Abstract
Sentence comprehension requires processing of argument structure information associated with verbs, i.e. the number and type of arguments that they select. Many individuals with agrammatic aphasia show impaired production of verbs with greater argument structure density. The extent to which these participants also show argument structure deficits during comprehension, however, is unclear. Some studies find normal access to verb arguments, whereas others report impaired ability. The present study investigated verb argument structure processing in agrammatic aphasia by examining event-related potentials associated with argument structure violations in healthy young and older adults as well as aphasic individuals. A semantic violation condition was included to investigate possible differences in sensitivity to semantic and argument structure information during sentence processing. Results for the healthy control participants showed a negativity followed by a positive shift (N400-P600) in the argument structure violation condition, as found in previous ERP studies (Friederici & Frisch, 2000; Frisch, Hahne, & Friederici, 2004). In contrast, individuals with agrammatic aphasia showed a P600, but no N400, response to argument structure mismatches. Additionally, compared to the control groups, the agrammatic participants showed an attenuated, but relatively preserved, N400 response to semantic violations. These data show that agrammatic individuals do not demonstrate normal real-time sensitivity to verb argument structure requirements during sentence processing.
Keywords: verb argument structure, event related potentials, language-related brain potentials, N400, P600, semantic processing, agrammatic aphasia
1. Introduction
Auditory sentence comprehension requires rapid analysis of complex auditory signals and construction of syntactic structures and meaning representations. Models of language processing suggest that phonological, syntactic and semantic information is accessed and coordinated within milliseconds in order to successfully understand sentences (Friederici, 2002, Friederici & Kotz, 2003; Hagoort, 2003; Kaan & Swaab, 2002). In particular, integration of information associated with verbs is essential for successful sentence comprehension. Verbs are central elements in this process because they specify the number of arguments that appear in the sentence, the thematic roles of these arguments (e.g., agent, the performer of an action; theme, the recipient of the action), the syntactic positions in which they occur (subject, direct object, etc.) and their syntactic realization (noun phrase, prepositional phrase, clause, etc.; This information is also referred to as subcategorization information). For instance, the intransitive verb sneeze requires only one, external, argument (an agent) as in: John sneezed. In contrast, the transitive verb fix requires two arguments (an agent and a theme) as in: John fixed the car. Other verbs take three arguments, requiring an agent, a theme, and a goal (e.g., John gave the book to the teacher).
A growing number of studies demonstrate that argument structure information of verbs is immediately and automatically activated during sentence processing (Carlson & Tannenhaus, 1988; MacDonald, Pearlmuter, & Seidenberg, 1994; Mauner & Konig, 2000; Shapiro, Brookins, Gordon, & Nagel, 1991; Shapiro, Gordon, Hack, & Killackey, 1993; Shapiro & Levine, 1990; Trueswell & Kim, 1998). For example, in a series of studies using a cross-modal lexical decision task, Shapiro and colleagues showed that lexical decision times to visually presented targets were longer when presented in the vicinity of verbs with more argument structure/subcategorization options. In addition, it has been shown that verbs prime for their arguments (Ferreti, McRae, & Hatherell, 2001), and that a verb’s thematic specifications are used to pro-actively restrict the domain of subsequent reference (Altmann & Kamide, 1999).
Verb argument structure processing ability in individuals with Broca’s (agrammatic) aphasia, however, is unclear. Several studies have found impairments in producing verbs and sentences with complex argument structures. For example, Kim and Thompson (2000) found that for English-speaking agrammatic individuals, three-argument verbs were more difficult to produce than two-argument verbs. A similar deficit was found in sentence production, evidenced by greater difficulty when producing sentences with more arguments as compared to those with fewer arguments (Thompson et al., 1997; Thompson, Dickey, Cho, Lee, & Griffin, 2007). These effects have been observed across different languages including English (Kim & Thompson, 2004; Thompson, 2003) German (De Blesser & Kauschke, 2003), Dutch (Jonkers & Bastiaanse, 1996, 1998), Italian (Luzzatti et al., 2002), Hungarian (Kiss, 2000), and Russian (Dragoy & Bastiaanse, 2009)
The nature of this deficit, however, is not completely understood. Some findings suggest that the lexical representation of verb argument structure may be preserved in individuals with agrammatic aphasia. For example, studies have shown normal activation of argument structure and subcategorization information in cross-modal lexical decision tasks such as the ones discussed above (Shapiro et al., 1990, 1993). In addition, the agrammatic participants in Kim & Thompson (2000, 2004) showed no effect of argument structure complexity on comprehension in a word-to-picture matching task, performing at normal levels.
In two studies, Kim and Thompson (2000, 2004) also tested patients’ ability to detect verb argument structure violations in a grammaticality judgment task in sentences with a missing obligatory argument (e.g. *The woman is giving the sandwich; *The boy is carrying) or with a noun phrase following an intransitive verb (e.g. *The dog is barking the girl). In both studies, agrammatic participants performed at near normal level (means 93.6% correct, 92.1% correct, respectively). Grodzinsky & Finkel (1998) found a similar pattern in a study testing verb argument structure (and other syntactic) violations. In that study some sentences contained violations similar to those of Kim and Thompson: transitive verbs lacking an obligatory direct object (*The children threw). In addition, they included verbs with semantically inappropriate complements (*The children sang the football over the fence). Results showed mean performance at 91% correct.
Notably, grammaticality judgment is an off-line task, where participants make their responses after sentences have been fully presented. In contrast, on-line measures allow observation of the sentence processing as it unfolds in time. Event-related potentials (ERPs), in particular, provide continuous records of cognitive activities associated with language processing, and can thus offer more direct information as to the underlying cause of sentence processing impairments in agrammatic aphasia.
ERPs have been used extensively to investigate the time course of semantic and syntactic processes involved in language comprehension. Processing of semantic anomalies has been shown in many studies to be associated with a centro-parietally distributed negativity peaking around 400 msec post stimulus onset, labeled the ‘N400’. In a pioneering study, Kutas & Hillyard (1980) found that the N400 component is elicited by presentation of semantic anomalies, indicating that it is sensitive to the semantic relations between individual words in the preceding language input. Subsequent studies in both auditory and visual domains found similar effects, suggesting that N400 amplitude reflects semantic processing, associated with restrictions resulting from sentence or discourse context (Connolly & Phillips, 1994; Friederici, Pfeifer, & Hahne, 1993; Holcomb & Neville, 1190; Munte, Heinze, & Mangun, 1993; Rosler et al., 1993; Osterhout & Nicol, 1999; vanBerkum et al., 1999). The N400 component also has been associated with lexical-semantic integration (Brown & Hagoort, 1993; Osterhout & Holcomb, 1992), with increasing semantic integration demands associated with increases in N400 amplitude.
In contrast to N400 semantic violations, manipulations of syntactic structure are associated with two main ERP components: a left anterior negativity (LAN) between 100 and 500 msec, and a late centro-parietal positivity peaking at around 600 msec, labeled the ‘P600’. The P600 component has been found to be sensitive to a variety of syntactic anomalies, including phrase structure violations (Neville, Nicol, Barss, Foster, & Garrett, 1991; Friederici, Hahne, & Mecklinger, 1996), errors of agreement (Coulson et al., 1998; Osterhout & Mobley, 1995), verb inflection (Gunter & Friederici, 1999; Osterhout & Nicol, 1999), and subcategorization violations (Ainsworth-Darnell, Shulman, & Boland, 1998; Osterhout, Holcomb, & Swinney, 1994). The P600 also has been found for garden-path sentences as well as other grammatical, but syntactically nonpreferred, constructions (Osterhout & Holcomb, 1992, 1993; Osterhout, Holcomb, & Swinney, 1994). More recently, the P600 component has been conceived of as an index of syntactic reprocessing cost (Osterhout, Holcomb, & Swinney, 1994), syntactic complexity or ambiguity (van Berkum, Brown, & Hagoort, 1999), or syntactic integration difficulty in general (Kaan, Harris, Gibson, & Holcomb, 2000). Based on these findings, it has been proposed that this late positivity reflects processes of syntactic reanalysis and repair (Friederici et al., 1996; Friedrici & Meyer, 2004; Friederici & Kotz, 2003; Friederici & Weissenborn, 2007; Gunter, Stowe, & Mulder, 1997).
Several ERP investigations have found distinct electrophysiological signatures for different aspects of verb processing. Specifically, violations of subcategorization requirements (e.g. *The cousin visited to the violinist instead of The cousin visited the violinist) are associated with a LAN-P600 pattern, related to syntactic processing, as mentioned above. In contrast, violations of the correct number of arguments (e.g. *The cousin dawdled the violinist) elicit a biphasic N400-P600 pattern (Friederici & Frisch, 2000; Frisch, Hahne, & Friederici, 2004; Friederici & Meyer, 2004), reflecting both semantic and syntactic violations. This latter pattern has been interpreted to reflect different aspects of lexical-thematic integration process. The N400 results from difficulty in integration of thematic information when obligatory arguments are missing (e.g., *John gives a car), or when illicit arguments are present (e.g., *John sleeps a bed), whereas the P600 reflects an attempt at syntactic reanalysis or repair following thematic integration failure. This interpretation is consistent with Frisch and Schlesewsky (2001) who found an N400-P600 pattern for German sentences with two noun phrases marked as grammatical subjects, arguably causing a similar failure of thematic integration. It is likewise consistent with the idea that the P600 effect does not reflect the detection of outright syntactic or semantic violations. Rather, it reflects accommodation processes arising from a surprising or dispreferred sentence continuation, as argued in Friederici & Frisch (2000).
Event-related potentials also have been used to study language deficits in aphasia. Several ERP studies investigated aphasic participants’ lexical-semantic processing. In one such study, Swaab, Brown, and Hagoort (1997) investigated ERP responses to auditorily presented sentences containing a semantically anomalous word in sentence final position (e.g., *The girl dropped the candy on the sky). The authors found that as a group, patients showed a preserved N400 effect to semantic violations. However, these effects were modulated according to the degree of comprehension deficit, such that individuals with severe comprehension deficits showed a reduced and delayed N400 effect, whereas those with mild deficits showed N400 patterns similar to those of normal controls. Similar results are reported in Hagoort, Brown, & Swaab (1996) in a study using unrelated and related word pairs. In another study in Dutch, Wassenaar and Hagoort (2005) reported a reduced and delayed N400 effect to semantic violations for patients with Broca’s aphasia in the visual modality. Finally, Kitade, Enai, Sei, & Morita (1999) found a delayed N400 effect in aphasic participants in a visual word recognition oddball paradigm. It seems thus to be the case that in general, aphasic participants display an N400 effect in the same conditions as healthy speakers do, but the effect is often delayed and attenuated, in correlation with the severity of their language deficits.
Syntax related ERP effects also have been shown to be modified in aphasic patients. In a series of studies, Hagoort and colleagues (Hagoort, Wassenaar, & Brown, 2003; Wassenaar, Brown, & Hagoort, 2004) recorded ERPs in healthy control and agrammatic participants while they listened to sentences that were either syntactically correct or contained violations of subject verb agreement (e.g., *The girls pay the baker and takes the bread home). They found that healthy adults showed a P600 effect in response to this manipulation, whereas Broca’s aphasic individuals did not. In addition, Broca’s aphasic individuals have been found to display a reduced and delayed P600 effect to phrase structure violations (Wassenaar & Hagoort, 2005).
To our knowledge, no previous studies have investigated electrophysiological responses to argument structure violations in aphasic individuals. However, the results of a recent ERP study by Wassenaar & Hagoort (2007), investigating on-line thematic role assignment in a sentence-picture matching is relevant in this respect. In this study, ERPs were recorded while participants decided whether an auditorily presented sentence (e.g. The cat licked the dog) matched a visually displayed picture (e.g. of a dog licking a cat). Neurologically unimpaired individuals showed on-line sensitivity to thematic role assignment, displaying an early negative effect followed by a later positive shift in response to thematic mismatches between the picture and the sentence. In contrast, the ERP effects for agrammatic individuals in response to the mismatches were either significantly delayed or absent. However, they showed off-line behavioral sensitivity to the sentence-picture mismatches, suggesting that although they were able to detect the mismatch, this detection did not occur on-line. In addition, prolonged reaction times in the Broca’s participant group indicate the need of these participants to use off-line response strategies for sentence interpretation.
