Seminal investigations by Frank1 and Starling2 provided the first evidence that the heart possesses inherent reserve capacity—a key principle that is a pillar of modern cardiology research and practice. After 150 years of research, we now understand that cardiovascular reserve capacity (CVRC) is determined by the integrative ability of cross-system mechanisms (eg, neurohormonal, central, and peripheral oxygen delivery3), which collectively possess remarkable adaptive capacity. Sequential as well as concurrent pathologic perturbations to either one or more of these mechanisms are offset by initial compensatory adaptive responses in other component systems to maintain whole-body homeostatic regulation—a process termed coordinated adaptation.4 Unfortunately, CVRC is finite, and continued insults ultimately lead to overt dysfunction (eg, acute coronary syndromes, left ventricular dysfunction). Pathologic impairments in CVRC are etiologic in many chronic disease conditions and are thus an integral consideration in daily practice. The purpose of this commentary is to provide an overview of the guiding principles and application of CVRC in the oncology setting using early breast cancer as an illustrative model.
Measurement of CVRC
In current oncology practice, evaluation of CVRC is almost exclusively determined via resting assessment of left ventricular ejection fraction (LVEF) via echocardiography or radionuclide angiography, typically before administration of potentially cardiotoxic adjuvant therapy. Using these approaches, the incidence of heart failure with modern anthracycline- and trastuzumab-containing regimens is < 5%, with corresponding rates of asymptomatic LVEF reductions between 10% and 50%.5–7 Resting LVEF, however, provides a snapshot of cardiac performance under optimal circumstances. Moreover, in addition to being load, rate, and contractility dependent, resting LVEF is not a sensitive measure of early myocyte (subclinical) damage8 and is not prognostic in patients with preserved LVEF (> 50%).9 Emerging techniques such as tissue Doppler imaging,10,11 speckle-tracking strain echocardiography,12 and magnetic resonance imaging13 may provide more sensitive detection of cardiac injury, although supporting evidence is currently limited. Impairments in other cardiac parameters such as diastolic relaxation and filling also occur after breast cancer treatment, despite preserved LVEF.14–16
The application of system stress is a hallmark method to detect subclinical myocardial impairments and coronary artery disease (CAD).17 Both nonpharmacologic (exercise) and pharmacologic (eg, dobutamine, dipyridamole) stress are commonly applied in conjunction with conventional imaging approaches to detect obstructive CAD.17,18 In this setting, exercise testing and determination of inotropic (contractile) reserve are independent predictors of prognosis beyond clinical factors, coronary anatomy, and LVEF.19,20
Extending Beyond the Heart
CVRC is determined by the integrative capacity of the cardiopulmonary system; thus it seems plausible that therapy-induced myocardial injury may occur in conjunction with (mal)adaptation in other organ components. Many anticancer therapies cause unique and varying degrees of injury to the cardiovascular system (ie, pulmonary-vascular/blood-skeletal muscle axis). For example, radiation and certain forms of systemic therapy (eg, chemotherapy, molecularly targeted therapies) can cause pulmonary dysfunction, anemia, endothelial dysfunction, and arterial stiffness and likely skeletal muscle dysfunction (eg, reduced oxidative phosphorylation).21–25 These direct insults occur in conjunction with indirect lifestyle perturbations (eg, physical inactivity) that synergistically cause marked impairments in CVRC. We have termed this phenomenon the multiple-hit hypothesis.21 Hence, tools with the ability to evaluate integrated cross talk between cardiovascular organ components may arguably provide the most accurate characterization of global (whole-body) CVRC.
