Abstract
Purpose
To determine the impact of longer periods between biopsy-confirmed breast cancer diagnosis and the initiation of treatment (Dx2Tx) on survival.
Patients and Methods
This study was a noninterventional, retrospective analysis of adult female North Carolina Medicaid enrollees diagnosed with breast cancer from January 1, 2000, through December, 31, 2002, in the linked North Carolina Central Cancer Registry–Medicaid Claims database. Follow-up data were available through July 31, 2006. Cox proportional hazards regression models were constructed to evaluate the impact on survival of delaying treatment ≥ 60 days after a confirmed diagnosis of breast cancer.
Results
The study cohort consisted of 1,786 low-income, adult women with a mean age of 61.6 years. A large proportion of the patients (44.3%) were racial minorities. Median time from biopsy-confirmed diagnosis to treatment initiation was 22 days. Adjusted Cox proportional hazards regression showed that although Dx2Tx length did not affect survival among those diagnosed at early stage, among late-stage patients, intervals between diagnosis and first treatment ≥ 60 days were associated with significantly worse overall survival (hazard ratio [HR], 1.66; 95% CI, 1.00 to 2.77; P = .05) and breast cancer–specific survival (HR, 1.85; 95% CI, 1.04 to 3.27; P = .04).
Conclusion
One in 10 women waited ≥ 60 days to initiate treatment after a diagnosis of breast cancer. Waiting ≥ 60 days to initiate treatment was associated with a significant 66% and 85% increased risk of overall and breast cancer–related death, respectively, among late-stage patients. Interventions designed to increase the timeliness of receiving breast cancer treatments should target late-stage patients, and clinicians should strive to promptly triage and initiate treatment for patients diagnosed at late stage.
INTRODUCTION
Longer waiting times between breast cancer diagnosis and the initiation of therapy are of prognostic concern if delay leads to stage progression, disease worsening, or treatment complications. A meta-analysis of 87 studies provided compelling evidence that women who initiate treatment 3 to 6 months after the appearance of breast cancer–related symptoms have significantly worse survival than women who wait less than 3 months.1 Sixty-two percent of the studies comprising that review, however, were published before 1970, and the most recent study included in the review was published more than a decade ago. Furthermore, recent studies conflict these older reports.2–6 In addition to previous reports being both outdated and contradictory, many of these studies used inconsistent definitions of delay. As a result, it is unclear whether worse survival is the result of women delaying physician consultation after the appearance of symptoms, longer waiting times for obtaining treatment after consultation and diagnosis,1,3,5–30 or both. Ultimately, studies conducted to date about the impact of time to treatment on survival in breast cancer care have raised as many questions as they have answered.
The majority of studies about survival and delays in obtaining care for breast cancer have examined the time between symptom appearance and initial consultation using self-reported data and suggested that delay may lead to later stage at diagnosis and correspondingly worse survival,1,2,14,17,26,31–38 although some studies reported no effect.3,12,39 However, few studies have specifically examined the relationship between the length of time from biopsy-confirmed diagnosis to the initiation of treatment (Dx2Tx) and survival. In fact, the review by Richards et al1 did not include any studies that specifically examined this period. Thus, additional research describing the impact of delaying treatment after a breast cancer diagnosis is warranted.
PATIENTS AND METHODS
Study Design
A retrospective cohort analysis was conducted to assess the effect of Dx2Tx length on survival using data from a cohort of adult women diagnosed with breast cancer in the North Carolina (NC) Medicaid system. Patients were NC Medicaid enrollees identified in the linked NC Central Cancer Registry (CCR)–Medicaid Claims database. The database was created by linking Medicaid claims with the CCR's information on all NC residents diagnosed with cancer. The CCR-Medicaid Claims–linked database includes all paid NC Medicaid claims and Medicare cross-over claims that originated under fee-for-service plans.40 For the study period, almost all NC Medicaid was under fee for service, with the exception of one small managed care organization that covered approximately 10,000 lives.41 Before analysis, data were de-identified, and approval was obtained from The Ohio State University's Institutional Review Board and the NC Department of Health and Human Services.
