Abstract
Pathologists and immunologists have collaborated over many years in their efforts to understand and properly diagnose cancer. The ability of pathologists to correctly diagnose this disease was facilitated by the development of immunohistology that utilized specificity of antibodies to distinguish between normal cells and cancer cells. Further boost was provided through the advent of monoclonal antibodies. The two disciplines are now together on the brink of a paradigm shift resulting from a better understanding of the importance for cancer diagnosis and prognosis to consider not only the characteristics of the cancer cells, but also the cancer microenvironment reflecting the host response to the disease. This new immunology and pathology alliance named “Immunoscore” will advance research in both disciplines as well as benefit patients.
Introduction
All patients that receive a message from their physician that symptoms that concerned them might potentially signal a cancerous growth, know that next comes an examination of the tissue specimen, most often a biopsy, by pathologists who are the ones to pronounce the final diagnosis. For many years this involved a careful examination of the morphology of putative cancer cells in the tissue sections that distinguished them from the surrounding normal tissue. In addition to abnormal cell morphology, useful information could also be derived from some of the stromal components surrounding the abnormal cell, in particular the degree of fibrosis, necrosis and lymphovascularization that suggested a more or a less established tumor or one that is likely to have already spread versus the one that might be still localized and potentially curable by surgery. These observations extended the role of pathology from purely diagnostic to some, albeit limited extent, prognostic (Leong and Zhuang, 2011).
Scientific and technical developments over the last several decades in every discipline concerned with cancer, including genetics, cell biology, molecular biology and immunology, have the potential to raise the contribution of pathology to that of an ultimate authority for diagnosis, prognosis and treatment selection. A lot of this has already happened in the realm of diagnosis but there is still a lot of room for improvement. Cancer geneticists and molecular biologists are identifying with an ever-increasing speed and lower and lower cost mutations in genes and pathways that can place a cell in a precise place from normal to various stages of premalignant and malignant transformation continuum. Some of the same mutations are predictive biomarkers of how fast or slowly cancer development might proceed, if at all, and thus who should be treated more aggressively and who should be only periodically examined. Other biomarkers clearly show that what was once a single cancer type, breast or prostate or lymphoma, can be divided into many different types, each deserving of a separate diagnosis and each potentially needing a different treatment option.
The avalanche of newly discovered genetic biomarkers has unfortunately produced only a few snowflakes for accepted clinical applications that would allow pathologists to provide personalized diagnosis and prognosis. The reasons are both conceptual and practical. Among the many candidate biomarkers, it is important to make an informed, scientifically based selection of a smaller panel potentially specific for every cancer type or subtype. Such decisions often require support from large studies requiring considerable resources. Even when those can be undertaken, the results need to be adopted by pharmaceutical companies who can produce standardized reagents for use by clinical labs. This is happening very slowly and, not surprisingly, only in the case of a few very robust biomarkers. A good example is breast cancer where thousands of biomarkers have been characterized and published but only three of them, estrogen receptor, progesterone receptor and Her-2/neu, are predominantly used to diagnose most breast cancers (Leong and Zhuang, 2011). This is in spite of continuing work of the research community that shows that using additional biomarkers leads to a diagnosis of very different breast cancer types and consequently different prognosis and treatment choices.
Immunology was the first discipline to push pathology into the new era of diagnosis and prognosis. With the advent of monoclonal antibodies (Kohler and Milstein, 1975), information obtained by studying cell morphology could be confirmed and extended by immunochemistry that used the exquisite specificity of antibodies to distinguished normal cells from cancer cells. Over the ensuing decades, hundreds if not thousands of antibodies were characterized that could improve the ability of a pathologist to make a more precise diagnosis (Leong and Leong, 2006). As in the case of molecular biomarkers, and for the same combination of conceptual and practical reasons, only relatively few antibodies, primarily those that have been commercialized, have entered the arena of diagnostic pathology.
