabetes, and some tumor systems, iTreg formation appears limited. The immunological contribution of iTreg is thus highly context dependent. Deciphering immune parameters responsible for iTreg formation and their role in modulating pathologic immune responses will be important.
Keywords: regulatory T lymphocyte, nTreg, iTreg, EAE, diabetes, tumor, colitis, TCR repertoire
1. Introduction
CD4+ regulatory T cells (Treg) that express the forkhead box p3 (Foxp3) transcription factor are essential for immune regulation[1]. Treg depletion, or absent or dysfunctional Foxp3 leads to fulminant, multi-organ autoimmunity and early death[2]. Tregs have a unique capacity to suppress the immune response, and operate through many mechanisms, including expression of inhibitory cell surface proteins such as CTLA-4, secretion of suppressive cytokines such as IL-10 and TGF-β, metabolic disruption, and direct cytolysis [3–5].
Treg can arise both from developing thymocytes, a population referred to as natural Treg (nTreg), or from activated conventional T cells (Tconv) that upregulate Foxp3 in the periphery, referred to as adaptive or induced Treg (iTreg). Foxp3 is induced in naive T cells after TCR stimulation in the presence of IL-2 and TGF-β [6, 7]. This is modulated by a variety of signaling pathways that impinge directly or indirectly on the Foxp3 promoter, (Table 1)[8, 9]. STAT5 is phosphorylated and translocates to the nucleus with IL2 stimulation, RUNX1/3 and other factors with the TCR, and Smad3 with TGF-β [10–16]. These bind Foxp3 promoter and enhancer elements to support Foxp3 transcription. Other transcription factors such as STAT3, activated in response to inflammatory cytokines including IL-6, IL-21, and IL-23, are repressive[17–21]. Interestingly, some of the same inflammatory signals that inhibit Foxp3 expression and hence Treg sustenance, are also necessary for Treg function. STAT3 is required for Treg suppression of Th17-mediated immunity. Treg similarly utilize Tbet for Th1, IRF4 for Th2, and Bcl6 for Tfh suppression[2, 8, 9]. Thus Treg formation, persistence, and activity exist in a dynamic balance established by local inflammatory and homeostatic inputs.
Table 1.
Molecules and stimuli positively or negatively regulating iTreg development are listed.
| Positive Regulators | Citation | Negative Regulators | Citation |
|---|---|---|---|
| IL-2 | [76–78] | IL-6 | [18, 19] |
| TGF-β | [79–82] | IL-4 | [83, 84] |
| TCR | [85–87] | IL-21 | [19, 20] |
| Low Dose or Chronic Antigen Exposure | [25, 88–90] | IL-23 | [21] |
| IL10 | [91, 92] | IL-27 | [17, 93] |
| RA | [31–33, 89, 94, 95] | TNFα | [96, 97] |
| AHR | [34, 35, 43] | OX40 | [96, 97] |
| Vitamin D3 | [98] | mTor, S1P1, PI3K | [99, 100] |
| Rapamycin | [6, 36, 37, 78, 101] | PCKθ | [102] |
| Foxp3 | [1, 5, 18, 22, 103, 104] | 4-1BB | [105, 106] |
| RORγt | [18, 107] | CD28 (Strong) | [108] |
| Smad3 | [10–12, 109] | RORγt | [18, 19] |
| Smad4 | [13, 109] | STAT3 | [17] |
| STAT5 | [14, 15] | STAT6 | [32] |
| STAT1* | [110] | NOTCH/Hes1* | [111–113] |
| Id3 | [114] | DNMT | [40, 115] |
| E2a | [114] | GATA3* | [114, 116] |
| Runx1/3 | [16] | ||
| c-rel | [117, 118] | ||
| NFAT | [12, 104, 118, 119] | ||
| AP-1 | [9, 120] | ||
| CREB | [39] | ||
| FoxO1/O3a | [102, 121] | ||
| Sp-1 | [40] | ||
| TIEG1 | [40, 122] |
Both positive and negative regulation documented.