Study Cohort
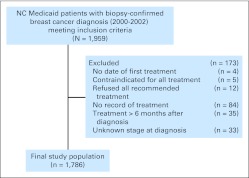
Patients were age 18 or older, female, and diagnosed with breast cancer from January 1, 2000, through December 31, 2002. Continuous enrollment in NC Medicaid for 6 months before the diagnosis of breast cancer was required. Patients without a valid date of initial treatment (n = 4), who died on or before the day their first treatment was received or had no record of any treatment (n = 84), and who had unknown cancer stage (n = 33) were excluded. Patients who refused recommended treatment (n = 12) or for whom treatment was contraindicated because of comorbidities or risk (determined by CCR variables describing reason for not receiving treatment; n = 5) and patients whose first treatment was later than 6 months after diagnosis (n = 35) were excluded (Fig 1). Treatment received more than 6 months after diagnosis could be for a second primary tumor (in the contralateral breast or elsewhere), or in the case of chemotherapy and radiation therapy, treatment could be for another site of metastasis.42 Sensitivity analyses of cutoffs at 9 and 12 months were similar.
Fig 1.
Study inclusion and exclusion criteria. NC, North Carolina.
Outcome Measures
Survival was calculated from the time of treatment initiation (claims or registry) to death listed in the CCR. Follow-up data were available through July 31, 2006. International Classification of Diseases, Oncology, Third Revision cause-of-death designations were used to determine breast cancer–specific (C500 to C509) survival.
Exposure of Interest
Dx2Tx was calculated as the number of days from biopsy-confirmed cancer diagnosis in the CCR to the day of first record of treatment in either the CCR or claims.
Baseline Covariates
Patient-level sociodemographic variables extracted from the CCR included age, race, marital status, year of diagnosis, and information about county of residence. Ethnicity was not analyzed because data about Hispanic ethnicity were available for less than 1% of the population. County of residence at diagnosis was categorized as metropolitan or nonmetropolitan based on US Office of Management and Budget designations.43 County of residence was also designated as a health professional shortage area if the entire county was defined as a health professional shortage area based on US Health and Human Services definitions.44
Tumor-specific characteristics collected from the CCR included cancer stage, tumor size, and tumor hormone receptor status. Breast cancer stage at diagnosis was categorized as in situ, localized (confined to the primary site; ie, no lymph nodes involved), regional (spread to regional lymph nodes or directly beyond primary site), distant (metastasized), or unstaged/unknown. Tumor hormone receptor status was defined as positive, if confirmed estrogen receptor or progesterone receptor positive; negative, if confirmed estrogen receptor negative and progesterone receptor negative; or undetermined/unknown, if the data were missing or incomplete. Data about human epidermal growth factor receptor 2 status were not available.
The D'Hoore et al45 version of the Charlson comorbidity index (CCI) was calculated for each patient from noncancer diagnosis codes in the 6 months before diagnosis. Claims data were also used to determine whether a patient was blind or disabled. Additionally, Medicaid records were used to determine whether a patient lived in an assisted-living facility or received home health care services in the 6 months before the time of cancer diagnosis. Data about whether or not patients had any claims for non–cancer-related hospitalizations or emergency department visits or consultations/procedures for additional biopsies, medical imaging, or genetic testing after diagnosis, but before the initiation of treatment, were also collected.
Treatment Information
Using available Current Procedural Terminology; International Classification of Diseases, Ninth Revision, Clinical Modification; National Drug Codes; and CCR codes in the linked data set, patient treatment histories were documented, including type of surgery at the primary site (breast-conserving surgery, mastectomy, or no record of surgery) and presence of radiation therapy, chemotherapy, and/or adjuvant hormonal therapy.