Immunology is currently poised to support pathologists in another giant leap forward, a large paradigm shift that deemphasizes the cancer cell and focuses the pathologist’s attention to the anti-tumor immune response of the patient. The well-established hallmarks of cancer that have served for many years as a guideline to pathologists (Hanahan and Weinberg, 2000) have been updated to include the ability of the cancer cell to evade effective acute inflammatory host response (Hanahan and Weinberg, 2011) and to benefit from ineffective chronic inflammatory response. This ability depends as much, if not more, on the host as it does on the tumor cell and thus a tumor cell centric view that has guided diagnosis and prognosis is beginning to change.
In this mini-review/personal perspective, examples will be provided using the author’s own work and experience in the immunobiology of the epithelial mucin MUC1 and anti-tumor immunity, to illustrate importance of both cancer cell biomarkers and the host response in the pathology of cancer, and how very often those two regulate one another and thus cannot be separated or individually evaluated if the true representation of the disease diagnosis, prognosis and response to therapy is to be achieved.
Epithelial cell antigen MUC1 in service to pathologists
Biomarker for cancer diagnosis
MUC1mucin is a high molecular weight transmembrane glycoprotein, detectable in low levels on the apical surface of normal polarized epithelia in the respiratory, genitourinary and digestive tract. MUC1 is overexpressed on most adenocarcinomas, including breast, lung, colon, pancreas, stomach, prostate, and ovary (Vlad et al., 2004). The extracellular domain of MUC1 is dominated by the VNTR variable number (between 20 and 200) of tandem repeats region VNTR consisting of a tandemly repeated sequence PDTRPAPGSTAPPAHGVTSA. This extracellular domain and in particular the VNTR, are involved in cell-cell and cell-matrix interactions. The smaller subunit of MUC1 (~ 20 kDa) contains a short extracellular portion, a transmembrane region and a short cytoplasmic tail with seven phosphorylation sites identifying MUC1 as a signaling molecule (Li et al., 1998; Li and Kufe, 2001; Li et al., 2001a; Li et al., 2001b).
Carbohydrate chains on MUC1 made by normal cells are typically long and branched terminated by sialic acid, giving the molecule a mucinous character. As an epithelial cell undergoes malignant transformation, however, it loses the normal apical-basolateral polarity and MUC1 begins to displays striking quantitative (overexpression) and qualitative (lack of polarization and hypoglycosylation: truncated glycan chains, immature glycosylated forms, increased sialylation, aberrant carbohydrate core-type expression (Brockhausen et al., 1995; Burchell et al., 2001) alterations. Numerous monoclonal antibodies have been produced and characterized over the last three decades against the tumor forms of MUC1 and (Hiraga et al., 1998)even though it is clear that MUC1 is expressed on virtually all adenocarcinomas, very few of these antibodies are part of the pathologists’ antibody armamentarium for diagnosis of human adenocarcinomas. One of the better known is antibody CA15-3 almost exclusively used in breast cancer diagnosis, even though MUC1 is expressed in all adenocarcinomas and could be useful in other diagnoses where it often shows superiority over other established biomarkers (Sangoi et al., 2009; Winter et al., 2012).
Differential expression of MUC1 has also been linked to prognosis (Baldus et al., 2002; Kraus et al., 2002; Leroy et al., 2002; Sagara et al., 1999; Sivridis et al., 2002). There is also evidence that MUC1 enhances the metastatic abilities of tumor cells (Guddo et al., 1998; Hiraga et al., 1998). Most importantly, however, surface expression of MUC1 is reported to be upregulated in preneoplastic conditions of virtually every tissue that gives rise to a MUC1 positive neoplasm (Arul et al., 2000; Cao et al., 1997; Jarrard et al., 1998; Lopez-Ferrer et al., 2001; Reis et al., 1999). Thus additional antibodies that already exist, which are directed to different MUC1 epitopes created by different tumor specific posttranslational modifications, could be useful for a more refined diagnosis and prognosis. In addition to MUC1, several other molecules from the mucin family have been explored for epithelial tumor diagnosis and a number of antibodies have been characterized for their expression on various tumors at different stages of disease (Goto et al., 2008). It will be important to not lose sight of these new reagents at this point in time when emphasis is being increasingly placed on personalized diagnoses and treatment rather than the “one antibody fits all” approach.
