Abstract
Previously, we reported that transformation of tobacco (Nicotiana tabacum L.) with a vector containing a potato cytosolic pyruvate kinase (PKc) cDNA generated two plant lines specifically lacking leaf PKc (PKc−) as a result of co-suppression. PKc deficiency in these primary transformants did not appear to alter plant development, although root growth was not examined. Here we report a striking reduction in root growth of homozygous progeny of both PKc− lines throughout development under moderate (600 μE m−2 s−1) or low (100 μE m−2 s−1) light intensities. When both PKc− lines were cultivated under low light, shoot and flower development were also delayed and leaf indentations were apparent. Leaf PK activity in the transformants was significantly decreased at all time points examined, whereas root activities were unaffected. Polypeptides corresponding to PKc were undetectable on immunoblots of PKc− leaf extracts, except in 6-week-old low-light-grown PKc− plants, in which leaf PKc expression appeared to be greatly reduced. The metabolic implications of the kinetic characteristics of partially purified PKc from wild-type tobacco leaves are discussed. Overall, the results suggest that leaf PKc deficiency leads to a perturbation in source-sink relationships.
PK is a key regulatory enzyme of glycolysis that catalyzes the irreversible synthesis of pyruvate and ATP from PEP and ADP. Substantial evidence indicates that PK is a primary control site of plant glycolytic flux to pyruvate (Plaxton, 1996). Plant PKc and PKp differ in their molecular, immunological, and kinetic characteristics (Plaxton, 1989, 1996; Blakeley et al., 1990, 1991). Tissue-specific isoforms of PKc and PKp having unique physical and/or kinetic and regulatory properties have also been reported (Podestá and Plaxton, 1991, 1994b; McHugh et al., 1995; Hu and Plaxton, 1996; Plaxton, 1996). PKc plays a major role in generating the precursor pyruvate for various biosynthetic pathways and mitochondrial respiration, whereas PKp is thought to be important in supplying pyruvate and ATP for several plastidic biosynthetic pathways.
Tobacco (Nicotiana tabacum L.) plants were transformed with a vector that was designed to examine the impact of overexpressing potato tuber PKc in plastids (Gottlob-McHugh et al., 1992). No transgenic plants were recovered that had increased expression of PKc. However, two primary transformants were unexpectedly produced that specifically lacked PKc in their leaves (PKc−). The reduction in tobacco PKc was likely attributable to the trans-inactivation phenomenon known as co-suppression or posttranscriptional homology-dependent gene silencing (Meyer and Saedler, 1996). Although PKc was immunologically undetectable in leaves, PKc protein levels similar to those of the wild type were observed in nonphotosynthetic tissues, including seeds and roots. Metabolite analyses indicated that the levels of PEP were substantially higher in the PKc− leaves than in wild-type leaves, consistent with a block in glycolysis at the step catalyzed by PK. Nevertheless, the transgenic tobacco plants lacking leaf PKc showed no alteration in shoot growth or leaf rates of photosynthetic O2 evolution and respiratory O2 consumption (Gottlob-McHugh et al., 1992). However, these plants were grown under optimal conditions and root growth was not examined.
In the present study, we report the analysis of homozygous progeny of these PKc− transformants grown under low or moderate light intensities. Our findings show that the absence of leaf PKc results in a striking decrease in root biomass and root:shoot ratios during growth under both light regimes, suggesting that an alteration in source:sink relationships was induced. Additionally, the altered leaf morphology and delayed development of PKc− tobacco grown under low light was noted. Finally, the characteristics of partially purified PKc from wild-type tobacco leaves lend support to other studies (Huppe and Turpin, 1994; Hu and Plaxton, 1996; Plaxton, 1996) suggesting a role for leaf PKc in regulating respiratory C flow for ATP production and in generating the C skeletons required for anabolic processes, including N assimilation.
MATERIALS AND METHODS
Tobacco (Nicotiana tabacum L. cv Petit Havana SR1) plants were raised in growth chambers with 16 h of light (100 or 600 μE m−2 s−1; 25°C)/8 h of dark (20°C) at 70% RH in 8-inch pots containing potting mixture no. 1. Plants were provided with nutrient solution containing 257 μm KH2PO4, 57 μm K2HPO4, 502 μm K2SO4, 243 μm MgSO4, 246 μm MgCl2, 748 μm CaCl2, 10 μm MnSO4, 1 μm CuSO4, 1 μm ZnSO4, 31 μm H3BO3, 0.5 μm Na2MoO4, 0.2 μm CoSO4, 37.6 μm Fe sequestrine, 3.8 μm Fe, and 10 mm KNO3− applied biweekly. Plants were harvested for growth analysis at the beginning of the photoperiod. Roots were separated from the potting mixture by careful washing over a meshed sieve (2-mm mesh). All measurements were made on the most recently fully expanded leaves or primary roots from plants of the indicated ages. Tissue for enzyme and immunoblot analysis was immediately frozen in liquid N2 and stored until use.