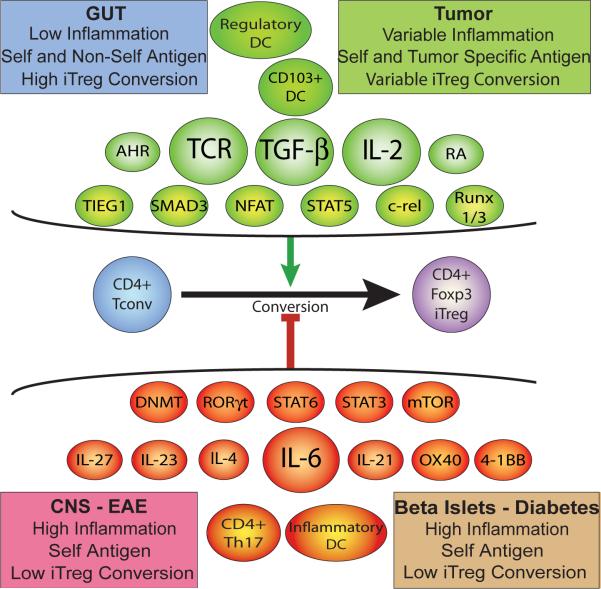
In vivo conditions that promote iTreg formation are not well resolved, though homeostatic expansion of T cells in lymphopenic conditions, provision or chronic exposure to low dose or oral antigen in non-inflammatory conditions, and antigen presentation by immature DCs are favorable[22–27]. Blocking studies indicate an in vivo role for IL-2 and TGF-β in iTreg induction[28–30]. Retinoic acid (RA) fosters iTreg formation in part by enhancing TGF-β production[31–33]. Additional pathways, including aryl hydrocarbon receptor signals and mTor inhibition further promote Foxp3 upregulation (Figure 1)[34–37]. Once induced, Foxp3 binds its own promoter, helping stabilize its own expression while also inhibiting effector T cell differentiation by, for example, antagonizing RORγt function[9, 38]. Foxp3 promoter elements are not demethylated in iTreg to the extent that they are in nTreg, and iTreg show significant instability[39, 40]. Indeed, though generally suppressive, in pathologic conditions, iTreg can revert to effector forms that contribute to immunopathology[22, 41].
Figure 1.
Foxp3 induction and iTreg formation is regulated by different antigen presenting cell types, cytokines and cell signaling molecules, signaling pathways, and transcriptions factors. The integration of positive and negative inductive signals will determine whether Foxp3 is upregulated. The distinct environments of the gastrointestinal tract, CNS, islets, and tumors will variably promote the formation of iTreg.
nTreg and iTreg share many regulatory properties, but are non-redundant[42, 43]. This in part reflects the distinct TCR repertoires of iTreg and nTreg. Whereas nTreg are derived from thymocytes selected for self-recognition, iTreg develop from conventional T cells and are more likely to recognize foreign antigens. This would be expected to guide these cells to different targets [44–46]. Their functional activities may also differ, and iTreg and nTreg have distinct gene expression profiles[47]. The formation and relative role of iTreg and nTreg within disease states is not well understood. Because phenotypic markers that definitively distinguish iTreg and nTreg are lacking, analysis of each population's presence and activity during disease has presented significant technical challenges. Here, we review data from several model systems exploring the generation, stability, and function of iTreg during pathologic conditions.
2. iTreg formation in gastrointestinal (GI) immunity
The GI mucosa interfaces with commensal microbiota and ingested food. Immune reactions to microbial and food antigens can lead to inflammatory bowel disease (IBD) or food intolerances. nTreg are positively selected in the thymus for self antigen recognition. As ingested and microbial antigens will not be expressed in the thymus, selection of nTreg specific for these will be limited[24]. Maturation of gut antigen-specific T cells into iTreg may therefore be important to preserve GI homeostasis. Indeed, iTreg specific for both microbes and fed antigens are found in the GI tract. These iTreg provide protection against colitis in a manner that complements rather than duplicates the effects of nTreg[42, 43, 48].
Mice deficient in the conserved nucleotide sequence 1 (CNS1) in the Foxp3 gene do not develop iTreg. CNS1 includes a TGF-β-NFAT response element and is required for iTreg but not nTreg formation. Systemic autoimmunity is not seen, but CNS-1 deficient mice are susceptible to spontaneous colitis[49]. Several GI-specific mechanisms may be involved. RA-induces iTreg that upregulate the gut homing receptors α4β7 integrin and CCR9, and can be found in the lamina propria[33, 50, 51]. CD103+ dendritic cells in the mesenteric lymph node (MLN) promote tolerance through the generation of iTreg in the context of TGF-β and RA [52, 53]. Interestingly, tolerance generated in the gut can also inhibit the progression of distant autoimmune diseases, including diabetes and experimental allergic encephalomyelitis (EAE)[24, 49, 54].
3. iTreg formation in central nervous system autoimmunity
Treg accumulate in the CNS of mice with EAE, and can comprise 20–40% of infiltrating CD4+ T cells late in disease. Transfer of iTreg or nTreg into mice inhibits EAE, whereas Treg depletion worsens disease[55]. In vitro, neurons stimulated the conversion of encephalitogenic T cells into iTreg in a B7 and TGF-β dependent manner[56]. Further, cells from nTreg-deficient Rag−/− mice transgenic for a myelin basic protein-specific TCR were able to differentiate into iTreg during homeostatic expansion[57]. This suggests that myelin specific iTreg may form in situ.
