Abstract
The intracellular α-synuclein (α-syn) protein, whose conformational change and aggregation have been closely linked to the pathology of Parkingson’s disease (PD), is highly populated at the presynaptic termini and remains there in the α-helical conformation. In this study, circular dichroism confirmed that natively unstructured α-syn in aqueous solution was transformed to its α-helical conformation upon addition of trifluoroethanol (TFE). Electrochemical and UV–visible spectroscopic experiments reveal that both Cu(I) and Cu(II) are stabilized, with the former being stabilized by about two orders of magnitude. Compared to unstructured α-syn (Binolfi et al., J. Am. Chem. Soc. 133 (2011) 194–196), α-helical α-syn stabilizes Cu(I) by more than three orders of magnitude. Through the measurements of H2O2 and hydroxyl radicals (OH•) in solutions containing different forms of Cu(II) (free and complexed by unstructured or α-helical α-syn), we demonstrate that the significantly enhanced Cu(I) binding affinity helps inhibit the production of highly toxic reactive oxygen species, especially the hydroxyl radicals. Our study provides strong evidence that, as a possible means to prevent neuronal cell damage, conversion of the natively unstructured α-syn to its α-helical conformation in vivo could significantly attenuate the copper-modulated ROS production.
Keywords: α-helix, α-synuclein, Parkinson’s disease, copper, binding affinity, reactive oxygen species
1.Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease (next only to Alzheimer’s disease or AD) and affects more than 1% of the people over the age of 65 in the United States [1]. One hallmark of PD is the progressive loss of dopaminergic neurons in the mid-brain region referred to as the substantia nigra [1]. The degenerating dopaminergic neurons develop a characteristic deposition of Lewy bodies (LB) comprised of abundant fibrils of α-synuclein (α-syn) [2]. The α-syn protein consists of 140 amino acids, whose sequence is shown in Scheme 1.
Scheme 1.
The sequence of α-syn with its N-terminus expressed in italics and its C-terminus underlined.
Metal ions have been suggested to play an important role in the etiology of PD because high concentrations of metal ions were detected in LB [3,4]. Among all the metal ions, copper has attracted extensive attention because abnormal homeostasis of Cu(II) has been found in PD patients [5] and Cu(II) has been demonstrated to accelerate the formation of the α-syn fibrils [6,7]. Furthermore, α-syn can sequester Cu(II) released from aberrant proteinns of PD patients [8]. In biological milieu, redox metal ions such as Cu(II) and Fe(II) are tightly regulated, because free forms of these ions can initiate redox cycles, leading to the production of reactive oxygen species (ROS) such as hydroxyl radicals (OH•) through the Harber-Weiss and Fenton-like reactions:[9]
| (1) |
| (2) |
| (3) |
Several in vitro studies have revealed that the natively unstructured α-syn protein is capable of binding Cu(II), with the strongest binding domain in the N-terminus and Met–1 as the anchoring site [10-16]. However, the dissociation constants (KD) reported range from sub-μM to sub-nM [10-16]. Such a wide discrepancy may stem from the differences in the electrolyte solutions (type of buffer, ionic stength, pH, etc.), choices of the competitive ligands, and the detection methods. We have recently shown that copper complexes of amyloidogenic proteins (e.g., α-syn [17], Aβ [18] and prion protein-derived peptides [9]), upon reduction by common cellular reductants, can generate H2O2 (another ROS) in the presence of oxygen. Using ascorbic acid (AA) oxidation to dehydroascorbate (DA) as an example, the following redox cycle [17] is thermodynamically favorable:
| (4) |
| (5) |
Binding of metal ions by α-helical α-syn might be different from that by natively unstructured α-syn. Studying Cu(II) binding by α-helical α-syn should be more biologically relevant, because α-syn is highly populated at the presynaptic termini (PST) and remains at such an interface in the α-helical conformation [19,20]. Moreover, release of metal ions by neurons during their depolarization and neurotransmission is also an interfacial process. Indeed, recently Lee and coworkers demonstrated that lipids (a good simulant of the presynaptic terminal) favor the formation of α-helical α-syn, resulting in an increase of binding affinity of α-syn to Cu(II) by 3.3 times [21]. However, whether Cu(I) reduced from Cu(II) can be stabilized by α-helical α-syn, and if so, how much it is stabilized, have not been examined. As can be seen from both of the aforementioned redox cycles involving copper, reoxidation of Cu(I) back to Cu(II) determines the amount and kinetics of ROS production.
The main objective of this work is centered on the study of the Cu(II) and Cu(I) binding affinity of α-helical α-syn (particularly the latter) with respect to that of the unstructured form, and the relationship between the binding affinity and the attenuation/inhibition of the production of reactive oxygen species (OH• and H2O2). Circular dichroism (CD) spectroscopy confirmed that α-helical α-syn was formed by adding trifluoroethanol (TFE) to an aqueous solution of α-syn. We employed UV-visible (UV-vis) spectroscopy to determine the affinity of α-helical α-syn to Cu(II), and utilized electrochemistry to measure the affinity to Cu(I). Furthermore, the concentrations of H2O2 and OH• in solutions containing different forms of Cu(II) (free and those complexed by unstructured and α-helical α-syn) were measured by electrochemical and fluorescence methods, respectively. We demonstrate, for the first time, that the dramatic stabilization of Cu(I) by α-helical α-syn leads to a significantly attenuated ROS production.