Southern-Blot Analysis
Total DNA from tobacco leaf tissue was isolated using a DNA isolation kit (GNOME, BIO 101, Vista, CA). After digestion with EcoRI, DNA (10 μg) was electrophoretically separated in 0.7% (w/v) agarose gels. DNA was transferred to Hybond-N nylon membrane (Amersham) and hybridized to a random-primer-labeled potato PKc-cDNA probe according to protocols supplied by the manufacturer.
Extraction and Measurement of Enzyme Activity
Enzymes were extracted from tobacco leaves and roots as described by Gottlob-McHugh et al. (1992); leaves were homogenized in three volumes and roots in four volumes of extraction buffer. PK activity was assayed spectrophotometrically as described by Plaxton (1989) and was corrected for PEP phosphatase activity by omitting ADP from the assays. Activities of ATP- and PPi-dependent phosphofructokinase and PEP carboxylase were determined as described by Podestá and Plaxton (1994a), whereas PEP phosphatase activity was measured as described by Duff et al. (1989). All enzyme assays were conducted at 30°C. Activity values are the means of triplicate determinations conducted on three to four separate extracts. One unit of enzyme activity is defined as the conversion of 1 μmol of substrate per min. Apparent Km values for PEP and ADP were calculated from the Michaelis-Menten equation fitted to a nonlinear, least-squares regression computer kinetics program (Brooks, 1992). All kinetic parameters are the means of three separate determinations and are reproducible to within ± 10% se.
Protein Determination
Protein concentration was determined by the dye-binding method of Bradford as described by Bollag and Edelstein (1991), with bovine γ-globulin as the standard.
Immunoblotting
SDS-PAGE was performed according to the method of Laemmli (1970) with a minigel apparatus (Bio-Rad) using 0.75-mm slab gels and a 7.5% (w/v) monomer concentration for the separating gel. Immunoblotting was performed using monospecific affinity-purified rabbit anti-castor endosperm PKc-IgG, and antigenic polypeptides were detected using an alkaline-phosphatase-conjugated secondary antibody as described previously (Plaxton, 1989). Immunological specificities were confirmed by immunoblots in which rabbit preimmune serum was substituted for the anti-PKc-IgG.
Partial Purification of Tobacco Leaf PKc
Fully expanded wild-type tobacco leaves (90 g) were homogenized in 200 mL of 100 mm imidazole-HCl, pH 7.1, containing 5 mm MgCl2, 20 mm NaF, 10 mm thiourea, 1 mm EDTA, 1 mm DTT, 1 mm PMSF, 20% (v/v) glycerol, 0.1% (v/v) Triton X-100, and 3% (w/v) insoluble PVP. The filtered extract was centrifuged for 20 min at 46,000g. Butyl-Sepharose, DEAE-Fractogel (VWR Canlab, Mississauga, Ontario, Canada), and ADP-agarose chromatographies were conducted as described by Hu and Plaxton (1996). The final preparation was concentrated to about 2.0 mL using an ultrafilter (YM-30, Amicon, Oakville, Ontario, Canada), divided into 200-μL aliquots, frozen in liquid N2, and stored at −80°C.
RESULTS
Selection of Transgenic Tobacco Plants with Reduced PK Activity
Two transgenic tobacco plant lines (1-121-35 and 7-121-35) that specifically lack PKc in their leaves were obtained after transformation of tobacco with a vector designed to overexpress potato tuber PKc in plastids. The absence of PKc activity in the leaves was related to the co-suppression of the potato PKc transgene and the endogenous tobacco PKc (Gottlob-McHugh et al., 1992).
Plant 1-121-35 initially appeared to have two T-DNA copies integrated at different loci (Gottlob-McHugh et al., 1992). Upon further analysis of more than 50 T1 progeny, this line was found to contain three segregating T-DNAs (results not shown). Analyses of leaf extracts using PK-activity assays and immunoblotting with affinity-purified anti-castor endosperm PKc-IgG revealed that about one-half of the progeny were PKc−, whereas the other half contained wild-type levels of PKc activity and amount (PKc+) (results not shown). Southern-blot analyses of these plants demonstrated that all of the progeny examined contained copies of the potato PKc transgene. The reversion to PKc+ generally appeared to be associated with a reduction in the number of potato PKc transgenes (i.e. reduction from all three transgenes to two or one transgene[s]), but this correlation was imperfect. A few plants with all three transgenes became PKc+ and several with fewer transgenes remained PKc−. The shoot and root growth of one progeny line (14-1) derived from the selfing of line 1-121-35 was examined (see below). All seedlings from this line were kanamycin resistant and all progeny tested were PKc−, suggesting that co-suppression in 14-1 was relatively stable.