Statistical Analysis
Univariate relationships between Dx2Tx length and baseline characteristics were assessed using χ2 tests. Separate Cox proportional hazards regression models were constructed to evaluate the effect of Dx2Tx length on both overall and breast cancer–specific survival after controlling for potentially confounding variables. Follow-up was measured from the first course of treatment until death or the end of the study (July 31, 2006), at which time all living participants were censored. Initially, Dx2Tx was modeled as a three-level dummy variable (0 to 29, 30 to 59, and ≥ 60 days) to ensure comparability with previous studies46–52; however, modeling results showed that survival was similar between patients with a Dx2Tx of 0 to 29 days and 30 to 59 days. Thus, we combined those two groups and modeled results as those who received initial treatment within ≥ 60 days versus 0 to 59 days. For Cox regression analyses, likelihood ratio χ2 tests were used to determine improved statistical fit. We decided a priori to assess cancer stage, race, US Office of Management and Budget–designated metropolitan status, and age for statistical interaction with Dx2Tx. In assessing stage for interaction, we dichotomized stage as early stage (in situ or local) and late (regional or distant) stage. Because of the large number of covariates available, in addition to the fully adjusted models, a final parsimonious model was constructed. In the final parsimonious model, we decided, a priori, to include only covariates that changed the hazard ratios (HRs) for Dx2Tx by at least 10% to 15% (ie, confounded),53 improved the precision of the estimated Dx2Tx parameter, were statistically significant at P < .05, or were known predictors of breast cancer survival (eg, age, stage, hormone receptor status). All P values were calculated using two-sided tests.
RESULTS
A total of 1,959 patients met inclusion criteria, of whom 173 (9%) were excluded (Fig 1). The final study population consisted of 1,786 women with a mean age of 61.6 years (standard deviation, 15.0 years) at the time of biopsy-confirmed breast cancer diagnosis (Table 1). The median time from biopsy-confirmed diagnosis to the initiation of the first course of treatment was 22 days (range, 0 to 177 days). The majority of women (66%) received their first course of treatment within 30 days of diagnosis, and nearly all of the women (90%) received initial treatment within 60 days of being diagnosed. Most women (81%) received surgery as their first course of treatment. However, the percentage of women receiving surgery as the first course of treatment was dependent on stage at diagnosis, with 87%, 87%, 78%, and 39% of women receiving surgery as initial treatment for in situ, local, regional, and distant stage tumors, respectively. Nonwhite women (P = .04), women who lived in metropolitan areas (P = .04), women who had not received home health care before diagnosis (P = .02), and women who received consultations/procedures for either additional biopsies (P < .001) or who were hospitalized (P < .001) or admitted to the emergency department (P < .001) between diagnosis and the initiation of treatment were more likely to have longer Dx2Tx (Table 2). Only one woman received genetic testing between diagnosis and treatment; thus, meaningful analysis could not be conducted. Moreover, women who had longer Dx2Tx were more likely to receive a nonsurgical intervention as a first course of treatment (P < .001) and to not receive chemotherapy (P = .01; Table 1).
Table 1.
Baseline Demographics and Clinical Characteristics of Final Study Population by Length of Time From Biopsy-Confirmed Diagnosis to Treatment