Modulator of the host response
MUC1 on cancer cells has also been shown to regulate chemotaxis of the cells of the innate immune system (Carlos et al., 2005; Oppenheim et al., 2005) thus interfering with the proper balance between innate and adaptive immune cells in the tumor microenvironment. More recently we have shown that the extracellular domain of MUC1 internalizes and forms a complex with NF-kB p65 that goes to the nucleus and activated promoters of numerous proinflammatory cytokines such as IL-6, TNF-a (Cascio et al., 2011). These cytokines, in particular, are responsible for driving chronic inflammation in the tumor microenvironment that has been associated with tumor progression rather than tumor rejection. We have shown that these complexes do not form in normal cells where the MUC1 extracellular domain gets fully glycosylated and thus prevented from binding p65. As cells begin to transform and hypoglycosylated MUC1 begins to emerge, the tumor microenvironment changes in favor of the tumor. As the pathologist makes a diagnosis of a MUC1 expressing tumor, the extension of that diagnosis that would also serve as prognosis, should be additional immunohistochemical analysis for the state of MUC1 glycosylation, the presence of predominantly innate immune infiltrate as well as increased production by the tumor of IL-6, TNF-a and other proinflammatory cytokines. All those together would create a tumor profile that could be ranked from the relatively benign, slow progressing and unlikely to metastasize, to invasive and most likely already metastasized phenotype.
“Immunoscore” as a new immunology tool for better pathology diagnosis
Immunologists have been aware for a long time that tumor growth is accompanied by a systemic anti-tumor immune response and they have pursued the question why is that immune response not effective in individuals who succumb to their disease. A frequent observation made in cancer patients was that their tumor was progressing in the presence of antibodies and T cells specific for one or more tumor antigens. With further development of knowledge in immunology and discoveries that elucidated multiple immune mechanisms that are required for an effective immune response against tumor, there has been a new appreciation of the importance of the composition and regulation of the immune microenvironment at the site of the primary tumor that might or might not be permissive to the destructive actions of anti-tumor immunity.
While there had been intermittent articles discussing interdependence between the host response and tumor development, and some factors identified that can tip the balance in favor of the host or the tumor, such as systemic activation of myelod cells and inhibition of effector T cells (Schmielau and Finn, 2001; Schmielau et al., 2001) or accumulation of regulatory T cells (Savage et al., 2012), the first comprehensive analysis of tumors as targets of patients immune responses and the high predictive value of such an analysis was published in 2006 (Galon et al., 2006). Large cohorts of human colorectal primary tumors were examined by gene expression profiling and immunohistochemistry for evidence of infiltrating immune cells. The type, density and localization of immune cells, and most importantly type 1 T cells, was found to correlate much more significantly with survival than other histopathological methods used for disease staging. In short, primary tumors found to be infiltrated with large numbers of activated T cells had a much slower course of development and patients survived much longer even if the tumor had been originally staged in the most advanced and thus most lethal category. The intensity and quality of the immune infiltrate was a superior predictor of survival than any of the other measures. These data were confirmed on additional colon cancer cohorts and their prognostic value compared with a whole panel of established prognostic factors (Mlecnik et al., 2011), showing repeatedly the superiority of the immune infiltrate into tumor as a prognostic biomarker (Fridman et al., 2011a; Fridman et al., 2011b; Galon et al., 2007). In the few years since these initial publications, many investigators have reported that this applies to many other tumors in addition to colon cancer, including different types of breast cancer (Liu et al., 2011; Ruffell et al., 2012), ovarian cancer (Milne et al., 2009), brain cancer (Yang et al., 2010) (Yang et al., 2011) and liver cancer (Chen et al., 2012). Meta analysis of data from over 100 published studies confirms a robust evidence that the presence and relative numbers of CD3,+ CD8,+ CD45RO+, FoxP3+ cells in addition to tumor associated macrophages, dendritic cells and neutrophils, play a key role in cancer progression and in patient’s survival (Roxburgh et al., 2012).
