Abstract
Transcatheter aortic valve implantation (TAVI) is indicated as an alternative treatment modality to surgical aortic valve replacement for high risk patients. The standard retrograde approach through the femoral artery is not feasible in the case of unfavorable iliofemoral anatomy or severe peripheral arterial disease (PAD). However, patients with aortic stenosis (AS) have a higher prevalence of for PAD because both diseases are consequences of atherosclerotic degenerative changes. Transsubclavian, transapical, and direct access to the ascending aorta by thoracotomy are alternative routes for the TAVI procedure. In this report, we present the first Korean patient with symptomatic severe AS and bilateral iliofemoral artery disease who was successfully treated with TAVI using a CoreValve (Medtronic, Minneapolis, MN, USA) by transsubclavian approach.
Keywords: Aortic valve stenosis, Catheters, Heart valve prosthesis, Prosthesis implantation
Introduction
Degenerative aortic valve disease is frequently found in elderly people. However, approximately 30% of elderly patients with severe aortic stenosis (AS) remain unreferred and untreated because these patients are considered too high risk or inappropriate for conventional open heart surgery.1) Transcatheter aortic valve implantation (TAVI) using a balloon expandable Sapien valve (Edwards Lifesciences, Irvine, CA, USA) or self-expandable CoreValve (Medtronic, Minneapolis, MN, USA) has demonstrated favorable outcomes in patients with high surgical risk. The most common approach for TAVI is the transfemoral approach because the relatively large profile (18 Fr) of the delivery catheter requires an access route with a diameter larger than 6 mm. However, peripheral arterial disease (PAD) is also relatively common in elderly patients with AS because degenerative aortic valve disease shares many characteristics with atherosclerotic disease.2) When a transfemoral approach is not feasible due to small or diseased iliofemoral arteries, alternative access routes such as the transapical approach, transsubclavian approach, as well as direct aortic access via minimal thoracotomy may be considered.3),4)
In this case report, we present the first Korean patient with symptomatic severe AS at high surgical risk accompanied by bilateral iliofemoral artery disease that was successfully treated with TAVI using a CoreValve by transsubclavian approach.
Case
An 82-year-old male patient presented with worsening symptoms of chest discomfort and exertional dyspnea {New York Heart Association (NYHA) III} for 2 months. He had past medical history of hypertension and dyslipidemia. Four years ago, the patient had been treated with percutaneous coronary intervention with implantation of stents at the left main artery and the distal left circumflex artery. At that time he also underwent percutaneous transluminal angioplasty with implantation of self-expandable nitinol stents at the bilateral iliac arteries. On admission, transthoracic and transesophageal echocardiography showed severe AS and mild aortic regurgitation (AR) due to degenerative change with calcification (Fig. 1). The aortic valve area was 0.44 cm2. The peak and mean pressure gradients across the aortic valve were estimated to be 97 and 59 mm Hg, respectively, while the diameter of the aortic annulus by echocardiography was 25 mm. The left ventricle (LV) showed a dilated enddiastolic dimension (64 mm) with LV hypertrophy. The LV systolic function was globally reduced with an LV ejection fraction (EF) of 39%. Based on the coronary angiography, the stent at the left main artery was patent. However, the stent implanted at the distal left circumflex artery showed in-stent restenosis, which was subsequently treated with a 3×30 mm paclitaxel-eluting balloon (SeQuent® Please, B. Braun Melsungen AG, Berlin, Germany). The previously inserted stents at the right and left iliac arteries were patent, however computed tomography (CT) angiography revealed that the minimal lumen diameter of the stented right iliac artery was 5.5 mm and that of the left iliac artery was 4.5 mm. Both common femoral arteries also showed significant stenosis with a minimum lumen diameter of 4.0 mm (Fig. 2). The diameter of the left subclavian artery was measured to be 7 mm on CT angiography. The calculated EuroSCORE was 44.08%.