The transgenic plant 7-121-35 contained two copies of the potato transgene. Selfing of 7-121-35 generated T1 progeny that segregated 3:1 for kanamycin resistance. Southern-blot analyses of 30 of these progeny demonstrated that 22 contained both copies of the transgene (Fig. 1, lane 1), whereas 8 contained no copies of the transgene (Fig. 1, lane 2), indicating that the two copies of the transgene co-segregate. The same two bands (Fig. 1, pot PK1 and pot PK2) that were detected using the potato cDNA as a probe were also detected using nptII as a probe (results not shown). Of the 22 progeny that contained the transgene, all were found to be PKc−, suggesting that the trans-inactivation event occurring in this line was more stable than in 1-121-35. The plants remained PKc− whether they were homozygous or hemizygous for the transgene. All 8 of the progeny that did not contain the transgene were found to be PKc+. The homozygous PKc− progeny line 15-7 was selected for developmental studies of growth, PK activity, and PKc expression under both low and moderate light intensities (see below). The PKc+ line 18-7, which was generated from the selfing of 7-121-35, was used as a control (control 1). An additional PKc+ control (control 2) was wild-type tobacco (cv Petit Havana SR1).
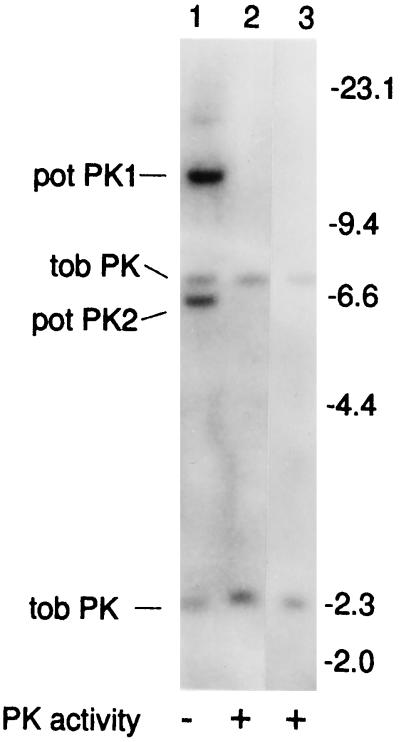
Figure 1.
Southern-blot analysis of T-DNA segregation in progeny of PKc− plants. Each lane contains 10 μg of EcoRI-digested tobacco DNA. The full-length potato cDNA (Blakeley et al., 1990) was used as the probe. Lane 1, Line 15-7 (PKc−); lane 2, line 18-7 (control 1); lane 3, wild type (control 2). The positions of the hybridizing T-DNA fragments (pot PK1 and pot PK2), the cross-hybridizing endogenous tobacco PK fragments (tob PK), and the λ-HindIII size markers (in kb) are indicated.
Growth and Development of Homozygous Progeny of Transgenic Plants
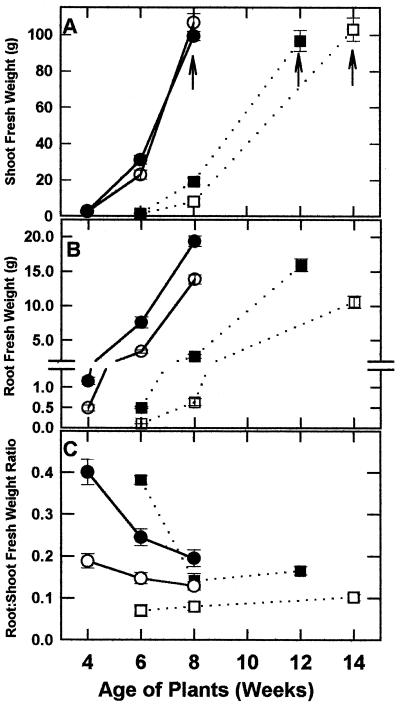
The effect of leaf PKc deficiency on tobacco growth was assessed throughout development under moderate and low light intensities (600 and 100 μE m−2 s−1, respectively). The 4-, 6-, and 8-week-old plants grown under moderate light correspond to the four-leaf, eight-leaf, and mature (onset of flowering) phases, respectively. As previously noted (Gottlob-McHugh et al., 1992), the PKc− plants grown under a moderate light intensity showed no alteration in shoot growth or shoot biomass relative to control plants (Fig. 2A). Both PKc− and PKc+ plants bolted at the same time and the first flower opened at about 8 weeks. However, root growth was significantly impaired in the moderate-light-grown PKc− (15-7) plants compared with PKc+ (control 1) plants at all three developmental stages (Fig. 2B). This resulted in a 32% reduction in the root:shoot ratio of mature plants (Fig. 2C). At maturity, the root:shoot ratios of the second PKc− line (14-1) were similarly reduced by 27% after growth under moderate light (n = 6).