| Demographic or Clinical Characteristic | 0 to 59 Days From Biopsy-Confirmed Diagnosis to Treatment (n = 1,603) |
≥ 60 Days From Biopsy-Confirmed Diagnosis to Treatment (n = 183) |
P* | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Demographic | |||||
| Age, years | .58 | ||||
| < 45 | 254 | 16 | 27 | 15 | |
| 45-54 | 280 | 17 | 40 | 22 | |
| 55-64 | 344 | 21 | 43 | 24 | |
| 65-74 | 336 | 21 | 39 | 21 | |
| 75-84 | 314 | 20 | 26 | 14 | |
| ≥ 85 | 75 | 5 | 8 | 4 | |
| Race | .04 | ||||
| White | 906 | 57 | 89 | 49 | |
| Nonwhite | 697 | 44 | 94 | 51 | |
| Marital status | .99 | ||||
| Married | 264 | 16 | 33 | 18 | |
| Single | 225 | 14 | 26 | 14 | |
| Divorced/separated | 252 | 16 | 28 | 15 | |
| Widowed | 392 | 24 | 44 | 24 | |
| Unknown | 470 | 29 | 52 | 28 | |
| Rural/urban status† | .04 | ||||
| Metropolitan | 891 | 56 | 113 | 62 | |
| Nonmetropolitan | 705 | 44 | 69 | 38 | |
| HPSA† | 1.00‡ | ||||
| HPSA | 42 | 3 | 4 | 2 | |
| Non-HPSA | 1,554 | 97 | 178 | 98 | |
| Year of diagnosis | .61 | ||||
| 2000 | 508 | 32 | 64 | 35 | |
| 2001 | 548 | 34 | 62 | 34 | |
| 2002 | 547 | 34 | 57 | 31 | |
| Tumor/cancer characteristics | |||||
| Stage at diagnosis | .15 | ||||
| In situ | 167 | 10 | 29 | 16 | |
| Localized | 783 | 49 | 86 | 47 | |
| Regional | 570 | 36 | 61 | 33 | |
| Distant | 83 | 5 | 7 | 4 | |
| Tumor size, mm | .70 | ||||
| 0-9 | 208 | 13 | 22 | 12 | |
| 10-19 | 411 | 26 | 52 | 28 | |
| 20-49 | 582 | 36 | 60 | 33 | |
| ≥ 50 | 166 | 10 | 17 | 9 | |
| Unknown | 236 | 15 | 32 | 18 | |
| Hormone receptor status | .09 | ||||
| Positive | 836 | 52 | 92 | 50 | |
| Negative | 298 | 19 | 25 | 14 | |
| Unknown/undetermined | 469 | 29 | 66 | 36 | |
| Morbidity indicators | |||||
| Charlson comorbidity index | .85 | ||||
| 0 | 726 | 45 | 82 | 45 | |
| 1-2 | 587 | 37 | 68 | 37 | |
| 3-5 | 214 | 13 | 22 | 12 | |
| ≥ 6 | 76 | 5 | 11 | 6 | |
| Blind or disabled§ | .36 | ||||
| Yes | 381 | 24 | 38 | 21 | |
| No | 1,222 | 76 | 145 | 79 | |
| Location of care | |||||
| Assisted living care | .70 | ||||
| Yes | 145 | 9 | 15 | 8 | |
| No | 1,458 | 91 | 168 | 92 | |
| Home health care | .02 | ||||
| Yes | 846 | 53 | 80 | 44 | |
| No | 757 | 47 | 103 | 56 | |
| Procedures conducted between diagnosis and treatment | |||||
| Additional biopsies | < .001 | ||||
| Any | 154 | 10 | 36 | 20 | |
| None | 1,449 | 90 | 147 | 80 | |
| Medical imaging | .27 | ||||
| Any | 83 | 5 | 13 | 7 | |
| None | 1,520 | 95 | 170 | 93 | |
| Hospitalization (not cancer related) | < .001 | ||||
| Any | 39 | 2 | 15 | 8 | |
| None | 1,564 | 98 | 168 | 92 | |
| Emergency department visit (not cancer related) | < .001 | ||||
| Any | 23 | 1 | 10 | 6 | |
| None | 1,580 | 99 | 173 | 95 | |
| Initial treatment information | |||||
| Type of treatment first received | < .001 | ||||
| Surgery | 1,334 | 83 | 117 | 64 | |
| Radiation | 63 | 4 | 14 | 8 | |
| Chemotherapy | 149 | 9 | 31 | 17 | |
| Hormonal or biologic | 57 | 4 | 21 | 12 | |
| Treatment types received | |||||
| Surgery | .19‡ | ||||
| Breast-conserving surgery | 579 | 36 | 77 | 42 | |
| Mastectomy | 989 | 62 | 101 | 55 | |
| None | 35 | 2 | 5 | 3 | |
| Radiation therapy | .79 | ||||
| Any | 824 | 51 | 96 | 53 | |
| None | 779 | 49 | 87 | 48 | |
| Chemotherapy | .05 | ||||
| Any | 683 | 43 | 64 | 35 | |
| None | 920 | 57 | 119 | 65 | |
| Hormonal or targeted biologic therapy | .38 | ||||
| Any | 458 | 29 | 58 | 32 | |
| None | 1,145 | 71 | 125 | 68 | |
NOTE. Percentages may not add to 100% because of rounding error.
Abbreviation: HPSA, health professional shortage area.
P value is from χ2 test.
Eight women did not have information about county of residence at diagnosis.
P value is from Fisher's exact test.
Only two women were blind.
Table 2.