To assess the origin of CNS infiltrating Treg in mice with a heterogeneous T cell repertoire, we monitored conventional and regulatory TCR sequences in the CNS and spleen of wild type mice or mice with a fixed TCRα chain during EAE using high-throughput sequencing. CNS CDR3 sequences from Treg and Tconv showed little commonality, but overlapped with the corresponding Treg or Tconv sequences in the spleen[58, 59]. Further, in the mice with a fixed TCRα, analysis of TCRβ sequences bearing defined Vβ and Jβ identified specific amino acids in the CDR3β of myelin-reactive receptors that were specifically and alternatively associated with Treg or Tconv lineage assignment. This indicates a distinct ontogeny for the populations, and argues against substantial iTreg formation in the setting of a diverse TCR repertoire[58]. Consistently, little iTreg formation was detected among Foxp3– T cells adoptively transferred into mice prior to EAE induction[60]. CNS1-deficiency, which impedes iTreg formation, also did not affect EAE severity, implying that iTreg are not required for EAE protection [49].
4. iTreg formation during diabetes
iTreg can be induced in NOD mice after immunization with insulin, and Treg transfer is able to block disease [61]. The involvement of iTreg in spontaneous diabetes in NOD mice has not been established. All trans retinoic acid (ATRA) increased Treg number and protected mice from insulitis[62]. Depletion of Treg prior to ATRA treatment abrogated protection, suggesting that ATRA-induced iTreg that may have formed were not sufficient to confer protection. In a model targeting a proinsulin mimiotope to DCs in NOD mice, iTreg also formed. These did not impact disease progression, but appeared to reduce the incidence of diabetes[63].
More data is available in the BDC2.5 TCR transgenic model of diabetes[63–65]. In a RAG−/− T cell transfer model anti-thymocyte globulin (mATG) led to immune tolerance that was dependent on the induction of CTLA4+ BDC2.5 iTreg effectors, indicating that in specific therapeutic contexts iTreg formation can be relevant[66, 67]. T cells in TCR transgenic models often express a second endogenously rearranged TCRα chain. Analysis of the repertoire of these second chains in BDC2.5 mice with diabetes indicated that islet-infiltrating Treg are unrelated to Tconv. This indicates that iTreg do not normally contribute significantly to the Treg response during diabetes in these mice[68].
5. iTreg formation in the tumor microenvironment
Tumors can create an immunosuppressive environment that may promote iTreg development through TGF-β production or other mechanisms[69]. Indeed, many tumors show large numbers of infiltrating Treg[70]. The extent to which these result from the induction of Foxp3 in conventional T cells or nTreg recruitment appears to be tumor dependent. In MO-5 (B16-ova) and TC-1 tumor models, repertoire analyses indicated that Treg infiltration was the result of homing and expansion of circulating Treg, and not intratumoral iTreg formation[71]. Foxp3+ iTreg also could not be recovered from OT-II mice implanted with TGF-β-producing B16-ova tumors, and Treg infiltrates in a murine glioblastoma (GBM) appeared to be predominantly of thymic origin[72, 73]. Repertoire analysis of carcinogen induced tumor-infiltrating Tconv and Treg displayed minimal TCR overlap. Greater similarity was observed between tumor infiltrating cells and those in the tumor draining lymph nodes, arguing against significant conversion[70].
In contrast with these reports, a separate study of B16F1 melanomas indicated substantial overlap between the tumor-responsive Tconv and Treg repertoires, implying that iTreg induction supplied a large component of the Treg response. A similar high level of repertoire overlap was seen in Treg isolated from patients with melanoma [74, 75]. Cumulatively, these data indicate tumor-specific variation in the origins of infiltrating Treg, with only some tumors showing substantial iTreg formation. Deciphering the environmental cues guiding the intratumoral induction of iTreg will be important to optimally counteract Treg suppression of the tumor-specific immune response.
6. Conclusions
Formation of iTreg during immunopathologic conditions shows regional and contextual variability. iTreg formation has been clearly identified within the GI tract. There is a strong rationale for iTreg generation at mucosal and integumentary interfaces based on specificity considerations. Continuous mucosal exposure to large quantities of predominantly innocuous environmental antigens leads to a strong risk for immunopathology due to overzealous immune responses. Diversion of reactive T cells to iTreg may help quell such responses.
In contrast with mucosal immunity, nTreg, which develop in response to thymic self antigens, will possess a repertoire biased toward recognition of a host's own tissues, one that should be capable of limiting autoreactive responses. The absence of robust markers that distinguish nTreg and iTreg prevents ontologic categorization of Treg by direct staining. Nevertheless, we and others have used indirect approaches and have not found a significant contribution of iTreg during some organ specific autoimmune responses where a heterogeneous TCR repertoire is present. Likewise, in some but not all studies, nTreg are the primary source for tumor-infiltrating Treg.