1.1 The Present Study
The present study investigated how individuals with agrammatic aphasia process verb argument structure information in real time, using ERPs and a grammaticality judgment task. A semantic violation condition was also included, to determine whether participants evinced the classical N400 effect to semantic anomalies, and to track possible differences in sensitivity to semantic and argument structure information during sentence processing. The ERP signatures of aphasic speakers were compared to those of neurologically intact young and older controls. ERPs were recorded while participants engaged in an auditory grammaticality judgment task.
The argument structure violation condition was realized by placing intransitive verbs (e.g., sneeze), in an NP-V-NP string (e.g., *John sneezed the doctor). We surmised that if argument structure information is automatically activated when the verb is presented, then the post-verbal NPs, which do not match the verb’s argument structure requirements, would not be lexically integrated, and this mismatch would be mirrored as a modulation of the ERP waveform. Following the results of Friederici et al. (2000, 2004) in German, it was predicted that, for healthy volunteers, detection of a mismatch between the number of arguments a verb requires and the actual number of nouns appearing in the sentence will be reflected by a negativity followed by a later positive shift. Furthermore, if agrammatic individuals are impaired in on-line access to argument structure information, then these participants should be less sensitive to argument structure violations and display a deviant pattern of ERP responses.
Following Kutas & Hillyard (1980) and Swaab et al. (1997), the semantic anomaly condition was developed by placing a contextually anomalous word in the sentence final position. We anticipated that difficulty integrating the semantically incongruent word with the preceding sentence context would elicit an N400 effect in unimpaired participants. With respect to the agrammatic participants, we hypothesized that they would show an N400 effect in response to semantic anomalies. However, this effect may be reduced or delayed reflecting impaired on-line use of lexical-semantic information, as documented in previous studies (Swaab et al., 1997; Wassenaar & Hagoort, 2005).
2. Methods
2.1 Participants
ERP responses and grammaticality judgments were acquired from three groups of participants: young adults (n = 15), older adults (n = 23), and agrammatic aphasic individuals (n = 15). The study was approved by the IRB at Northwestern University and all participants gave their written informed consent prior to the study. All volunteers were compensated for their participation in the study.
Non-brain-damaged volunteers were recruited from the Northwestern University community and the greater Chicago area. Both groups of neurologically unimpaired participants were native speakers of English. All young (age: M = 22 years, SD = 3; education: M = 15.3 years, SD = 1.4) and older controls (age: M = 60 years, SD = 11; education: M = 17 years, SD = 3) were right handed and had normal hearing and normal or corrected-to-normal vision. Participants had no history of neurological, psychiatric, speech, language, or learning disorders and none were taking neuroleptic or mood altering medications at the time of the study.
Aphasic participants (10 males) were recruited from the subject pool of the Aphasia and Neurolinguistics Research Laboratory at Northwestern University. Mean age was 55.4 years (SD = 12), and mean education was 16.6 years (SD = 3). All of the aphasic participants suffered left-hemisphere strokes, on average 6 years and 4 months prior to the study. All were right handed, native English-speaking individuals with normal hearing and normal or corrected-to-normal vision. Aphasic participants were matched with the group of healthy older individuals for age (F (1, 37) = 1.47, p > .05) and education (F < 1).
Participants were classified as agrammatic based on their performance on the Western Aphasia Battery (WAB, Kertesz, 2007), and the Northwestern Assessment of Verbs and Sentences (NAVS, Thompson, 2011), as well as narrative language samples and other tests. Aphasic participants presented with effortful spontaneous speech, consisting of simple phrases and marked by omission and substitution of grammatical morphemes. Analysis of their narrative production revealed reduced phrase length (MLU (words): M = 6.47, SD =1.98; range: 3–10), and high proportions of ungrammatical sentences (grammatical sentences produced: M = 42%, SD =15%). Participants presented with mild-to-moderate aphasia (WAB-AQ: M = 76.1, Range: 56.4 – 93), with relatively preserved verb (M = 98%, SD = 6%) and sentence comprehension (M = 72%, SD = 15%, see Table 1).
Table 1.
Language scores for agrammatic participants
| Participant | WAB- AQ | NAVS | Narrative production | |||
|---|---|---|---|---|---|---|
|
| ||||||
| SCT | VCT | ASPT | MLU (words) | % grammatical sentences | ||
| P1 | 60.2 | 77 | 100 | 66 | 4.83 | 50 |
| P2 | 66.3 | 73 | 77 | 81 | 4.5 | 0 |
| P3 | 93 | 80 | 100 | 100 | 7.09 | 60.56 |
| P4 | 56.4 | 50 | 100 | 94 | 3 | 0 |
| P5 | 80.6 | 87 | 100 | 94 | 7.27 | 57.14 |
| P6 | 89 | 70 | 94 | 88 | 4.09 | 33.33 |
| P7 | 86 | 97 | 100 | 100 | 8.77 | 78.57 |
| P8 | 81.2 | NC | 100 | 100 | 6.8 | 61.29 |
| P9 | 81.4 | 53 | 100 | 97 | 8.29 | 47.54 |
| P10 | 74 | 80 | 100 | 94 | 6.07 | 11.54 |
| P11 | 75.2 | 47 | 100 | 84 | 7.9 | 56 |
| P12 | 76.7 | 90 | 100 | 81 | 8.04 | 53.57 |
| P13 | 78 | 60 | 100 | 88 | 6.07 | 64.29 |
| P14 | 83 | 73 | 100 | 97 | 9.93 | 78.57 |
| P15 | 65 | NC | 100 | 56 | 4.47 | 0 |
|
| ||||||
| Mean (SD) | 76.4 (10.49) | 72.1 (15.7) | 98.1 (6.03) | 88 (12.9) | 6.47 (1.98) | 42.1 (14.65) |
Abbreviations: WAB = Western Aphasia Battery; AQ = aphasia quotient; NAVS = Northwestern Assessment of Verbs and Sentences; SCT = sentence comprehension test; VCT = verb comprehension test; ASPT = argument structure production test; MLU = mean length of utterance; NC = not completed
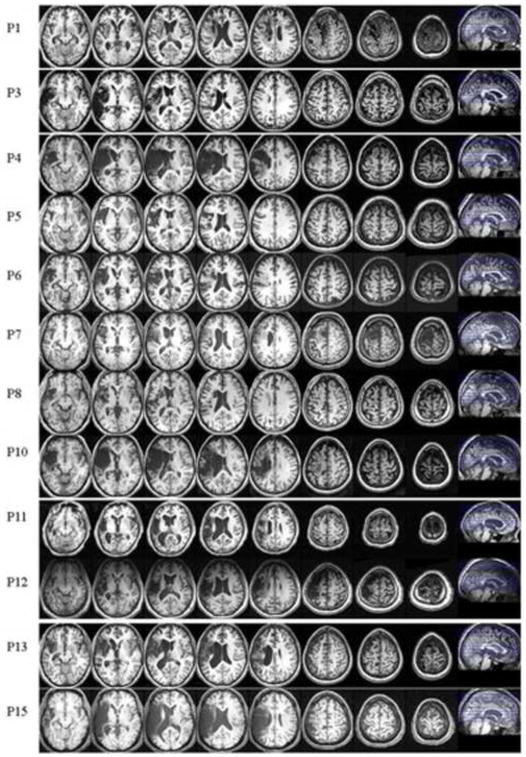
All aphasic participants presented with left hemisphere lesions due to a cerebral vascular accident in the vicinity of the left middle cerebral or anterior temporal artery. Anatomical scans were performed for 12 of the 15 participants, showing variation in the location of the lesion and its extent across participants. Four of the patients presented with large lesions involving frontal, parietal and temporal regions, and one presented with additional subcortical involvement. Selected slices from these patients’ T1 MRI images are presented in Figure 1, and a brief description of their lesions is provided in Table 2.
Figure 1.
Selected slices from T1 MRI images of aphasic participants showing lesion sites. MRI scans were not available for patients P2, P9 and P14
Table 2.
Lesion characteristics for agrammatic participants
| Participant | Lesion localization |
|---|---|
| P1 | Left hemisphere superior frontal and premotor cortex (BAs 6 and 8) extending to subcortical regions |
| P3 | Left pars orbitalis of the IFG, middle and superior temporal gyrus, extending to the fusiform gyrus |
| P4 | Extensive lesion involving left hemisphere inferior frontal (BAs 44, 45, 47) gyrus, middle superior frontal gyri (BA 46), premotor cortex, extending to the anterior superior temporal gyrus |
| P5 | Left pars opercularis, pars orbitalis of IFG and premotor cortex, extending to the anterior temporal lobe |
| P6 | Left pars opercularis, premotor cortex, inferior precentral and postcentral gyri, extending to the anterior temporal lobe |
| P7 | Left pars opercularis of the IFG, superior frontal cortex, including primary motor and premotor cortex |
| P8 | Left pars orbitalis, pars opercularis of the IFG, middle and anterior superior temporal gyri, extending to subcortical regions |
| P10 | Large left hemisphere lesion involving inferior frontal gyrus (BAs 45 and 47) and prefrontal cortex, extending to the primary somatosensory cortex (BA 2), and including anterior middle and superior temporal gyri, |
| P11 | Left pars opercularis, premotor cortex, including part of primary auditory cortex |
| P12 | Left hemisphere superior frontal gyrus, pre- and postcentral gyrus, and premotor cortex |
| P13 | Left hemisphere inferior frontal and prefrontal gyri, extending to the anterior middle and superior temporal gyri, |
| P15 | Large lesion involving inferior frontal gyrus, extending to the motor and premotor cortex, and left hemisphere somatosensory cortex, involving superior temporal gyrus, angular and supramarginal gyri, as well as primary and association auditory cortex |
2.2 Materials
Thirty-five transitive and 35 intransitive verbs were selected to form sentence stimuli. The transitive verbs were obligatory two-argument verbs that take either animate or inanimate objects (e.g., pull). The intransitive verbs all were obligatory one-argument verbs with no possible direct or indirect object arguments (e.g., sneeze). Note that intransitive verbs can be followed by a noun phrase superficially resembling a direct object in two constructions – the cognate object construction (1a) and the intransitive resultative construction (1b):
-
John sneezed a cute baby sneeze.
John sneezed the napkin off the table.
Despite appearances, linguistic evidence reveals that in both cases, the post-verbal NP is not a direct object of the verb, but rather an adjunct (see e.g. Carrier & Randall, 1992, 1993; Jones, 1988; Mittwoch, 1998; Zubizarreta, 1987). For example, unlike true objects of transitive verbs, these NPs cannot be passivized (2), pronominalized (3) or questioned (4). There is thus a clear distinction in the argument structure information associated with the two verb types.
A baby carriage was pulled.
*A cute baby sneeze was sneezed.
John pulled a baby carriage and then his brother pulled it.
*John sneezed a cute baby sneeze and then his brother sneezed it.
What did John pull?
*What did John sneeze?
Both transitive and intransitive verbs required a human agent in subject position (i.e., unaccusative verbs, psych verbs, etc. were excluded). The complete list of the verbs used in the experiment is provided in the Appendix.
The verbs were embedded in sentences to comprise the experimental stimuli. All sentences were active constructions that included a human/animate subject and an object, which was realized as a coordinated NP (the N and the N). Correct sentences (e.g. Anne visited the doctor and the nurse) contained a transitive verb and a semantically congruent object. Sentences with argument structure violations (e.g., *Anne sneezed the doctor and the nurse) were realized by replacing the transitive verb in the well-formed sentence with an intransitive verb. In the semantic violation condition (e.g., *Anne visited the doctor and the socks), the terminal word of each sentence was semantically inappropriate, resulting in a semantic mismatch. There were 35 sentences in each condition, for a total of 105 critical sentences.
All critical verbs were matched across conditions with respect to frequency (p > .05; log10 lemma frequency of occurrence per million according to the CELEX database, Baayen, Piepenbrock, & Van Rijn, 1993), and number of syllables (1–2 syllables, p > .05). Nouns used to form the final NP were also matched for frequency and number of syllables across conditions (ps > .05).
One hundred sixty additional filler sentences were created in order to counterbalance the number of correct and incorrect sentences and to avoid strategic effects. Out of these, 67 sentences contained violations, resulting from an illicit omission of an obligatory object or of the conjunction and. Ninety three sentences were grammatical sentences with intransitive, transitive or ditransitive verbs.