Overall Survival: Cox Proportional Hazards Modeling Results for Time From Biopsy-Confirmed Diagnosis to Treatment ≥ 60 Days Versus 0 to 59 Days Stratified by Stage at Diagnosis (N = 1,786)
| Variables Added Into Model* | Early Stage (n = 1,065) |
Late Stage (n = 721) |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Crude | 0.72 | 0.33 to 1.55 | .40 | 1.28 | 0.79 to 2.09 | .32 |
| Factor adjustment | ||||||
| Patient sociodemographics | ||||||
| Age, race, marital status, and year at diagnosis | 0.76 | 0.35 to 1.64 | .48 | 1.23 | 0.75 to 2.01 | .42 |
| County-level metropolitan and HPSA status† | 0.76 | 0.35 to 1.65 | .49 | 1.22 | 0.74 to 2.00 | .44 |
| Patient tumor-specific characteristics | ||||||
| Stage, tumor size, and hormone receptor status† | 0.78 | 0.36 to 1.71 | .54 | 1.43 | 0.87 to 2.36 | .16 |
| Patient comorbidities | ||||||
| Charlson comorbidity index and disability status‡ | 0.74 | 0.34 to 1.61 | .44 | 1.47 | 0.89 to 2.44 | .13 |
| Setting of care | ||||||
| Assisted living and home health† | 0.76 | 0.34 to 1.66 | .48 | 1.47 | 0.89 to 2.44 | .13 |
| Treatment type | ||||||
| Surgery type, chemotherapy, radiation, and hormonal therapy† | 0.76 | 0.35 to 1.68 | .51 | 1.66 | 0.99 to 2.77 | .05 |
| Procedures/consultations between diagnosis and treatment | ||||||
| Additional biopsies, medical imaging, hospitalizations, and ED visits†‡ | 0.67 | 0.30 to 1.48 | .32 | 1.56 | 0.94 to 2.62 | .08 |
| Final parsimonious model§ | 0.70 | 0.31 to 1.54 | .37 | 1.66 | 1.00 to 2.77 | .05 |
Abbreviations: ED, emergency department; HPSA, health professional shortage area; HR, hazard ratio.
Each model includes the variables from the model above it.
N = 1,778 because eight patients were missing information related to county of residence.
Fully adjusted model.
The final parsimonious model included age, stage, tumor size, hormone receptor status, Charlson comorbidity index, surgery type, radiation therapy, and information about procedures/consultations between diagnosis and treatment.
During a median follow-up period of 4.7 years (range, 1 day to 6.6 years), 247 deaths occurred, of which 144 (58%) were breast cancer deaths. The majority of deaths from all causes (160 of 247 deaths, 65%) and from breast cancer (114 of 144 deaths, 79%) occurred among patients diagnosed at late stage. Crude results showed no effect of Dx2Tx length on breast cancer–specific or overall survival. Adjusted results showed that the relationship between Dx2Tx length and both overall and breast cancer–specific survival was modified by stage at diagnosis (early v late; P for interaction = .05 for overall survival and P for interaction = .04 for breast cancer–specific survival). Therefore, we presented stratified models to allow for statistical control of stage differences within early-stage (local v in situ) and late-stage (distant v regional) models.
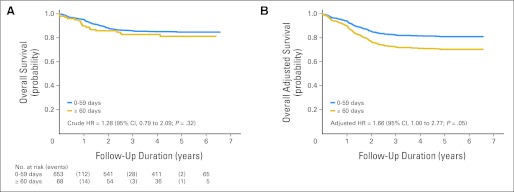
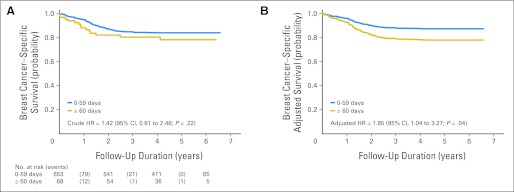
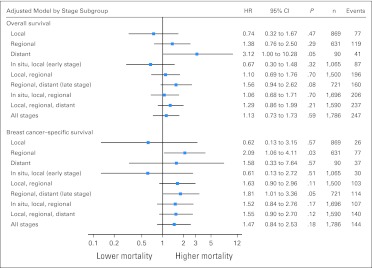
Parsimoniously adjusted, stage-stratified models revealed that although Dx2Tx length did not affect overall survival (P = .37) or breast cancer–specific survival (P = .49) among patients diagnosed at early stage, Dx2Tx ≥ 60 days was associated with worse overall survival (HR, 1.66; 95% CI, 1.00 to 2.77; P = .05; Table 2 and Fig 2) and breast cancer–specific survival (HR, 1.85; 95% CI, 1.04 to 3.27; P = .04; Table 3 and Fig 3) among patients diagnosed at late stage. Other than Dx2Tx length, the only other factor that was predictive of worse breast cancer–specific survival among patients diagnosed at late stage was being diagnosed with distant (v regional) disease (HR, 3.38; 95% CI, 2.09 to 5.56; P < .001). There was no evidence of violation of the proportional hazards assumption. Sensitivity analysis confirmed that women diagnosed at late stage seemed to be the subgroup most affected by Dx2Tx ≥ 60 days (Fig 4).
