SUMMARY
RCK domains control activity of a variety of K+ channels and transporters through binding of cytoplasmic ligands. To gain insight toward mechanisms of RCK domain activation, we solved the structure of the RCK domain from the Ca2+-gated K+ channel, MthK, bound with Ba2+, at 3.1 Å resolution. The Ba2+-bound RCK domain was assembled as an octameric gating ring, as observed in structures of the full-length MthK channel, and shows Ba2+ bound at several positions. One of the Ba2+ sites, termed C1, overlaps with a known Ca2+-activation site, determined by residues D184 and E210. Functionally, Ba2+ can activate reconstituted MthK channels as observed in electrophysiological recordings, whereas Mg2+ (up to 100 mM) was ineffective. Ba2+ activation was abolished by the mutation D184N, suggesting that Ba2+ activates primarily through the C1 site. Our results suggest a working hypothesis for a sequence of ligand-dependent conformational changes that may underlie RCK domain activation and channel gating.
Keywords: K channel, barium, lipid bilayer, allosteric
INTRODUCTION
Regulator of K+ conductance (RCK) domains are structurally-conserved ligand-binding domains that control the activity of a diverse array of K+ channels and transporters, including large-conductance Ca2+-activated K+ channels (BK channels), in response to binding of cytoplasmic ligands, such as nucleotides, Na+, Ca2+, Mg2+, and H+ (Bellamacina, 1996; Butler et al., 1993; Golowasch et al., 1986; Jiang et al., 2002a; Jiang et al., 2001; Kroning et al., 2007; Kuo et al., 2005; Parfenova et al., 2007; Salkoff et al., 2006; Schreiber et al., 1998; Xia et al., 2004; Yuan et al., 2003; Zhang et al., 2006a). Consequently, RCK-containing channels and transporters couple binding of ligands to regulation of membrane potential or K+ uptake. For example, the bacterial KtrAB complex couples nucleotide binding with K+ uptake, whereas BK channels link binding of Ca2+ and Mg2+ to K+ efflux to control smooth muscle contractilty and neuronal activity (Brenner et al., 2005; Brenner et al., 2000; Girouard et al., 2010; Imlach et al., 2008; Meredith et al., 2006).
MthK is a prototypical RCK-containing K+ channel whose crystal structure has provided insight toward mechanisms of channel gating by RCK domains (Hou et al., 2008; Jiang et al., 2002a, b; Niu et al., 2004; Pau et al., 2010; Pau et al., 2011; Ye et al., 2006). In MthK, binding of Ca2+ to an octameric ring of RCK domains (the “gating ring”), which is tethered to the pore of the channel, leads to a series of conformational changes that facilitates channel opening and K+ conduction (Figure 1A) (Jiang et al., 2002a; Li et al., 2007; Parfenova et al., 2006; Pau et al., 2010; Zadek and Nimigean, 2006). The Ca2+-activation mechanism of MthK involves three different Ca2+ binding sites per RCK domain, for a total of 24 Ca2+ sites per channel (Pau et al., 2011); thus a complete quantitative description of the gating mechanism could be complex. To dissect the mechanism and quantify energetic relationships among modulatory binding sites and the gate of the channel, it is useful to have access to experimental tools that permit channel activation through specific sites. In addition, it will be of value to understand the structural basis of ion coordination and selectivity at specific binding sites, as this information may provide insight toward ion coordination mechanisms in other proteins.
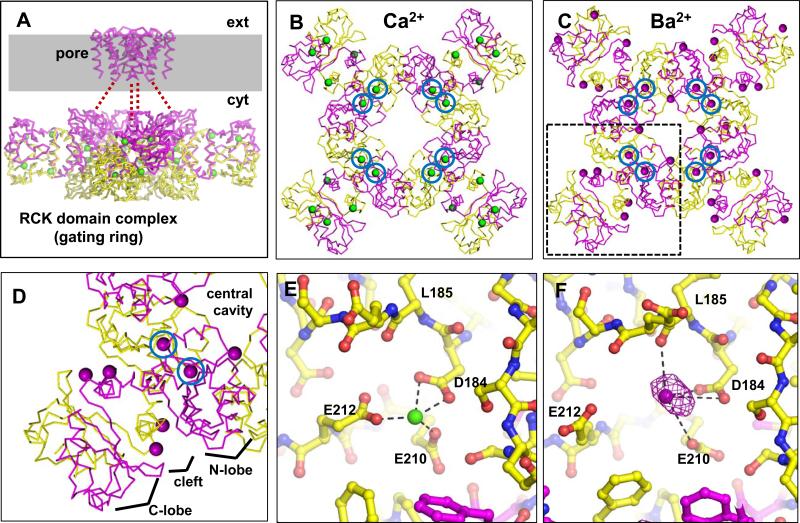
Figure 1. Comparison of Ca2+- and Ba2+-bound MthK gating rings.
A, C-α trace of the biological structure of the MthK channel (PDB accession no. 3RBZ) . Presumed boundary of the plasma membrane is shaded gray, with extracellular and cytoplasmic sides of the membrane indicated by ext and cyt, respectively. Subunits that form the pore region and their associated RCK domains are colored magenta (unresolved linker residues are represented by red dashes); associated RCK domains are colored yellow. Ca2+ ions are shown as green spheres. B, C, Side-by-side comparison of the Ca2+-bound (B) and Ba2+-bound gating ring (C; PDB accession number 4EI2), viewed from above; in B, the transmembrane domains have been removed for clarity. In C, Ba2+ ions are shown as purple spheres. Ions at the C1 sites are circled. D, Magnified view of the boxed region in C, showing a dimer of RCK domains with associated Ba2+ ions. Each dimer consists of an N-lobe, a cleft, and a C-lobe, radiating from the central cavity of the gating ring. Blue circled regions show the location of the C1 site. E, Ca2+ coordination at the C1 site. F, Structure of the Ba2+-bound C1 site. Structure is overlayed with NCS-averaged anomalous difference map contoured at 20σ (purple mesh). Ba2+-O distances, indicated by dashed lines, are: D184(Oδ1), 2.6 Å; D184(Oδ2), 4.0 Å; E210(Oε1), 3.8 Å; L185(O), 2.8 Å. See also Figure S1.
In this work, we use Ba2+ to pan for native metal binding sites in the MthK RCK domain. Use of Ba2+ as a probe in X-ray crystallographic analysis has several advantages; Ba2+ is electron-dense, and thus in general can be distinguished from other protein atoms based on its strong electron density peak (Jiang and MacKinnon, 2000). Also, Ba2+ can yield anomalous scattering that is detectable with moderate resolution diffraction data, further enabling its unambiguous distinction from other cations, water or protein atoms.
A specific advantage in using Ba2+ to probe MthK RCK domain structure and function is that MthK contains native Ca2+ binding sites. Ba2+, although slightly larger than Ca2+ in ionic radius, can sometimes act as a functional surrogate for Ca2+, for example as a charge-carrying permeant ion in the analysis of Ca2+ channels, as well as activation of BK channels (DiPolo et al., 1983; Hagiwara and Ohmori, 1982; Hille, 2001; Lee and Tsien, 1983; Randall and Tsien, 1995; Todorovic and Lingle, 1998; Zhou et al., 2012). On the other hand, Ba2+ cannot effectively replace Ca2+ in triggering neurotransmitter release at the frog neuromuscular junction (Zengel and Magleby, 1977, 1980). Thus some Ca2+ binding sites may bind Ba2+, whereas other may exclude it.