Figure 2.
Growth curves of PKc− (line 15-7) and PKc+ (control 1; line 18-7) plants cultivated under moderate and low light intensities. A, Developmental profiles for shoot fresh weight; the arrows indicate the age at which maturation (i.e. onset of flowering) occurred. B, Developmental profiles for root fresh weight. C, Developmental profiles for root:shoot fresh weight ratios. •, ▪, PKc+ plants; ○, □, PKc− plants; •, ○, moderate light; ▪, □, low light. All values represent the means ± se for 8 to 16 independent determinations.
Under low light, both root biomass and root:shoot ratios were again significantly decreased in the PKc− plants at all time points (Figs. 2, B and C, and 3A). In addition, however, differences were seen in the shoot growth and morphology of the PKc− plants relative to the PKc+ controls (Figs. 2A and 3). PKc− shoots were smaller in 8-week-old plants, but after bolting were equivalent to those of the control plants at maturity (Figs. 2A and 3B). The reduced shoot biomass of the 8-week-old PKc− plants was apparently caused by a developmental impediment, because flowering was delayed by approximately 2 weeks (Figs. 2A and 3C). Although maturation of the PKc− low-light-grown plants was impeded, there were no observable differences in their flower morphology or ability to set seed. No differences in growth were observed between control 1 and control 2 or between the two PKc− lines (Fig. 3). Seed fresh weights, seed viability, and germination rates of the PKc− and PKc+ plants were identical (results not shown). However, leaf morphology was altered such that pronounced indentations were apparent in fully expanded leaves of the mature PKc− plants grown under low light (Fig. 3D).
Figure 3.
Impact of the absence of leaf PKc on the growth and development of tobacco cultivated under a low light intensity. Two PKc− lines, 15-7 and 14-1, are shown on the right, and the two PKc+ lines, 18-7 (control 1) and wild type (control 2), are shown on the left in A through C. A and B, Reduced root and shoot growth, respectively, of 6-week-old PKc− plants. C, Delayed flowering of 12-week-old PKc− plants; D, Altered leaf morphology of flowering (14-week-old) PKc− (15-7) plants.
Enzyme Activities and Immunoblotting
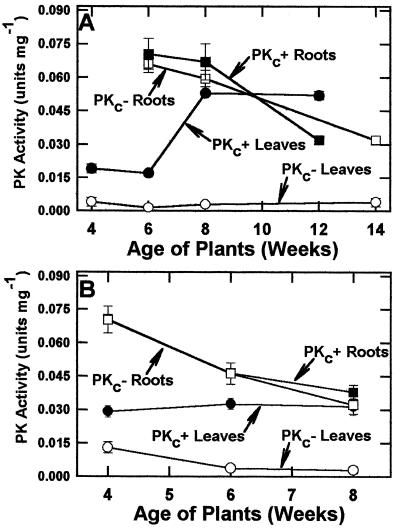
Developmental profiles for PK activity in leaves and roots of PKc+ (control 1) and PKc− (15-7) tobacco plants grown under both light regimes are shown in Figure 4. PK specific activity in the most recently fully expanded leaves of control plants grown under low light increased from 0.019 ± 0.003 units/mg at 4 weeks to 0.052 ± 0.004 units/mg at 12 weeks (Fig. 4A). Root PK activity, however, decreased with age. The PKc− plants were similar to controls with respect to PK activity in roots but showed significantly reduced PK activity in fully expanded leaves at all developmental stages examined.
Figure 4.
Developmental profiles for PK activity in leaves and roots of PKc− (line 15-7) and PKc+ (control 1; line 18-7) plants grown under low (A; 100 μE m−2 s−1) or moderate (B; 600 μE m−2 s−1) light intensities. All values represent the means ± se of triplicate determinations conducted with four separate extracts.