Fig 2.
(A) Crude and (B) adjusted overall survival curves for late-stage patients (time from biopsy-confirmed diagnosis to the initiation of treatment ≥ 60 days v 0 to 59 days; n = 721). HR, hazard ratio.
Table 3.
Breast Cancer–Specific Survival: Cox Proportional Hazards Modeling Results for Time From Biopsy-Confirmed Diagnosis to Treatment ≥ 60 Days Versus 0 to 59 Days Stratified by Stage at Diagnosis (N = 1,786)
| Variables Added Into Model* | Early Stage (n = 1,065) |
Late Stage (n = 721) |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Crude | 0.58 | 0.14 to 2.45 | .46 | 1.42 | 0.81 to 2.48 | .22 |
| Factor adjustment | ||||||
| Patient sociodemographics | ||||||
| Age, race, marital status, and year at diagnosis | 0.58 | 0.14 to 2.44 | .46 | 1.37 | 0.78 to 2.41 | .27 |
| County-level metropolitan and HPSA status† | 0.60 | 0.35 to 2.55 | .49 | 1.35 | 0.77 to 2.37 | .30 |
| Patient tumor-specific characteristics | ||||||
| Stage, tumor size, and hormone receptor status† | 0.56 | 0.13 to 2.38 | .43 | 1.68 | 0.95 to 2.98 | .07 |
| Patient comorbidities | ||||||
| Charlson comorbidity index and disability status† | 0.54 | 0.13 to 2.31 | .41 | 1.66 | 0.94 to 2.95 | .08 |
| Setting of care | ||||||
| Assisted living and home health† | 0.56 | 0.13 to 2.40 | .44 | 1.67 | 0.94 to 2.97 | .08 |
| Treatment type | ||||||
| Surgery type, chemotherapy, radiation, and hormonal therapy† | 0.56 | 0.12 to 2.53 | .45 | 1.90 | 1.06 to 3.41 | .03 |
| Procedures/consultations between diagnosis and treatment | ||||||
| Additional biopsies, medical imaging, hospitalizations, and ED visits†‡ | 0.61 | 0.13 to 2.72 | .51 | 1.81 | 1.01 to 3.36 | .05 |
| Final parsimonious model§ | 0.60 | 0.14 to 2.61 | .49 | 1.85 | 1.04 to 3.27 | .04 |
Abbreviations: ED, emergency department; HPSA, health professional shortage area; HR, hazard ratio.
Each model includes the variables from the model above it.
N = 1,778 because eight patients were missing information related to county of residence.
Fully adjusted model.
The final parsimonious model included age, stage, tumor size, hormone receptor status, Charlson comorbidity index, surgery type, and radiation therapy.
Fig 3.
(A) Crude and (B) adjusted breast cancer–specific survival curves for late-stage patients (time from biopsy-confirmed diagnosis to the initiation of treatment ≥ 60 days v 0 to 59 days; n = 721). HR, hazard ratio.
Fig 4.