Tumor infiltrating cells: memory of previous challenges
In our attempt to better understand why some patients could better control their tumors as characterized by vigorous T cell infiltration, slow progression and increased survival, while other patients could not, we explored one specific anti-tumor immune response directed against the MUC1 antigen described above. We had initially described the tumor form of MUC1, the hypoglycosylated overexpressed non-polarized molecule on epithelial adenocarcinomas, as the specific target of T cell responses found in the tumor draining lymph nodes of pancreatic cancer patients (Barnd et al., 1989). Subsequently we observed that some healthy individuals might also have circulating anti-MUC1 antibodies. That led us to examine conditions other than cancer that might expose the immune system to abnormal, hypoglycosylated forms of MUC1. We discovered that at sites of acute or chronic inflammation resulting from viral or bacterial infections, such as in the salivary gland during mumps infection or the breast duct affected by mastitis, MUC1 was overexpressed and hypoglycosylated phenocopying tumor MUC1. Those conditions were similarly accompanied by the development of MUC1 specific immune responses and immune memory (Cramer and Finn, 2011; Cramer et al., 2005; Cramer et al., 2010). It is our current hypothesis that tumors that start to grow in an individual who has had multiple previous events that generated anti-MUC1 immune memory (and likely a memory for many other abnormally expressed molecules found later on tumor cells) are more likely to get infiltrated faster and more abundantly with memory T cells than tumors in “naive” individuals.
The importance of the “Immunoscore” and the correlations we are establishing between adaptive memory for abnormal cell molecules and tumor infiltration, suggest that it will be possible to raise the Immunoscore by eliciting immune memory through vaccination against some of the better defined tumor antigens of individuals at high risk for developing cancer. Using again MUC1 as an example, individuals with an increased risk for developing MUC1+ tumors such as colon cancer (family history or history of polyps), breast cancer (BRCA-1 and BRCA-2 mutation, family history), pancreatic cancer (chronic pancreatitis, K-ras mutation, pancreatic cysts, family history), could be vaccinated with a MUC1 vaccine that elicits MUC1-specific T cells and antibodies. The expectation would be that the MUC1-specific immune memory cells would accumulate at the site of an abnormal MUC1 expression that would mark a new tumor and control its growth or lead to its elimination. There is plenty of evidence from animal models that tumors growing in pre-vaccinated animals are more infiltrated, grow slower or are completely eliminated.
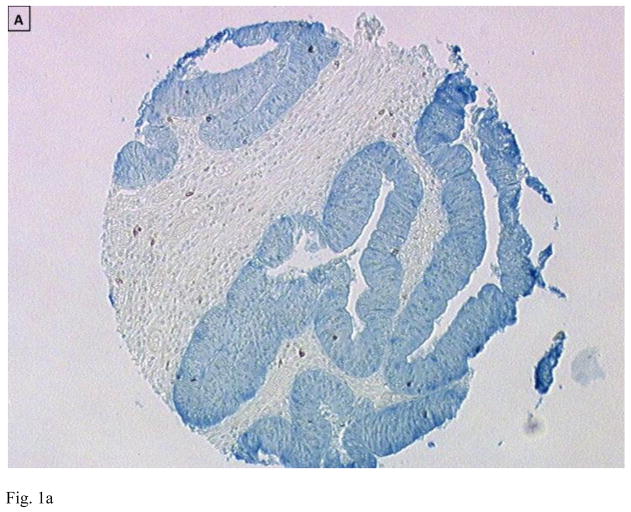
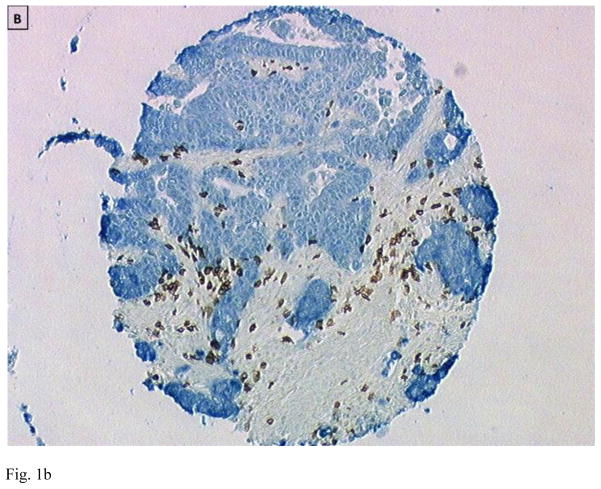
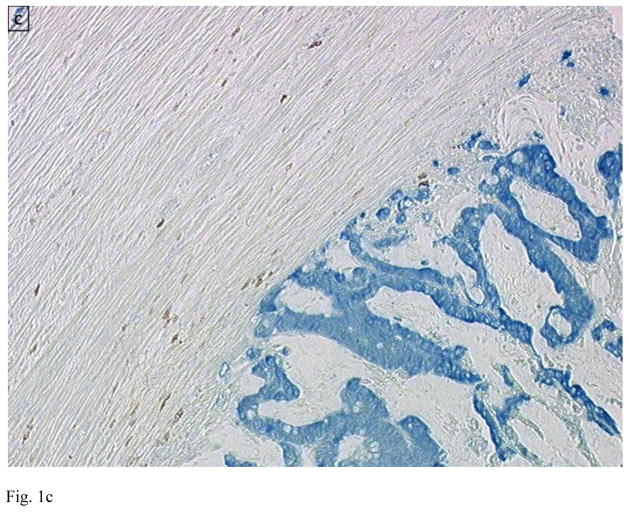
Figure 1.
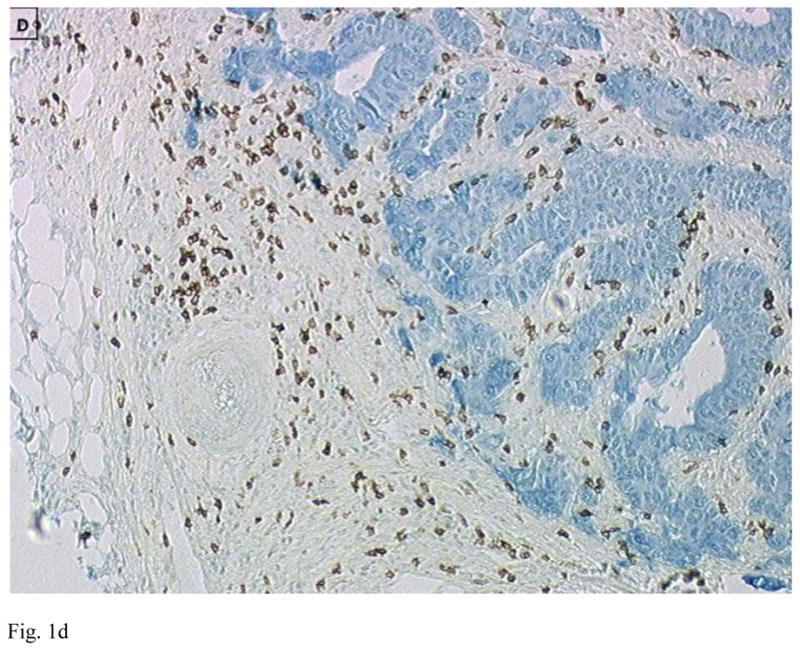