To this end, incremental exercise tolerance testing, which evaluates cardiorespiratory fitness (ie, efficiency of the cardiopulmonary system to deliver and use oxygen to resynthesize ATP), is a powerful predictor of cardiovascular and all-cause mortality in a broad range of adult populations.26,27 Of relevance, we found that despite preserved LVEF ≥ 50%, cardiorespiratory fitness was significantly impaired in patients with early breast cancer a mean of 3 years after the completion of adjuvant therapy, compared with women of the same age without a history of breast cancer.28 Specifically, patients reached a predicted cardiorespiratory fitness for a particular age group (eg, 40 years) approximately 20 to 30 years earlier than age-matched women without a history of cancer. The combination of global CVRC assessment with cardiac biomarkers could also provide a powerful approach. Cardiac troponin T, troponin I, and N-terminal pro-brain natriuretic peptide are independent predictors of cardiovascular disease (CVD) mortality in healthy populations29,30 and of cardiotoxicity in patients with hematologic and solid malignancies.31,32
Clearly, a number of tools are available to oncology professionals to both detect and monitor therapy-induced cardiovascular injury. When used appropriately, such tools should accurately characterize CVRC and provide additional decision-making information beyond that provided by current established techniques, allowing more accurate prognostication and early intervention. Evidence-based recommendations to guide the selection of method(s) are not available in the oncology setting but are well established in cardiovascular medicine.33 For example, the American College of Cardiology/American Heart Association recommend that CVRC evaluation of patients presenting with heart failure include subjective symptomatic classifications (ie, New York Heart Association functional classification) as well as exercise tolerance testing.33 Although guidelines are not currently available, we contend that evaluation of CVRC should be initially considered for patients receiving antitumor agents/regimens known to cause cardiac damage or for those presenting with CAD risk factors that increase the risk of cardiotoxicity. However, anticancer agents likely cause unique and varying degrees of injury to all components of the cardiovascular system; thus assessment of CVRC could arguably be considered for all patients initiating adjuvant therapy. Such recommendations are not evidence based at present, and the ideal method(s) for predicting acute and/or late-occurring CVD in the oncology setting has not been established.34 Additional studies are now required to determine the feasibility, cost, and clinical importance of CVRC testing in breast as well as other cancer populations.
Increasing Importance of Evaluating CVRC in Early Breast Cancer
The Childhood Cancer Survivor Study (CCSS), a prospective cohort of more than 20,000 adult survivors of childhood cancer, has demonstrated that significant improvements in cancer-specific survival come at the expense of considerable increased risk of competing causes of morbidity and mortality.35 Specifically, in comparison with a sibling comparison group, the relative risk of congestive heart failure, CAD, and cerebrovascular events was 15.1 (95% CI, 4.8 to 47.9), 10.4 (95% CI, 4.1 to 25.9), and 9.3 (95% CI, 4.1 to 21.1), respectively, 25 years after primary diagnosis.36 The long-term follow-up (> 25 years) results highlight the prolonged latency required from initial exposure to development of major events. Of similar importance, significant improvements in early detection and adjuvant therapy have also resulted in dramatic reductions in the risk of cancer-specific mortality after a diagnosis of early breast cancer.37 Consequently, younger and middle-age patients with breast cancer now have sufficient survival to be at risk for competing mortality. Thus, is it plausible to ask whether the CCSS experience provides a relevant benchmark to estimate the future extent and magnitude of cardiovascular late effects in the estimated 2.5 million adult breast cancer survivors?
Clearly, the mechanisms of injury are likely unique between children and adults, given that anticancer therapy is administered during cardiac development in the pediatric oncology population. Nevertheless, juvenile hearts possess tremendous reserve capacity, which is in contrast to adult patients with cancer, in whom anticancer therapy is generally initiated when CVRC is already significantly diminished because of aging and comorbid conditions (especially in those age ≥ 65 years).38,39 As such, it could be speculated that therapy initiated in elderly patients could cause equal or possibly accelerated manifestation of CVD. Intriguingly, emerging evidence indicates that CVD is now the predominant cause of mortality in the population of women diagnosed with early-stage breast cancer, especially in those diagnosed at age > 50 years.40–43 Moreover, it seems that patients with breast cancer may have excess CVD risk compared with age-matched control women without a history of cancer.44,45 It is important to remember, however, that the burden of CVD in recently published studies reflects the management patterns for early-stage breast cancer 15 to 20 years ago, not those associated with modern therapeutic approaches. On one hand, the risk of late-occurring CVD may be lower because of the introduction of newer radiotherapy techniques and wide recognition of anthracycline-induced cardiotoxicity. On the other hand, these advances could be offset by more aggressive use of anthracyclines (higher doses and shorter intervals between cycles), approval of newer adjuvant cytotoxic agents (taxane-based regimens) and hormonal regimens (aromatase inhibitors), and introduction of molecularly targeted therapies (human epidermal growth factor receptor 2– directed therapies), all of which have different cardiovascular safety profiles than historical regimens. Modern adjuvant therapy is also generally administered for longer durations, with a growing trend toward extended adjuvant therapy, which increases the period of exposure and possibly the extent of cardiovascular injury.