2. Materials and methods
2.1. Materials
Sodium hydroxide, sodium chloride, LB broth, ammonium sulfate, cupric sulfate, guanidine hydrochloride, ampicillin, 3-(N-morpholino)-propanesulfonic acid (MOPS), TFE, isopropyl β-D-thioga-lactopyranoside (IPTG), trifluoroacetic acid, and other organic solvents were obtained from Thermo Fisher Scientific, Inc. (Pittsburgh, PA). Wang resin, Fmoc-protected amino acids, triisopropylsilane, diisopropylcarbodiimide, 1-hydroxylbenzotriazole, and piperidine were purchased from Anaspect Inc. (San Jose, CA). The chromophoric chelator, Mag-Fura-2 (MF), was obtained from Invitrogen Corp (Carlsbad, CA). Coumarin-3-carboxylic acid (3-CCA) and ferrocene monocarboxylic acid (Fc-COOH) were received from Sigma-Aldrich (Milwaukeee, WI). All aqueous solutions were prepared with deionized water (18 MΩ · cm) treated with a water purification system (Simplicity Plus, Millipore Corp., Billerica, MA). The synthesis and purification of α-syn(1–19) have been described in our previous work [17].
2.2. Expression and purification of α-syn
The wild-type human α-syn was expressed and purified following our published procedure [17]. Briefly, the pRK172 plasmid was transformed to E. coli strain BL21 (DE3) and IPTG was used to induce the expression. E. coli cells were harvested by centrifugation and subsequently resuspended in 10 mM phosphate buffer solution (pH 7.4). This step was followed by incubation with lysozyme. The α-syn-containing supernatant was separated via precipitation, centrifugation, and freeze-drying. Purification was performed using a semi-preparative reversed-phase HPLC (Shimadzu 6AD, Columbia, MO) system equipped with a column (Jupiter-10μm-C18-300Ǻ, dimension of 250 × 4.6 mm i.d.) from Phenomenex (Torrance, CA). The mobile phases were 0.1% trifluoroacetic acid in water (v/v, mobile phase A) and 0.1% trifluoroacetic acid in acetonitrile (v/v, mobile phase B). The flow rate was set at 4.75 mL/min, and the elution gradient was 25–75% B for 20 min.
2.3. Circular dichroism spectroscopy
The CD spectra were collected on a Jasco J-815 spectropolarimeter (Jasco, Tokyo, Japan). A 0.1 mm path length quartz cell with a 300 μL capacity was used to record data between 185 and 280 nm at a scan speed of 20 nm min−1 and a response time of 8 s. The experiments were performed at room temperature in 0.1 mg mL−1 protein solution. The samples were prepared by dissolving lyophilized α-Syn in aqueous MOPS (10 mM, pH = 7.4) or that containing 50% TFE (v/v).
2.4. Ultraviolet-visible spectroscopy
Ultraviolet-visible absorbance measurements were performed using a Cary 100 UV-vis spectrometer (Agilent Technologies, Palo Alto, CA). The binding affinity of α-syn to Cu(II) was determined through the well-established competitive binding assay using MF [10]. The 1 mM CuSO4 stock solution was prepared with 1 mM H2SO4. Aliquots of the stock Cu(II) solution were added to the MOPS buffer containing 5 μM MF. The dissociation constant of the MF–Cu(II) complex (KD) was determined from the absorbance at 370 nm plotted as a function of the Cu(II) concentration, [L], using the following equation:
| (Eq.1) |
where A0 and AL are the absorbance readings of MF at 370 nm in the absence and presence of Cu(II), respectively. Aα is the steady-state absorbance of the MF solution that is saturated with Cu(II). [M0] is the formal concentration of MF.
To determine the α-syn–Cu(II) dissociation constant K’D(MF–Cu(II)), Cu(II) stock solution was added to a mixture of 2.5 μM MF and 50 μM of α-syn. The apparent dissociation constant K’D(MF–Cu(II)) was determined using Eq. 1. The dissociation constant (K’D(MF–Cu(II))) was then calculated from Eq. 2:
| (Eq.2) |
where K’D(MF–Cu(II)) is the real dissociation constant of the Cu(II) complex of α-syn.
2.5. Electrochemical measurement
Voltammetric measurements of the Cu(II) complexes of unstructured and α-helical α-syn were carried out on a CHI832 electrochemical workstation (CH Instruments, Austin, TX) in a homemade plastic electrochemical cell. A glassy carbon disk (3 mm in diameter), a platinum wire, and a Ag/AgCl electrode served as the working, auxiliary, and reference electrodes, respectively. The electrolyte solution was a 10 mM MOPS buffer (pH 7.4) comprising 0.1 M Na2SO4 in water or in 50% TFE (v/v).
2.6. Binding affinity between Cu(I) and α-helical α-syn
We measured the dissociation constant of the Cu(I) complex of α-helical α-syn from the shift of the redox potential of α-helical α-syn–Cu(II) with respect to that of free Cu(II)/Cu(I):[22]
| (Eq.3) |
Where is the stadard potential of the Cu(II)/Cu(I) couple and E0a-syn–Cu(II)/a-syn–Cu(I) is that of the α-syn–CU(II)/α-syn–Cu(I) couple, which was assumed to be the average (0.097 V) of the oxidation (0.137 V) and reduction (0.056 V) potentials. KD(a-syn–Cu(II)) is the dissociation constant of the Cu(II) complex of α-helical α-syn, which can be determined from the mf assay. Thus, the reciprocal of KD(a-syn–Cu(I)), which is also KA(a-syn–Cu(I)) (binding affinity of α-syn to Cu(I)), can be deduced.