Immunohistology of primary colon tumors in a tissue microarray (TMA) showing examples of tumors with low (A and C) and high (B and D) CD8 T cell infiltration. Tumor is stained blue (cytokeratin) and CD8+ T cells brown. Images are courtesy of Dr. Jerome Galon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arul GS, et al. Mucin gene expression in Barrett’s oesophagus: an in situ hybridisation and immunohistochemical study. Gut. 2000;47:753–61. doi: 10.1136/gut.47.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldus SE, et al. Comparative evaluation of the prognostic value of MUC1, MUC2, sialyl-Lewis(a) and sialyl-Lewis(x) antigens in colorectal adenocarcinoma. Histopathology. 2002;40:440–9. doi: 10.1046/j.1365-2559.2002.01389.x. [DOI] [PubMed] [Google Scholar]
- Barnd DL, et al. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci U S A. 1989;86:7159–63. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhausen I, et al. Mechanisms underlying aberrant glycosylation of MUC1 mucin in breast cancer cells. Eur J Biochem. 1995;233:607–17. doi: 10.1111/j.1432-1033.1995.607_2.x. [DOI] [PubMed] [Google Scholar]
- Burchell JM, et al. O-linked glycosylation in the mammary gland: changes that occur during malignancy. J Mammary Gland Biol Neoplasia. 2001;6:355–64. doi: 10.1023/a:1011331809881. [DOI] [PubMed] [Google Scholar]
- Cao Y, et al. Immunodetection of epithelial mucin (MUC1, MUC3) and mucin-associated glycotopes (TF, Tn, and sialosyl-Tn) in benign and malignant lesions of colonic epithelium: apolar localization corresponds to malignant transformation. Virchows Arch. 1997;431:159–66. doi: 10.1007/s004280050083. [DOI] [PubMed] [Google Scholar]
- Carlos CA, et al. Human tumor antigen MUC1 is chemotactic for immature dendritic cells and elicits maturation but does not promote Th1 type immunity. J Immunol. 2005;175:1628–35. doi: 10.4049/jimmunol.175.3.1628. [DOI] [PubMed] [Google Scholar]
- Cascio S, et al. MUC1 protein expression in tumor cells regulates transcription of proinflammatory cytokines by forming a complex with nuclear factor-kappaB p65 and binding to cytokine promoters: importance of extracellular domain. J Biol Chem. 2011;286:42248–56. doi: 10.1074/jbc.M111.297630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KJ, et al. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol. 2012;29:1817–26. doi: 10.1007/s12032-011-0006-x. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Finn OJ. Epidemiologic perspective on immune-surveillance in cancer. Curr Opin Immunol. 2011;23:265–71. doi: 10.1016/j.coi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer DW, et al. Conditions associated with antibodies against the tumor-associated antigen MUC1 and their relationship to risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1125–31. doi: 10.1158/1055-9965.EPI-05-0035. [DOI] [PubMed] [Google Scholar]