Our goal in the present studies was to gain further insight toward properties of binding sites that may contribute to Ba2+/Ca2+ selectivity, and also to exploit potential selectivity to gain further insight toward channel activation through ligand binding to specific sites. Our results suggest that Ba2+ can distinguish among activation sites in the MthK RCK domain, and selectively binds to a single activation site in each RCK domain, facilitating opening of the MthK channel. Comparison with previous MthK gating ring structures suggests that the Ba2+-bound gating ring exists in an intermediate-activated conformation, and binding of additional ligands is required for maximal stability of the fully-activated conformation in the channel gating mechanism.
Results
Ba2+-coordination in the MthK gating ring
The MthK channel is formed by a pore domain that yields a pathway for conduction of K+ through the plasma membrane, and a tethered RCK domain complex (the “gating ring”; Figure 1A) (Jiang et al., 2002a; Pau et al., 2011). Biologically, the gating ring is comprised of eight RCK domains, four of which are directly tethered to the pore via a 19-residue linker (represented by dashed lines in Figure 1A), and four of which are expressed as soluble domains that are co-assembled with the tethered domains (Jiang et al., 2002a). Here we focus on the structure of the gating ring to determine conformations that may underlie activation of the channel by divalent cations, to gate transmembrane K+ conduction.
Using Ba2+ as a probe for divalent binding sites in the MthK RCK domain, we crystallized the MthK RCK domain protein in the presence of 100 mM barium chloride and obtained crystals that diffracted X-rays to 3.1 Å, in space group P212121 (Table 1). The structure was solved by molecular replacement and refined to an Rwork/Rfree of 0.209/0.243. Two essentially identical gating rings (16 RCK domains) are present per asymmetric unit (Figures 1 and S1), which results in 16-fold non-crystallographic symmetry (NCS). The final refined electron density maps were of excellent quality owing in part to 16-fold NCS-averaging, and allowed building of the entire RCK domain for all 16 chains in the asymmetric unit. Ba2+ ions bound to the RCK gating ring were identified in the structure based on a combination of strong electron density peaks, coinciding peaks in the anomalous difference density map, and favorable local chemistry for Ba2+ coordination at the site (i.e. close contact with oxygen atoms, and lack of close contacts with basic or hydrophobic sidechains). Based on these data, we identified positions of several Ba2+ ions in each gating ring (Figure 1C), which we categorized into several classes based on their locations in each RCK domain.
Table 1.
Data collection and refinement statistics
| Data collection | |
| Space group | P212121 |
| Cell dimensions | |
| a, b, c (Å) | 110.0, 136.4, 498.4 |
| α, β, γ (°) | 90, 90, 90 |
| Resolution (Å) | 30-3.1 (3.22-3.1) |
| No. total / unique reflections | 3,132,635 / 131,081 |
| R sym | 0.118 (0.556) |
| I / σI | 24.4 (4.2) |
| Completeness (%) | 97.5 (96.4) |
| Redundancy | 4.9 (4.4) |
| Refinement | |
| Resolution (Å) | 30-3.1 |
| No. unique reflections (non-anomalous) | 131,021 |
| Rwork / Rfree$ | 0.209 / 0.243 |
| No. atoms | |
| Protein | 27,716 |
| Ligand/ion | 57 |
| Water | 32 |
| B-factors | |
| Protein | 141.2 |
| Ligand/ion | 257.4 |
| Water | 101.8 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.006 |
| Bond angles (°) | 0.996 |
*Values in parentheses are for highest-resolution shell.
Rfree was calculated using 3,758 reflections selected in thin resolution shells
Most notably, each RCK domain contained a Ba2+ ion coordinated by sidechain oxygens from residues D184 and E210 and the main-chain carbonyl oxygen of residue L185, at a site that coincides in part with a Ca2+ binding site identified in previous Ca2+-bound structures of the MthK RCK domain, here termed the “C1” site (Dong et al., 2005; Jiang et al., 2002a). Comparison of ion coordination in the Ca2+-bound and Ba2+-bound RCK domain structures illustrates differences between Ca2+ and Ba2+ coordination at C1, principally the shorter metal-oxygen distances for Ca2+ (ranging from 2.4 – 2.7 Å) vs. Ba2+ (ranging from 2.6 – 4.4 Å) (Figure 1E and F). Mean Ba2+-O distances for residues D184, L185, and E210 were: D184(Oδ1), 2.7 Å; D184(Oδ2), 4.0 Å; E210(Oε1), 3.6 Å; and L185(O), 2.9 Å, which is within the range of Ba2+-O distances observed in structures of other proteins at comparable resolution (Chaptal et al., 2011; Inanobe et al., 2011). Unlike Ca2+ coordination observed in previous MthK structures, the E212 sidechain does not appear to participate directly in Ba2+ coordination in this gating ring structure; the mean distance between Ba2+ and the nearest sidechain oxygen from E212 was 4.5 Å (Figure 1E, F).
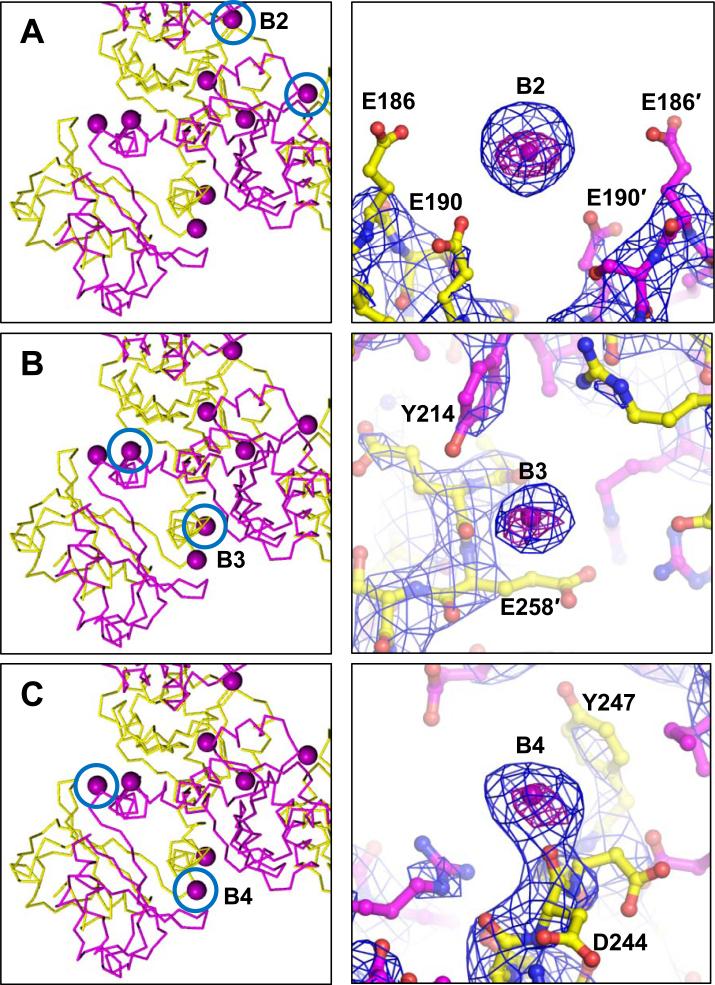
Aside from C1, Ba2+ ions were located at several other sites on the gating ring, though these other Ba2+ ions interacted directly with fewer protein oxygens than observed in C1 based on Ba2+-O distances at these sites (Figure 2). These sites were termed B2 (or the “cavity site”), formed by an electronegative pocket of E190 residues from adjacent subunits; B3, formed by the sidechain hydroxyl group of Y214 and the main chain carbonyl from E258, also in adjacent subunits; and B4, formed in part by the main chain carbonyl from D244. These sites were characterized by strong electron density, anomalous peaks, and favorable local chemistry, though they did not exhibit multiple close protein contacts as observed at the C1 site. For site B2, the mean distance to E190(Oε2) was 4.6 Å; for B3, mean distances to Y214(OH) and E258(O) were 4.6 and 4.5 Å, respectively; for B4, the mean distance to the only apparent close protein contact (D244(O)) was 3.2 Å. Although multiple close protein contacts (<4 Å) were not observed at these sites, each site was characterized by a relatively high density of oxygen-containing sidechains (Figure 2), which likely provides a favorable electrostatic environment for cation-binding.