2.7. Determination of hydrogen peroxide concentrations
H2O2 in TFE-containing and TFE-free solutions was determined in a thin-layer electrochemical flow cell (Bioanalytical System Inc., West Lafayette, IN) using a hydrogel-peroxidase-modified glassy carbon electrode, as described in our previous study [17]. Both solutions contained 10 μM α-syn, 5 μM Cu(II) and 200 μM AA. Upon incubation of these solutions for predetermined times, they were diluted five-fold with 10 mM MOPS buffer prior to injections into the electrochemical flow cell.
2.8. Hydroxyl radical detection
Fluorescence measurements were carried out at room temperature using a Varian fluorescence spectrophotometer (Cary Eclipse). A modified version of the coumarin-3-carboxylic acid (3-CCA) assay was used to detect the hydroxyl radical [23]. Hydroxyl radicals react with 3-CCA to produce 7-OHCCA, which emits fluorescence at ~450 nm upon excitation at 395 nm. The blank solution was MOPS buffer comprising only 1 mM 3-CCA and 200 μM AA and its background signal had been subtracted from all of the fluorescence values in this study.
3. Results and discussion
3.1. The α-syn protein adopts an α-helical conformation in a mixture of TFE and water
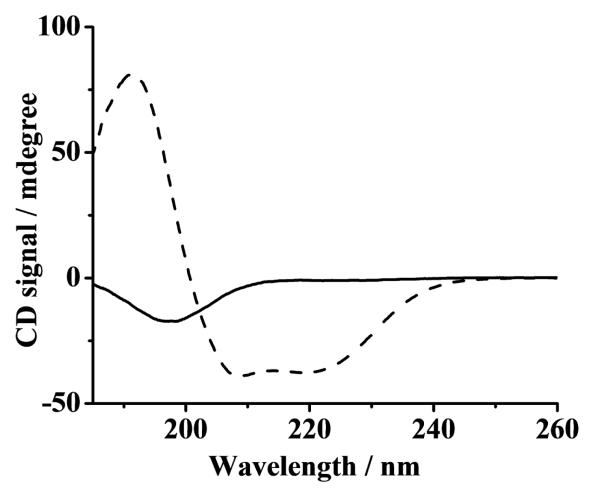
As mentioned in the introduction, in vivo α-syn adopts the α-helical conformation at presynaptic termini. However, thus far, information about α-syn binding to copper in the α-helical conformation, particularly to the reduced form of copper (Cu(I)) is rather limited. The mixed TFE/water system has been used to induce conformational changes of proteins [24] and to mimic the cell membrane environment [25]. We therefore adopted this mixed solvent system to convert the natively unstructured conformation of α-syn to its α-helical conformation by adding 50% TFE to an aqueous solution. Circular dichroism spectroscopy was exploited to confirm such a conversion in the secondary structure. The CD spectra of α-syn in aqueous solution was characterized by a negative peak at 199 nm (solid curve in Fig.1), which is indicative of the unstructured conformation. When TFE was added to the solution, two negative peaks (at 222 and 209 nm) and a positive peak at 192 nm (dashed curve) [26] were observed. Therefore, in this mixed solvent system, the stable conformation of α-syn is α-helical.
Fig. 1.
CD spectra of 0.1 mg mL−1 α-syn in water (solid curve) and in a TFE/water mixture (v/v = 50/50; dashed curve). Both solutions contained 10 mM MOPS with pH 7.4.
3.2. Cu(II) binding by α-helical α-syn is enhanced over binding by the unstructured conformation
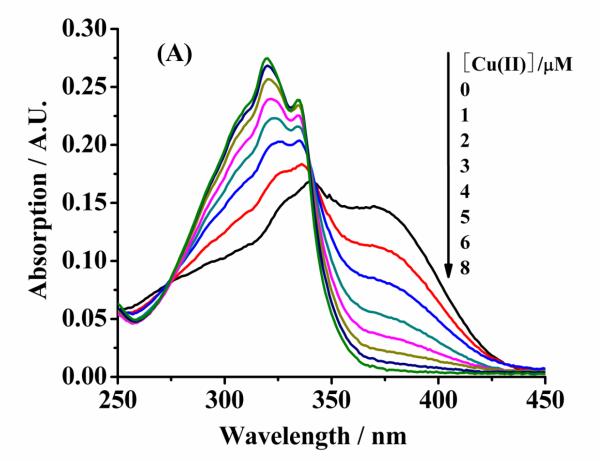
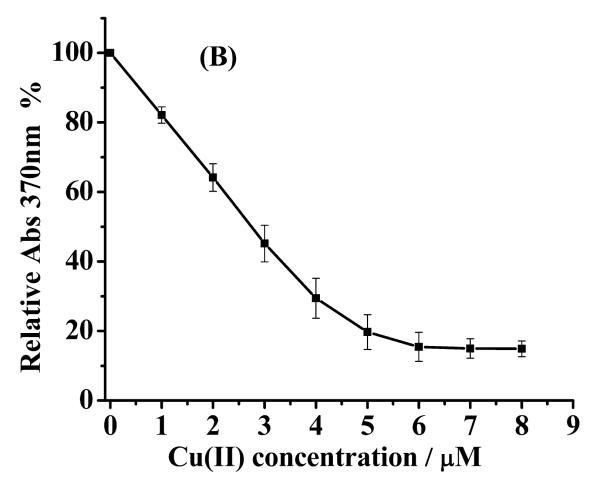
Because wild-type α-syn does not contain any fluorescent amino acid residues at its N-terminus, we used the metal chelator, MF, in a competitive binding assay [10] to determine KD(a-syn–Cu(II)) of unstructured or α-helical α-syn. As shown in Fig. 2A, pure MF shows a maximum UV-vis peak at 340 nm and a shoulder peak at 370 nm. The shoulder peak at 370 nm decreases with the increase of Cu(II) concentration, whereas two new peaks appear at 320 and 335 nm. As mentioned in the Experimental section, the peak intensity at 370 nm can be substituted into Eq. 1 to calculate KD(MF–Cu(II)). KD(MF–Cu(II)) was determined to be 25 ± 3 nM (Fig. 2B).