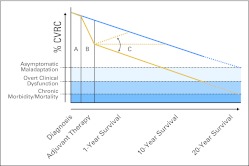
A conceptual model to illustrate the suspected trajectories of therapy-induced declines/recovery in CVRC, based on prior work in early breast cancer,28,47,48 is presented in Figure 1. As outlined, the trajectory of change in CVRC across the breast cancer treatment continuum is contingent on the interaction between patient CVRC at diagnosis, which is determined by nonmodifiable (eg, age, genetic predisposition) and modifiable (eg, lifestyle, CVD risk factors) determinants, and the direct as well as indirect effects of the selected treatment management plan. After adjuvant therapy, a proportion of patients will experience an acute spontaneous recovery in CVRC49,50; a larger proportion, however, will sustain a marked, potentially irreversible,49 impairment in CVRC. As with healthy women, breast cancer survivors are ultimately subjected to the normal age-related increases in risk factor burden and comorbidities that contribute to the established trajectory declines in CVRC.46 Unfortunately, those women experiencing irreversible CVRC impairments during adjuvant therapy will lack the required CVRC to withstand normal age-related pathologies. As a result, the incidence of clinical overt dysfunction occurs at a much earlier age (Fig 1) than that observed in age-matched women without a history of breast cancer.
Fig 1.
Trajectory decline in cardiovascular reserve capacity (CVRC) across the breast cancer survivorship continuum. The postulated trajectories of decline in CVRC in patients with early-stage breast cancer (gold lines) compared with age-matched decline in women without a history of breast cancer (blue line). Specifically, (A) at diagnosis, baseline CVRC is determined by nonmodifiable (age, genetic predisposition) and modifiable risk factors (eg, hypertension, obesity, physical inactivity). (B) After initiation of adjuvant therapy, the extent and magnitude of decline in CVRC is determined by the direct and indirect effects of the selected treatment management plan and its interaction with modifiable and nonmodifiable risk factors. Several trajectories of change in CVRC, as assessed by left ventricular ejection fraction and cardiorespiratory fitness, have been observed (C) after completion of adjuvant therapy: continued declines in CVRC,28,49 spontaneous acute recovery,50 and plateau.51 Ultimately, however, similar to age-matched women without a history of cancer, patients with breast cancer are subjected to normal age-related and comorbid pathologies. As CVRC depletes below a critical threshold, asymptomatic maladaptation occurs, which, without intervention, leads to overt clinical dysfunction (eg, heart failure, myocardial infarction) and ultimately chronic morbidity and premature mortality.
Where to Go Next
If the tenets of the multiple-hit hypothesis are true, why is there not a greater extent of acute and long-term cardiovascular toxicity in recent phase III trials51–53 and meta-analyses?54,55 Several potential explanations may explain this incongruence: one, evaluation of subclinical cardiotoxicity or CVRC is seldom studied or reported in the adjuvant setting; two, most trials, with few exceptions, have limited cardiac follow-up and variable cardiac end point reporting; three, adjudication of cause of death can be unreliable; four, few trials report even 10-year outcomes; five, long-term outcomes are not yet available for adjuvant trastuzumab or third-generation aromatase inhibitor trials; and six, data on the real-world incidence of cardiovascular toxicity are limited in a nontrial context and could be expected to be higher given the preponderance to exclude women with more unfavorable cardiovascular risk profiles.56
Clearly, many questions remain to be addressed, and the clinical utility of CVRC in the oncology setting is in its infancy. Of relevance, the CCSS investigators recently established the St Jude Lifetime Cohort, which will, among other things, evaluate physiologic mechanisms underlying therapy-induced late effects in adult childhood cancer survivors using a wide battery of quantitative assessments together with collection of patient-reported outcomes and tissue specimens.57 The establishment of similar cohort studies in adult patients with breast and other cancers that include evaluation of CVRC are urgently required to fully understand the prevalence, magnitude, and pathophysiologic mechanisms of therapy-induced late cardiovascular effects. Such efforts, in conjunction with existing tools used in the oncology setting, should inform treatment stratification, mortality-risk prediction, and surveillance of therapy-induced toxicity/recovery across the cancer survivorship continuum. Furthermore, this information, in turn, can guide the design and implementation of preventive and early-intervention strategies to abrogate or reverse impairments in CVRC.
In conclusion, it is becoming increasingly apparent that surviving early-stage breast cancer comes with the risk of late-occurring CVD. The importance of CVD is likely to further increase with continual improvements in breast cancer–specific outcomes, along with the rapidly aging population. If current trends continue, adjuvant therapy–associated CVD may hinder further improvement in overall survival after a diagnosis of early breast cancer. The landscape of breast cancer prognosis and survivorship has and will continue to change dramatically over the next two decades; with such changes, the central tenets and implications of CVRC are poised to become increasingly important concepts in individualizing the curative-intent management and long-term surveillance of breast cancer and other adult oncology populations.