Figure 2. Ba2+ sites in the MthK gating ring cavity and cleft region.
Locations of Ba2+ sites in the RCK domain are indicated by blue circles (left panels); representative sites are shown as ball-and-stick (right panels), overlayed with NCS-averaged 2Fo-Fc maps (blue mesh) and anomalous difference maps (purple mesh). A, Ba2+ site in the gating ring cavity determined by E190 residues from adjacent subunits (indicated by ′) Ba2+-O distances in Å: E190(Oε2), 4.9; E190′(Oε), 4.9. B, Ba2+ site determined by the Y214 sidechain and E258 carbonyl from adjacent subunits. Ba2+-O distances in Å: Y214(OH), 4.6; E258′(O), 4.5. C, Ba2+ site determined by the main-chain carbonyl of D244. Ba2+-O distance to D244(O), 2.6 Å. NCS-averaged 2Fo-Fc maps are contoured at 5σ; NCS-averaged anomalous difference maps are contoured at 20σ (A, C) or 14σ (B). See also Figure S2.
Additional weaker Ba2+ sites were modeled in the vicinities of residues D202, D287, and D284 (Figure S2). Because strong anomalous peaks were not observed at these sites in all RCK subunits, identification of these sites was mainly based on strong electron density peaks that could not be adequately modeled as water molecules or Na+ ions. At one of these sites (formed by D284/D287), Ba2+ ions may provide a countercharge to stabilize lattice contacts between gating rings (Figures S1, S2C). No density corresponding to Ba2+ was observed in the other previously-identified MthK Ca2+-activation sites, formed between residue E248 and E266, and between residues D305 and E326 (Pau et al., 2011).
Thus, the most well-defined Ba2+ site in the RCK domain was observed at site C1, which overlaps with a key Ca2+ activation site in the MthK channel (Jiang et al., 2002a; Pau et al., 2011).
Conformation of the Ba2+-bound gating ring and its relation to MthK channel activation
Comparison of MthK gating ring structures shows that the Ba2+-bound gating ring is in a different overall conformation from the Ca2+-bound gating ring (Figure 1B, C). Because the Ca2+-bound gating ring is tethered to the apparently open MthK pore, it has been hypothesized that the Ca2+-bound form represents the “activated” gating ring conformation (Jiang et al., 2002a). Because the Ba2+-bound gating ring structure is in a different overall conformation, initially we hypothesized that this conformation may not be in an fully-activated form. We therefore tested whether Ba2+ could activate MthK channels by recording MthK currents with Ba2+ (instead of Ca2+) at the cytoplasmic side of the channel; in these experiments, the pore-blocking effect of Ba2+ was minimized by analyzing MthK currents recorded at -100 mV, at which little Ba2+ blockade would be expected to occur (Thomson and Rothberg, 2010).
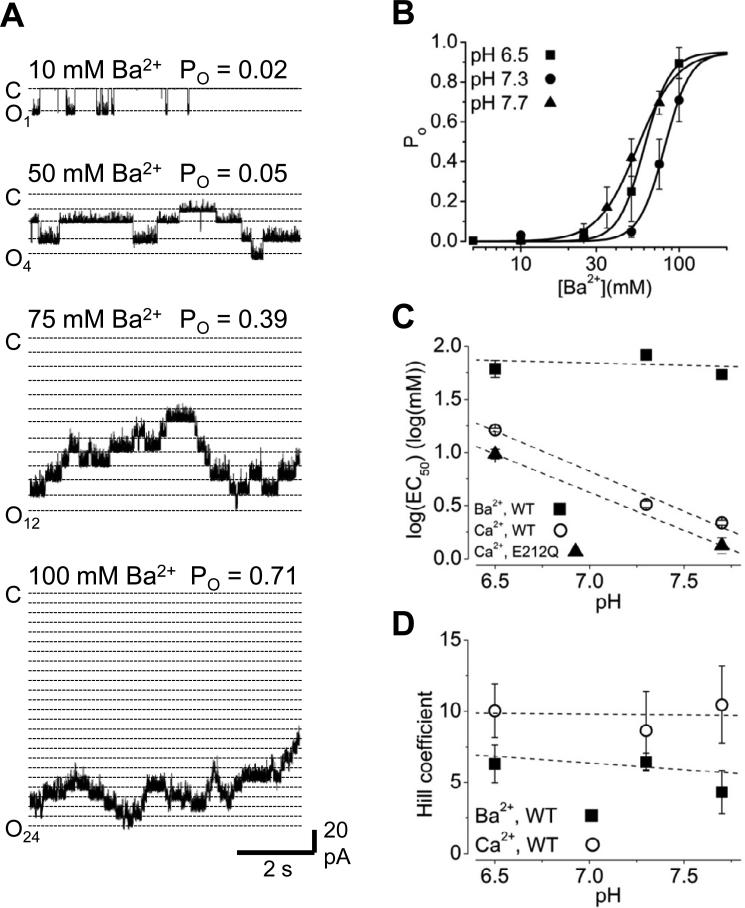
Despite the apparent conformational differences between the Ca2+- and Ba2+-bound gating rings, we found that MthK channels can be activated by Ba2+ (Figure 3A, B). With 10 mM Ba2+, low levels of activity are observed, and increasing [Ba2+] at the cytoplasmic side of the channel increases Po, in a manner that was completely reversible (Figure S3). Increasing [Ba2+] also leads to decrease in single-channel current amplitude, which appears to arises from fast blockade, similar to that elicited by Ca2+ and other divalent cations in K+ channels (Ferguson, 1991; Li et al., 2007; Zhang et al., 2006b).
Figure 3. Ba2+-activates MthK channels.
A, Current traces illustrating activation of MthK channels at pH 7.3 with Ba2+ at the indicated concentrations. Traces are from the same bilayer, which contained 24 active channels. Open channel current levels are indicated by dotted lines; note that increasing [Ba2+] decreased the size of the single-channel current, through an apparent fast-blocking effect. B, Po vs. [Ba2+] relations determined at pH 6.5, 7.3 and 7.7. Curves represent Hill equation fits, with EC50 = 60.5, 81.1, and 54.8 mM Ba2+, and nH = 5.3, 5.4, and 3.5 for pH 6.5, 7.3, and 7.7, respectively. C, Means of log(EC50) values determined from fitting Po vs. [Ba2+] relations of MthK wild-type channels from individual bilayers, plotted as a function of pH. Log(EC50) values for Ca2+-activation of MthK wild-type channels (circles) are plotted on the same axes for comparison, and illustrate the steep pH-dependence for Ca2+ activation. Ca2+-activation of E212Q mutant channels (triangles) exhibits pH-dependence comparable to wild-type. Log(EC50) values for Ca2+ activation correspond to EC50 (in mM) of 16 ± 0.6, 3.0 ± 0.2, and 2.2 ± 0.1 for wild-type (pH 6.5, 7.3, and 7.7, respectively) and 9.9 ± 0.2 and 1.3 ± 0.1 for E212Q channels (pH 6.5 and 7.7). D, Means of Hill coefficients, also determined from fitting Po vs. [Ba2+] relations of individual bilayers, plotted with Hill coefficients for Ca2+-activation. See also Figure S3.