Fig. 2.
(A) UV-Vis spectra of 5 μm mf in the presence of different Cu(II) concentrations in mops. (B) A plot of absorbance at 370 nm vs. [Cu(II)] (solid squares, which are the averages of three replicates). the fitted curve was obtained using EQ. 1.
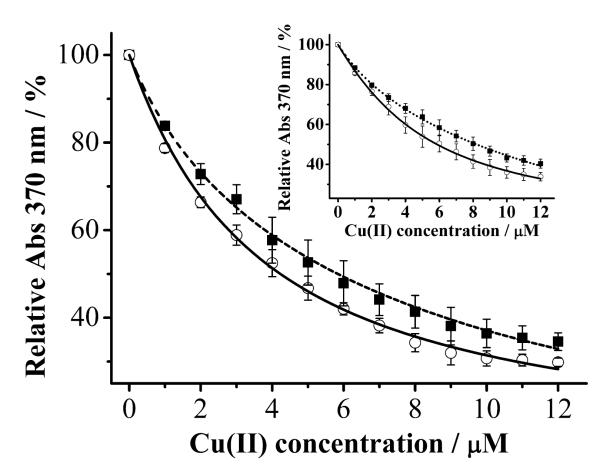
In the presence of α-syn, the apparent dissociation constant K’D(MF–Cu(II)) can also be calculated by fitting the data acquired at 370 nm. The solid curve in Fig. 3 shows the decay of the MF peak intensity at 370 nm upon addtion of various amounts of Cu(II) into an aqueous α-syn solution, whereas the dashed curve corresponds to that in the mixed solvent system. By fitting the data with Eqs. 1 and 2, values of KD(a-syn–Cu(II)) for unstructured α-syn and α-helical α-syn were determined to be 420 ± 30 and 56 ± 12 nM, respectively. The KD(a-syn–Cu(II)) for unstructured α-syn is in good agreement with those reported by Lee and co-workers [11,12] and Fernández and co-workers [7,10]. However, our value are about three orders of magnitude higher (weaker binding) than those determined from electron paramagnetic resonance and isothermal titration calorimetric measurements [13,16]. As mentioned in the Introduction, the discrepancy might be originated from the different experimental conditions and detection principles. We also studied the interaction of α-syn(1–19) and Cu(II) in TFE-free and TFE-containing solutions (inset) and found KD of the Cu(II) complex of α-syn(1–19) in the former solution (347 ± 32 nM) to be higher than in the latter (52 ± 9 nM). In a separate CD measurement (data not shown), we confirmed that α-syn(1–19) is also in the α-helical conformation in the TFE-containing solution. Therefore, the analougous behaviors between the full-length α-syn and α-syn(1–19) suggests in both the natively unstructured and α-helical conformations, the major Cu(II)-binding site is located in the N-terminus of α-syn. This point is consistent with findings by Lee and co-workers [11,21].
Fig. 3.
Absorbance of MF (2.5 μM) at 370 nm plotted as a function of [Cu(II)] in an aqueous α-syn solution (50 μM; solid curve) or an α-syn solution containing 50% TFE (dashed curve). Inset: The same experiments performed with α-syn(1–19) in aqueous solution (50 μM; solid curve) and an α-syn(1–19) solution containing 50% TFE (dotted curve). The curve fitting was obtained using Eq. 1.