While it is clear that adaptive upregulation of Foxp3 on T cells occurs, it remains to be determined why this is variably supported in different anatomic locations and environmental conditions. Possibly, the small number of iTreg that do form during autoimmune diseases expand to fill a niche made available by the absence of nTreg. Both iTreg and nTreg will be supported by the same tropic cytokines, and competition between these populations is possible. Alternatively or in a complementary manner, iTreg, which arise from Tconv with a broad specificity for foreign antigens, may fill specificity gaps left in the nTreg repertoire, which is skewed toward self-specificity. A better understanding of the signals that induce and sustain iTreg will be critical for the development of enhanced therapeutics capable of manipulating these essential regulators of the immune system.
Highlights
iTreg and nTreg are non-redundant, and have distinct roles and antigen specificity.
iTreg are important for the induction of gastrointestinal tolerance.
iTreg formation is inconsistent in models of autoimmune or neoplastic disease.
The role of iTreg in immunopatholgic conditions is context and location dependent.
Acknowledgements
Supported by the National Institutes of Health Grant R01 AI056153 (to TLG) and the American Lebanese Syrian Associated Charities (ALSAC)/St. Jude Children's Research Hospital (to all authors).
Abbreviations
- Foxp3
forkhead box p3 transcription factor
- Treg
regulatory T lymphocyte
- nTreg
natural Treg
- iTreg
induced Treg
- Tconv
conventional T cell
- TCR
T cell receptor
- EAE
experimental allergic encephalomyelitis
- CNS
central nervous system
- CDR
complementarity determining region.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu. Rev. Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Geiger TL, Tauro S. Nature and nurture in Foxp3(+) regulatory T cell development, stability, and function. Hum Immunol. 2012;73:232–239. doi: 10.1016/j.humimm.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv Immunol. 2003;81:331–371. doi: 10.1016/s0065-2776(03)81008-8. [DOI] [PubMed] [Google Scholar]
- [4].Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- [6].Dons EM, Raimondi G, Cooper DKC, Thomson AW. Induced regulatory T cells: mechanisms of conversion and suppressive potential. Hum Immunol. 2012;73:328–334. doi: 10.1016/j.humimm.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shevach EM, Davidson TS, Huter EN, Dipaolo RA, Andersson J. Role of TGF-Beta in the induction of Foxp3 expression and T regulatory cell function. J Clin Immunol. 2008;28:640–646. doi: 10.1007/s10875-008-9240-1. [DOI] [PubMed] [Google Scholar]
- [8].Xu L, Kitani A, Strober W. Molecular mechanisms regulating TGF-beta-induced Foxp3 expression. Mucosal Immunol. 2010;3:230–238. doi: 10.1038/mi.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gao Y, Lin F, Su J, Gao Z, Li Y, Yang J, Deng Z, Liu B, Tsun A, Li B. Molecular mechanisms underlying the regulation and functional plasticity of FOXP3(+) regulatory T cells. Genes Immun. 2012;13:1–13. doi: 10.1038/gene.2011.77. [DOI] [PubMed] [Google Scholar]
- [10].Lu L, Wang J, Zhang F, Chai Y, Brand D, Wang X, Horwitz DA, Shi W, Zheng SG. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Martinez GJ, Zhang Z, Chung Y, Reynolds JM, Lin X, Jetten AM, Feng X-H, Dong C. Smad3 differentially regulates the induction of regulatory and inflammatory T cell differentiation. J Biol Chem. 2009;284:35283–35286. doi: 10.1074/jbc.C109.078238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- [13].Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng X-H, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol. 2011;186:6329–6337. doi: 10.4049/jimmunol.1100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lin J-X, Li P, Liu D, Jin HT, He J, Ata Ur Rasheed M, Rochman Y, Wang L, Cui K, Liu C, Kelsall BL, Ahmed R, Leonard WJ. Critical Role of STAT5 transcription factor tetramerization for cytokine responses and normal immune function. Immunity. 2012;36:586–599. doi: 10.1016/j.immuni.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kitoh A, Ono M, Naoe Y, Ohkura N, Yamaguchi T, Yaguchi H, Kitabayashi I, Tsukada T, Nomura T, Miyachi Y, Taniuchi I, Sakaguchi S. Indispensable role of the Runx1-Cbfbeta transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity. 2009;31:609–620. doi: 10.1016/j.immuni.2009.09.003. [DOI] [PubMed] [Google Scholar]