All sentences were spoken by a female native speaker of English and recorded onto a digital recorder in a sound-attenuating booth. Sound files were separated into words, digitized at a sampling rate of 44 kHz and stored as separate files. The duration of the sound files varied with word length, with determiner files taking between 400 and 500 ms, and longer words taking up to 750 ms. Praat software (Boersma & Weenink, 2009) was used to normalize sound files. Each experimental sentence was seven words long, with each word file presented for 900 msec, and sentence duration was 6300 msec.
2.3 Procedure
The experiment was programmed and presented using E-prime experimental presentation software (Schneider, Eschman, & Zuccolotto, 2007) on a Lenovo desktop computer running Windows XP Professional with an Intel Core 2Quad CPU processor. Participants were seated in a dimly illuminated sound-attenuating booth and were instructed to move as little as possible. On each trial a fixation cross was displayed at the center of the computer screen, while an auditory sentence was presented over loudspeakers. Eight hundred milliseconds after the offset of the sentence, a visual cue presented in the center of the screen instructed the participants to perform an acceptability judgment task. They were instructed to press the green button if they thought the sentence was correct, and the red button if they thought it was incorrect. The visual response cue was presented for 5000 msec or until a response was made. The next trial started 1500 msec after the participants’ button press. This delayed judgment task was used to eliminate effects of motor response preparation on the ERPs of interest. The order of presentation was randomized and counterbalanced across participants. Before the main experiment, participants performed a 10-item practice session with feedback to ensure that they understood the task. Practice items were not used during the experiment.
2.4 ERP data Recording and Analysis
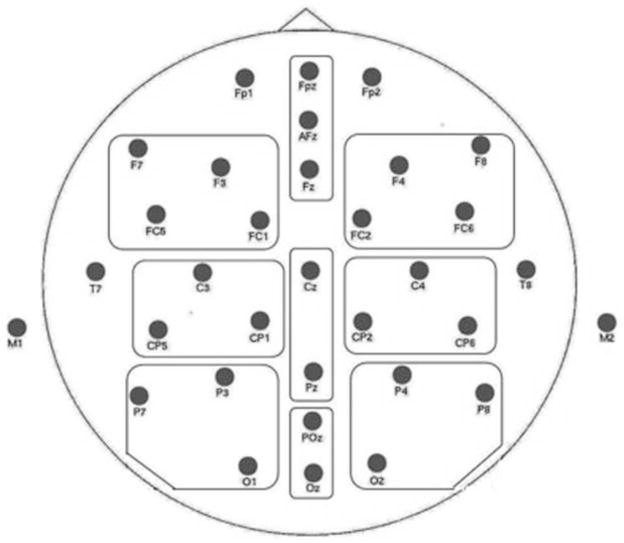
EEG data were acquired using a high impedance physiological measurement system (ANT: Advanced NeuroTechnology). ERPs were recorded from 32 scalp sites by means of Ag/AgCl electrodes attached to an elastic cap according to the International 10–20 System (Fp1, FPz, FP2, F7, F5, F4, F3, F8, Fz, FC5, FC1, FC2, FC6, T7, T8, C3, C2, C4, CP5, CP1, CP2, CP6, P7, P3, P4, P8, Pz, POz, O1, O2, Oz, M1, M2). AFz served as ground electrode (see Figure 2 for channel layout). Recordings were referenced to the average of two mastoid electrodes (M1 and M2).
Figure 2.
Electrode layout. The 32 channel montage representing grouping of the electrodes into 9 regions. The data from the electrodes indicated by the solid line were used in the analysis: FC (Fz, Fpz), CC (Cz, Pz), PC (POz, Oz), RCP (C4, CP2, CP6), LCP (C3, CP5, CP1), RP (P4, P8, O2), LP (P3, P7, O1), RF (F4, F8, FC2, FC6), LF (F7, F3, FC1, FC5). The data from the remaining electrodes were used for visualization of the ERP effect using isovoltage maps.
In order to control for eye movements, horizontal electro-oculogram (EOG) was monitored by placing electrodes at the outer cantus of each eye. Vertical eye movements were recorded by placing two electrodes above and below participants’ left eye. Electrode impedances were kept at or below 5 kΩ. EEG and EOG signals were amplified using an ANT Refa-8 32-channel EEG amplifier by TMSI, and data were recorded continuously with a low pass filter of 100 Hz, and digitization rate of 256 Hz.
The waveforms were screened for amplifier and movement artifacts, which were rejected prior to averaging. Single trials with eye blinks were corrected for blink artifacts using the Principal Component Analysis method described by Ille, Berg, & Scherg (2002). Overall rejection rate was 9.02% for young participants, 8.91% for older controls, and 10.32% for agrammatic participants. For all participant groups, rejected trials were evenly distributed among experimental conditions.
ERPs were time locked to the onset of the critical word in each sentence. In the argument structure violation condition the waveforms were time locked both to the determiner immediately following the verb (e.g. John sneezed the doctor) and to the noun following it (e.g. John sneezed the doctor). In principle, when the verb in the sentence is intransitive, it specifies that it cannot take a direct object, and thus, the violation can be detected at “the”. As explained in section 2.2., at “the”, a grammatical continuation of the sentence is still possible (as in e.g., the intransitive resultative construction, John sneezed the napkin off the table). In this case, “the” does not signal a violation of grammaticality, but rather of preference (see also Friederici & Frisch, 2000). However, by the time the following noun is heard, a clear violation arises. The waveform at the determiner was compared to that of the determiner in the grammatical sentence condition, and the waveform of the noun was compared to that of the corresponding noun in the grammatically correct condition. For the semantic violation condition waveforms were time locked to the sentence final noun, and compared to the ERPs elicited by the congruent sentence final noun in the grammatical sentences.
ERPs were obtained by dividing trials into epochs from −100 to 900 msec relative to the target word onset. ERP averages were baseline corrected to the 100 ms pre-stimulus interval and low-pass filtered at 30 Hz using a 24dB/oct zero phase shift digital filter. On the basis of visual inspection and previous studies, two latency windows from 300 to 700 msec were defined for statistical analysis: 300–500 msec for negativity effects (LAN/N400), and 500–700 msec for late positivities (P600). Where warranted by visual inspection of the waveforms, an additional time window of 700–800 msec was included.
Statistical analyses were performed using repeated measures ANOVAs on the mean ERP amplitudes computed for each participant, violation condition and electrode site in the selected latency windows. To investigate the different topographical distribution of ERP effects and to reduce the number of multiple comparisons performed, electrodes were divided into eight subsets by region: FC (Fz, Fpz), CC (Cz, Pz), PC (POz, Oz), RCP (C4, CP2, CP6), LCP (C3, CP5, CP1), RP (P4, P8, O2), LP (P3, P7, O1), RF (F4, F8, FC2, FC6), and LF (F7, F3, FC1, FC5). To protect against Type I error resulting from a large number of comparisons, the Huynh & Feld (1970) correction was applied when evaluating effects with more than one degree of freedom in the numerator. In these cases the original degrees of freedom are reported with the corrected probability levels.
To test for possible differences in variability between the different participant groups (e.g. whether responses of the young control group were more homogenous than those of the older group and patients), we compared the variances in the three groups on the mean amplitude of the difference waves and the time-to-peak, using Levene’s test for homogeneity of variance. Comparisons were carried out for each electrode and condition in each time window where between-group differences were found.
3. Results
3.1 Behavioral Results
Mean response latencies and accuracy for the three participant groups across different experimental conditions are presented in Table 3.
Table 3.
Mean (standard deviation) accuracy (%) and mean reaction times (msec) for control groups and agrammatic participants across the different experimental conditions.
| Participant group | Grammatical | AS violations | Semantic violations | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Accuracy | RT | Accuracy | RT | Accuracy | RT | |
| Young controls | 95.9 (4.4) | 537.54 (160.93) | 97.7 (5.2) | 533.57 (185.85) | 98.8 (2.7) | 508.33 (178.14) |
| Older controls | 96.8 (5.2) | 596.36 (256.88) | 96.9 (5.1) | 580.58 (188.70) | 98.9 (1.8) | 566.19 (176.72) |
| Agrammatic | 84.3 (13.2) | 1124.82 (348.54) | 59.7 (27.3) | 1166.25 (330.31) | 81.4 (17.4) | 1147.62 (336.49) |
Abbreviations: AS = argument structure
A group (young, older, agrammatic) x condition (correct sentences, argument structure violations, semantic violations) repeated measures ANOVA revealed a significant main effect of group (F (2, 50) = 40.72, p < .001) and condition (F (2, 100) = 11.65, p < .001), and a condition x group interaction (F (4,100) = 10.46, p < .001), indicating that aphasic participants and controls showed different patterns of response accuracy across different experimental conditions. Post-hoc comparisons revealed a significant difference between the agrammatic patients and the two control groups (ps < .001), such that the overall accuracy of the agrammatic participants was lower than that of control participants, but no difference was found between the two control groups (p = .975). Further, in the agrammatic group, argument structure violations were judged significantly less accurately than grammatical sentences (F (1, 14) = 10.34, p < .01) and than semantic violations (F (1, 14) = 19.48, p < .01). However, there was no significant difference in accuracy between grammatical sentences and sentences with semantic violations (F < 1) for these participants.1 The two control groups were equally accurate in responding to the different sentence types (all ps > .05, corrected for multiple comparisons).
Similar analysis for the reaction time data revealed a main effect of group (F (2, 50) = 41.44, p < .001), but no effect of condition and no interaction between condition and participant group (both Fs < 1). Post-hoc comparisons revealed that agrammatic participants had significantly prolonged response latencies, compared to both control groups (all ps < .01, corrected for multiple comparisons), but there were no significant differences in reaction times between the two control groups (p > .05).
3.2 ERP results
The results of omnibus ANOVAs with condition and electrode site as within subject factors for each participant group at the two latency ranges are presented in Table 4. Table 5 summarizes the results of statistical tests performed for each participant group at different electrode regions and conditions in the specified time intervals.
Table 4.
Results of statistical tests performed on the mean amplitude of ERP waveforms for different experimental conditions and participant groups in the specified latency ranges
| Time window | Young Controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Condition | Electrode | C*E | |||||||
| df | F | p | df | F | p | df | F | p | |
| AS violation (‘the’) | |||||||||
| 300–400 | 1, 14 | 4.08 | 0.063 | 29, 406 | 1.13 | 0.355 | 29, 406 | 1.11 | 0.358 |
| 500–700 | 1, 14 | 0.23 | 0.636 | 29, 406 | 0.66 | 0.647 | 29, 406 | 0.5 | 0.723 |
| AS violation (‘doctor’) | |||||||||
| 300–500 | 1, 14 | 2.22 | 0.158 | 29, 406 | 2.89 | 0.025 | 29, 406 | 0.85 | 0.512 |
| 500–700 | 1, 14 | 2.878 | 0.112 | 29, 406 | 3.05 | 0.021 | 29, 406 | 0.56 | 0.718 |
| Semantic violation | |||||||||
| 300–500 | 1, 14 | 18.12 | 0.001 | 29, 406 | 1.1 | 0.361 | 29, 406 | 4.2 | 0.003 |
| 500–700 | 1, 14 | 19.16 | 0.001 | 29, 406 | 3.63 | 0.003 | 29, 406 | 4.3 | 0.001 |
|
| |||||||||
| Age Matched | |||||||||
|
| |||||||||
| Condition | Electrode | C*E | |||||||
| df | F | p | df | F | p | df | F | p | |
|
| |||||||||
| AS violation (‘the’) | |||||||||
| 300–500 | 1, 22 | 0.35 | 0.56 | 29, 638 | 1.87 | 0.094 | 29, 638 | 0.96 | 0.42 |
| 500–700 | 1, 22 | 7.17 | 0.014 | 29, 638 | 1.2 | 0.315 | 29, 638 | 1.93 | 0.089 |
| 700–800 | 1, 22 | 14.89 | 0.001 | 29, 638 | 2.21 | 0.044 | 29, 638 | 4.02 | 0.012 |
| AS violation (‘doctor’) | |||||||||
| 300–500 | 1, 22 | 1.5 | 0.233 | 29, 638 | 12.11 | <.001 | 29, 638 | 3.00 | 0.038 |
| 500–700 | 1, 22 | 3.79 | 0.064 | 29, 638 | 5.54 | <.001 | 29, 638 | 1.68 | 0.174 |
| Semantic violation | |||||||||
| 300–500 | 1, 22 | 10.98 | 0.003 | 29, 638 | 5.96 | 0.001 | 29, 638 | 4.81 | 0.002 |
| 500–700 | 1, 22 | 7.67 | 0.011 | 29, 638 | 3.51 | 0.018 | 29, 638 | 2.53 | 0.049 |
|
| |||||||||
| Nonfluent | |||||||||
|
| |||||||||
| Condition | Electrode | C*E | |||||||
| df | F | p | df | F | p | df | F | p | |
|
| |||||||||
| AS violation (‘the’) | |||||||||
| 300–500 | 1, 14 | 0 | 0.965 | 29, 406 | 1.93 | 0.069 | 29, 406 | 0.59 | 0.672 |
| 500–700 | 1, 14 | 0.18 | 0.676 | 29, 406 | 2.33 | 0.036 | 29, 406 | 1.08 | 0.379 |
| AS violation (‘doctor’) | |||||||||
| 300–500 | 1, 14 | 0.05 | 0.833 | 29, 406 | 1.13 | 0.354 | 29, 406 | 0.56 | 0.672 |
| 500–700 | 1, 14 | 0.01 | 0.94 | 29, 406 | 1.48 | 0.209 | 29, 406 | 0.45 | 0.775 |
| Semantic violation | |||||||||
| 300–500 | 1, 14 | 5.02 | 0.042 | 29, 406 | 1.28 | 0.278 | 29, 406 | 0.991 | 0.44 |
| 500–700 | 1, 14 | 4.54 | 0.051 | 29, 406 | 1.67 | 0.156 | 29, 406 | 0.82 | 0.498 |
Table 5.