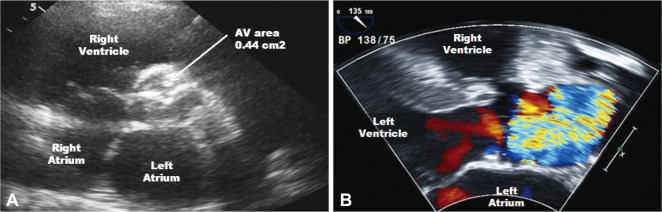
Fig. 1.
Transthoracic echocardiography showed severely narrowed aortic valve area (0.44 cm2) measured by the continuity equation (A), and color Doppler revealed aliasing due to severe aortic stenosis (B).
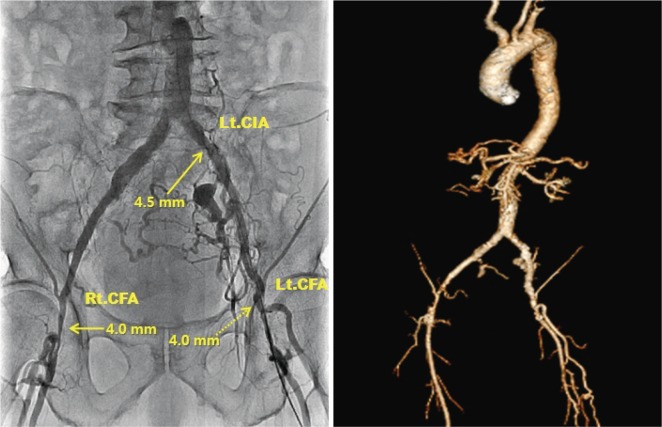
Fig. 2.
Angiography and computed tomography image showed iliofemoral arteries with severe peripheral arterial occlusive disease. Previous stent at Lt. common iliac artery was patent but showed a minimum diameter of 4.5 mm and both common femoral arteries showed a minimum diameter of 4.0 mm. Lt. CIA: left common iliac artery, Rt. CFA: right common femoral artery, Lt. CFA: left common femoral artery.
In this patient we decided to perform TAVI by transsubclavian approach. The procedure was carried out in a hybrid operating room under general anesthesia. After surgical exposure of the left subclavian artery, a 0.035 inch Amplatz Super Stiff wire (Boston Scientific, Natick, MA, USA) was inserted into the LV through an 18 Fr introducer sheath (Fig. 3). Balloon dilation of the stenotic aortic valve was performed with a 23 mm balloon (Z-med, NuMED Inc., Hopkinton, NY, USA) under rapid pacing using a temporary pacemaker. Under angiographic guidance, a 29 mm CoreValve was slowly deployed at the aortic annulus. An immediate post-procedural aortogram showed good position of the CoreValve with mild AR (Fig. 4). The vascular access site at the left subclavian artery was closed surgically without any complications. The post-procedural echocardiography demonstrated well functioning bioprosthetic aortic valve with mild paravalvular AR. The peak and mean pressure gradients across the aortic valve decreased from 97 and 59 mm Hg to 42 and 23 mm Hg, respectively. The aortic valve area increased from 0.44 cm2 to 2.86 cm2. The LVEF improved from 39% to 52%. Four days post procedure, the patient was discharged with improved symptoms (NYHA I). There was no sign of stroke or any conduction abnormality as indicated by the electrocardiogram. The patient has been free from any major cardiovascular events or symptom aggravation for a total follow-up duration of 6 months.