Acknowledgment
Supported in part by research Grants No. CA143254, CA142566, CA138634, CA133895, and CA164751 from the National Cancer Institute and by George and Susan Beischer (L.W.J.).
Contributor Information
Graeme J. Koelwyn, School of Health and Exercise Sciences, University of British Columbia, Kelowna, British Columbia, Canada.
Michel Khouri, Duke University Medical Center, Durham, NC.
John R. Mackey, Cross Cancer Institute, Edmonton, Alberta, Canada.
Lee W. Jones, Duke University Medical Center, Durham, NC.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: John R. Mackey, Roche, sanofi-aventis Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Frank O. Zur Dynamik des Herzmuskels [in German] Z Biol. 1895;32:370–437. [Google Scholar]
- 2.Starling EH, Visscher MB. The regulation of the energy output of the heart. J Physiol. 1927;62:243–261. doi: 10.1113/jphysiol.1927.sp002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brilla CG, Maisch B. Regulation of the structural remodelling of the myocardium: From hypertrophy to heart failure. Eur Heart J. 1994;15(suppl D):45–52. doi: 10.1093/eurheartj/15.suppl_d.45. [DOI] [PubMed] [Google Scholar]
- 4.Hsia CC. Coordinated adaptation of oxygen transport in cardiopulmonary disease. Circulation. 2001;104:963–969. doi: 10.1161/hc3401.094928. [DOI] [PubMed] [Google Scholar]
- 5.Meinardi MT, van Veldhuisen DJ, Gietema JA, et al. Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol. 2001;19:2746–2753. doi: 10.1200/JCO.2001.19.10.2746. [DOI] [PubMed] [Google Scholar]
- 6.Perez EA, Suman VJ, Davidson NE, et al. Effect of doxorubicin plus cyclophosphamide on left ventricular ejection fraction in patients with breast cancer in the North Central Cancer Treatment Group N9831 Intergroup adjuvant trial. J Clin Oncol. 2004;22:3700–3704. doi: 10.1200/JCO.2004.03.516. [DOI] [PubMed] [Google Scholar]
- 7.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2–overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 8.Ewer MS, Ali MK, Mackay B, et al. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving adriamycin. J Clin Oncol. 1984;2:112–117. doi: 10.1200/JCO.1984.2.2.112. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Evans JC, Benjamin EJ, et al. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 10.Dokainish H, Sengupta R, Pillai M, et al. Assessment of left ventricular systolic function using echocardiography in patients with preserved ejection fraction and elevated diastolic pressures. Am J Cardiol. 2008;101:1766–1771. doi: 10.1016/j.amjcard.2008.02.070. [DOI] [PubMed] [Google Scholar]
- 11.Weidemann F, Jamal F, Sutherland GR, et al. Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am J Physiol Heart Circ Physiol. 2002;283:H792–H799. doi: 10.1152/ajpheart.00025.2002. [DOI] [PubMed] [Google Scholar]
- 12.Neilan TG, Jassal DS, Perez-Sanz TM, et al. Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury. Eur Heart J. 2006;27:1868–1875. doi: 10.1093/eurheartj/ehl013. [DOI] [PubMed] [Google Scholar]
- 13.Lightfoot JC, D'Agostino RB, Jr, Hamilton CA, et al. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. 2010;3:550–558. doi: 10.1161/CIRCIMAGING.109.918540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho E, Brown A, Barrett P, et al. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: A speckle tracking echocardiographic study. Heart. 2010;96:701–707. doi: 10.1136/hrt.2009.173997. [DOI] [PubMed] [Google Scholar]
- 15.Nagy AC, Cserép Z, Tolnay E, et al. Early diagnosis of chemotherapy-induced cardiomyopathy: A prospective tissue Doppler imaging study. Pathol Oncol Res. 2008;14:69–77. doi: 10.1007/s12253-008-9013-4. [DOI] [PubMed] [Google Scholar]
- 16.Tassan-Mangina S, Codorean D, Metivier M, et al. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: Early and late alterations of left ventricular function during a prospective study. Eur J Echocardiogr. 2006;7:141–146. doi: 10.1016/j.euje.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Sicari R, Nihoyannopoulos P, Evangelista A, et al. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC) Eur J Echocardiogr. 2008;9:415–437. doi: 10.1093/ejechocard/jen175. [DOI] [PubMed] [Google Scholar]
- 18.Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography: A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Med, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance endorsed by the American College of Chest Physicians. J Am Coll Cardiol. 2011;57:1126–1166. doi: 10.1016/j.jacc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Mark DB, Hlatky MA, Harrell FE, Jr, et al. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med. 1987;106:793–800. doi: 10.7326/0003-4819-106-6-793. [DOI] [PubMed] [Google Scholar]
- 20.Meluzín J, Cerný J, Frélich M, et al. Prognostic value of the amount of dysfunctional but viable myocardium in revascularized patients with coronary artery disease and left ventricular dysfunction: Investigators of this multicenter study. J Am Coll Cardiol. 1998;32:912–920. doi: 10.1016/s0735-1097(98)00324-6. [DOI] [PubMed] [Google Scholar]