Statistical analyses of ERP effects across experimental conditions and electrode regions in the specified time intervals
| Time window | Electrode Region
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CC | PC | LCP | RCP | LP | RP | LF | RF | |
| Young controls
|
||||||||
| AS violation (‘the’) | ||||||||
| 300–500msec | n.s. | 4.73* | 4.82* | n.s. | 4.33* | n.s. | n.s. | 4.85* |
| 500–700msec | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| AS violation (‘doctor’) | ||||||||
| 300–500msec | 5.78* | n.s. | n.s. | n.s. | n.s. | 6.80* | n.s. | n.s. |
| 500–700msec | 5.72* | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Semantic violation | ||||||||
| 300–500msec | 11.80** | 8.84* | 9.86** | n.s. | 13.47** | 20.90** | n.s. | 7.66* |
| 500–700msec | 8.61* | 7.02* | 22.48*** | 7.80* | 9.99** | n.s. | n.s. | 7.53* |
|
| ||||||||
| Age-matched controls
|
||||||||
| AS violation (‘the’) | ||||||||
| 300–500msec | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| 500–700msec | 11.16** | 6.67* | 5.29* | 5.10* | n.s. | 7.69* | n.s. | n.s. |
| 700–800msec | 33.98*** | 8.74** | 11.33** | 9.89** | n.s. | 6.05* | 8.46** | n.s. |
| AS violation (‘doctor’) | ||||||||
| 300–500msec | 5.52* | n.s. | 6.97* | n.s. | 9.18** | 6.44* | n.s. | 6.03* |
| 500–700msec | 7.00* | n.s. | 10.54** | n.s. | 7.12* | 5.84* | n.s. | 4.83* |
| Semantic violation | ||||||||
| 300–500msec | 9.18** | 13.74** | 9.17** | 23.07*** | 9.07** | 8.06* | n.s. | 7.93* |
| 500–700msec | n.s. | 6.87* | n.s. | 26.90*** | 8.43** | n.s. | n.s. | 5.85* |
|
| ||||||||
| Agrammatic
|
||||||||
| AS violation (‘the’) | ||||||||
| 300–500msec | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| 500–700msec | 6.36* | n.s. | n.s. | 5.07* | 6.12* | n.s. | n.s. | n.s. |
| AS violation (‘doctor’) | ||||||||
| 300–500msec | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| 500–700msec | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Semantic violation | ||||||||
| 300–500msec | n.s. | n.s. | 7.64* | n.s. | n.s. | 4.81* | n.s. | n.s. |
| 500–700msec | n.s. | n.s. | 5.11* | 4.34* | n.s. | 7.77* | n.s. | n.s. |
Note:
p < 05;
p < .01,
p < .001.
3.2.1 Argument structure violations
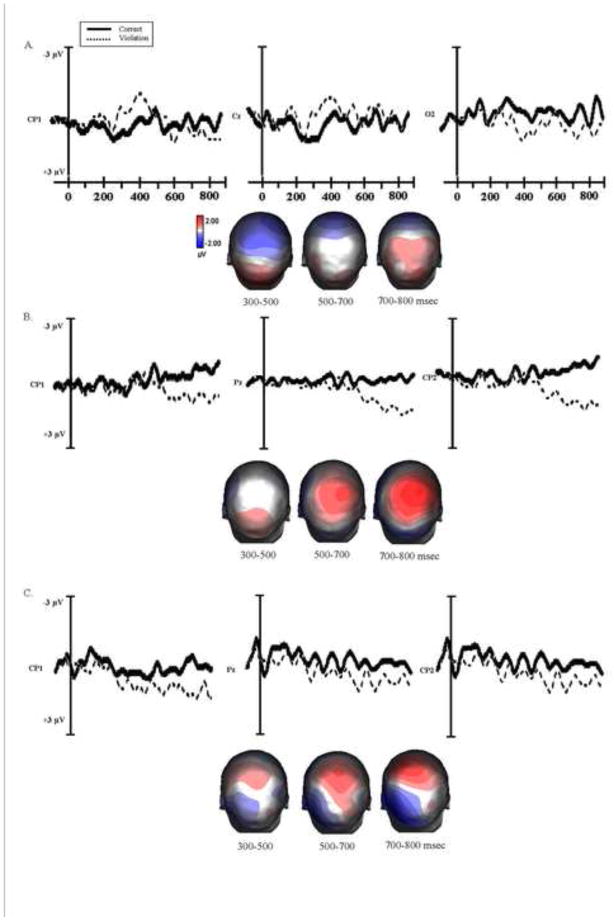
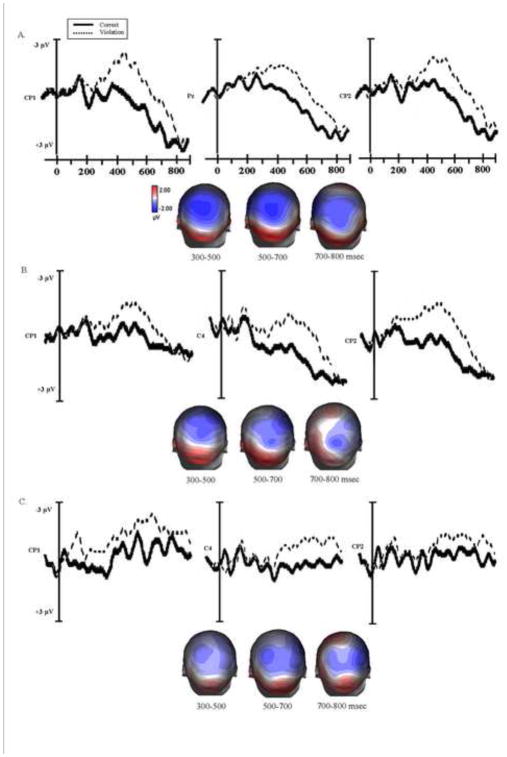
The ERP effects for the argument structure violation for each participant group at the position of the determiner are presented in Figure 3 and results at the critical noun are shown in Figure 4.
Figure 3.
Grand average ERPs at the representative electrodes for each participant group (A. Young Controls B. Older Adults and C. Agrammatic patients) elicited by the correct sentences (solid line) and verb argument structure violation (broken line) at the position of the deteminer. The voltage maps show topographical distribution of ERP effects across the scalp based on the difference waveforms (violation-correct) at 300–500, 500–700 and 700–800 msec time intervals.
Figure 4.
Grand average ERPs at the representative electrodes for each participant group (A. Young Controls B. Older Adults and C. Agrammatic patients) elicited by the correct sentences (solid line) and verb argument structure violation (broken line) at the position of the critical noun. The voltage maps show topographical distribution of ERP effects across the scalp based on the difference waveforms (violation-correct) at 300–500, 500–700 and 700–800 msec time intervals.
Young control participants
For the target word “the”, in the early time window (300–400 msec) an omnibus ANOVA revealed an effect of condition, F (1, 14) = 4.08, p = .06. The regional analyses revealed a significant negativity at the right frontal-central sites (RFC, F (1, 14) = 4.85, p < .05), and left central-parietal sites (LCP, F (1, 14) = 4.82, p < .05). This negativity was followed by a positive shift at the posterior sites (PC, F (1, 14) = 4.73, p < .05; LP, F (1, 14) = 4.33, p = .05). No significant ERP effects were found in the 500–700 time window.
Analysis of the waveforms time locked to the critical noun following the verb revealed a significant main effect of electrode site in the two time windows (300–500 msec: F (29, 406) = 2.89, p < .05; 500–700 msec: F (29, 406) = 3.05, p < .05), but no significant main effect of condition or interaction was found. The regional analyses revealed negativity restricted to the central and right parietal electrodes in the 300–500 msec time window (CC, F (1, 14) = 5.78, p < .05; RP, F (1, 14) = 6.80, p < .05), and to central sites in the 500–700 msec time interval (CC, F (1, 14) = 5.72, p < .05).
Older control participants
For the target word ‘the’ in the early time window the omnibus ANOVA revealed no significant main effect of condition or electrode and no interaction. However, at the later time window (500–700 msec) there was a significant main effect of condition, F (1, 22) = 7.17, p < .01. The regional analyses revealed that the argument structure violations at the determiner elicited a positive shift with a predominantly central-parietal distribution (CC, F (1, 22) = 11.16 p < .05; LCP, F (1, 22) = 5.29, p < .05; RCP, F (1, 22) = 5.10, p < .05; PC, F (1, 22) = 6.67, p < .01; RP, F (1, 22) = 7.69, p < .05).
In the 700–800 msec time window there was a significant main effect of condition, F (1, 22) = 14.89, p < .01, and electrode, F (29, 638) = 2.21, p < .05, and an electrode x condition interaction, F (29, 638) = 4.02, p < .05, indicating that the positive effect elicited by the argument structure violation continued to be present in this time window, and was distributed mainly over central and parietal sites (CC, F (1, 22) = 33.98, p < .01; PC, F (1, 22) = 8.74, p < .01; LCP, F (1, 22) = 11.33, p < .01; RCP, F (1, 22) = 9.89, p < .01; RP, F (1, 22) = 6.05, p < .05).
The analysis of the waveform time locked to the critical noun revealed a significant main effect of electrode, F (29, 638) = 12.11, p < .01, and a condition x electrode interaction, F (29, 638) = 3.00, p < .05, in the 300–500 msec time window. The violation elicited a sustained negativity, which was most pronounced at the central and parietal sites (CC, F (1, 22) = 5.52, p < .05; LCP, F (1, 22) = 6.97, p < .05; LP, F (1, 22) = 9.18, p < .01; RP, F (1, 22) = 6.44, p < .05). In the 500–700 msec time interval the omnibus ANOVA showed a main effect of electrode, F (29, 638) = 5.54, p < .01. The regional analyses revealed a negative shift that was largest at the central and parietal electrodes (CC, F (1, 22) = 7.00, p < .05; LCP, F (1, 22) = 10.54, p < .01; RCP, F (1, 22) = 5.84, p < .05; LP, F (1, 22) = 7.12, p < .05).
Agrammatic participants
For the determiner in the argument structure violation, at the early time window the omnibus ANOVA revealed no significant main effect of condition or electrode and no interaction. However, in the 500–700 msec latency interval there was a significant main effect of electrode, F (29, 406) = 2.33, p < .05. The regional ANOVAs revealed that the violation elicited a positive shift. This effect was not widely distributed, but restricted mainly to the central sites (CC, F (1, 14) = 6.36, p < .05; RCP, F (1, 14) = 5.07, p < .05). The critical noun in the verb argument structure violation condition did not elicit significant ERP effects at any of the time windows for the agrammatic participants.