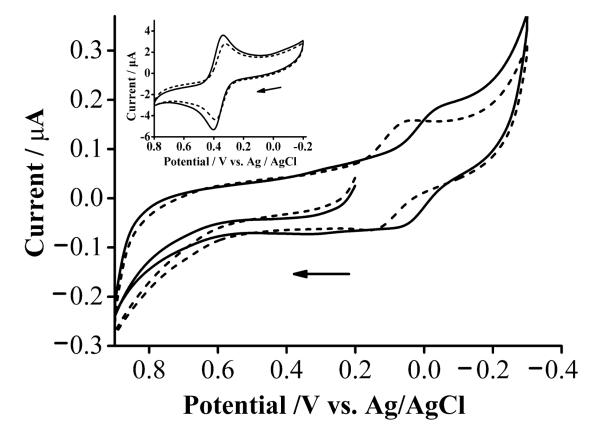
3.3. Alpha-helical α-syn stabilizes Cu(I) over Cu(II)
We collected a cyclic voltammogram of the Cu(II) complex of α-helical α-syn (solid cuve in Fig. 4) and compared it to that of the complex formed between Cu(II) and unstructured α-syn (dashed curve). The former complex exhibits a greater reversibility [27], as reflected by a smaller peak potential difference (0.081 V) and a peak current ratio (0.93) greater than the latter (0.088 V and 0.88, respectively). The redox wave of the former (E1/2 = 0.097 V, which is the average of the oxidation and reduction peak potentials) is shifted in the anodic direction by 0.093 V with respect to that of the latter (E1/2 = 0.004V). To account for possible effects caused by the change in the dielectric constant (or uncompensated solution resistance), we have conducted voltammetric measurements of Fc-COOH in TFE-containing and TFE-free solutions (inset of Fig. 4). The redox wave of Fc-COOH in the mixture of TFE and water is shifted by 0.014 V with respect to that of Fc-COOH in water. Therefore, we estimated the actual shift to be 0.093 ± 0.014 V. From eq. 3, such a shift corresponds to a ratio of KD(a-syn–Cu(I)) over KD(a-syn–Cu(II)) in the range of 100–300. Thus, our results indicate that the stabilization of Cu(I) by α-helical α-syn is about two orders of magnitude more than that of Cu(II). Previously we published a procedure for estimating the KD value of the iron complex of unstructured α-syn [22]. Similarly, KD(a-syn–Cu(I)) of α-helical α-syn was estimated to be between 0.19 and 0.56 nM, which is more than three orders of magnitude lower than that of the Cu(I) complex of unstructured α-syn (~ 2 μM determined with NMR by Binolfi et al. [28]). We should caution that the absolute KD(a-syn–Cu(I)) value is dependent on the exact KD(a-syn–Cu(II)) value reported in the literature [10-16], as KD(a-syn–Cu(I)) is deduced from the ratio of these two dissociation constants. Given the wide range of variability of the KD(a-syn–Cu(II)) values reported thus far, the final KD(a-syn–Cu(I)) might need be corrected when the field settles on the final KD(a-syn–Cu(II)) value. Nevertheless, our derivation and conclusion are internally consistent in that KD(a-syn–Cu(I)) is always 100–300 lower than KD(a-syn–Cu(II)). Such a range of elevation is significant and indicates the Cu(I) center is strongly coordinated by α-syn in the α-helical conformation.
Fig. 4.
Cyclic voltammograms of the α-syn–Cu(II) complex (formed using 100 μM Cu(II) and 100 μM α-syn) in an aqueous solution (solid curve) and in a TFE/water solution (dashed curve). Inset: cyclic voltammograms of 1.0 mM Fc-COOH in an aqueous solution (solid) and a TFE/water mixture solution (dashed). All solutions contained 0.1 M Na2SO4, with pH 7.4 maintained by MOPS. The scan rate was 5 mV s−1 and the solid arrow indicates the initial scan direction.
3.4. Alpha-helical α-syn impedes copper redox cyclying and attenuates ROS production
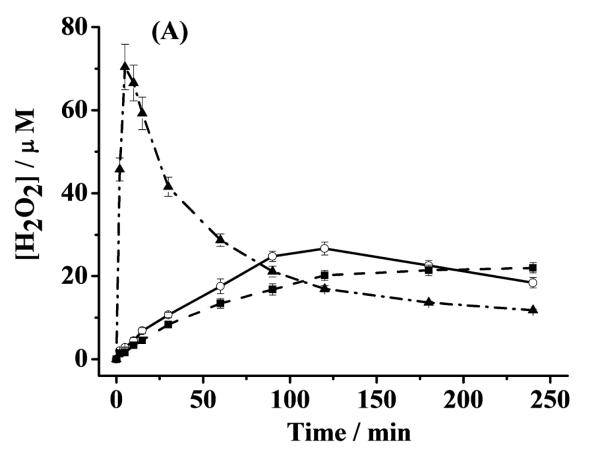
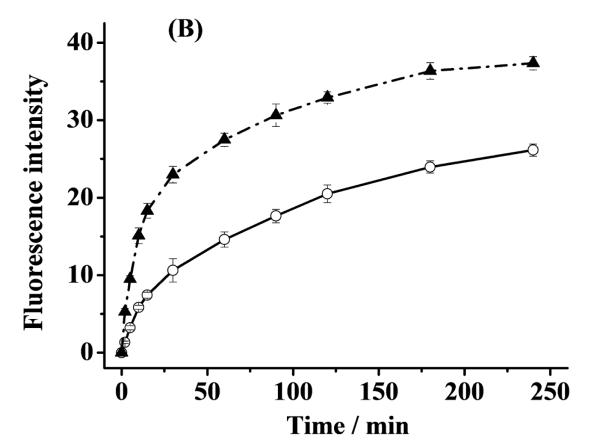
The significant stabilization of Cu(I) by α-helical α-syn suggests that α-helical α-syn should be able to attenuate or inhibit ROS production by slowing or shutting down the turn-over of Cu(I) to Cu(II) (e.g., in reaction 3 or 5). To verify this, we measured both the H2O2 and OH• concentrations in aerated solutions containing α-helical α-syn–Cu(II), unstructured α-syn–Cu(II), and free Cu(II) over a span of 4 h. To initiate the redox cycle, AA was used to reduce the complexed and free Cu(II) to their Cu(I) counterparts. As shown by the dash-dotted curve in Fig. 5A, in the presence of free Cu(II), the amount of H2O2 increases sharply at the beginning but decays precipitiously at ca. 5 min. In the unstructured α-syn–Cu(II) solution, the trend is similar, though the amount of H2O2 produced is considerably smaller and the decay is also much more gradual. Furthermore, the decay did not begin until ca. 120 min. In contrast, the H2O2 concentration in the α-helical α-syn–Cu(II) solution rises even more gradually and levels off (instead of decreasing) after about 125 min. These trends are highly comparable to those