It was previously observed that Ca2+-activation of MthK is inhibited by cytoplasmic H+, through a mechanism in which H+ specifically inhibits Ca2+-dependent gating and not the intrinsic gating of the MthK pore (Pau et al., 2010). The effect of [H+] on Ca2+-activation of MthK is substantial, resulting in a 5.6-fold change in Ca2+ EC50 per 10-fold change in [H+] over the range of 20 to 316 nM H+ (pH 6.5 to 7.7; Figure 3C). To determine whether Ba2+ activates MthK through a pH-sensitive mechanism, we assessed Ba2+-activation over a range of pH (6.5 to 7.7). Figure 3B shows that, interestingly, Ba2+ activation of MthK is much less H+-sensitive than Ca2+-activation, with an 11% decrease in Ba2+ EC50 per 10-fold increase in [H+] over the range of 20 to 316 nM H+ (pH 7.7 to 6.5; Figure 3C).
Because Ca2+ activates MthK channels through other sites in addition to C1, our electrophysiological results suggest that pH modulation of MthK channels does not arise through direct modulation of C1, but instead through the other Ca2+ sites (Pau et al., 2011). However, the lack of pH modulation with Ba2+ activation could also arise from non-participation of the E212 sidechain, which contributes to Ca2+ coordination but not Ba2+ coordination. Therefore to determine whether protonation of E212 might contribute to pH modulation of Ca2+-dependent activation, we recorded activity from E212Q mutant channels over a range of [H+] and [Ca2+]. If pH-dependence arises from protonation of E212, then the E212Q mutation should abolish or substantially reduce the shift in EC50 for Ca2+ activation. We observe, however, that E212Q channels retain pH-sensitivity comparable to MthK wild-type channels, with a 5.2-fold change in Ca2+ EC50 per 10-fold change in [H+] over the range of 20 to 316 nM H+ (Figure 3C). This further supports the idea that pH modulation of MthK channels does not arise through titration of Ca2+-coordinating residues at C1 and may arise through effects on other sites, or effects on residues that couple these sites to channel opening.
Comparison of EC50 values for Ca2+ and Ba2+ activation also illustrates that overall, higher [Ba2+] is required to activate the channel than [Ca2+] (Pau et al., 2010). This difference may reflect a lower binding affinity for Ba2+ at its activation sites and/or overall weak energetic coupling between Ba2+-binding and channel opening. In addition to the higher EC50 for Ba2+-activation, we also observe generally lower Hill coefficients for Ba2+-activation compared to Ca2+-activation of the channel (mean Hill coefficients from data in Figure 3D were 5.3 ± 0.7 for Ba2+ activation, 9.5 ± 1.3 for Ca2+ activation, from 10 and 16 different bilayers, respectively), which may reflect a weaker energetic coupling between Ba2+ binding and channel opening in MthK compared with Ca2+-activation (Pau et al., 2010; Zadek and Nimigean, 2006).
Ba2+ activation occurs primarily through binding to the C1 site
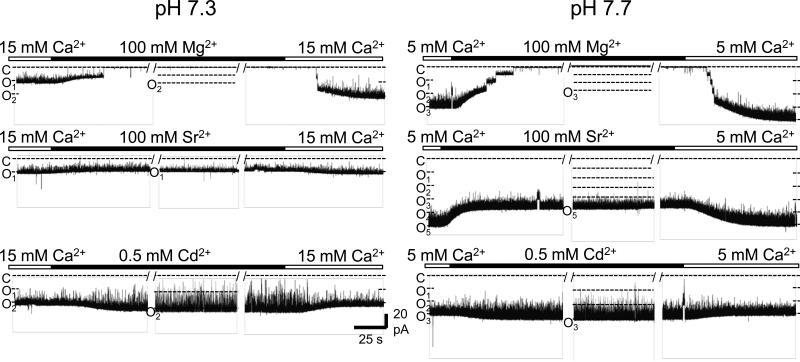
Our identification of Ba2+ binding sites in the MthK gating ring and functional observations of steep relation between Po and [Ba2+] in reconstituted MthK channels are consistent with the idea that Ba2+ activation may arise through binding to a specific site in the MthK RCK domain. However, because Ba2+ appears to decorate many sites on the RCK domains (at 100 mM), it is alternatively possible that channel activation may occur through Ba2+ acting as a non-specific counter-charge at one or more of these identified sites. We next tested whether Ba2+ activation might arise from a general effect of increasing ionic strength, first by assessing the effects of other divalent ions on channel gating. Of the ions tested, we found that 100 mM Mg2+ elicited no detectable activation of MthK channels, while 100 mM Sr2+ or 0.5 mM Cd2+ resulted in near-maximal levels of channel activity, over pH ranging from 7.3 to 7.7 (Figure 4). The observation that Mg2+ is essentially ineffective, while Cd2+ appears to be an even more potent activator of MthK than Ca2+ under otherwise identical ionic conditions, is consistent with the idea that divalent cation activation of MthK arises through a set of selective binding sites. These results agree well with previous findings that the activation sites of MthK are highly-selective for Ca2+ over Mg2+ (Dong et al., 2005; Parfenova et al., 2007; Zadek and Nimigean, 2006). In contrast, Cd2+ is known to bind to at least two sites in the MthK RCK domain; the C1 site, as well as an additional site determined by residues D305 and E326, which binds Ca2+ but not Ba2+ (Dvir et al., 2010; Pau et al., 2011).
Figure 4. MthK channels can be activated by Cd2+ or Sr2+, but not Mg2+.
Representative current traces at -100 mV showing activation of wild-type MthK channels at pH 7.3 (left) and 7.7 (right) with Ca2+ at the cytoplasmic side of a bilayer, followed by replacement with a solution containing either Mg2+, Sr2+, or Cd2+, followed by replacement back to Ca2+ (at the indicated concentrations). At either pH, 100 mM Sr2+ or 0.5 mM Cd2+ was sufficient to maximally activate MthK channels (Po = 0.95 ± 0.02 for Sr2+; 0.92 ± 0.04 for Cd2+), whereas no activation was observed with 100 mM Mg2+ (bottom traces; 0 openings observed). Breaks in the recordings, indicated by “/ /”, were introduced manually and represent a gap of ~3 sec in each instance.