- Cramer DW, et al. Mumps and ovarian cancer: modern interpretation of an historic association. Cancer Causes Control. 2010;21:1193–201. doi: 10.1007/s10552-010-9546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman WH, et al. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011a;71:5601–5. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- Fridman WH, et al. Immunosurveillance in human non-viral cancers. Curr Opin Immunol. 2011b;23:272–8. doi: 10.1016/j.coi.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Galon J, et al. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883–6. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- Goto Y, et al. Human high molecular weight-melanoma-associated antigen: utility for detection of metastatic melanoma in sentinel lymph nodes. Clin Cancer Res. 2008;14:3401–7. doi: 10.1158/1078-0432.CCR-07-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guddo F, et al. MUC1 (episialin) expression in non-small cell lung cancer is independent of EGFR and c-erbB-2 expression and correlates with poor survival in node positive patients. J Clin Pathol. 1998;51:667–71. doi: 10.1136/jcp.51.9.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hiraga Y, et al. Immunoreactive MUC1 expression at the deepest invasive portion correlates with prognosis of colorectal cancer. Oncology. 1998;55:307–19. doi: 10.1159/000011868. [DOI] [PubMed] [Google Scholar]
- Jarrard JA, et al. MUC1 is a novel marker for the type II pneumocyte lineage during lung carcinogenesis. Cancer Res. 1998;58:5582–9. [PubMed] [Google Scholar]
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Kraus S, et al. MUC1 mucin and trefoil factor 1 protein expression in renal cell carcinoma: correlation with prognosis. Hum Pathol. 2002;33:60–7. doi: 10.1053/hupa.2002.29682. [DOI] [PubMed] [Google Scholar]
- Leong AS, Leong TY. Newer developments in immunohistology. J Clin Pathol. 2006;59:1117–26. doi: 10.1136/jcp.2005.031179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong AS, Zhuang Z. The changing role of pathology in breast cancer diagnosis and treatment. Pathobiology. 2011;78:99–114. doi: 10.1159/000292644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy X, et al. MUC1 expression is correlated with nuclear grade and tumor progression in pT1 renal clear cell carcinoma. Am J Clin Pathol. 2002;118:47–51. doi: 10.1309/1F99-BPDY-7DHH-9G97. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Interaction of glycogen synthase kinase 3beta with the DF3/MUC1 carcinoma-associated antigen and beta-catenin. Mol Cell Biol. 1998;18:7216–24. doi: 10.1128/mcb.18.12.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kufe D. The Human DF3/MUC1 carcinoma-associated antigen signals nuclear localization of the catenin p120(ctn) Biochem Biophys Res Commun. 2001;281:440–3. doi: 10.1006/bbrc.2001.4383. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3 beta and beta-catenin. J Biol Chem. 2001a;276:6061–4. doi: 10.1074/jbc.C000754200. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. J Biol Chem. 2001b;276:35239–42. doi: 10.1074/jbc.C100359200. [DOI] [PubMed] [Google Scholar]
- Liu F, et al. CD8(+) cytotoxic T cell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130:645–55. doi: 10.1007/s10549-011-1647-3. [DOI] [PubMed] [Google Scholar]