Sensitivity analysis of fully adjusted overall and breast cancer–specific survival for time from biopsy-confirmed diagnosis to the initiation of treatment ≥ 60 days versus 0 to 59 days by stage at diagnosis subgroup. Models for the in situ–only subgroup are not presented because there were too few events to build a valid model. All model results are adjusted for age, race, marital status, year of diagnosis, metropolitan status, and health professional shortage area status of a patient's county of residence at diagnosis, stage at diagnosis (where > one stage is included), tumor size, hormone receptor status, Charlson comorbidity index, disability status, whether or not the patient lived in home health care or assisted living care in the year before diagnosis, whether or not the patient had any claims for consultations/procedures for biopsies or medical imaging or for hospitalizations or emergency department visits between diagnosis and treatment, type of surgery received, and whether or not chemotherapy, radiation therapy, or hormonal therapy was administered. RR, relative risk.
DISCUSSION
On the basis of the study results, ensuring that women diagnosed at late stage receive their first course of treatment in less than 60 days may be one strategy to impact breast cancer outcomes. Women enrolled in NC Medicaid diagnosed with breast cancer at late stage who waited ≥ 60 days (v 0 to 59 days) to initiate their first course of treatment after diagnosis had 66% and 85% increases in risk of all-cause and breast cancer–specific mortality, respectively, after statistical adjustment for treatment type(s) received and patient sociodemographic, tumor-specific, comorbidity, and setting-of-care characteristics. The National Breast and Cervical Cancer Early Detection Program previously was successful in using treatment initiation benchmarks and case-management strategies to ensure treatment within 60 days after a diagnosis of breast cancer,54,55 and future studies should explore the effectiveness of similar programs in other populations.
To our knowledge, only three previous studies have specifically examined the impact on survival of longer Dx2Tx (confirmed diagnosis to first treatment).4–6 None of the studies found a significant relationship between survival and Dx2Tx length. The reasons for the discrepancies between previous studies and ours could be many. Patients in NC Medicaid likely differ from the populations of the three previous studies (eg, higher proportion nonwhite, unemployed, and below federal poverty level), which included patients in the South Carolina cancer registry,6 the Surveillance, Epidemiology, and End Results populations of New Mexico and San Francisco (1975 to 1984),4 and patients diagnosed in a Spanish hospital.5
Furthermore, Redondo et al5 and Smith et al6 analyzed Dx2Tx as ≥ 1 month versus less than 1 month, rather than the 60-day (2-month) cut point that we used to compare survival. We found no difference in survival between patients with a Dx2Tx of 0 to 29 days versus 30 to 59 days. Thus, previous studies may have used comparison cut points that were too short to detect a meaningful difference in survival. Additionally, although Smith et al6 and Delgado et al4 did control for treatment type received, Redondo et al5 did not. Treatment type(s) received was the largest confounder of the effect on survival of Dx2Tx length in our study and should be considered in future studies. In addition, we were able to parse out breast cancer–specific survival estimates for longer time-to-treatment intervals, which showed a heightened effect compared with overall survival.
Finally, and perhaps most importantly, this study is the first to our knowledge to stratify patients by stage at diagnosis when examining the effect of Dx2Tx length on survival. In the absence of stratification, the effect of initial treatment delay on survival was diminished. This finding could partially explain why previous studies that did not present stage-stratified results failed to find a significant effect on survival of longer Dx2Tx.4–6 Additionally, our finding that longer Dx2Tx only influenced the survival of late-stage patients is consistent with previous research that reported no effect of delay on survival in stage I patients.17,31,33 Even though the goal of treatment for patients diagnosed at late stage may not always be curative, early treatment still seems to prolong survival. Future studies should carefully consider effect modification by stage when assessing the effect on survival of time-to-treatment intervals, because most studies have examined all stages combined.1–6
This study is not without limitations. Primarily, our findings should not be generalized without future replication in more diverse study populations and time intervals. Whether these findings are uniquely applicable to the NC Medicaid population or more broadly valid to the general population must be evaluated. NC Medicaid is a unique population of patients, and the findings of this study may not represent individuals with higher incomes or in other states. Women diagnosed with breast cancer in NC Medicaid, compared with women with breast cancer nationally, were more likely to be nonwhite (44% v 17%, respectively), at least age 65 years (45% v 40%, respectively), and diagnosed at late stage (40% v 31%, respectively).41,56,57 However, patients in this study had similar insurance coverage and incomes as a result of Medicaid eligibility status requirements, which helped prevent confounding by differences in socioeconomic status and health insurance coverage.