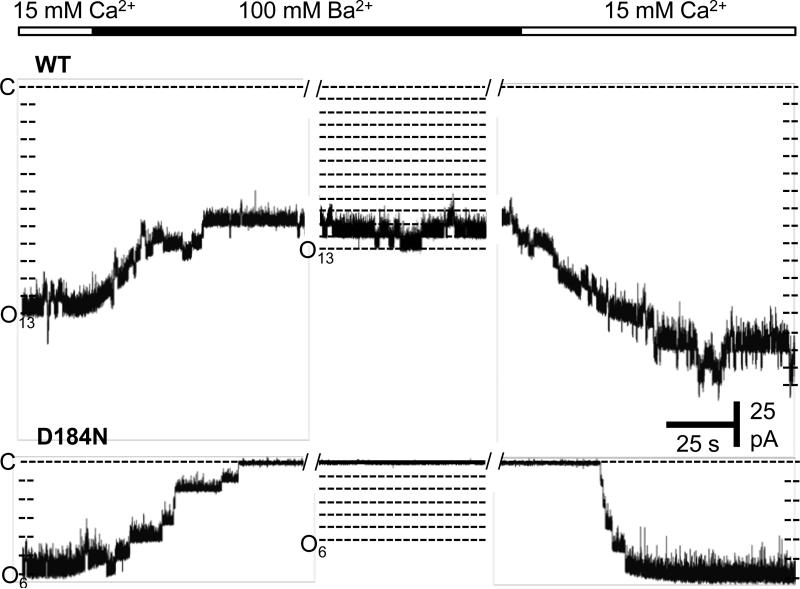
Coordination of Ba2+ at the C1 site led us to further hypothesize that Ba2+ activation may arise specifically through binding of Ba2+ at this site. To test this idea, we obtained recordings from D184N mutant channels with [Ba2+] sufficient to elicit near-maximal activation of wild-type MthK channels. The D184N mutation was previously shown to decrease Ca2+ activation of MthK, and apparently knock out Ca2+ binding at the C1 site, as indicated by crystallographic data (Pau et al., 2011). Figure 5 illustrates that no detectable Ba2+ activation is observed in D184N channels at [Ba2+] up to 100 mM, consistent with Ba2+ activation occuring primarily through the C1 site.
Figure 5. The D184N mutation effectively abolishes Ba2+ activation of MthK.
Representative current traces at -100 mV showing MthK channel activation at pH 7.3 with 15 mM Ca2+ at the cytoplasmic side of a bilayer, followed by replacement with a solution containing 100 mM Ba2+ (with no added Ca2+), and then back to 15 mM Ca2+. The top series of traces shows that 100 mM Ba2+ was sufficient to maximally activate wild-type MthK channels (Po = 0.9), whereas no Ba2+ activation was observed with D184N mutant channels (0 openings observed, n=5 different bilayer experiments).
DISCUSSION
Working hypothesis for the physical basis of Ba2+ recognition
Here we have exploited structural analysis of MthK to dissect mechanisms underlying metal-activation of RCK domains. We observe principally that Ba2+ can bind to a site on each RCK domain that overlaps with a Ca2+-activation site (C1, determined by residues D184 and E210), and through binding to this site, Ba2+ can activate the RCK domain to facilitate opening of the channel.
Ba2+ has a slightly larger ionic radius than Ca2+, and apart from general chemical similarities, Ba2+ and Ca2+ are known to have quantitatively different characteristics. For example, Ba2+ water substitution rate has been estimated to be around an order of magnitude greater for Ba2+ than that of Ca2+ (Hille, 2001). It is known that Ba2+ permeates rapidly through voltage-dependent Ca2+ channels, and can bind to and partially activate calmodulin, whereas it seems to be a poor substitute for Ca2+ in other physiological processes (such as neurotransmitter release) (DiPolo et al., 1983; Kursula and Majava, 2007; Satoh et al., 1987; Zengel and Magleby, 1980, 1981). Our results suggest that Ba2+ can bind to and activate MthK channels through selective action at the C1 site, whereas Ba2+ is effectively excluded from binding at the other known MthK Ca2+ sites. Because Ba2+ coordination at the C1 site incorporates additional protein oxygens that do not participate in Ca2+ coordination, it is likely that the presence of these oxygens at favorable positions, and the absence of such oxygens at other sites, contributes to the apparent exclusion of Ba2+ from the other MthK Ca2+ sites.
In addition to its similarities to Ca2+, Ba2+ also is nearly identical in ionic radius to K+, and Ba2+ can flow through K+ channels (albeit much more slowly than K+)(Bello and Magleby, 1998; Neyton and Miller, 1988a, b; Rothberg et al., 1996). Because, in contrast to site C1, Ba2+ ions at sites B2 through B4 do not form multiple close contacts with protein oxygens and likely interact with the protein in near fully-hydrated states (Figure 2 and S2), one may speculate that under physiological ionic conditions with 150 mM (or more) K+ in the cytoplasm, K+ ions may occupy these sites as countercharges. Similar cation binding sites have been observed within the electronegative pockets in the cytoplasmic domain of the Kir channel (Xu et al., 2009).
Mechanism of Ba2+ activation and implications for the Ca2+ activation mechanism
We observed that the overall conformation of the Ba2+-bound gating ring is different from that of the fully-activated, Ca2+-bound full-length MthK channel (Figure 1B, C) (Jiang et al., 2002a, b). In previous work, it was found that MthK channels in which two of the three Ca2+ binding sites the RCK domain have been mutated can diminish Ca2+-activation, suggesting that MthK channels can be weakly activated through single Ca2+ binding sites (Pau et al., 2011). Thus it is possible that Ba2+ activates MthK channels through binding to the C1 site (Figures 1, 3 and 5), and hence that the Ba2+-bound gating ring represents an intermediate, singly-liganded conformation in the domain's activation pathway.
To further explore whether the Ba2+-bound gating ring might represent an intermediate, singly-liganded conformation, we compared the conformation of a dimeric unit from the Ba2+-bound gating ring with other previously-determined MthK RCK domain structures by aligning the structures to yield the minimal root-mean-square deviation (rmsd). We reasoned that comparisons yielding the lowest rmsd values would indicate structures in the same conformation, and higher rmsd values would indicate conformational differences.