3.2.2 Semantic Violations
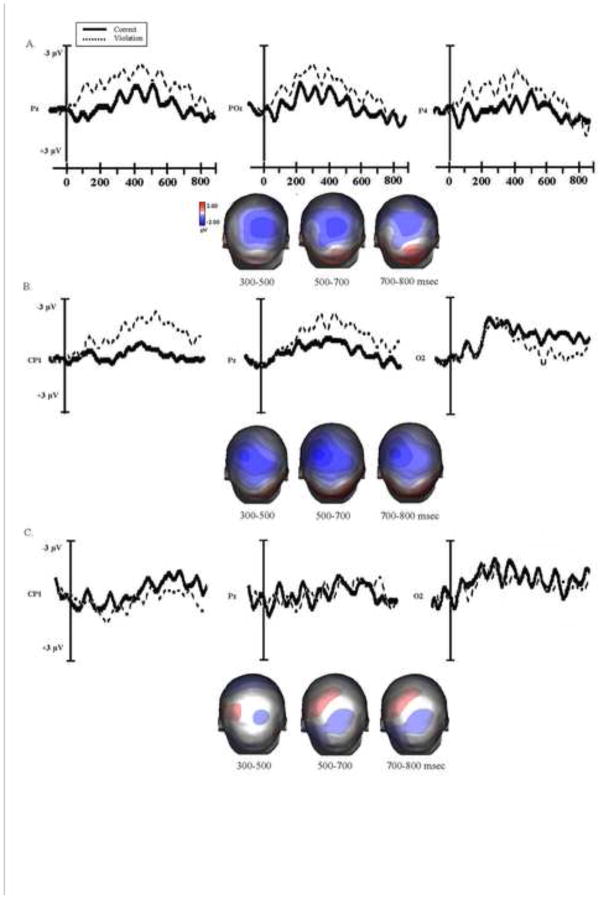
The ERP effects for the semantic violation for each participant group are presented in Figure 5.
Figure 5.
Grand average ERPs at the representative electrodes for each participant group (A. Young Controls B. Older Adults and C. Agrammatic patients) elicited by the correct sentences (solid line) and semantic violation (broken line) at the position of the critical word. The voltage maps show topographical distribution of ERP effects across the scalp based on the difference waveforms (violation-correct) at 300–500, 500–700 and 700–800 msec time intervals.
Young control participants
In response to semantic violations young participants displayed a negative-going wave with a centro-parietal distribution, peaking at about 400 msec. For the 300–500 msec latency window, the omnibus ANOVA revealed a significant main effect of condition, F (1, 14) = 18.12, p < .01, and a condition x electrode interaction, F (29, 406) = 4.20, p < .01. The subsequent regional ANOVAs revealed a negativity, largest at the central and parietal sites (CC, F (1, 14) = 11.80, p < .01; LCP, F (1, 14) = 9.86, p < .01, PC, F (1, 14) = 8.84, p < .05; LP, F (1, 14) = 13.47, p < .01; RP, F (1, 14) = 20.90, p < .01).
In the 500 to 700 msec time window, the omnibus ANOVA revealed significant main effects of condition, F (1, 14) = 19.16, p < .01 and of electrode, F (29, 406) = 3.63, p < .01, and a significant electrode x condition interaction, F (29, 406) = 4.30, p < .01. The regional analyses indicated that the negativity elicited by the semantic violation continued into this time-window, and was the strongest at the central and parietal sites (CC, F (1, 14) = 8.61, p < .05; PC, F (1, 14) = 7.02, p < .05; LCP, F (1, 14) = 22.48, p < .01; RCP, F (1,14) = 7.80, p < .05; LP, F (1, 14) = 9.99, p < .01).
Older control participants
A centro-parietal negativity peaking at around 400 msec was also observed for older adults. For the early latency window the omnibus ANOVA revealed significant main effects of condition, F (1, 22) = 10.98, p < .01 and electrode, F (29, 638) = 5.96, p < .01, and a significant condition x electrode interaction, F (29, 638) = 4.81, p < .01. The negativity elicited by the semantically anomalous word was most pronounced at the central and parietal sites (CC, F (1, 22) = 9.18, p < .01; RCP, F (1, 22) = 23.07, p < .01; LCP, F (1, 22) = 9.17, p < 01; PC, F (1, 22) = 13.74, p < .01; RP, F (1, 22) = 8.06, p < .05), and was observed also over the right frontal-central sites (RF, F (1, 22) = 7.93, p < .05). In addition, there was a positive shift at the left parietal sites (LP, F (1, 22) = 9.07, p < .01).
In the 500–700 msec latency window, the ANOVA again showed a significant main effect of condition, F (1, 22) = 7.67, p < .05 and electrode, F (29, 638) = 3.51, p < .05, and an electrode x condition interaction, F (29, 638) = 2.53, p < .05. The negativity elicited by the semantic violation was distributed mainly over central and parietal sites (PC, F (1, 22) = 6.87, p < .05; RCP, F (1, 22) = 26.90, p < .01), as well as right frontal-central sites (RFC, F (1, 22) = 5.85, p < .05), and the positive shift at the left parietal sites was also significant (LP, F (1, 22) = 8.43, p < .01).
Agrammatic participants
In the early latency window, the omnibus ANOVA revealed a significant main effect of condition, F (1, 14) = 5.02, p < .05. Planned comparisons revealed a negativity elicited by the semantic violation, at the left central and right parietal sites, (LC, F (1, 14) = 7.64, p < .05; RP, F (1, 14) = 4.81, p < .05). Similarly, there was a significant main effect of condition (F (1, 14) = 4.54, p = .05) in the 500–700 msec time interval. Once again, regional analyses revealed that the negative shift elicited by the semantic violation condition was significant at the central and parietal electrodes (LC, F (1, 14) = 5.11, p < .05; RCP, F (1, 14) = 4.34, p = .05; RP, F (1, 14) = 7.77, p < .05).
3.2.3 Between Group Analyses
To explore between group differences in ERP effects, pairwise comparisons between the agrammatic group and the two control groups were performed at different electrode regions, as described below. Table 6 summarizes the results of statistical tests performed for each violation condition at different electrode regions in the different time intervals. The results are presented in detail below.
Table 6.
Results of group comparisons between the agrammatic and the two control groups across experimental conditions and electrode regions in the specified time intervals
| msec | Effect | Electrode Region
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CC | PC | LCP | RCP | LP | RP | LF | RF | ||
| Agrammatic vs. Young Controls
|
|||||||||
| AS violation (‘the’) | |||||||||
| 300–500 | GxC | ns | ns | 4.09* | ns | ns | ns | ns | ns |
| Group | ns | ns | 4.34* | ns | ns | ns | ns | ns | |
| 500–700 | ns | ns | ns | ns | ns | ns | ns | ns | |
| AS violation (‘doctor’) | |||||||||
| 300–500 | GxC | ns | ns | ns | ns | ns | ns | ns | ns |
| Group | 8.98** | ns | 10.39** | 4.64* | ns | ns | ns | ns | |
| 500–700 | ns | ns | ns | ns | ns | ns | ns | ns | |
| Semantic violation | |||||||||
| 300–500 | GxC | ns | 5.46* | ns | ns | 4.54* | 6.13* | ns | ns |
| Group | ns | ns | ns | ns | ns | ns | ns | ns | |
| 500–700 | GxC | ns | ns | ns | ns | ns | ns | ns | 5.40* |
| Group | 6.10* | ns | 5.49* | ns | 12.72* | ns | ns | ns | |
|
| |||||||||
| Agrammatic vs. Age Matched
|
|||||||||
| AS violation (‘the’) | |||||||||
| 300–500 | GxC | ns | ns | ns | ns | ns | ns | ns | ns |
| Group | ns | ns | 5.86* | ns | 6.80* | ns | ns | ns | |
| 500–700 | GxC | ns | ns | ns | ns | ns | ns | ns | ns |
| Group | ns | ns | ns | ns | ns | ns | ns | ns | |
| 700–800 | GxC | ns | ns | 4.19* | ns | ns | ns | ns | ns |
| Group | ns | ns | 5.64* | ns | ns | Ns | 9.61** | 7.03* | |
| AS violation (‘doctor’) | |||||||||
| 300–500 | GxC | ns | ns | ns | ns | 4.67* | ns | ns | ns |
| Group | 14.70*** | 10.51 | 10.92** | 12.60*** | 7.68** | 10.49** | ns | ns | |
| 500–700 | GxC | ns | ns | 8.58** | ns | 4.45* | ns | ns | ns |
| Group | ns | ns | ns | ns | ns | ns | ns | ns | |
| Semantic violation | |||||||||
| 300–500 | ns | ns | ns | ns | ns | ns | ns | ns | |
| 500–700 | GxC | ns | ns | ns | ns | ns | ns | ns | ns |
| Group | ns | ns | ns | 6.15* | ns | ns | ns | 7.88** | |
Note:
p < 05;
p < .01,
p < .001
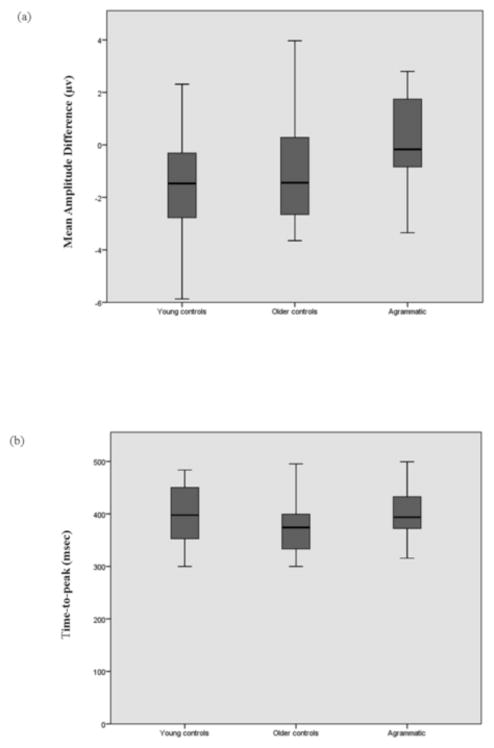
Variability in amplitude and latency is characteristic of ERPs of abnormal populations (as reported for example in Iraqui, Kutas, & Salmon, 1996; Swaab et al., 1997). To assess whether differences in variability around the mean might have influenced our results, we compared the variances in the three participant groups on the mean amplitude of the difference waves and on the time-to-peak measure. These measures were calculated for each electrode over each participant group, and group variances were compared using Levene’s test for homogeneity of variance. The results for relevant electrodes, namely electrodes showing an effect of condition in any of the participant groups, are presented in Table 7. Results for a representative electrode and condition are illustrated in Figure 6. As can be seen in Table 7, in the vast majority of cases, the variance around the mean was not significantly different between the three participant groups, neither for the mean amplitude of the difference wave nor for the time-to-peak, suggesting that the between-group differences to be discussed below are not due to differences in variance (namely homogenous responses in one group and variable responses in another).
Table 7.