in our previous studies on the attenuation or inhibition of H2O2 production by peptides derived from prion protein (PrP) with high and low copper occupancies [9]. In Fig. 5A, the highest H2O2 concentration in the free Cu(II) soution is ca. 60% greater than that in the unstructured α-syn–Cu(II) solution, whereas the peak H2O2 concentration in the unstructured α-syn–Cu(II) solution is 24% higher than the highest amount of H2O2 produced in the α-helical α-syn–Cu(II) solution. As suggested in our previous work [9], the initial sharp rise of H2O2 in the free Cu(II)-containing solution can be partially attributed to the rapid production of H2O2 by free Cu(II), and the drastic decay suggests that the Fenton-like reaction (cf. reaction 3 in Introduction), in which the freshly generated H2O2 begins to react with free Cu(II) at an appreciable rate to produce OH•, has become predominant. In other words, as H2O2 accumulates in the solution, reactions 1–3 will accelerate and [OH•] increases at the expense of [H2O2]. Unlike our previous work on copper complexes of the PrP peptides wherein only [H2O2] was measured [9], for this study we determined [OH•] in solutions that had been incubated for predetermined times. The occurrence of the Fenton-like reaction is confirmed by the dash-dotted curve in Fig. 5B, which shows that a rapid rise of [OH•] (measured by the increased CCA fluorescence) is followed by a gradual change. Interestingly, when Cu(II) is complexed by unstructured α-syn, [OH•] produced by the Fenton-like reaction (solid curve in Fig. 5B) is smaller. After about 30 min, [OH•] in the unstructured α-syn–Cu(II) solution is typically 35% less than that in the solution wherein Cu(II) is uncomplexed. This suggests that complexation of Cu(II) by unstructured α-syn helps scavenge free Cu(II) in solution and slows down the ROS production.
Fig. 5.
(A) Concentrations of H2O2 generated by α-helical α-syn–Cu(II)(dashed curve), unstructured α-syn–Cu(II)(solid curve) and free Cu(II) (dash-dotted curve). [AA], [α-syn] and [Cu(II)] were 200, 10 and 5 μM, respectively. (B) Changes of [OH•] plotted as the 7-OHCCA fluorescence intensity in the presence of free Cu(II) (5 μM; dash-dotted curve) and the Cu(II) complex of unstructured α-syn (solid curve; 5 μM Cu(II) and 10 μM α-syn). Both solutions contained 200 μM AA and 1 mM 3-CCA. The error bars are relative standard deviations of three replicates.
That α-helical α-syn is more effective in impeding the H2O2 production in copper redox cycling is interesting. We also attempted to measure the CCA fluorescence in solution containing α-helical α-syn–Cu(II) but did not detect any CCA flurescence signal. This observation suggests that either production of OH• through reactions 1–3 is completely inhibited by α-helical α-syn or any trace amount of OH• might have reacted away with TFE. We believe that the former process is more likely because the shape of the dashed curve in Fig. 5A is sigmoidal (i.e., no decay was observed). The absence of a decay suggests that conversion of H2O2 to OH• probably does not take place. We attribute the substaintial retardation or complete inhibition of the OH• production to the significant stabilization of Cu(I) by α-helical α-syn.
3.5. Biological implications
In vivo, the α-syn concentration in plasma is rather low (~ 20 nM) [29]. Presynaptic terminals help convert the natively unstructured α-syn into the α-helical conformation, allowing α-syn to be compacted or enriched [20]. It is interesting to note that at the cell membrane/cytosol interface, depolarization of cell membrane often accompanies metal ion release. It is possible that Cu(II) released from such a process or bound by lipid [13] can be sequestered by α-helical α-syn. Given the nM affinity constants of unstructured [13,16] and α-helical α-syn [21] to Cu(II), it is possible that α-helical α-syn can also seize Cu(II) from other copper-transport proteins [30,31]. In brain, abundant antioxidants exist to counter the effects of oxidative stress/damage, these species (e.g., AA and glutathione) have rather low reduction potentials and can readily reduce the Cu(II) center in the α-helical α-syn-copper complex to Cu(I).