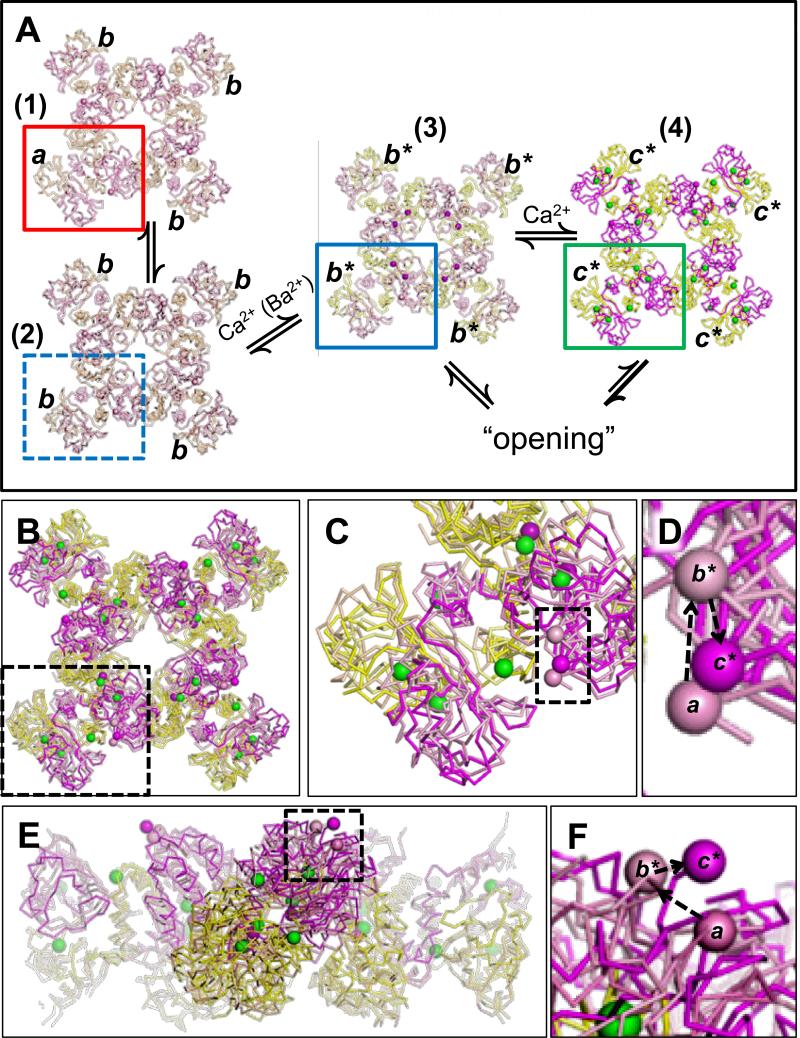
Through these comparisons, a physical picture of conformational transitions that may underlie MthK gating emerges (Figure 6; Table S1). We first noted that the structure of the unliganded MthK gating ring (with the D184N mutation; PDB accession no. 2FY8; (Ye et al., 2006)) showed that the unliganded ring can exist in two different overall conformations (Figure 6A, (1) and (2)). Unliganded ring (1) contains component RCK domains in two different conformations, indicated in Figure 6A by “a” (red box) and “b”, whereas unliganded ring (2) contains all RCK domains in the b form (Figure 6A, blue dashed box). Our alignments revealed that form a is most similar to a wild-type unliganded RCK domain (PDB accession no. 2AEJ; rmsd = 0.77 Å; Figure S4A; (Dong et al., 2005)), whereas b is most similar to the Ba2+-bound conformation, indicated by b* in Figure 6A (rmsd = 0.74 Å; Figure S4B). In contrast, alignment of form a with the fully-liganded RCK domain (c*, green box in Figure 6A) yielded a higher rmsd value, consistent with a conformational difference between these structures (rmsd = 1.76 Å; Table S1). Based on these data, we hypothesize that for wild-type MthK channels, RCK domains within the gating ring may undergo sequential transitions from form a to b, where b can be stabilized by binding of Ba2+ (or potentially Ca2+) to yield b*, followed by additional Ca2+ binding to yield c* (Figure 6A; see Supplemental Discussion). The ligand-bound states (b* and c*) would underlie facilitation of channel opening through allosteric coupling.
Figure 6. Working hypothesis of elementary steps in MthK gating ring activation.
A, RCK domains undergo transitions from inactive (a) to partially-activated/singly-liganded (b / b*), then to fully-liganded/activated (c*). Detailed explanation provided in Discussion. B, Aligned and overlayed unliganded (1), Ba2+-bound (3) and Ca2+-bound gating rings (4). C, Expanded view of the region indicated by black dashed box in B. Spheres within the dashed box indicate the Cα of residue R116, which in turn would be linked to the transmembrane pore. Changes in position of R116 may indicate RCK domain movements coupled to channel opening. D, Expanded view of the boxed region from C. Dashed arrows indicate the sequence a→b*→c*. E, Aligned and overlayed structures, viewed normal to the plane of the membrane. F, Expanded view of the boxed region from E, indicating the sequence a→b*→c*. See also Figure S4 and Table S1.
Alignment of the complete gating ring structures (1), (3), and (4) reveals a potential sequence of positions for the N-terminal ends of RCK domains (Cα's of R116, indicated by spheres in Figure 6D and F). In the context of the full-length channel, these N-termini are linked to the pore and are thus likely to impact pore conformation. These alignments suggest that binding of Ba2+ may lead to a relative translation of the R116 Cα in a direction normal to the membrane (a→b*; Figure 6F), whereas binding of Ca2+ to additional activation sites may move the R116 Cα in a plane parallel to the membrane (b*→c*, Figure 6D, F), to further stabilize the open state (Pau et al., 2011; Ye et al., 2006). Further experiments will be required to directly assess the impact of these movements on pore structure.
Relation to gating mechanisms in eukaryotic channels
Activation by metal cations, including Ba2+, has been used previously to dissect gating mechanisms of eukaryotic BK channels, yielding insight toward the specificity of putative metal binding sites within the BK channel RCK domains, defined through mutational analysis (Shi et al., 2002; Xia et al., 2002; Zeng et al., 2005; Zhang et al., 2001; Zhou et al., 2012). Despite determination of the structure of the BK channel gating ring, however, the structural basis of metal binding is clear only in the case of Ca2+ binding at the “Ca2+ bowl site”, determined principally by residues D985 and D897 (in the human BK channel)(Yuan et al., 2011; Yuan et al., 2010). Thus the structural basis for preference of specific metals for putative activation sites within the BK channel RCK domain is yet unclear. Results obtained through structural analysis of MthK channels may continue to provide useful insights toward the physical basis of RCK domain activation, complementing ongoing analysis of BK channels.
EXPERIMENTAL PROCEDURES
Crystallization
Purification of the MthK RCK domain was performed as described previously (Pau et al., 2011). The crystal analyzed for this study formed in a 2 μl hanging drop consisting of equal volumes of protein solution (6 mg/ml of purified MthK RCK domain, 250 mM NaCl, 20 mM Tris, pH 8.0) and crystallization buffer (28% polyethylene glycol (PEG) 300, 100 mM BaCl2, 100 mM Tris pH 8.5). This crystal was mounted in a nylon loop and rapidly frozen in liquid N2 without additional cryoprotection, and then stored in liquid N2 until the time of diffraction data collection.
X-ray data collection and analysis
Diffraction data were collected at beamline X6A at the National Synchrotron Light Source, at -180°C under a nitrogen stream, at a wavelength of 1.1 Å. Data were processed and scaled to 3.1 Å using HKL2000, and phasing was done by molecular replacement with Phaser (McCoy et al., 2007; Otwinowski and Minor, 1997). The best scoring molecular replacement solution used the structure of the “closed” MthK gating ring (PDB accession no. 2FY8). This solution contained two complete gating rings (16 RCK domain chains) per asymmetric unit. Examination of electron density maps and additional model building was performed using COOT (Emsley and Cowtan, 2004). The model was refined by positional and isotropic B-factor refinement in Phenix (Adams et al., 2010). To increase the observation/parameter ratio, 16-fold torsion-angle non-crystallographic symmetry (NCS) restraints were included in refinement and found to significantly decrease the Rfree. Additionally, from the early stages of model building, the model was divided into 50 Translation/Libration/Screw (TLS) groups, and TLS refinement was performed using Phenix. Bias in the assignment of the Rfree test set (arising from the high NCS) was minimized by use of thin resolution shells in test set selection, also performed in Phenix.refine. The final model contains 27,716 protein atoms (3,557 residues), 57 Ba2+ ions, and 32 water molecules and was refined to final working and free R values of 20.9% and 24.3%, respectively, at 3.1 Å resolution (Table 1). Ramachandran plot analysis showed 92.2% in most favored regions, 7.2% in additional allowed regions, 0.6% in additional allowed regions, and 0.0% in disallowed regions. Figures were prepared using PyMOL, and pairwise structural alignments were performed using the “align” command in PyMOL, as described in Supplemental Methods.