PK specific activities in fully expanded leaves of control 1 and PKc− (15-7) plants grown for 4 weeks under a moderate light intensity were 0.029 ± 0.003 and 0.014 ± 0.002 units/mg, respectively (Fig. 4B). Whereas leaf PK activities of moderate-light control 1 plants were relatively constant over the time course examined, the PK activity of PKc− plants decreased with age. At maturity, the activities measured in extracts prepared from leaves of control 1 and PKc− plants were lower than those of the respective flowering plants grown under low light (Fig. 4). PK activities in roots of the plants grown under moderate light were in the same range and followed a similar pattern to those grown under low light.
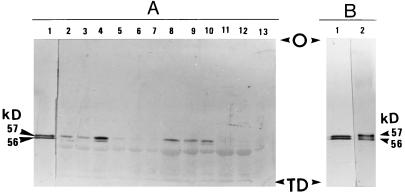
Immunoblotting using anti-castor endosperm PKc-IgG was employed to assess the relative abundance of PKc in extracts prepared from expanded leaves of PKc+ (control 1) and PKc− (15-7) plants cultivated under low and moderate light intensities. Two to three separate leaf extracts of the PKc− and PKc+ plants at each developmental stage were analyzed and representative results are shown in Figure 5A. With the exception of 6-week-old low-light-grown plants, the PKc− leaves appeared to lack PKc protein throughout development under both low and moderate light (Fig. 5A, lanes 5–8 and 11–13). The faint 57-kD immunoreactive band observed on the immunoblot of an extract from leaves of 6-week-old, low-light-grown PKc− plants (Fig. 5A, lane 5) was far less intense relative to that obtained with the respective PKc+ (control 1) extract (Fig. 5A, lane 2), and is consistent with the respective PK activities (Fig. 4A). In control 1 low-light-grown leaves, immunoreactive staining corresponding to PKc was less intense at 6 or 8 weeks than at maturity (Fig. 5A, lanes 2–4), consistent with the lower PK activities observed at the earlier developmental stages (Fig. 4). As with PK activity (Fig. 4B), the relative amount of immunoreactive PKc in leaf extracts derived from moderate-light-grown control 1 plants was relatively constant over the time course examined (Fig. 5A, lanes 8–10).
Figure 5.
Immunological detection of castor and tobacco leaf PKc. Extracts were electrophoresed on 7.5% (w/v) SDS-polyacrylamide minigels and transferred to a PVDF membrane. Immunoblotting was performed using affinity-purified anti-castor endosperm PKc-IgG (Plaxton, 1989). A, Immunoblot analysis of extracts prepared from leaves of tobacco plants grown under low (lanes 2–7) and moderate (lanes 8–13) light intensities. Lane 1 contains 30 ng of homogeneous castor leaf PKc (Hu and Plaxton, 1996), whereas all other lanes contain 15 μg of protein from a tobacco leaf extract. Lanes 2, 3, and 4 represent 6-, 8-, and 12-week (mature) control 1 (line 18-7) leaf extracts, respectively; lanes 5, 6, and 7 represent 6-, 8-, and 14-week (mature) PKc− (line 15-7) leaf extracts, respectively; lanes 8, 9, and 10 represent 4-, 6-, and 8-week (mature) control 1 (line 18-7) leaf extracts, respectively; and lanes 11, 12, and 13 represent 4-, 6-, and 8-week (mature) PKc− (line 15-7) leaf extracts, respectively. B, Immunoblot of 30 ng of homogeneous castor leaf PKc (lane 1) (Hu and Plaxton, 1996) and 300 ng of partially purified PKc from wild-type tobacco leaves (lane 2). O, Origin; TD, tracking dye front.
At maturity, extractable activities of PEP carboxylase, PEP phosphatase, and the PPi- or ATP-dependent phosphofructokinases were not significantly different in leaves or roots of control 1 versus PKc− (15-7) plants grown under low light (not shown). There were no significant differences in soluble protein concentration between PKc− and PKc+ plants in either roots or leaves of plants grown under low or moderate light.
Characterization of Partially Purified PKc from Wild-Type Tobacco Leaves
Approximately 8 units of PKc having a final specific activity of 2.1 units/mg were isolated from wild-type tobacco leaves (overall yield, 10%; purification, 75-fold). The final preparation was devoid of PEP-phosphatase activity. The partially purified enzyme exhibited a broad pH-activity optimum of approximately 6.5 and a native molecular mass of about 220 kD, as estimated by gel filtration on a calibrated Superose 6 fast-protein liquid chromatography column. Immunoblotting using anti-castor endosperm PKc-IgG indicated that as in castor leaf PKc (Fig. 5B, lane 1), the tobacco leaf enzyme is composed of equal proportions of subunits having molecular masses of 57 and 56 kD (Fig. 5B, lane 2). Although at pH 7.6 the enzyme exhibited 70% of the Vmax at pH 6.5, its affinity for PEP and ADP was higher at pH 7.6 than at pH 6.5. Apparent Km values for PEP and Mg-ADP were 0.071 and 0.041 mm at pH 7.6, and 0.118 and 0.098 mm at pH 6.5, respectively. Mg-ATP, magnesium citrate, malate, 2-oxoglutarate, glutamate, and Pi inhibited the enzyme by about 40 to 60% when assayed at pH 6.5 or 7.6 with subsaturating concentrations of PEP and ADP (0.15 and 0.1 mm, respectively).