Comparison of variability around the mean of time-to-peak (TTP) and mean amplitude of difference waves (MAdiff) between the three participant groups
| Condition | Electrode site | Levene Statistic | Significance | |
|---|---|---|---|---|
| Argument Structure Determiner, 300–500 |
CP1 (LCP) | TTP | 0.867 | 0.427 |
| Madiff | 1.751 | 0.184 | ||
| CP5 (LCP) | TTP | 4.465 | 0.016 | |
| Madiff | 0.141 | 0.868 | ||
| C3 (LCP) | TTP | 1.307 | 0.280 | |
| Madiff | 3.473 | 0.039 | ||
| CP6 (RCP) | TTP | 0.694 | 0.504 | |
| Madiff | 2.117 | 0.131 | ||
| Pz(CC) | TTP | 1.648 | 0.203 | |
| Madiff | 2.88 | 0.066 | ||
| POZ (PC) | TTP | 1.069 | 0.351 | |
| Madiff | 2.155 | 0.127 | ||
| Oz (PC) | TTP | 1.031 | 0.364 | |
| Madiff | 0.317 | 0.729 | ||
| P3 (LP) | TTP | 0.723 | 0.496 | |
| Madiff | 0.252 | 0.779 | ||
| P7 (LP) | TTP | 5.156 | 0.009 | |
| Madiff | 0.048 | 0.953 | ||
| P4 (RP) | TTP | 0.805 | 0.453 | |
| Madiff | 0.126 | 0.882 | ||
| P8 (RP) | TTP | 0.945 | 0.407 | |
| Madiff | 2.395 | 0.102 | ||
|
| ||||
| Argument Structure Determiner, 500–700 |
CP1 (LCP) | TTP | 1.013 | 0.371 |
| Madiff | 0.071 | 0.932 | ||
| CP2 (RCP) | TTP | 2.342 | 0.107 | |
| Madiff | 0.095 | 0.91 | ||
| CP6 (RCP) | TTP | 2.814 | 0.70 | |
| Madiff | 0.986 | 0.38 | ||
| CZ (CC) | TTP | 0.184 | 0.833 | |
| Madiff | 2.923 | 0.063 | ||
| PZ (CC) | TTP | 1.465 | 0.241 | |
| Madiff | 0.86 | 0.241 | ||
| POZ (PC) | TTP | 1.55 | 0.221 | |
| Madiff | 2.74 | 0.074 | ||
| Oz (PC) | TTP | 0.885 | 0.419 | |
| Madiff | 0.217 | 0.806 | ||
| P3 (LP) | TTP | 0.036 | 0.965 | |
| Madiff | 0.217 | 0.806 | ||
| P7 (LP) | TTP | 0.192 | 0.826 | |
| Madiff | 0.791 | 0.459 | ||
| P8 (RP) | TTP | 0.659 | 0.522 | |
| Madiff | 0.496 | 0.612 | ||
|
| ||||
| Argument Structure Noun, 300–500 msec |
CP1 (LCP) | TTP | 0.646 | 0.529 |
| Madiff | 1.138 | 0.329 | ||
| CP2 (RCP) | TTP | 0.078 | 0.925 | |
| Madiff | 0.354 | 0.704 | ||
| CP6 (RCP) | TTP | 0.411 | 0.665 | |
| Madiff | 0.366 | 0.695 | ||
| CZ (CC) | TTP | 0.809 | 0.451 | |
| Madiff | 1.71 | 0.191 | ||
| PZ(CC) | TTP | 0.084 | 0.92 | |
| Madiff | 1.992 | 0.147 | ||
| POZ(PC) | TTP | 0.03 | 0.971 | |
| Madiff | 3.468 | 0.039 | ||
| Oz(PC) | TTP | 1.647 | 0.203 | |
| Madiff | 0.377 | 0.688 | ||
| P3(LP) | TTP | 1.332 | 0.273 | |
| Madiff | 0.204 | 0.816 | ||
| P7(LP) | TTP | 2.382 | 0.103 | |
| Madiff | 0.322 | 0.726 | ||
| P8(RP) | TTP | 0.326 | 0.723 | |
| Madiff | 0.302 | 0.741 | ||
|
| ||||
| Semantic Violation 300–500 msec |
CP1 (LCP) | TTP | 0.011 | 0.989 |
| MAdiff | 1.361 | 0.266 | ||
| CP2 (RCP) | TTP | 0.462 | 0.633 | |
| Madiff | 3.072 | 0.055 | ||
| CP6 (RCP) | TTP | 2.146 | 0.128 | |
| Madiff | 1.642 | 0.204 | ||
| CZ (CC) | TTP | 1.182 | 0.315 | |
| Madiff | 0.451 | 0.639 | ||
| PZ (CC) | TTP | 0.473 | 0.626 | |
| Madiff | 0.013 | 0.987 | ||
| POZ (PC) | TTP | 0.032 | 0.968 | |
| Madiff | 0.921 | 0.405 | ||
| Oz (PC) | TTP | 2.151 | 0.127 | |
| Madiff | 1.844 | 0.169 | ||
| P3 (LP) | TTP | 0.526 | 0.594 | |
| Madiff | 0.628 | 0.538 | ||
| P7 (LP) | TTP | 0.756 | 0.475 | |
| Madiff | 6.161 | 0.004 | ||
| P4 (RP) | TTP | 4.753 | 0.013 | |
| Madiff | 1.331 | 0.273 | ||
| P8 (RP) | TTP | 0.318 | 0.729 | |
| Madiff | 2.611 | 0.083 | ||
Figure 6.
Variability of the three participant groups in the semantic violation condition, in the 300–500 msec window, at electrode Pz: (a) Mean amplitude of the difference wave (microvolts); (b) Time-to-peak (msec).
Agrammatic participants vs. young controls
For the argument structure violation at the determiner, a group (young, agrammatic) by condition (correct, semantic violation) ANOVA revealed a significant main effect of group (F (1, 28) = 4.34, p < .05) and a marginally significant group by condition interaction (F (1, 28) = 4.09, p = .053) at the left central-parietal sites in the 300–500 msec time window. These results indicate that in response to verb argument structure violations, negativity was present for younger controls, whereas participants with aphasia showed a positive shift. At the critical noun, the analysis showed a significant main effect of group at the central and parietal sites (CC, F (1, 28) = 8.98, p < .01; LCP, F (1, 28) = 10.39, p < .01; RCP, F (1, 28) =, p < .05), which reflected presence of negativity for young participants but not for participants with aphasia.
For the semantic violation, the ANOVA revealed a significant interaction between group and condition at the central and parietal sites in the 300–500 msec time window (RP, F (1, 28) = 6.13, p < .05; PC, F (1, 28) = 5.46, p < .05; LP, F (1, 28) = 4.54, p < .05). This interaction was due to the reduced negativity elicited by semantic violations in this time window for the agrammatic participants. In addition, significant group effects were obtained in the 500–700 msec time window, indicating significantly larger negativity for young controls compared to agrammatic participants at the central and parietal electrodes (CC, F (1, 28) = 6.10, p < .05; LCP, F (1, 28) = 5.49, p < .05, LP, F (1, 28) = 12.72, p < .01).
Agrammatic vs. older controls
For the argument structure violation at the determiner, the between group ANOVAs revealed a significant effect of group at the central and parietal sites (LCP, F (1, 36) = 5.86, p < .05; LP, F (1, 36) = 6.80, p < .05) in the early time window, and a significant group x condition interaction at the left central sites (LC, F (1, 36) = 4.19, p < .05) in the 700–800 msec time interval. These results reflected larger positivity observed at this word in response to verb argument structure violation for older control participants.
At the critical noun, significant group effects were obtained in the 300–500 msec window at the parietal and central sites, reflecting greater negativity present for older controls compared to aphasic participants (CC: F (1, 36) = 14.69, p < .01; PC: F (1, 36) = 10.51, p < .01; LCP: F (1, 36) = 10.92, p < .01; RCP: F (1, 36) = 12.60, p < .01; LP: F (1, 36) = 7.68, p < .01; RP: F (1, 36) = 10.49, p < .01). Similarly, in the 500–700 msec time interval, significant group x condition interactions were observed at the central and parietal sites (LCP: F (1, 36) = 8.58, p < .05; LP: F (1, 36) = 4.45, p < .05). This interaction reflected the finding that a negativity was elicited by verb argument structure violations at the position of the noun in older healthy controls, but not in participants with aphasia.
For the semantic violation condition, statistical comparison of the agrammatic and age-matched participants revealed a significant group effect in the 500–700 msec time window (RCP, F(1, 36) = 6.15, p < .05), indicating larger negativity for older controls compared to agrammatic participants.
4. Discussion
In this study we investigated event-related potentials (ERPs) associated with verb argument structure and semantic violations in sentence contexts. The main goal was to investigate how individuals with agrammatic aphasia process verb argument structure information in real-time during sentence comprehension. We also wanted to examine possible differences in sensitivity to semantic and argument structure information during sentence processing. Argument structure violations were realized by introducing a mismatch between the verb’s thematic properties and the number of arguments in the sentence, whereas semantic violations were realized by placing a contextually incongruous noun in the sentence final position.
Behaviorally, agrammatic participants were less accurate than control participants in their grammaticality judgments. The difference was most pronounced in the argument structure violation condition, where grammaticality judgment accuracy was at chance. These results contrast with previous studies, showing near normal performance on grammaticality judgments of structures with argument structure violations, e.g. Grodzinsky & Finkel (1998), Kim & Thompson (2000, 2004). Low accuracy in the argument structure condition in the present study may be explained by the fact that argument structure violations occurred at mid-sentence (whereas in previous studies some violations were sentence final), and were followed by a grammatical five-word noun phrase. Additionally, there were 800 msec of silence beginning at sentence offset, only after which participants were allowed to respond. In contrast, in previous studies participants could provide their judgments at any point during the presentation of the sentence. The delayed judgment required in our task may have caused a working memory load or allowed for interference effects.
The ERP results showed that argument structure and semantic violations led to different electrophysiological responses, and they elicited a distinct pattern of electrophysiological signatures in healthy participants and individuals with aphasia. Young healthy adults showed online sensitivity to the mismatch between the number of arguments required by the verb and the actual arguments appearing in the sentence. As soon as the post-verbal determiner following an intransitive verb was heard, signaling an illegal theme argument (noun phrase), a fronto-central negativity was elicited at central sites, and a positive shift was seen at posterior sites. These results indicate that during normal processing, as sentence structure is built on-line, the verb’s argument structure properties place restrictions on the arguments that can follow it. The response pattern found in our study is similar to the N400-P600 pattern found in previous studies investigating verb argument structure processing, and in particular violation of the number of arguments (Friederici & Frisch, 2000). As suggested in previous studies (Friederici & Frisch, 2000; Frisch et al., 2004), when arguments cannot be assigned thematic roles by the verb, lexical-semantic integration problems arise, which are reflected in the N400 effect, whereas subsequent attempts at reanalysis and repair of the sentence structure are reflected in the P600 effect. Friederici & Meyer (2004) take this late positivity to reflect second-pass controlled processes of revision after violations have been detected. Note that for young participants, this pattern was observed at the determiner immediately following the verb, while the waveforms time-locked to the onset of the subsequent noun did not show this pattern, indicating that young healthy adults show sensitivity to verb argument structure information as soon as an inconsistent word is heard and that they resolve this anomaly in real time. The present findings are thus consistent with previous ERP and behavioral studies showing an effect of verb information on the processing of incoming arguments (Ferreti et al., 2001; Osterhout et al., 1994; Trueswell & Kim, 1998).
Results for the older control group indicated somewhat different processing patterns compared to young healthy listeners, albeit the ERP data showed online sensitivity to verb argument structure processing. The older control group did not show an N400 effect on the determiner, indicating that assignment of thematic roles and lexical integration is less automatic and immediate in older listeners. At the determiner site the older participants showed a centro-parietal positive shift, reflecting reanalysis or repair processes arising from a dispreferred sentence continuation, but failed to acknowledge an outright inconsistency between the verb’s thematic specifications and the incoming material, as evidenced by the lack of negativity at the determiner. In addition, a left-parietal negative effect was observed on the following noun, which may suggest that the acknowledgement of the violation was delayed in this group until the full lexical item was processed. It can be noted that negativities on the noun in some electrode sites may have been influenced by the sustained effect on the preceding word, i.e., the determiner, which was used as a baseline for the noun event. Since the positivity observed on the determiner for older participants extended even up to 800 msec post stimulus onset on some sites, it is possible that the negativity found on the following noun was exaggerated. Note however that a strong negativity was observed also in left parietal-central sites, where no sustained effect was found in the determiner condition.
Delayed ERP effects related to aging have been reported in previous studies (Kutas & Iragui, 1998; Federmeier & Kutas, 2005; see review in Wlotko, Lee, & Federmeier, 2010). For example, in Federmeier et al. (2003), older adults displayed a qualitatively similar but substantially delayed response to constraints imposed by sentence context. As concluded by Wlotko et al. (2010), older adults are less likely to use active prediction during comprehension, and are thus less efficient in using sentence context information to facilitate processing. Note, that reaction time data cannot reflect this delayed processing, since responses were obtained only after the entire sentence was heard.
The ERP responses elicited by the verb argument structure violations in the agrammatic group were different from those of both young and older controls, indicating that participants with agrammatic aphasia are impaired in on-line processing of verb argument structure information. The agrammatic participants showed an attenuated and restricted positivity at the position of the determiner, and no negative shift. The finding of a late positive shift at the position of the determiner indicates that, as older controls, these participants attempted to integrate the surplus incoming material with the preceding sentence context. This result is similar to that of Friederici et al. (1998) in which a Broca’s patient was presented with sentences containing word-category violations. In contrast to healthy controls showing a biphasic LAN-P600 response to these violations, the agrammatic participant displayed only a late positivity. The authors suggested that the absence of the early negativity indicates a loss of fast and automatic processing, but the presence of a P600 reveals that secondary syntactic processes are still available.
In addition, no ERP responses were elicited at the critical noun in the argument structure violation condition, suggesting that aphasic participants were not able to detect the mismatch between the requirements of the verb and the actual incoming arguments even at this later stage, as reflected also by their higher error rates in this condition. These findings indicate that agrammatic individuals are impaired in using verb information on-line to integrate individual arguments into an overall sentence context. Verb information may not be available with the sufficient level of activation to enable integration between sentence constituents at a normal speed. This interpretation is consistent with Wassenaar and Hagoort (2005) who suggested that incomplete or delayed availability of word-class information hinders Broca’s aphasic listeners’ ability to construct a phrasal structure of the sentence.