- [17].Huber M, Steinwald V, Guralnik A, Brüstle A, Kleemann P, Rosenplänter C, Decker T, Lohoff M. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- [18].Ziegler SF, Buckner JH. FOXP3 and the regulation of Treg/Th17 differentiation. Microbes Infect. 2009;11:594–598. doi: 10.1016/j.micinf.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun C-M, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- [21].Kanakasabai S, Casalini E, Walline CC, Mo C, Chearwae W, Bright JJ. Differential regulation of CD4(+) T helper cell responses by curcumin in experimental autoimmune encephalomyelitis. The Journal of nutritional biochemistry. 2012;23(11):1498–1507. doi: 10.1016/j.jnutbio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- [22].Murai M, Krause P, Cheroutre H, Kronenberg M. Regulatory T-cell stability and plasticity in mucosal and systemic immune systems. Mucosal Immunol. 2010;3:443–449. doi: 10.1038/mi.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Winstead CJ, Reilly CS, Moon JJ, Jenkins MK, Hamilton SE, Jameson SC, Way SS, Khoruts A. CD4+CD25+Foxp3+ regulatory T cells optimize diversity of the conventional T cell repertoire during reconstitution from lymphopenia. J Immunol. 2010;184:4749–4760. doi: 10.4049/jimmunol.0904076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Verginis P, McLaughlin KA, Wucherpfennig KW, von Boehmer H, Apostolou I. Induction of antigen-specific regulatory T cells in wild-type mice: visualization and targets of suppression. Proc Natl Acad Sci USA. 2008;105:3479–3484. doi: 10.1073/pnas.0800149105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Daniel C, Ploegh H, von Boehmer H. Antigen-specific induction of regulatory T cells in vivo and in vitro. Methods Mol Biol. 2011;707:173–185. doi: 10.1007/978-1-61737-979-6_11. [DOI] [PubMed] [Google Scholar]
- [27].Nakayama T, Yamashita M. The TCR-mediated signaling pathways that control the direction of helper T cell differentiation. Semin Immunol. 2010;22:303–309. doi: 10.1016/j.smim.2010.04.010. [DOI] [PubMed] [Google Scholar]
- [28].Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Petrausch U, Jensen SM, Twitty C, Poehlein CH, Haley DP, Walker EB, Fox BA. Disruption of TGF-beta signaling prevents the generation of tumor-sensitized regulatory T cells and facilitates therapeutic antitumor immunity. J Immunol. 2009;183:3682–3689. doi: 10.4049/jimmunol.0900560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sawamukai N, Satake A, Schmidt AM, Lamborn IT, Ojha P, Tanaka Y, Kambayashi T. Cell-autonomous role of TGFβ and IL-2 receptors in CD4+ and CD8+ inducible regulatory T-cell generation during GVHD. Blood. 2012;119:5575–5583. doi: 10.1182/blood-2011-07-367987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O'Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Takaki H, Ichiyama K, Koga K, Chinen T, Takaesu G, Sugiyama Y, Kato S, Yoshimura A, Kobayashi T. STAT6 Inhibits TGF-beta1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. J Biol Chem. 2008;283:14955–14962. doi: 10.1074/jbc.M801123200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pot C. Aryl hydrocarbon receptor controls regulatory CD4+ T cell function. Swiss Med Wkly. 2012;142:w13592. doi: 10.4414/smw.2012.13592. [DOI] [PubMed] [Google Scholar]
- [35].Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah A-M, Burns EJ, Weiner HL. From the Cover: An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O'Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Apostolou I, Verginis P, Kretschmer K, Polansky J, Hühn J, von Boehmer H. Peripherally induced Treg: mode, stability, and role in specific tolerance. J Clin Immunol. 2008;28:619–624. doi: 10.1007/s10875-008-9254-8. [DOI] [PubMed] [Google Scholar]
- [39].Polansky JK, Schreiber L, Thelemann C, Ludwig L, Krüger M, Baumgrass R, Cording S, Floess S, Hamann A, Huehn J. Methylation matters: binding of Ets-1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells. J Mol Med. 2010;88:1029–1040. doi: 10.1007/s00109-010-0642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, Reid SP, Levy DE, Bromberg JS. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol. 2009;182:259–273. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Beres A, Komorowski R, Mihara M, Drobyski WR. Instability of Foxp3 expression limits the ability of induced regulatory T cells to mitigate graft versus host disease. Clin Cancer Res. 2011;17:3969–3983. doi: 10.1158/1078-0432.CCR-10-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Haribhai D, Lin W, Edwards B, Ziegelbauer J, Salzman NH, Carlson MR, Li S-H, Simpson PM, Chatila TA, Williams CB. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol. 2009;182:3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li S-H, Relland LM, Wise PM, Chen A, Zheng Y-Q, Simpson PM, Gorski J, Salzman NH, Hessner MJ, Chatila TA, Williams CB. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Föhse L, Suffner J, Suhre K, Wahl B, Lindner C, Lee C-W, Schmitz S, Haas JD, Lamprecht S, Koenecke C, Bleich A, Hämmerling GJ, Malissen B, Suerbaum S, Förster R, Prinz I. High TCR diversity ensures optimal function and homeostasis of Foxp3+ regulatory T cells. Eur J Immunol. 2011;41:3101–3113. doi: 10.1002/eji.201141986. [DOI] [PubMed] [Google Scholar]