Channel purification and reconstitution
The full-length MthK channel was expressed in E.coli XL-1 Blue cells on induction with 0.4 mM IPTG, and purified by metal affinity chromatography as described previously (Pau et al., 2010). Channels were reconstituted into liposomes composed of E.coli lipids (Avanti) (Heginbotham et al., 1998), and the proteoliposomes were rapidly frozen in liquid N2 and stored at -80°C until use. Mutations were generated using the QuickChange Site-directed Mutagenesis Kit (Stratagene) and confirmed by DNA sequencing.
Electrophysiology
Recordings were obtained using planar lipid bilayers of POPE:POPG (3:1), in a horizontal bilayer chamber, at 22-24°C. Solution in the cis (top) chamber contained 200 mM KCl and 10 mM HEPES, pH 7.0; solution in the trans (bottom) chamber contained 200 mM KCl, with either 10 mM HEPES (to buffer solutions to pH 7.3 or 7.7) or 10 mM MES (to buffer solutions to pH 6.5), and MgCl2, CdCl2, CaCl2, SrCl2 or BaCl2 at the indicated concentrations. Recordings analyzed for this work were obtained at -100 mV transmembrane voltage, though currents through each bilayer were typically observed at additional voltages to confirm the reversal potential and other MthK channel properties. Within each bilayer, multiple solution changes were performed using a gravity-fed perfusion system, and to ensure completeness of solution changes, the trans chamber was washed with a minimum of ten chamber-volumes of solution during each solution change.
Currents were amplified using a Dagan PC-ONE patch clamp amplifier with low-pass filtering to give a final effective filtering of 1 kHz (dead time of 0.18 ms), and sampled by computer at a rate of 10 kHz. Currents were analyzed by measuring durations of channel openings and closings at each current level by 50% threshold analysis, using pClamp9. These measurements were used to quantify channel activity as , where i is the open level and Pi is the percent of time at that level. The mean channel open probability (Po) is then calculated by dividing NPo times N, which is the number of active channels in the bilayer, determined by recording under conditions where maximal channel opening is observed. Data are presented as mean ± SEM. Electrophysiological results are based on observations from a minimum of three different experiments for each recording condition, and includes a total of 264 individual data sets from 45 different lipid bilayer patches.
Because we could observe the effect of fast Ba2+ blockade (i.e. Ba2+-dependent reduction of single-channel current amplitude) in our recordings, we wondered whether slow Ba2+ blockade might also occur. Slow Ba2+ blockade can produce long closed events of several seconds in duration, which would have a substantial impact on Po (Rothberg et al., 1996; Thomson and Rothberg, 2010). To estimate the potential impact of slow Ba2+ blockade, we activated MthK channels to Po of >0.9 using 15 mM Ca2+, and then changed the solution to include both 15 mM Ca2+ and 50 mM Ba2+ (Figure S5). In three different experiments of this type (ranging from 2.5 to 8 min of continuous recording for each experiment), we observed 0 closings >1 sec, suggesting that slow Ba2+ blockade has a nominal impact on Po in recordings at -100 mV.
Supplementary Material
Research Highlights.
We present the structure of a Ba2+-bound RCK gating ring at 3.1 Å.
Ba2+ binds to the RCK domain at a site in common with a known Ca2+-activation site.
Ba2+ can substitute for Ca2+ to activate reconstituted MthK channels.
These results suggest a working hypothesis for ligand-activation of MthK channels.
ACKNOWLEDGEMENTS
We thank Chris Lingle (Washington University in St. Louis) and Andrew Thomson (Temple University) for helpful discussions and comments on the manuscript, and the X-ray Core Facility of Thomas Jefferson University / Kimmel Cancer Center for technical support. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01 GM068523 to B.S.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamacina CR. The nicotinamide dinucleotide binding motif: a comparison of nucleotide binding proteins. FASEB J. 1996;10:1257–1269. doi: 10.1096/fasebj.10.11.8836039. [DOI] [PubMed] [Google Scholar]
- Bello RA, Magleby KL. Time-irreversible subconductance gating associated with Ba2+ block of large conductance Ca2+-activated K+ channels. J Gen Physiol. 1998;111:343–362. doi: 10.1085/jgp.111.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- Chaptal V, Kwon S, Sawaya MR, Guan L, Kaback HR, Abramson J. Crystal structure of lactose permease in complex with an affinity inactivator yields unique insight into sugar recognition. Proc Natl Acad Sci U S A. 2011;108:9361–9366. doi: 10.1073/pnas.1105687108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R, Caputo C, Bezanilla F. Voltage-dependent calcium channel in the squid axon. Proc Natl Acad Sci U S A. 1983;80:1743–1745. doi: 10.1073/pnas.80.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Shi N, Berke I, Chen L, Jiang Y. Structures of the MthK RCK domain and the effect of Ca2+ on gating ring stability. J Biol Chem. 2005;280:41716–41724. doi: 10.1074/jbc.M508144200. [DOI] [PubMed] [Google Scholar]
- Dvir H, Valera E, Choe S. Structure of the MthK RCK in complex with cadmium. J Struct Biol. 2010;171:231–237. doi: 10.1016/j.jsb.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Ferguson WB. Competitive Mg2+ block of a large-conductance, Ca(2+)-activated K+ channel in rat skeletal muscle. Ca2+, Sr2+, and Ni2+ also block. J Gen Physiol. 1991;98:163–181. doi: 10.1085/jgp.98.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J, Kirkwood A, Miller C. Allosteric effects of Mg2+ on the gating of Ca2+-activated K+ channels from mammalian skeletal muscle. J Exp Biol. 1986;124:5–13. doi: 10.1242/jeb.124.1.5. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L, Kolmakova-Partensky L, Miller C. Functional reconstitution of a prokaryotic K+ channel. J Gen Physiol. 1998;111:741–749. doi: 10.1085/jgp.111.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. 3rd edn Sinauer Associates, Inc.; Sunderland, MA: 2001. [Google Scholar]
- Hou S, Xu R, Heinemann SH, Hoshi T. Reciprocal regulation of the Ca2+ and H+ sensitivity in the SLO1 BK channel conferred by the RCK1 domain. Nat Struct Mol Biol. 2008;15:403–410. doi: 10.1038/nsmb.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlach WL, Finch SC, Dunlop J, Meredith AL, Aldrich RW, Dalziel JE. The molecular mechanism of ‘ryegrass staggers’ a neurological disorder of K+ channels. J Pharmacol Exp Ther. 2008 doi: 10.1124/jpet.108.143933. [DOI] [PubMed] [Google Scholar]
- Inanobe A, Nakagawa A, Kurachi Y. Interactions of cations with the cytoplasmic pores of inward rectifier K(+) channels in the closed state. J Biol Chem. 2011;286:41801–41811. doi: 10.1074/jbc.M111.278531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002a;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. The open pore conformation of potassium channels. Nature. 2002b;417:523–526. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- Jiang Y, MacKinnon R. The barium site in a potassium channel by x-ray crystallography. J Gen Physiol. 2000;115:269–272. doi: 10.1085/jgp.115.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Pico A, Cadene M, Chait BT, MacKinnon R. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 2001;29:593–601. doi: 10.1016/s0896-6273(01)00236-7. [DOI] [PubMed] [Google Scholar]
- Kroning N, Willenborg M, Tholema N, Hanelt I, Schmid R, Bakker EP. ATP binding to the KTN/RCK subunit KtrA from the K+-uptake system KtrAB of Vibrio alginolyticus: Its role in the formation of the KtrAB complex and its requirement in vivo. J Biol Chem. 2007;282:14018–14027. doi: 10.1074/jbc.M609084200. [DOI] [PubMed] [Google Scholar]