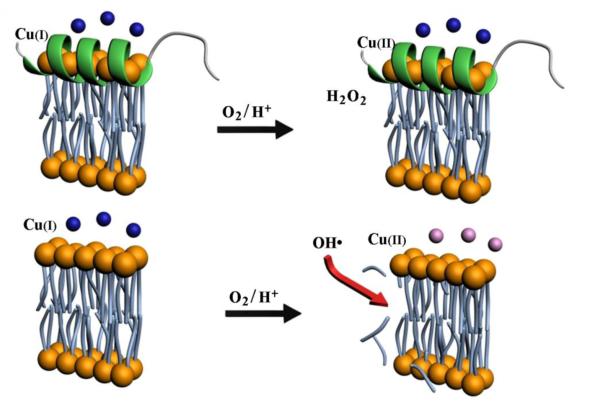
OH• is highly reactive and can modify unsaturated lipids, damage nucleic acids, and oxidize proteins, all of which have been detected in PD brain [32]. On the other hand, H2O2 has a dual function: at high concentration (mM or sub-mM levels) it is a ROS that can inflict oxidative damage, but at low concentration (μM level) it can serve as a signaling molecule [33]. The significant stabilization of Cu(I) by α-helical α-syn leads to a gradual and controlled production of H2O2 (cf. Fig. 3A) and possibly a complete quenching of the OH•-producing copper redox cycle (i.e., reactions 1–3). Such a stabilization has at least two beneficial outcomes. First, α-helical α-syn probably plays a protective role in maintaining the integrity of the neuronal cells (i.e., keeping nucleic acids, proteins, and lipids intact) by complexing copper and stabilizing copper in the Cu(I) state so that H2O2 is produced in a controlled fashion and generation of the highly reactive OH• is substainially retarded. Second, it is well established that the PD pathology is closely associated with loss of dopaminergic neurons. Since dopamine is released from dopaminergic neurons at PST and in a OH•-rich milieu can be chemically modified into other forms (e.g., 6-hydroxydopamine, which is highly neurotoxic), we posit that another benefitial effect of stabilizing Cu(I) by α-helical α-syn could be that dopamine released from vesicles will remain intact in a OH•-deficient environment. Consequently, intact dopamine molecules released by PST can be properly captured by dopamine receptors at PST, be internalized by the signal-transmitting neurons, or be quickly cleared from the extracellular milieu. We should also note that the integrity of dopamine-encapsulating vesicles can also be affected by OH• through the lipid peroxidation process, which affects their proper fusion with neuronal membrane [34]. The inhibition/attenuation of free copper-induced ROS production by α-helical α-syn and the circumvention of the abovementioned damages at a cell membrane are pictorially shown in Fig. 6.
Fig. 6.
A model showing the stabilization of Cu(I)by α-helical α-syn at part of a vesicle or cell membrane (top; shown as a lipid bilayer). The stabilization can significantly attenuate the copper-induced OH• production. Without α-syn, copper could generate a high level of OH• via the Harber-Weiss and/or Fenton-like reactions, which in turn would damage cell membrane via lipid peroxidation or cleavage of unsaturated lipids, as shown at the bottom of the model. The OH• radicals can also modify dopamine (denoted by blue spheres) into other forms such as 6-hydroxydopamine (denoted by pink spheres).
4. Conclusions
Using UV-visible spectroscopy and electrochemistry, we have shown that α-helical α-syn binds Cu(II) and Cu(I) more strongly than its unstructured conformation. To the best of our knowledge, this is the first study that demonstrates the binding affinity between α-helical α-syn and Cu(I) is substantially greater than that between α-helical α-syn and Cu(II). Compared to the complex formed between Cu(I) and unstructured α-syn [28], the affinity constant (~1010 M−1) of the α-helical α-syn–Cu(I) complex is more than four orders of magnitude higher. In line with this finding, H2O2 generated through the copper redox cycling is at a tolerable level (< 30 μM as shown in Fig. 5A), while OH• produced via the Fenton-like reaction is kept at a minimal concentration. Our study provides strong evidence that, as a possible means to prevent neuronal cell damage/death, conversion of the natively unstructured α-syn to its α-helical conformation significantly attenuates the copper-modulated ROS production.
Highlights.
Both Cu(I) and Cu(II) are strongly coordinated by α-helical α-syn.
α-helical α-syn stabilizes Cu(I) 104 time more than unstructured α-syn does.
Stabilization of Cu(I)by α-helical α-syn leads to a controlled production of H2O2.
OH• production via copper redox cycling is attenuated by Cu(I) coordination with α-syn.
Acknowledgment
Partial support of this work by an NIH grant (No. SC1NS070155-01 to FZ), an NSF-RUI grant (1112105 to FZ) and the Natural Science Foundation of China (Nos. 20876179 and 21076232 to YL) is gratefully acknowledged.