The lack of online sensitivity to argument structure information in the agrammatic group contrasts with evidence for retained sensitivity to semantic information, as revealed by their responses to semantic violations. In the semantic condition, the three participant groups showed qualitatively similar ERP responses, though the effects formed a continuum with regard to distribution and the size of the effects. Younger adults showed a broadly distributed centro-parietal negative shift, most pronounced from 300 to 500 msec, in keeping with the classic N400 effect found in correlation with lexical-semantic integration difficulties (Connolly & Phillips, 1994; Kutas & Hillyard, 1980; Rosler et al., 1993).2 The amplitude of the N400 effect was attenuated in older adults, consistent with previous reports of decreases in amplitude of the N400 effect (as well as other ERP components) with advancing age (Faustmann, Murdoch, Finnigan, & Copland, 2007; Gunter, Jackson, & Mulder, 1992; Kutas & Iragui, 1998). This decrease in N400 amplitude with advanced age has been associated with less efficient integration of lexical items with the semantic context (Iraqui et al., 1996; Wassenaar & Hagoort, 2005), possibly due to slower access to semantic memory, which becomes larger and more interconnected with age, or due to reduced neural processing speed (Kutas & Iraqui, 1998). Another related possibility is that less effective inhibitory mechanisms in older adults lead to poor integration resulting in smaller N400 effects (Cameli & Phillips, 1999).3 The N400 response to semantic violations was further attenuated in the agrammatic group, and restricted to the central sites. The effects were also flatter, without clearly defined peaks. A reduction of the N400 amplitude has been reported in other studies of sentence comprehension in individuals with aphasia (Swaab, Brown, & Hagoort, 1997, 1998; Wassenaar & Hagoort, 2005). Hagoort and colleagues (Hagoort et al., 1996; Swaab et al., 1997) argued that slower and inefficient lexical integration processes could cause a degraded or incomplete representation of the sentence preceding the final word. This may lead to a less defined representation of the sentence context, and reduce the difference between congruent and incongruent conditions, hence causing an attenuated N400 effect. Importantly, however, the N400, though attenuated, was found in the agrammatic group. This finding is in line with the agrammatic participants’ behavioral results, showing above chance accuracy on grammaticality judgments for semantic violations.
ERP studies using linguistic anomaly paradigms are a powerful way to examine online language processing in healthy and impaired systems. More recently it has been recognized that other types of information are embedded in the EEG signal. In particular, spectral analysis techniques can reveal changes in the amplitude of ongoing oscillations within specific frequency bands. Time-frequency analysis of oscillatory activity can detect task-related neural activity that is not necessarily phase-locked to a stimulus, and would not be detectable in time-domain averaging (Mouraux & Iannetti, 2008; Le Van Quyen & Bragin, 2007). Therefore, it is possible that, using such fine-grained methods, neural activity of aphasic participants, not detectible using time-domain averaging, may be present but not precisely time locked to the onset of a temporal event. We are currently investigating how different violation conditions may trigger transient power modulations in the ongoing EEG activity by mapping the ERP signal into a two dimensional time-frequency representation. In the aphasic participants, the non-phase-locked modulations of the ERP signal should be reflected in changes in the power of oscillatory activity as a function of time and frequency.
5. Conclusions
Sentence comprehension requires fast and efficient access to and integration of lexical-semantic and syntactic information. Information encoded in the verb’s lexical entry is crucial for this process because verbs specify the characteristics of their arguments, such as their number and type. In the present study, we used ERPs to investigate the sensitivity of healthy controls and agrammatic aphasic individuals to argument structure violations embedded in sentence contexts. Results showed that the agrammatic participants were impaired on grammaticality judgments of sentences, especially those with argument structure violations, to an extent exceeding the deficits reported in previous behavioral studies (Grodzinsky & Finkel, 1998; Kim & Thompson, 2000, 2004). Furthermore, agrammatic participants displayed reduced electrophysiological responses to argument structure violations compared to control participants. Both control groups displayed an N400-P600 pattern (although this effect appeared on the determiner for the young group and on the noun for the older group). In contrast, argument structure violations elicited only an attenuated P600 effect on the determiner in participants with aphasia, and no negativity was observed. In contrast to argument structure violations, the agrammatic participants’ response to semantic violations was not qualitatively different from control participants’. Less impaired semantic processing was reflected also in agrammatic participants’ grammaticality judgments to semantic violations, which were significantly more accurate than those for argument structure violations.
The present results show that agrammatic individuals do not demonstrate normal real-time sensitivity to mismatches between the argument structure requirements of a verb and the incoming linguistic information accompanying it. While these participants are aware of semantic incongruity in real time, the same does not hold for verb-argument incongruity, as evidenced by the lack of an N400 component, and by grammaticality judgment accuracy. The present results suggest that sentence comprehension difficulties in individuals with aphasia may result from incomplete or inefficient access to lexical information associated with verbs, and impaired online integration of that information within the context of sentences.
Highlights
An ERP acceptability judgment experiment was run with aphasic and control subjects.
Semantic violations elicited an N400 effect in young and older controls.
Argument structure violations elicited a biphasic N400-P600 pattern in controls.
Aphasics exhibited a reduced, delayed N400 in response to semantic violations.
Aphasics did not show normal biphasic responses to argument structure violations.
Acknowledgments
This research was supported by the NIH/NIDCD-R01-DC01948-18 to C. K. Thompson. The authors wish to thank Sladjana Lukic and Elena Barbieri for assistance with data collection. We also thank an anonymous reviewer for numerous helpful comments.
Appendix Verbs used in the experiment
| Transitive | Intransitive |
|---|---|
| bite | blink |
| bury | bowl |
| carry | jog |
| catch | cough |
| chase | crawl |
| cover | cry |
| drag | frown |
| follow | fish |
| grab | travel |
| hit | kneel |
| hold | laugh |
| hug | sweat |
| join | march |
| kick | nod |
| kiss | pray |
| lick | run |
| lift | scream |
| paint | shiver |
| pet | sit |
| pull | sleep |
| punch | smile |
| push | snore |
| save | stand |
| scrub | swim |
| spend | walk |
| squeeze | weep |
| touch | whisper |
| visit | sneeze |
| wash | wink |
| watch | yawn |
| weigh | dive |
| injure | drool |
| poke | ski |
| betray | skate |
| pinch | spit |
Footnotes
This indicates that participants did not exhibit a general ‘yes-bias’, leading them to accept ungrammatical sentences.
The negativity observed in response to semantic violations in our participants was sustained, remaining significant at the later time window (500–700 msec). Similar effect was observed in previous studies that presented violations at the end of the sentence (Connolly & Phillips, 1994; Connolly et al., 1992).
In addition, for older participants, the N400 was followed by a positivity at the posterior sites. A similar pattern has been observed in a few previous studies in correlation with processing of semantic anomalies (Friederici & Frisch, 2000; Gunter et al., 1997; Wassenaar & Hagoort, 2005). It has been suggested that this late positive effect reflects processes of reanalysis and repair, which can be elicited in response to both semantic and syntactic anomalies. A similar account has been put forward by Kaan, Harris, Gibson, & Holcomb (2000), who consider this late positivity a reflection of general integration difficulty resulting from syntactic or thematic processing difficulties. The finding that older, but not young adults, showed this positive effect in response to semantic incongruity indicates that for older adults lexical-semantic integration processes are less efficient, the late positive shift reflecting an attempt at repair of semantic incongruity after it has been detected.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainsworth-Darnell K, Shulman HG, Boland JE. Dissociating brain responses to syntactic and semantic anomalies: Evidence from event-related potentials. Journal of Memory and Language. 1998;38:112–130. [Google Scholar]
- Altmann GTM, Kamide Y. Incremental interpretation at verbs: Restricting the domain of subsequent reference. Cognition. 1999;73:247–264. doi: 10.1016/s0010-0277(99)00059-1. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, van Rijn H. The CELEX lexical database (Release 1) [CD-ROM] Philadelphia, PA: Linguistic Data Consortium, University of Pennsylvania; 1993. [Google Scholar]
- Band GPH, Kok A. Age effects on the response monitoring in a mental-rotation task. Biological Psychology. 2000;51:201–221. doi: 10.1016/s0301-0511(99)00038-1. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat: doing phonetics by computer [computer program] 2009 [Google Scholar]
- Brown CM, Hagoort P. The processing nature of the N400: Evidence from masked priming. Journal of Cognitive Neuroscience. 1993;5:34–44. doi: 10.1162/jocn.1993.5.1.34. [DOI] [PubMed] [Google Scholar]
- Cameli L, Phillips NA. Age-related differences in semantic priming: Evidence from event-related brain potentials. Brain and Cognition. 1999;41:179–183. [PubMed] [Google Scholar]
- Carlson GN, Tanenhaus MK. Thematic roles and language comprehension. In: Wilkins W, editor. Thematic relations. New York: Academic Press; 1988. [Google Scholar]
- Carrier J, Randall J. The argument structure and syntactic structure of resultatives. Linguistic Inquiry. 1992;23:173–234. [Google Scholar]
- Carrier J, Randall J. Lexical Mapping. In: Reuland E, Abraham W, editors. Knowledge and Language Volume II: Lexical and Conceptual Structure. Dordrecht: Kluwer Academic Publishers; 1993. pp. 119–142. [Google Scholar]
- Connolly JF, Phillips NA. Event-related potential components reflect phonological and semantic processing of the terminal word of spoken sentences. Journal of Cognitive Neuroscience. 1994;6:256–266. doi: 10.1162/jocn.1994.6.3.256. [DOI] [PubMed] [Google Scholar]
- Coulson S, King J, Kutas M. Expect the unexpected: Event-related brain responses to morphosyntactic violations. Language and Cognitive Processes. 1998;13:21–58. [Google Scholar]
- De Bleser R, Kauschke C. Acquisition and loss of nouns and verbs: Parallel or divergent patterns? Journal of Neurolinguistics. 2003;16:213–229. [Google Scholar]
- Dragoy O, Bastiaanse R. Verb production and word order in Russian agrammatic speakers. Aphasiololgy. 2009;24:28–55. [Google Scholar]
- Faustmann A, Murdoch BE, Finnigan S, Copland DA. Effects of advancing age on the processing of semantic anomalies in adults: Evidence from event-related brain potentials. Experimental Aging Research. 2007;33:439–460. doi: 10.1080/03610730701525378. [DOI] [PubMed] [Google Scholar]
- Federmeier K, Kutas M. Aging in context: age-related changes in context use during language comprehension. Psychophysiology. 2005;42:133–141. doi: 10.1111/j.1469-8986.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- Federmeier K, van Petten C, Schwartz TJ, Kutas M. Sounds, words, sentences: Age-related changes across levels of language processing. Psychology and Aging. 2003;18:858–872. doi: 10.1037/0882-7974.18.4.858. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends in Cognitive Sciences. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Frisch S. Verb argument structure processing: The role of verb-specific and argument-specific information. Journal of Memory and Language. 2000;43:476–507. [Google Scholar]