DISCUSSION
Growth and Development of Transgenic Tobacco Lacking Leaf PKc
Our results are consistent with the previous observations (Gottlob-McHugh et al., 1992) that the transgenic tobacco plants resulting from transformation with sense constructs of the potato tuber PKc cDNA and placed under the control of the cauliflower mosaic virus 35S promoter had a specific elimination of PKc in their leaves. There was no significant difference in shoot or flower development of the homozygous progeny of the primary transformants lacking leaf PKc during growth under moderate light (Fig. 2A). This result agrees with those previously obtained with the primary transformants (Gottlob-McHugh et al., 1992). Of interest, however, was the significant reduction in root growth, and thus root:shoot ratios, that occurred when the PKc− plants were cultivated under moderate or low light (Figs. 2, B and C, and 3A).
Furthermore, shoot growth of the PKc− plants was significantly reduced by 8 weeks of growth under low light (Figs. 2A and 3B). This effect appears to be attributable to a developmental delay, because the shoot fresh weights of PKc− and control low-light-grown plants were equivalent when the two groups matured (i.e. when they began to flower) at about 12 and 14 weeks, respectively (Figs. 2A and 3C). The reason for the pronounced indentations in leaves of the PKc− plants grown under low light (Fig. 3D) is unknown. However, the normal seed development and germination of the PKc− plants illustrates that the absence of leaf PKc was not lethal under the conditions used.
Enzyme Activities and Protein Expression
At 4 and 6 weeks of growth under a low light intensity, PK activity in leaves of homozygous PKc− progeny of the primary transformants was reduced by up to 75%, but was much less than the differences measured at maturity (Fig. 4A). Immunoblot analyses confirmed that with the exception of 6-week-old, low-light-grown plants (in which the amount of leaf PKc appeared to be greatly reduced), PKc was undetectable in leaf extracts of the PKc− plants (Fig. 5A). Taken together, the data of Figures 4A and 5A also indicate that the expression of leaf PKc in PKc+ plants is relatively lower during earlier phases of growth under low light than at the onset of flowering. In 4-week-old, moderate-light-grown plants, the difference between PK activities in PKc− and control leaves was only about 2-fold, whereas this difference was much greater (10-fold) in leaves of mature, 8-week-old plants (Fig. 4B).
The residual PK activities in PKc− leaves lacking PKc protein has been demonstrated to arise from the presence of chloroplastic PK (Gottlob-McHugh et al., 1992). The loss of PKc, an important site of cytosolic substrate-level phosphorylation of ADP to ATP, could be expected to be more deleterious during growth under the very low light irradiance of 100 μE m−2 s−1, when photosynthetic ATP production is severely compromised (photosynthesis in tobacco saturates at greater than 1200 μE m−2 s−1). This provides a rationale for the delayed development of the PKc− plants grown under low, but not moderate (i.e. 600 μE m−2 s−1), light intensities (Figs. 2A and 3B).
In contrast to leaves, the control and PKc− tobacco lines yielded similar developmental profiles for PK activity in roots (Fig. 4). This finding is consistent with the previous observation that the primary PKc− transformants and wild-type plants contained an equivalent amount of immunologically detectable PKc in their roots (Gottlob-McHugh et al., 1992). Therefore, the co-suppression of PKc appears to be specifically confined to leaf tissue. Enzymological studies have indicated that, similar to animal systems, higher plant PKc exists as a tissue-specific isozyme exhibiting substantial differences in several kinetic/regulatory properties that reflect the distinctive metabolic requirements of leaf versus seed tissues (Podestá and Plaxton 1991, 1994b; Hu and Plaxton, 1996; Plaxton, 1996). Further work is needed to assess the mechanism(s) whereby transformation of tobacco with a cDNA encoding potato tuber PKc results in the co-suppression or gene silencing of the putative leaf-specific isozyme of PKc.