- Kuo MMC, Haynes WJ, Loukin SH, Kung C, Saimi Y. Prokaryotic K+ channels: From crystal structures to diversity. FEMS Microbiology Reviews. 2005;29:961–985. doi: 10.1016/j.femsre.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Kursula P, Majava V. A structural insight into lead neurotoxicity and calmodulin activation by heavy metals. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007;63:653–656. doi: 10.1107/S1744309107034525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Tsien RW. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983;302:790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Li Y, Berke I, Chen L, Jiang Y. Gating and inward rectifying properties of the MthK K+ channel with and without the gating ring. J Gen Physiol. 2007;129:109–120. doi: 10.1085/jgp.200609655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Miller C. Discrete Ba2+ block as a probe of ion occupancy and pore structure in the high-conductance Ca2+ -activated K+ channel. J Gen Physiol. 1988a;92:569–586. doi: 10.1085/jgp.92.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Miller C. Potassium blocks barium permeation through a calcium-activated potassium channel. J Gen Physiol. 1988b;92:549–567. doi: 10.1085/jgp.92.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X, Qian X, Magleby KL. Linker-gating ring complex as passive spring and Ca(2+)-dependent machine for a voltage- and Ca(2+)-activated potassium channel. Neuron. 2004;42:745–756. doi: 10.1016/j.neuron.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Parfenova LV, Abarca-Heidemann K, Crane BM, Rothberg BS. Molecular architecture and divalent cation activation of TvoK, a prokaryotic potassium channel. J Biol Chem. 2007;282:24302–24309. doi: 10.1074/jbc.M703650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenova LV, Crane BM, Rothberg BS. Modulation of MthK potassium channel activity at the intracellular entrance to the pore. J Biol Chem. 2006;281:21131–21138. doi: 10.1074/jbc.M603109200. [DOI] [PubMed] [Google Scholar]
- Pau VP, Abarca-Heidemann K, Rothberg BS. Allosteric mechanism of Ca2+ activation and H+-inhibited gating of the MthK K+ channel. J Gen Physiol. 2010;135:509–526. doi: 10.1085/jgp.200910387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau VP, Smith FJ, Taylor AB, Parfenova LV, Samakai E, Callaghan MM, Abarca-Heidemann K, Hart PJ, Rothberg BS. Structure and function of multiple Ca2+-binding sites in a K+ channel regulator of K+ conductance (RCK) domain. Proc Natl Acad Sci U S A. 2011;108:17684–17689. doi: 10.1073/pnas.1107229108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg BS, Bello RA, Song L, Magleby KL. High Ca2+ concentrations induce a low activity mode and reveal Ca2(+)-independent long shut intervals in BK channels from rat muscle. J Physiol. 1996;493(Pt 3):673–689. doi: 10.1113/jphysiol.1996.sp021414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- Satoh S, Kubota Y, Itoh T, Kuriyama H. Mechanisms of the Ba2+-induced contraction in smooth muscle cells of the rabbit mesenteric artery. J Gen Physiol. 1987;89:215–237. doi: 10.1085/jgp.89.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M, Wei A, Yuan A, Gaut J, Saito M, Salkoff L. Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J Biol Chem. 1998;273:3509–3516. doi: 10.1074/jbc.273.6.3509. [DOI] [PubMed] [Google Scholar]
- Shi J, Krishnamoorthy G, Yang Y, Hu L, Chaturvedi N, Harilal D, Qin J, Cui J. Mechanism of magnesium activation of calcium-activated potassium channels. Nature. 2002;418:876–880. doi: 10.1038/nature00941. [DOI] [PubMed] [Google Scholar]
- Thomson AS, Rothberg BS. Voltage-dependent inactivation gating at the selectivity filter of the MthK K+ channel. J Gen Physiol. 2010;136:569–579. doi: 10.1085/jgp.201010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic SM, Lingle CJ. Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: effects of anticonvulsant and anesthetic agents. J Neurophysiol. 1998;79:240–252. doi: 10.1152/jn.1998.79.1.240. [DOI] [PubMed] [Google Scholar]
- Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418:880–884. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- Xia XM, Zhang X, Lingle CJ. Ligand-dependent activation of Slo family channels is defined by interchangeable cytosolic domains. J Neurosci. 2004;24:5585–5591. doi: 10.1523/JNEUROSCI.1296-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Shin HG, Szep S, Lu Z. Physical determinants of strong voltage sensitivity of K(+) channel block. Nat Struct Mol Biol. 2009;16:1252–1258. doi: 10.1038/nsmb.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Li Y, Chen L, Jiang Y. Crystal structures of a ligand-free MthK gating ring: insights into the ligand gating mechanism of K+ channels. Cell. 2006;126:1161–1173. doi: 10.1016/j.cell.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, Nonet M, Kaczmarek L, Crowder CM, Salkoff L. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron. 2003;37:765–773. doi: 10.1016/s0896-6273(03)00096-5. [DOI] [PubMed] [Google Scholar]
- Yuan P, Leonetti MD, Hsiung Y, MacKinnon R. Open structure of the Ca2+ gating ring in the high-conductance Ca2+-activated K+ channel. Nature. 2011;481:94–97. doi: 10.1038/nature10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Leonetti MD, Pico AR, Hsiung Y, MacKinnon R. Structure of the human BK channel Ca2+-activation apparatus at 3.0 A resolution. Science. 2010;329:182–186. doi: 10.1126/science.1190414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadek B, Nimigean CM. Calcium-dependent gating of MthK, a prokaryotic potassium channel. J Gen Physiol. 2006;127:673–685. doi: 10.1085/jgp.200609534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XH, Xia XM, Lingle CJ. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J Gen Physiol. 2005;125:273–286. doi: 10.1085/jgp.200409239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel JE, Magleby KL. Transmitter release during repetitive stimulation: selective changes produced by Sr2+ and Ba2+. Science. 1977;197:67–69. doi: 10.1126/science.17160. [DOI] [PubMed] [Google Scholar]
- Zengel JE, Magleby KL. Differential effects of Ba2+, Sr2+, and Ca2+ on stimulation-induced changes in transmitter release at the frog neuromuscular junction. J Gen Physiol. 1980;76:175–211. doi: 10.1085/jgp.76.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel JE, Magleby KL. Changes in miniature endplate potential frequency during repetitive nerve stimulation in the presence of Ca2+, Ba2+, and Sr2+ at the frog neuromuscular junction. J Gen Physiol. 1981;77:503–529. doi: 10.1085/jgp.77.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Solaro CR, Lingle CJ. Allosteric regulation of BK channel gating by Ca(2+) and Mg(2+) through a nonselective, low affinity divalent cation site. J Gen Physiol. 2001;118:607–636. doi: 10.1085/jgp.118.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zeng X, Xia XM, Lingle CJ. pH-regulated Slo3 K+ channels: properties of unitary currents. J Gen Physiol. 2006a;128:301–315. doi: 10.1085/jgp.200609551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Niu X, Brelidze TI, Magleby KL. Ring of negative charge in BK channels facilitates block by intracellular Mg2+ and polyamines through electrostatics. J Gen Physiol. 2006b;128:185–202. doi: 10.1085/jgp.200609493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zeng XH, Lingle CJ. Barium ions selectively activate BK channels via the Ca2+-bowl site. Proc Natl Acad Sci U S A. 2012;109:11413–11418. doi: 10.1073/pnas.1204444109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.