- Lopez-Ferrer A, et al. Mucins as differentiation markers in bronchial epithelium. Squamous cell carcinoma and adenocarcinoma display similar expression patterns. Am J Respir Cell Mol Biol. 2001;24:22–29. doi: 10.1165/ajrcmb.24.1.4294. [DOI] [PubMed] [Google Scholar]
- Milne K, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlecnik B, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–8. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- Oppenheim JJ, et al. Autoantigens act as tissue-specific chemoattractants. J Leukoc Biol. 2005;77:854–61. doi: 10.1189/jlb.1004623. [DOI] [PubMed] [Google Scholar]
- Reis CA, et al. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999;59:1003–7. [PubMed] [Google Scholar]
- Roxburgh CS, et al. Determinants of short- and long-term outcome in patients undergoing simultaneous resection of colorectal cancer and synchronous colorectal liver metastases. Int J Colorectal Dis. 2012;27:363–9. doi: 10.1007/s00384-011-1339-9. [DOI] [PubMed] [Google Scholar]
- Ruffell B, et al. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2796–801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara M, et al. Expression of mucin 1 (MUC1) in esophageal squamous-cell carcinoma: its relationship with prognosis. Int J Cancer. 1999;84:251–7. doi: 10.1002/(sici)1097-0215(19990621)84:3<251::aid-ijc9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Sangoi AR, et al. Immunohistochemical comparison of MUC1, CA125, and Her2Neu in invasive micropapillary carcinoma of the urinary tract and typical invasive urothelial carcinoma with retraction artifact. Mod Pathol. 2009;22:660–7. doi: 10.1038/modpathol.2009.16. [DOI] [PubMed] [Google Scholar]
- Savage PA, et al. Basic principles of tumor-associated regulatory T cell biology. Trends Immunol. 2012 doi: 10.1016/j.it.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–60. [PubMed] [Google Scholar]
- Schmielau J, et al. Suppressed T-cell receptor zeta chain expression and cytokine production in pancreatic cancer patients. Clin Cancer Res. 2001;7:933s–939s. [PubMed] [Google Scholar]
- Sivridis E, et al. Patterns of episialin/MUC1 expression in endometrial carcinomas and prognostic relevance. Histopathology. 2002;40:92–100. doi: 10.1046/j.1365-2559.2002.01316.x. [DOI] [PubMed] [Google Scholar]
- Vlad AM, et al. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–93. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- Winter JM, et al. A novel survival-based tissue microarray of pancreatic cancer validates MUC1 and mesothelin as biomarkers. PLoS One. 2012;7:e40157. doi: 10.1371/journal.pone.0040157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I, et al. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: evidence of distinct immunological microenvironments that reflect tumor biology. J Neurosurg. 2011;115:505–11. doi: 10.3171/2011.4.JNS101172. [DOI] [PubMed] [Google Scholar]
- Yang I, et al. CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J Clin Neurosci. 2010;17:1381–5. doi: 10.1016/j.jocn.2010.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]