Tobacco Leaf PKc Is Feedback Inhibited by End Products of Respiration and N Assimilation
To further assess the potential function and regulation of tobacco leaf PKc, the enzyme was partially purified from wild-type leaves. The marked inhibition of tobacco leaf PKc by Mg-ATP, citric-acid-cycle intermediates, and glutamate is consistent with the suggestion (Hu and Plaxton, 1996; Plaxton, 1996) that PKc plays a key role in C3 leaves in the regulation of the glycolytic flux for both mitochondrial respiration and the provision of C skeletons for anabolism. Any reduction in the levels of citric-acid-cycle intermediates by enhanced respiration and/or anabolic processes should relieve feedback inhibition of PKc and, consequently, stimulate the overall flux of cytosolic glycolysis to pyruvate. Because glutamate is the primary product of N assimilation by Gln synthetase/Gln 2-oxoglutarate aminotransferase, the inhibition of tobacco leaf PKc by glutamate should exert considerable feedback control on this enzyme. The elimination of PKc could therefore be expected to alter the leaf's ability to regulate glycolysis for respiration and to produce the C skeletons required for anabolic processes such as N assimilation.
Plants Contain Metabolic Bypasses to PKc
The findings of the present and previous (Gottlob-McHugh et al., 1992) studies are compatible with the proposals (Black et al., 1987; Theodorou and Plaxton, 1995; Plaxton, 1996) that, because of their use of alternative enzymes and pathways, plants are able to maintain C flow through cytosolic glycolysis even in the absence of an “essential” enzyme such as PKc. Pathways unique to plants that can potentially circumvent the reaction catalyzed by PKc include PKp, PEP phosphatase, or the combined activities of PEP carboxylase, malate dehydrogenase, and NAD-malic enzyme (Duff et al., 1989; Plaxton, 1996). PEP phosphatase and PEP carboxylase have been suggested to be important PKc bypasses during periods of nutritional Pi starvation, when PKc activity may be curtailed owing to ADP limitation (Theodorou and Plaxton, 1995; Plaxton, 1996). There was no significant up-regulation in the leaf or root activities of the putative PKc bypass enzymes PEP phosphatase and PEP carboxylase (or other key regulatory enzymes of cytosolic glycolysis such as ATP- and PPi-dependent phosphofructokinase) to compensate for the elimination of leaf PKc. Therefore, fine regulation of preexisting enzymes may allow the PKc− leaves to compensate to some degree for the physical elimination of PKc. Further work is needed to determine if this is the case, and if so, whether it is possibly achieved by the constitutive posttranslational modification of leaf PEP carboxylase into its phosphorylated, more active state (Chollet et al., 1996).
CONCLUDING REMARKS
To the best of our knowledge the present study is the first to show that specific “knockout” of an enzyme in one tissue (i.e., leaves) exerts a maximal detrimental effect on the development of another tissue (i.e. roots), in which the enzyme appears to be expressed normally. Preliminary in planta, pulse-chase 14CO2 radiolabeling studies have indicated that the stunted root growth of the low-light-grown transgenic tobacco plants deficient in leaf PKc may be caused by a 40% reduction in the nighttime export of recently assimilated 14CO2 from fully expanded source leaves (Jiao et al., 1997). This agrees with the fact that root growth is absolutely dependent on imported photosynthate, but not on imported amino acids, because inorganic N can be assimilated locally (Geigenberger et al., 1996). The reduced nighttime export of recently assimilated 14CO2 has been correlated with a doubling in the nighttime loss of respiratory 14CO2 by the PKc− leaves (Jiao et al., 1997). Presumably, the PKc-bypass enzyme that is being used by the PKc− leaves to compensate for the absence of PKc cannot regulate glycolytic flux in support of dark respiration to the same extent as PKc.
ACKNOWLEDGMENTS
We thank Drs. Stefan Falk, Bernie Grodzinski, and David Layzell for helpful discussions and Ms. Hélène Labbé for her technical help in producing and maintaining transgenic plants.
Abbreviations:
- PK
pyruvate kinase
- PKc and PKp
cytosolic and plastidic pyruvate kinase isozymes, respectively
- PKc− and PKc+
tobacco plants that are and are not deficient in leaf PKc, respectively
- T-DNA
transfer DNA
Footnotes
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada. This is Eastern Cereal and Oilseed Research Centre contribution no. 971,184.