5. Abbreviations
- α-syn
α-synuclein
- PD
Parkinson’s disease
- TFE
trifluoroethanol
- LB
Lewy bodies
- ROS
reactive oxygen species
- OH•
hydroxyl radicals
- AA
ascorbic acid or ascorbate
- DA
Dehydroascorbate
- PST
presynaptic termini
- MOPS
3-(N-morpholino)-propanesulfonic acid
- IPTG
isopropyl β-D-thioga-lactopyranoside
- MF
mag-Fura-2
- 3-CCA
coumarin-3-carboxylic acid
- Fc-COOH
ferrocene monocarboxylic acid
- CD
circular dichroism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fink AL. Acc. Chem. Res. 2006;39:628–634. doi: 10.1021/ar050073t. [DOI] [PubMed] [Google Scholar]
- 2.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellani RJ, Siedlak SL, Perry G, Smith MA. Acta Neuropathol. 2000;100:111–114. doi: 10.1007/s004010050001. [DOI] [PubMed] [Google Scholar]
- 4.Gaeta A, Hider RC. Br. J. Pharmacol. 2005;146:1041–1059. doi: 10.1038/sj.bjp.0706416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pall HS, Blake DR, Gutteridge JM, Williams AC, Lunec J, Hall M, Taylor A. Lancet. 1987;2:238–241. doi: 10.1016/s0140-6736(87)90827-0. [DOI] [PubMed] [Google Scholar]
- 6.Khan A, Ashcroft AE, Higenell V, Korchazhkina OV, Exley C. J. Inorg. Biochem. 2005;99:1920–1927. doi: 10.1016/j.jinorgbio.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Rasia RM, Bertoncini CW, Marsh D, Hoyer W, Cherny D, Zweckstetter M, Griesinger C, Jovin TM, Fernandez CO. Proc. Natl. Acad. Sci. U.S.A. 2005;102:4294–4299. doi: 10.1073/pnas.0407881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim KS, Choi SY, Kwon HY, Woo MH, Kang TC, Kang JH. Free Radical Bio. Med. 2002;32:544–550. doi: 10.1016/s0891-5849(02)00741-4. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Jiang D, McDonald A, Hao Y, Millhauser GL, Zhou F. J. Am. Chem. Soc. 2011;133:12229–12237. doi: 10.1021/ja2045259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binolfi A, Lamberto GR, Duran R, Quintanar L, Bertoncini CW, Souza JM, Cervenansky C, Zweckstetter M, Griesinger C, Fernandez CO. J. Am. Chem. Soc. 2008;130:11801–11812. doi: 10.1021/ja803494v. [DOI] [PubMed] [Google Scholar]
- 11.Jackson MS, Lee JC. Inorg. Chem. 2009;48:9303–9307. doi: 10.1021/ic901157w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JC, Gray HB, Winkler JR. J. Am. Chem. Soc. 2008;130:6898–6899. doi: 10.1021/ja711415b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudzik CG, Walter ED, Millhauser GL. Biochemistry. 2011;50:1771–1777. doi: 10.1021/bi101912q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Prudent M, Fauvet B, Lashuel HA, Girault HH. ACS Chem. Neurosci. 2011;2:667–675. doi: 10.1021/cn200074d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas HR, Lee JC. J. Inorg. Biochem. 2010;104:245–249. doi: 10.1016/j.jinorgbio.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong L, Simon JD. J. Phys. Chem. B. 2009;113:9551–9561. doi: 10.1021/jp809773y. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Liu L, Zhang L, Peng Y, Zhou F. Biochemistry. 2010;49:8134–8142. doi: 10.1021/bi1010909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang D, Men L, Wang J, Zhang Y, Chickenyen S, Wang Y, Zhou F. Biochemistry. 2007;46:9270–9282. doi: 10.1021/bi700508n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jao CC, Hegde BG, Chen J, Haworth IS, Langen R. Proc. Natl. Acad. Sci. U.S.A. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Shah N, Thakur G, Zhou F, Leblanc RM. Chem. Commun. 2010;46:6702–6704. doi: 10.1039/c0cc02098b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucas HR, Lee JC. Metallomics. 2011;3:280–283. doi: 10.1039/c0mt00088d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng Y, Wang C, Xu H, Liu Y, Zhou F. J. Inorg. Biochem. 2010;104:365–370. doi: 10.1016/j.jinorgbio.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadal RC, Rigby SEJ, Viles JH. Biochemistry. 2008;47:11653–11664. doi: 10.1021/bi8011093. [DOI] [PubMed] [Google Scholar]
- 24.Jayaraman G, Kumar TK, Arunkumar AI, Yu C. Biochem. Biophys. Res. Commun. 1996;222:33–7. doi: 10.1006/bbrc.1996.0693. [DOI] [PubMed] [Google Scholar]
- 25.Munishkina LA, Phelan C, Uversky VN, Fink AL. Biochemistry. 2003;42:2720–2730. doi: 10.1021/bi027166s. [DOI] [PubMed] [Google Scholar]
- 26.Homoelle BJ, Beck WF. Biochemistry. 1997;36:12970–5. doi: 10.1021/bi971121n. [DOI] [PubMed] [Google Scholar]
- 27.Bard AJ, Faulkner LR. Fundamentals and applications. John Wiley & Sons; New York: 2001. Electrochemical methods. [Google Scholar]
- 28.Binolfi A, Valiente-Gabioud AA, Duran R, Zweckstetter M, Griesinger C, Fernandez CO. J. Am. Chem. Soc. 2011;133:194–196. doi: 10.1021/ja107842f. [DOI] [PubMed] [Google Scholar]
- 29.Fjorback AW, Varming K, Jensen PH. Scand. J. Clin. Lab. Invest. 2007;67:431–435. doi: 10.1080/00365510601161497. [DOI] [PubMed] [Google Scholar]
- 30.Madsen E, Gitlin JD. Annu. Rev. Neurosci. 2007;30:317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- 31.Wernimont AK, Yatsunyk LA, Rosenzweig AC. J. Biol. Chem. 2004;279:12269–12276. doi: 10.1074/jbc.M311213200. [DOI] [PubMed] [Google Scholar]
- 32.Uttara B, Singh AV, Zamboni P, Mahajan RT. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veal EA, Day AM, Morgan BA. Mol. Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Urano S, Asai Y, Makabe S, Matsuo M, Izumiyama N, Ohtsubo K, Endo T. Eur. J. Biochem. 1997;245:64–70. doi: 10.1111/j.1432-1033.1997.00064.x. [DOI] [PubMed] [Google Scholar]