- Friederici AD, Hahne A, Mecklinger A. The temporal structure of syntactic parsing: Event-related potentials during speech perception and word-by-word reading. Journal of Experimental Psychology: Learning, Memory & Cognition. 1996;22:1–31. doi: 10.1037//0278-7393.22.5.1219. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Hahne A, von Cramon DY. First-pass versus second-pass parsing processes in a Wernicke’s and a Broca’s aphasic: Electrophysiological evidence for a double dissociation. Brain and Language. 1998;62:311–341. doi: 10.1006/brln.1997.1906. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Kilborn K. Temporal constraints on language processing: Syntactic priming in Broca’s aphasia. Journal of Cognitive Neuroscience. 1989;1:262–272. doi: 10.1162/jocn.1989.1.3.262. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA. The brain basis of syntactic processes: Functional imaging and lesion studies. NeuroImage. 2003;20:S8–S17. doi: 10.1016/j.neuroimage.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Meyer M. The brain knows the difference: two types of grammatical violations. Brain Research. 2004;1000:72–77. doi: 10.1016/j.brainres.2003.10.057. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Pfeifer E, Hahne A. Event-related brain potentials during natural speech processing: Effects of semantic, morphological and syntactic violations. Cognitive Brain Research. 1993;1:183–192. doi: 10.1016/0926-6410(93)90026-2. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Weissenborn J. Mapping sentence form onto meaning: The syntax-semantics interface. Brain Research. 2007;1146:50–58. doi: 10.1016/j.brainres.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Frisch S, Hahne A, Friederici AD. Word category and verb-argument structure information in the dynamics of parsing. Cognition. 2004;91:191–219. doi: 10.1016/j.cognition.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y, Finkel L. The neurology of empty categories. Journal of Cognitive Neuroscience. 1998;10:281–292. doi: 10.1162/089892998562708. [DOI] [PubMed] [Google Scholar]
- Gunter TC, Friederici AD. Concerning the Automaticity of Syntactic Processing. Psychophysiology. 1999;36:126–137. doi: 10.1017/s004857729997155x. [DOI] [PubMed] [Google Scholar]
- Gunter TC, Jackson JL, Mulder G. An electrophysiological study of semantic processing in young and middle aged academics. Psychophysiology. 1992;27:28–53. doi: 10.1111/j.1469-8986.1992.tb02009.x. [DOI] [PubMed] [Google Scholar]
- Gunter TC, Stowe LA, Mulder G. When syntax meets semantics. Psychophysiology. 1997;34:660–676. doi: 10.1111/j.1469-8986.1997.tb02142.x. [DOI] [PubMed] [Google Scholar]
- Haarmann HJ, Kolk HHJ. On-line sensitivity to subject-verb agreement violations in Broca’s aphasia: The role of syntactic complexity and time. Brain and Language. 1994;46:493–516. doi: 10.1006/brln.1994.1028. [DOI] [PubMed] [Google Scholar]
- Hagoort P. Impairments of lexical-semantic processing in Aphasia: Evidence from the processing of lexical ambiguities. Brain and Language. 1993;45:189–232. doi: 10.1006/brln.1993.1043. [DOI] [PubMed] [Google Scholar]
- Hagoort P. How the brain solves the binding problem for language: A neurocomputational model of syntactic processing. Neuroimage. 2003;20:S18–S19. doi: 10.1016/j.neuroimage.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Brown C, Swaab TY. Lexical-semantic event-related potential effects in patients with left hemisphere lesions and aphasia, and patients with right hemisphere lesions without aphasia. Brain. 1996;119:627–649. doi: 10.1093/brain/119.2.627. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Wassenaar MED, Brown CM. Real-time semantic compensation in patients with agrammatic comprehension: electrophysiological evidence for multiple-route plasticity. Proceedings of the National Academy of Science of the United States of America. 2003;100:4340–4345. doi: 10.1073/pnas.0230613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb PJ, Neville HJ. Auditory and visual semantic priming in lexical decision: A comparison using event-related brain potentials. Language and Cognitive Processes. 1990;5:281–312. [Google Scholar]
- Huynh H, Feldt LS. Conditions under which mean square ratios in repeated measurements have exact F-distributions. Journal of the American Statistical Association. 1970;65:1582–1589. [Google Scholar]
- Ille N, Berg P, Scherg M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. Journal of Clinical Neuropsychology. 2002;19:113–124. doi: 10.1097/00004691-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Iragui V, Kutas M, Salmon DP. Event-related potentials during semantic categorization in normal aging and senile dementia of the Alzheimer’s type. Electroencephalography and Clinical Neurophysiology. 1996;100:392–406. [PubMed] [Google Scholar]
- Jones MA. Cognate objects and the case filter. Journal of Linguistics. 1988;24:89–110. [Google Scholar]
- Jonkers R, Bastiaanse R. The influence of instrumentality and transitivity on action naming in Broca’s and anomic aphasia. Brain and Language. 1996;55:37–39. doi: 10.1016/j.bandl.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kaan E, Harris A, Gibson E, Holcomb P. The P600 as an index of syntactic integration difficulty. Language and Cognitive Processes. 2000;15:159–201. [Google Scholar]
- Kaan E, Swaab TY. The brain circuitry of syntactic comprehension. Trends in Cognitive Science. 2002;6:350–356. doi: 10.1016/s1364-6613(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Kawohl K, Bunse S, Willmes K, Hoffrogge A, Buchner H, Huber W. Semantic event-related potential components reflect severity of comprehension deficits in aphasia. Neurohabilitation and Neural Repair. 2010;24:282–289. doi: 10.1177/1545968309348311. [DOI] [PubMed] [Google Scholar]
- Kertesz A. The Western Aphasia Battery. New York: Grune and Stratton; 2007. [Google Scholar]
- Kim M, Thompson CK. Patterns of comprehension and production of nouns and verbs in agrammatism: Implications for lexical organization. Brain and Language. 2000;74:1–25. doi: 10.1006/brln.2000.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Thompson CK. Verb deficits in Alzheimer’s disease and agrammatism: Implications for lexical organization. Brain and Language. 2004;88:1–20. doi: 10.1016/s0093-934x(03)00147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss K. Effects of verb complexity on agrammatic aphasic’s sentence production. In: Bastiaanse R, Gordzinsky Y, editors. Grammatical disorders in aphasia. London: Whurr Publishers; 2000. pp. 152–170. [Google Scholar]
- Kitade S, Enai T, Sei H, Morita Y. The N400 event-related potential in aphasia. Journal of Medical Investigation. 1999;46:87–95. [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends in Cognitive Science. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science. 1980;207:203–208. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Brain potentials during reading reflect word expectancy and semantic association. Nature. 1984;307:161–163. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- Kutas M, Iragui V. The N400 in a semantic categorization task across six decades. Electroencephalography and Clinical Neurophysiology. 1998;108:456–471. doi: 10.1016/s0168-5597(98)00023-9. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Bragin A. Analysis of dynamic brain oscillations: methodological advances. TRENDS in Neuroscience. 2007;30:365–373. doi: 10.1016/j.tins.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Luzzatti C, Raggi R, Zonca G, Pistarini C, Contardi A, Pinna GD. Verb-noun double dissociation in aphasic lexical impairments: The role of word frequency and imageability. Brain and Language. 2002;81:432–444. doi: 10.1006/brln.2001.2536. [DOI] [PubMed] [Google Scholar]
- MacDonald MC, Pearlmutter NJ, Seidenberg MS. Lexical nature of syntactic ambiguity resolution. Psychological Review. 1994;101:676–703. doi: 10.1037/0033-295x.101.4.676. [DOI] [PubMed] [Google Scholar]
- Mauner G, Koenig JP. Linguistic vs. conceptual sources of implicit agents in sentence comprehension. Journal of Memory and Language. 2000;43:110–134. [Google Scholar]
- Mittwoch A. Cognate objects as reflections of davidsonian event arguments. In: Rothstein S, editor. Events and Grammar. Dordrecht: Kluwer Academic Publishers; 1988. pp. 309–332. [Google Scholar]
- Mouraux A, Iannetti GD. Across-trial averaging of event-related EEG responses and beyond. Magnetic Resonance Imaging. 2008;26:1041–1054. doi: 10.1016/j.mri.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Munte TF, Heinze HJ, Mangun GR. Dissociation of negative ERP components produced by incongruities in grammatical and semantic judgment tasks. Journal of Cognitive Neuroscience. 1993;5:335–344. doi: 10.1162/jocn.1993.5.3.335. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Nicol J, Barss A, Forster KI, Garrett MF. Syntactically based sentence processing classes: Evidence from event-related brain potentials. Journal of Cognitive Neuroscience. 1991;3:151–165. doi: 10.1162/jocn.1991.3.2.151. [DOI] [PubMed] [Google Scholar]
- Osterhout L, Holcomb PJ. Event-related brain potentials elicited by syntactic anomaly. Journal of Memory and Language. 1992;31:785–806. [Google Scholar]
- Osterhout L, Holcomb PJ. Event-related potentials and syntactic anomaly: Evidence of anomaly detection during the perception of continuous speech. Language and Cognitive Processes. 1993;8:413–438. [Google Scholar]
- Osterhout L, Holcomb PJ, Swinney DA. Brain potentials elicited by garden-path sentences: Evidence of the application of verb information during parsing. Journal of Experiment Psychology: Learning, Memory, & Cognition. 1994;20:786–803. doi: 10.1037//0278-7393.20.4.786. [DOI] [PubMed] [Google Scholar]
- Osterhout L, Mobley LA. Event-related brain potentials elicited by failure to agree. Journal of Memory and Language. 1995;34:739–773. [Google Scholar]
- Osterhout L, Nicol J. On the distinctiveness, independence, and time course of the brain responses to syntactic and semantic anomalies. Language and Cognitive Processes. 1999;14:283–317. [Google Scholar]
- Rösler F, Friederici AD, Pütz P, Hahne A. Event-related brain potentials while encountering semantic and syntactic constraint violations. Journal of Cognitive Neuroscience. 1993;5:345–362. doi: 10.1162/jocn.1993.5.3.345. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschmann A, Zuccolotto A. E-prime user’s guide. Pittsburgh, PA: Psychology Software Tools; 2002. [Google Scholar]
- Shapiro LP, Brookins B, Gordon B, Nagel N. Verb effects during sentence processing. Journal of Experimental Psychology: Learning, Memory & Cognition. 1991;17:983–996. doi: 10.1037//0278-7393.17.5.983. [DOI] [PubMed] [Google Scholar]
- Shapiro LP, Gordon B, Hack N, Killackey J. Verb argument processing in complex sentences in Broca and Wernicke’s aphasia. Brain and Language. 1993;45:423–447. doi: 10.1006/brln.1993.1053. [DOI] [PubMed] [Google Scholar]
- Shapiro LP, Levine B. Verb processing during sentence comprehension in aphasia. Brain and Language. 1990;38:21–47. doi: 10.1016/0093-934x(90)90100-u. [DOI] [PubMed] [Google Scholar]
- Swaab TY, Brown C, Hagoort P. Spoken sentence comprehension in aphasia: Event-related potential evidence for a lexical integration deficit. Journal of Cognitive Neuroscience. 1997;9:39–66. doi: 10.1162/jocn.1997.9.1.39. [DOI] [PubMed] [Google Scholar]
- Swaab TY, Brown CM, Hagoort P. Understanding ambiguous words in sentence contexts: Electrophysiological evidence for delayed contextual selection in Broca’s aphasia. Neuropsychologia. 1998;36:737–761. doi: 10.1016/s0028-3932(97)00174-7. [DOI] [PubMed] [Google Scholar]
- Thompson CK. Diagnostic Test. Northwestern University; 2011. Northwestern Assessment of Verbs in Sentences. (available at www.flintbox.com) [Google Scholar]
- Thompson CK, Dickey MW, Cho S, Lee J, Griffin Z. Verb argument structure encoding during sentence production in agrammatic aphasic speakers: An eye-tracking study. Brain and Language. 2007;103:24–26. [Google Scholar]
- Thompson CK, Lange K, Schneider S, Shapiro L. Agrammatic and non-brain-damaged subjects’ verb and verb argument structure production. Aphasiology. 1997;11:473–490. [Google Scholar]
- Trueswell JC, Kim AE. How to prune a garden-path by nipping it in the bud: Fast-priming of verb argument structures. Journal of Memory and Language. 1998;39:102–123. [Google Scholar]
- Van Berkum JJA, Brown CM, Hagoort P. Early referential context effects in sentence processing: Evidence from event-related brain potentials. Journal of Memory and Language. 1999;41:147–182. [Google Scholar]
- Wassenaar MED, Brown CM, Hagoort P. ERP Effects of Subject-Verb Agreement Violations in Patients with Broca’s Aphasia. Journal of cognitive neuroscience. 2004;16:553–576. doi: 10.1162/089892904323057290. [DOI] [PubMed] [Google Scholar]
- Wassenaar MED, Hagoort P. Word-category violations in patients with Broca’s aphasia: An ERP study. Brain and language. 2005;92:117–137. doi: 10.1016/j.bandl.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Wassenaar MED, Hagoort P. Thematic role assignment in patients with Broca’s aphasia: Sentence-picture matching electrified. Neuropsychologia. 2007;45:716–740. doi: 10.1016/j.neuropsychologia.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Wlotko EW, Lee CL, Federmeier KD. Language of the aging brain: Event-related potential studies of comprehension in older adults. Language and Linguistics Compass. 2010;4:623–638. doi: 10.1111/j.1749-818X.2010.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubizarreta M. Levels of representation in the lexicon and in the syntax. Dordrecht: Foris Publications; 1987. [Google Scholar]