LITERATURE CITED
- Black CC, Mustardy L, Sung SS, Kormanik PP, Xu D-P, Paz N. Regulation and roles for alternative pathways of hexose metabolism in plants. Physiol Plant. 1987;69:387–394. [Google Scholar]
- Blakeley SD, Plaxton WC, Dennis DT. Cloning and characterisation of a cDNA for the cytosolic isozyme of plant pyruvate kinase: the relationship between the plant and nonplant enzyme. Plant Mol Biol. 1990;15:665–669. doi: 10.1007/BF00017842. [DOI] [PubMed] [Google Scholar]
- Blakeley SD, Plaxton WC, Dennis DT. Relationship between the subunits of leucoplast pyruvate from Ricinus communis and a comparison with the enzyme from other sources. Plant Physiol. 1991;96:1283–1288. doi: 10.1104/pp.96.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag DM, Edelstein SJ. Protein concentration determination. In: Bollag DM, Edelstein SJ, editors. Protein Methods. New York: Wiley-Liss; 1991. pp. 50–55. [Google Scholar]
- Brooks SPJ. A simple computer program with statistical tests for the analysis of enzyme kinetics. BioTechniques. 1992;13:906–911. [PubMed] [Google Scholar]
- Chollet R, Vidal J, O'Leary MH. Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:273–297. doi: 10.1146/annurev.arplant.47.1.273. [DOI] [PubMed] [Google Scholar]
- Duff SMG, Lefebvre DD, Plaxton WC. Purification and characterization of a phosphoenolpyruvate phosphatase from Brassica nigra suspension cell. Plant Physiol. 1989;90:734–741. doi: 10.1104/pp.90.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Lerchl J, Stitt M, Sonnewald U. Phloem-specific expression of pyrophosphatase inhibits long-distance transport of carbohydrates and amino acids in tobacco plants. Plant Cell Environ. 1996;19:43–55. [Google Scholar]
- Gottlob-McHugh SG, Sangwan RS, Blakeley SD, Vanlerberghe GC, Ko K, Turpin DH, Plaxton WC, Miki BL, Dennis DT. Normal growth of transgenic tobacco plants in the absence of cytosolic pyruvate kinase. Plant Physiol. 1992;100:820–825. doi: 10.1104/pp.100.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Plaxton WC. Purification and characterization of cytosolic pyruvate kinase from leaves of the castor oil plant. Arch Biochem Biophys. 1996;333:298–307. doi: 10.1006/abbi.1996.0394. [DOI] [PubMed] [Google Scholar]
- Huppe HC, Turpin DH. Integration of carbon and nitrogen metabolism in plant and algal cells. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:577–607. [Google Scholar]
- Jiao J, Knowles VL, Plaxton WC, Grodzinski B. Leaf photosynthesis, export and C-partitioning in transgenic tobacco plants lacking leaf cytosolic pyruvate kinase and grown in greenhouse and growth chambers (abstract no. 315) Plant Physiol. 1997;114:S-81. [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed]
- McHugh SG, Knowles VL, Blakeley SD, Sangwan RS, Miki BL, Dennis DT, Plaxton WC. Differential expression of cytosolic and plastid pyruvate kinase isozymes in tobacco. Physiol Plant. 1995;95:507–514. [Google Scholar]
- Meyer P, Saedler H. Homology-dependent gene silencing in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:23–48. doi: 10.1146/annurev.arplant.47.1.23. [DOI] [PubMed] [Google Scholar]
- Plaxton WC. Molecular and immunological characterization of plastid and cytosolic pyruvate kinase isozymes from castor-oil-plant endosperm and leaf. Eur J Biochem. 1989;181:443–451. doi: 10.1111/j.1432-1033.1989.tb14745.x. [DOI] [PubMed] [Google Scholar]
- Plaxton WC. The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:185–214. doi: 10.1146/annurev.arplant.47.1.185. [DOI] [PubMed] [Google Scholar]
- Podestá FE, Plaxton WC. Kinetic and regulatory properties of cytosolic pyruvate kinase from germinating castor oil seeds. Biochem J. 1991;279:495–501. doi: 10.1042/bj2790495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podestá FE, Plaxton WC. Regulation of carbon metabolism in germinating Ricinus communis cotyledons. I. Developmental profiles for the activity, concentration, and molecular structure of the pyrophosphate- and ATP-dependent phosphofructokinases, phosphoenolpyruvate carboxylase, and pyruvate kinase. Planta. 1994a;194:374–380. [Google Scholar]
- Podestá FE, Plaxton WC. Regulation of carbon metabolism in germinating Ricinus communis cotyledons. II. Properties of phosphoenolpyruvate carboxylase and cytosolic pyruvate kinase associated with the regulation of glycolysis and nitrogen assimilation. Planta. 1994b;194:381–387. [Google Scholar]
- Theodorou ME, Plaxton WC (1995) Adaptations of plant respiratory metabolism to nutritional phosphate deprivation. In N Smirnoff, ed, Environment and Metabolism: Flexibility and Acclimation. BIOS Scientific, London, pp 79–109