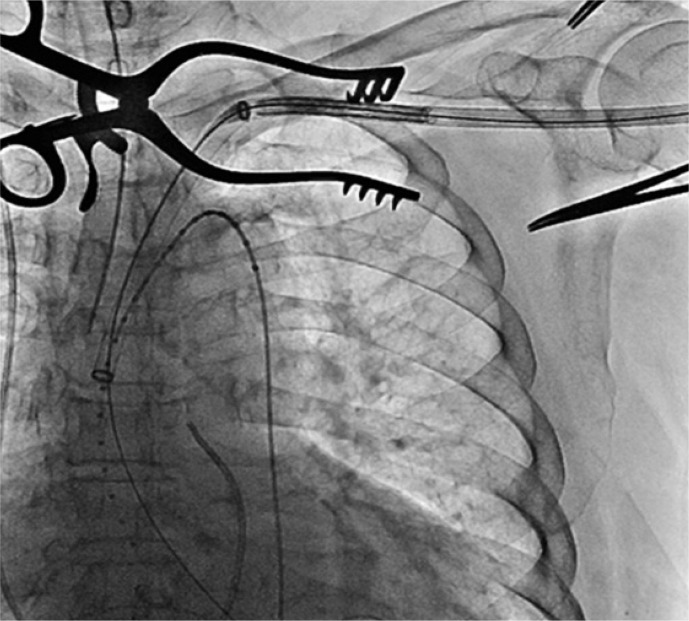
Fig. 3.
CoreValve delivery system is advanced through 18 Fr sheath inserted into the Lt. subclavian artery.
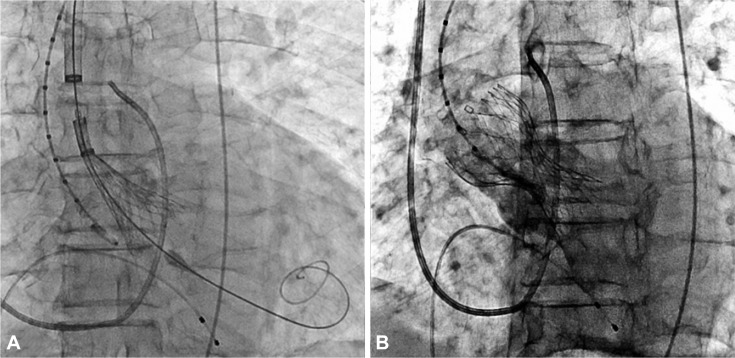
Fig. 4.
CoreValve delivery catheter and bioprosthetic valve deployed across the aortic valve (A) and spontaneously expanded. An aortogram showed good positioning of the CoreValve with mild aortic regurgitation (B).
Discussion
Transcatheter aortic valve implantation has emerged as a promising alternative treatment modality to surgical aortic valve replacement for patients with severe AS at high surgical risk. Patients with an estimated mortality risk >20% by logistic EuroSCORE or >10% by the Society of Thoracic Surgeons score system are generally considered candidates for the TAVI procedure. In addition, combined respiratory failure, pulmonary hypertension, previous cardiac surgery, right ventricular failure, hostile thorax (such as radiation, burns, previous thoracic pleurodesis, or multiple thoracotomies), severe connective tissue disease, liver cirrhosis, cachexia, and porcelain aorta are further indications for TAVI. Currently, two types of stented valves, the balloon-expandable Edwards SAPIEN valve and the self-expandable CoreValve system are commonly used for the percutaneous treatment of severe AS. There has been no randomized study comparing the two systems to date. However, the procedural success rates (>95%) have been reported to be high with both valves. The mortality rates are similar at 30 days (Edwards 12% vs. CoreValve 11%, p=0.99) and one year (Edwards 18% vs. CoreValve 21%) for both systems. The need for pacemaker appears to be significantly higher with the CoreValve.5)
Commonly, the transfemoral route is used for the TAVI procedure due to the vessel diameter requirement of >6 mm for the 18 Fr device introducer sheath and delivery catheter. However, when transfemoral approach is not amenable due to the concomitant PAD, alternative routes by transapical, transsubclavian or direct aortic access through thoracotomy can be utilized for the TAVI procedure. The patient we observed here is the first Korean case of TAVI by transsubclavian approach using a CoreValve. Recent studies have shown that the safety of transsubclavian TAVI is not inferior to transfemoral TAVI.6) Petronio et al.7) reported a 100% procedural success rate and 0% intraprocedural mortality according to the analysis of 54 transsubclavian approach cases. Thirty-day mortality was 0% (vs. 6.1% in transfemoral approach group, p=0.13) and the six-month mortality rate was 9.4% (vs. 15.8%, p=0.44) showing no difference compared with transfemoral TAVI.