- 21.Jones LW, Haykowsky MJ, Swartz JJ, et al. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Lakoski SG, Eves ND, Douglas PS, et al. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol. 2012;9:288–296. doi: 10.1038/nrclinonc.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 24.Chaosuwannakit N, D'Agostino R, Jr, Hamilton CA, et al. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J Clin Oncol. 2010;28:166–172. doi: 10.1200/JCO.2009.23.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckman JA, Thakore A, Kalinowski BH, et al. Radiation therapy impairs endothelium-dependent vasodilation in humans. J Am Coll Cardiol. 2001;37:761–765. doi: 10.1016/s0735-1097(00)01190-6. [DOI] [PubMed] [Google Scholar]
- 26.Jones LW, Watson D, Herndon JE, 2nd, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116:4825–4832. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kavanagh T, Mertens DJ, Hamm LF, et al. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation. 2002;106:666–671. doi: 10.1161/01.cir.0000024413.15949.ed. [DOI] [PubMed] [Google Scholar]
- 28.Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels LB, Laughlin GA, Clopton P, et al. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: Results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008;52:450–459. doi: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zethelius B, Johnston N, Venge P. Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men: A community-based cohort study. Circulation. 2006;113:1071–1078. doi: 10.1161/CIRCULATIONAHA.105.570762. [DOI] [PubMed] [Google Scholar]
- 31.Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: Clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–3916. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 32.Lipshultz SE, Miller TL, Scully RE, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: Associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30:1042–1049. doi: 10.1200/JCO.2010.30.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines—Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 34.Lipshultz SE, Colan SD. Cardiovascular trials in long-term survivors of childhood cancer. J Clin Oncol. 2004;22:769–773. doi: 10.1200/JCO.2004.12.937. [DOI] [PubMed] [Google Scholar]
- 35.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 36.Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: A review of published findings. J Clin Oncol. 2009;27:2339–2355. doi: 10.1200/JCO.2008.21.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 38.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises—Part I: Aging arteries—A “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 39.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises—Part II: The aging heart in health—Links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 40.Chapman JA, Meng D, Shepherd L, et al. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst. 2008;100:252–260. doi: 10.1093/jnci/djn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, et al. Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. J Clin Oncol. 2007;25:4952–4960. doi: 10.1200/JCO.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- 42.Hanrahan EO, Valero V, Gonzalez-Angulo AM, et al. Prognosis and management of patients with node-negative invasive breast carcinoma that is 1 cm or smaller in size (stage 1; T1a,bN0M0): A review of the literature. J Clin Oncol. 2006;24:2113–2122. doi: 10.1200/JCO.2005.02.8035. [DOI] [PubMed] [Google Scholar]
- 43.Schairer C, Mink PJ, Carroll L, et al. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96:1311–1321. doi: 10.1093/jnci/djh253. [DOI] [PubMed] [Google Scholar]
- 44.Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 45.Hooning MJ, Aleman BM, van Rosmalen AJ, et al. Cause-specific mortality in long-term survivors of breast cancer: A 25-year follow-up study. Int J Radiat Oncol Biol Phys. 2006;64:1081–1091. doi: 10.1016/j.ijrobp.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 46. Reference deleted.
- 47.Patnaik JL, Byers T, DiGuiseppi C, et al. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Telli ML, Hunt SA, Carlson RW, et al. Trastuzumab-related cardiotoxicity: Calling into question the concept of reversibility. J Clin Oncol. 2007;25:3525–3533. doi: 10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]
- 49.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J Clin Oncol. 2005;23:2900–2902. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 50.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 51.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eiermann W, Pienkowski T, Crown J, et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol. 2011;29:3877–3884. doi: 10.1200/JCO.2010.28.5437. [DOI] [PubMed] [Google Scholar]
- 53.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 54.Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McArthur HL, Chia S. Cardiotoxicity of trastuzumab in clinical practice. N Engl J Med. 2007;357:94–95. doi: 10.1056/NEJMc070065. [DOI] [PubMed] [Google Scholar]
- 57.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]