- [45].Davidson TS, Shevach EM. Polyclonal Treg cells modulate T effector cell trafficking. Eur J Immunol. 2011;41:2862–2870. doi: 10.1002/eji.201141503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shevach EM. Biological functions of regulatory T cells. Adv Immunol. 2011;112:137–176. doi: 10.1016/B978-0-12-387827-4.00004-8. [DOI] [PubMed] [Google Scholar]
- [47].Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci USA. 2010;107:5919–5924. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, Brand D, Liu Z, Zheng SG. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. The Journal of Immunology. 2010;185:2675–2679. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Laffont S, Siddiqui KRR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J Immunol. 2010;40:1877–1883. doi: 10.1002/eji.200939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- [54].Badami E, Sorini C, Coccia M, Usuelli V, Molteni L, Bolla AM, Scavini M, Mariani A, King C, Bosi E, Falcone M. Defective differentiation of regulatory FoxP3+ T cells by small-intestinal dendritic cells in patients with type 1 diabetes. Diabetes. 2011;60:2120–2124. doi: 10.2337/db10-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Selvaraj RK, Geiger TL. Mitigation of experimental allergic encephalomyelitis by TGF-beta induced Foxp3+ regulatory T lymphocytes through the induction of anergy and infectious tolerance. J Immunol. 2008;180:2830–2838. doi: 10.4049/jimmunol.180.5.2830. [DOI] [PubMed] [Google Scholar]
- [56].Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- [57].Shen S, Ding Y, Tadokoro CE, Olivares-Villagómez D, Camps-Ramírez M, Curotto de Lafaille MA, Lafaille JJ. Control of homeostatic proliferation by regulatory T cells. J Clin Invest. 2005;115:3517–3526. doi: 10.1172/JCI25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu X, Nguyen P, Liu W, Cheng C, Steeves M, Obenauer JC, Ma J, Geiger TL. T cell receptor CDR3 sequence but not recognition characteristics distinguish autoreactive effector and Foxp3(+) regulatory T cells. Immunity. 2009;31:909–920. doi: 10.1016/j.immuni.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nguyen P, Liu W, Ma J, Manirarora JN, Liu X, Cheng C, Geiger TL. Discrete TCR repertoires and CDR3 features distinguish effector and Foxp3+ regulatory T lymphocytes in myelin oligodendrocyte glycoprotein-induced experimental allergic encephalomyelitis. The Journal of Immunology. 2010;185:3895–3904. doi: 10.4049/jimmunol.1001550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Bäckström BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhang W, Jin H, Hu Y, Yu Y, Li X, Ding Z, Kang Y, Wang B. Protective response against type 1 diabetes in nonobese diabetic mice after coimmunization with insulin and DNA encoding proinsulin. Human Gene Therapy. 2010;21:171–178. doi: 10.1089/hum.2009.095. [DOI] [PubMed] [Google Scholar]
- [62].Van Y-H, Lee W-H, Ortiz S, Lee M-H, Qin H-J, Liu C-P. All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes. 2009;58:146–155. doi: 10.2337/db08-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Petzold C, Riewaldt J, Koenig T, Schallenberg S, Kretschmer K. Dendritic cell-targeted pancreatic beta-cell antigen leads to conversion of self-reactive CD4(+) T cells into regulatory T cells and promotes immunotolerance in NOD mice. Rev Diabet Stud. 2010;7:47–61. doi: 10.1900/RDS.2010.7.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kornete M, Sgouroudis E, Piccirillo CA. ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J Immunol. 2012;188:1064–1074. doi: 10.4049/jimmunol.1101303. [DOI] [PubMed] [Google Scholar]
- [65].Thomas DC, Mellanby RJ, Phillips JM, Cooke A. An early age-related increase in the frequency of CD4+ Foxp3+ cells in BDC2.5NOD mice. Immunology. 2007;121:565–576. doi: 10.1111/j.1365-2567.2007.02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].D'Addio F, Yuan X, Habicht A, Williams J, Ruzek M, Iacomini J, Turka LA, Sayegh MH, Najafian N, Ansari MJ. A novel clinically relevant approach to tip the balance toward regulation in stringent transplant model. Transplantation. 2010;90:260–269. doi: 10.1097/tp.0b013e3181e64217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lu Y, Suzuki J, Guillioli M, Umland O, Chen Z. Induction of self-antigen-specific Foxp3+ regulatory T cells in the periphery by lymphodepletion treatment with anti-mouse thymocyte globulin in mice. Immunology. 2011;134:50–59. doi: 10.1111/j.1365-2567.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wong J, Mathis D, Benoist C. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J Exp Med. 2007;204:2039–2045. doi: 10.1084/jem.20070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu. Rev. Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- [70].Hindley JP, Ferreira C, Jones E, Lauder SN, Ladell K, Wynn KK, Betts GJ, Singh Y, Price DA, Godkin AJ, Dyson J, Gallimore A. Analysis of the T-cell receptor repertoires of tumor-infiltrating conventional and regulatory T cells reveals no evidence for conversion in carcinogen-induced tumors. Cancer Res. 2011;71:736–746. doi: 10.1158/0008-5472.CAN-10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sainz-Perez A, Lim A, Lemercier B, Leclerc C. The T-cell receptor repertoire of tumor-infiltrating regulatory T lymphocytes is skewed towards public sequences. Cancer Res. 2012;72(14):3557–69. doi: 10.1158/0008-5472.CAN-12-0277. [DOI] [PubMed] [Google Scholar]
- [72].Wainwright DA, Sengupta S, Han Y, Lesniak MS. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro-oncology. 2011;13:1308–1323. doi: 10.1093/neuonc/nor134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Quatromoni JG, Morris LF, Donahue TR, Wang Y, McBride W, Chatila T, Economou JS. T cell receptor transgenic lymphocytes infiltrating murine tumors are not induced to express foxp3. J Hematol Oncol. 2011;4:48. doi: 10.1186/1756-8722-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kuczma M, Kopij M, Pawlikowska I, Wang C-Y, Rempala GA, Kraj P. Intratumoral convergence of the TCR repertoires of effector and Foxp3+ CD4+ T cells. PLoS ONE. 2010;5:e13623. doi: 10.1371/journal.pone.0013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fourcade J, Sun Z, Kudela P, Janjic B, Kirkwood JM, El-Hafnawy T, Zarour HM. Human tumor antigen-specific helper and regulatory T cells share common epitope specificity but exhibit distinct T cell repertoire. J Immunol. 2010;184:6709–6718. doi: 10.4049/jimmunol.0903612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Schallenberg S, Tsai P-Y, Riewaldt J, Kretschmer K. Identification of an immediate Foxp3(−) precursor to Foxp3(+) regulatory T cells in peripheral lymphoid organs of nonmanipulated mice. J Exp Med. 2010;207:1393–1407. doi: 10.1084/jem.20100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- [78].Long SA, Buckner JH. Combination of rapamycin and IL-2 increases de novo induction of human CD4(+)CD25(+)FOXP3(+) T cells. J Autoimmun. 2008;30:293–302. doi: 10.1016/j.jaut.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- [80].Chen W, Jin W, Hardegen N, Lei K-J, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J Immunol. 2007;178:7667–7677. doi: 10.4049/jimmunol.178.12.7667. [DOI] [PubMed] [Google Scholar]
- [82].Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- [83].Hadjur S, Bruno L, Hertweck A, Cobb BS, Taylor B, Fisher AG, Merkenschlager M. IL4 blockade of inducible regulatory T cell differentiation: the role of Th2 cells, Gata3 and PU.1. Immunol Lett. 2009;122:37–43. doi: 10.1016/j.imlet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- [84].Beal AM, Ramos-Hernández N, Riling CR, Nowelsky EA, Oliver PM. TGF-β induces the expression of the adaptor Ndfip1 to silence IL-4 production during iTreg cell differentiation. Nat Immunol. 2012;13:77–85. doi: 10.1038/ni.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio C-W, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh C-S. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh C-S. Antigen-specific peripheral shaping of the natural regulatory T cell population. J Exp Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- [88].Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- [89].Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- [90].Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med. 2011;208:1501–1510. doi: 10.1084/jem.20110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Khattar M, Chen W, Stepkowski SM. Expanding and converting regulatory T cells: a horizon for immunotherapy. Arch Immunol Ther Exp (Warsz) 2009;57:199–204. doi: 10.1007/s00005-009-0021-1. [DOI] [PubMed] [Google Scholar]
- [92].Geng S, Yu Y, Kang Y, Pavlakis G, Jin H, Li J, Hu Y, Hu W, Wang S, Wang B. Efficient induction of CD25- iTreg by co-immunization requires strongly antigenic epitopes for T cells. BMC Immunol. 2011;12:27. doi: 10.1186/1471-2172-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR, Neurath MF. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- [94].Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- [97].Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, Killeen N, Ishii N, Li XC. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kang SW, Kim SH, Lee N, Lee W-W, Hwang K-A, Shin MS, Lee S-H, Kim W-U, Kang I. 1,25-Dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. J Immunol. 2012;188:5276–5282. doi: 10.4049/jimmunol.1101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, Chi H. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lee JH, Lydon JP, Kim CH. Progesterone suppresses the mTOR pathway and promotes generation of induced regulatory T cells with increased stability. Eur J Immunol. 2012;42:1–14. doi: 10.1002/eji.201142317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, Finney CAM, Greenwood EJD, Knox DP, Wilson MS, Belkaid Y, Rudensky AY, Maizels RM. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ma J, Ding Y, Fang X, Wang R, Sun Z. Protein kinase C-θ inhibits inducible regulatory T cell differentiation via an AKT-Foxo1/3a-dependent pathway. J Immunol. 2012;188:5337–5347. doi: 10.4049/jimmunol.1102979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–1821. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- [105].Smith SE, Hoelzinger DB, Dominguez AL, Van Snick J, Lustgarten J. Signals through 4-1BB inhibit T regulatory cells by blocking IL-9 production enhancing antitumor responses. Cancer Immunol Immunother. 2011;60:1775–1787. doi: 10.1007/s00262-011-1075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Madireddi S, Schabowsky R-H, Srivastava AK, Sharma RK, Yolcu ES, Shirwan H. SA-4-1BBL Costimulation Inhibits Conversion of Conventional CD4(+) T Cells into CD4(+)FoxP3(+) T Regulatory Cells by Production of IFN-γ. PLoS ONE. 2012;7:e42459. doi: 10.1371/journal.pone.0042459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zhou L, Lopes JE, Chong MMW, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Semple K, Nguyen A, Yu Y, Wang H, Anasetti C, Yu X-Z. Strong CD28 costimulation suppresses induction of regulatory T cells from naive precursors through Lck signaling. Blood. 2011;117:3096–3103. doi: 10.1182/blood-2010-08-301275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Feng X-H, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- [110].Chang J-H, Kim Y-J, Han S-H, Kang C-Y. IFN-gamma-STAT1 signal regulates the differentiation of inducible Treg: potential role for ROS-mediated apoptosis. Eur J Immunol. 2009;39:1241–1251. doi: 10.1002/eji.200838913. [DOI] [PubMed] [Google Scholar]
- [111].Haque R, Lei F, Xiong X, Bian Y, Zhao B, Wu Y, Song J. Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity. J Immunol. 2012;189:1228–1236. doi: 10.4049/jimmunol.1200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ostroukhova M, Qi Z, Oriss TB, Dixon-McCarthy B, Ray P, Ray A. Treg-mediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-beta. J Clin Invest. 2006;116:996–1004. doi: 10.1172/JCI26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Asano N, Watanabe T, Kitani A, Fuss IJ, Strober W. Notch1 signaling and regulatory T cell function. J Immunol. 2008;180:2796–2804. doi: 10.4049/jimmunol.180.5.2796. [DOI] [PubMed] [Google Scholar]
- [114].Maruyama T, Li J, Vaque JP, Konkel JE, Wang W, Zhang B, Zhang P, Zamarron BF, Yu D, Wu Y, Zhuang Y, Gutkind JS, Chen W. Control of the differentiation of regulatory T cells and T(H)17 cells by the DNA-binding inhibitor Id3. Nat Immunol. 2011;12:86–95. doi: 10.1038/ni.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wong CP, Nguyen LP, Noh SK, Bray TM, Bruno RS, Ho E. Induction of regulatory T cells by green tea polyphenol EGCG. Immunol Lett. 2011;139:7–13. doi: 10.1016/j.imlet.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Mantel P-Y, Kuipers H, Boyman O, Rhyner C, Ouaked N, Rückert B, Karagiannidis C, Lambrecht BN, Hendriks RW, Crameri R, Akdis CA, Blaser K, Schmidt-Weber CB. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ruan Q, Chen YH. Nuclear factor-κB in immunity and inflammation: the Treg and Th17 connection. Adv Exp Med Biol. 2012;946:207–221. doi: 10.1007/978-1-4614-0106-3_12. [DOI] [PubMed] [Google Scholar]
- [118].Ruan Q, Kameswaran V, Tone Y, Li L, Liou H-C, Greene MI, Tone M, Chen YH. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Li Q, Shakya A, Guo X, Zhang H, Tantin D, Jensen PE, Chen X. Constitutive nuclear localization of NFAT in Foxp3+ regulatory T cells independent of calcineurin activity. J Immunol. 2012;188:4268–4277. doi: 10.4049/jimmunol.1102376. [DOI] [PubMed] [Google Scholar]
- [120].Mantel P-Y, Ouaked N, Rückert B, Karagiannidis C, Welz R, Blaser K, Schmidt-Weber CB. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176:3593–3602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- [121].Kerdiles YM, Stone EL, Beisner DR, Beisner DL, McGargill MA, Ch'en IL, Stockmann C, Katayama CD, Hedrick SM. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Glisic S, Ehlenbach S, Jailwala P, Waukau J, Jana S, Ghosh S. Inducible regulatory T cells (iTregs) from recent-onset type 1 diabetes subjects show increased in vitro suppression and higher ITCH levels compared with controls. Cell Tissue Res. 2010;339:585–595. doi: 10.1007/s00441-009-0900-0. [DOI] [PubMed] [Google Scholar]