Moynagh et al.8) reported that the transsubclavian TAVI achieved better outcomes compared with the transfemoral approach in optimal valve positioning (88.6% vs. 60.5%, p<0.0001) and in major adverse cardiovascular and cerebrovascular events (2.9% vs. 13.4%, p=0.09), even though transsubclavian TAVI patients had significantly higher EuroSCOREs because they were frequently accompanied by PAD, coronary artery disease, prior myocardial infarct, and prior percutaneous coronary intervention experience. CoreValve is currently not available for the transapical approach. However, patients treated by transapical approach using Edwards SAPIEN showed significantly lower one-year survival than those treated by transfemoral approach. More renal failure and stroke events were observed in the transapical TAVI group compared with transfemoral TAVI group.5) Transapical approach generally accompanies the risk from general anesthesia, thoracotomy and the incision of the LV apex. Moreover, patients with structural change of the LV due to remodeling and distorted angle, the risk of transapical TAVI may increase.9) By contrast, transsubclavian approach can be safely performed via local anesthesia combined with administration of a mild systemic sedative and analgesic agent. Therefore, if the transfemoral approach is not applicable due combined PAD in a patient with severe AS requiring TAVI procedure, and if the subclavian artery has a diameter >6 mm, a transsubclavian approach should be considered as a primary alternative route.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–2720. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- 2.Aronow WS, Ahn C, Kronzon I. Association of valvular aortic stenosis with symptomatic peripheral arterial disease in older persons. Am J Cardiol. 2001;88:1046–1047. doi: 10.1016/s0002-9149(01)01990-7. [DOI] [PubMed] [Google Scholar]
- 3.Bruschi G, De Marco F, Fratto P, et al. Direct aortic access through right minithoracotomy for implantation of self-expanding aortic bioprosthetic valves. J Thorac Cardiovasc Surg. 2010;140:715–717. doi: 10.1016/j.jtcvs.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Da Gama Ribeiro V, Vouga L, Markowitz A, et al. Vascular access in transcatheter aortic valve implantation. Int J Cardiovasc Imaging. 2011;27:1235–1243. doi: 10.1007/s10554-011-9900-8. [DOI] [PubMed] [Google Scholar]
- 5.Bosmans JM, Kefer J, De Bruyne B, et al. Procedural, 30-day and one year outcome following CoreValve or Edwards transcatheter aortic valve implantation: results of the Belgian National Registry. Interact Cardiovasc Thorac Surg. 2011;12:762–767. doi: 10.1510/icvts.2010.253773. [DOI] [PubMed] [Google Scholar]
- 6.Witkowski A, Dąbrowski M, Chmielak Z, et al. Transcatheter aortic valve implantation using transfemoral/transsubclavian or transapical approach: 30-day follow-up of the initial 30 patients. Kardiol Pol. 2011;69:105–114. [PubMed] [Google Scholar]
- 7.Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv. 2010;3:359–366. doi: 10.1161/CIRCINTERVENTIONS.109.930453. [DOI] [PubMed] [Google Scholar]
- 8.Moynagh AM, Scott DJ, Baumbach A, et al. CoreValve transcatheter aortic valve implantation via the subclavian artery: comparison with the transfemoral approach. J Am Coll Cardiol. 2011;57:634–635. doi: 10.1016/j.jacc.2010.08.642. [DOI] [PubMed] [Google Scholar]
- 9.Fraccaro C, Napodano M, Tarantini G, et al. Expanding the eligibility for transcatheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system. JACC Cardiovasc Interv. 2009;2:828–833. doi: 10.1016/j.jcin.2009.06.016. [DOI] [PubMed] [Google Scholar]