Another limitation, inherent in any administrative claims database analysis, is the potential for coding errors; however, it is not expected that these would occur differentially. Moreover, no data about the provider- and health care–level components (eg, information about the health care facilities and physician practices that patients were first seen in, referred to, or treated in or about the physicians or staff themselves) were available. Likewise, no data about patient adherence to recommended treatment or patient psychosocial characteristics including fear, anxiety, fatalism, depression, knowledge, beliefs, or barriers to obtaining treatment were available. Future studies should assess the effects of these unmeasured factors that may contribute to delays and/or affect survival.
A fundamental difficulty in studying various time-to-treatment intervals in breast cancer is that investigators cannot ethically randomly assign how long a patient must wait to receive care. In the absence of random assignment, this study was a robust alternative that allowed for statistical control for a wealth of variables related to patient demographics and medical history, tumor-specific characteristics, comorbid conditions, and information about the types of treatment received in a large cohort of patients with ample follow-up data. Moreover, the Dx2Tx interval has been largely under-researched, and it is important that studies continue to parse out which elements of the detection-diagnosis-treatment pathway most affect survival. Approaches to reduce the length of time from symptom appearance to consultation, from consultation to diagnosis, and from diagnosis to treatment are quite different from one another. For example, strategies to shorten the amount of time a patient takes to seek care after noticing a suspicious finding or symptom would be quite different than strategies targeted at ensuring physicians perform the first course of treatment in a timely manner after diagnosis. Thus, the various treatment periods along the breast cancer care continuum should be examined separately. In the future, researchers should also examine whether survival effects are dependent on which particular type of treatment is delayed (eg, local v systemic therapies) and comprehensively examine predictors of longer initial treatment (and other) periods. We are currently developing separate papers that address these topics using the same data set.
In conclusion, results of this study are novel and suggest that future research analyzing how delays in initiating treatment after a breast cancer diagnosis affect survival should carefully examine effect modification by stage at diagnosis. Results suggest that interventions should target late-stage patients to increase the timeliness of receiving breast cancer treatments and that clinicians should structure their practice settings to promptly triage and initiate treatment for patients diagnosed at late stage.
Acknowledgment
John M. McLaughlin, PhD, MSPH, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank Fabian Camacho, MS, MA, for his programming support. Fabian Camacho is Senior Instructor in the Division of Health Services Research, Department of Public Health Sciences, Penn State College of Medicine and the Hershey Cancer Institute, Hershey, PA.
Footnotes
See accompanying article on page 4485
Supported by National Cancer Institute Grant No. 1R01CA121317.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Roger T. Anderson, Abbott Laboratories (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Electra D. Paskett
Provision of study materials or patients: Roger T. Anderson
Collection and assembly of data: John M. McLaughlin, Roger T. Anderson, Rajesh Balkrishnan
Data analysis and interpretation: John M. McLaughlin, Eric E. Seiber, Rajesh Balkrishnan
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Richards MA, Westcombe AM, Love SB, et al. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet. 1999;353:1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 2.Afzelius P, Zedeler K, Sommer H, et al. Patient's and doctor's delay in primary breast cancer: Prognostic implications. Acta Oncol. 1994;33:345–351. doi: 10.3109/02841869409098427. [DOI] [PubMed] [Google Scholar]
- 3.Sainsbury R, Johnston C, Haward B. Effect on survival of delays in referral of patients with breast-cancer symptoms: A retrospective analysis. Lancet. 1999;353:1132–1135. doi: 10.1016/s0140-6736(99)02374-0. [DOI] [PubMed] [Google Scholar]
- 4.Delgado DJ, Lin WY, Coffey M. The role of Hispanic race/ethnicity and poverty in breast cancer survival. P R Health Sci J. 1995;14:103–116. [PubMed] [Google Scholar]
- 5.Redondo M, Rodrigo I, Pereda T, et al. Prognostic implications of emergency admission and delays in patients with breast cancer. Support Care Cancer. 2009;17:595–599. doi: 10.1007/s00520-008-0513-2. [DOI] [PubMed] [Google Scholar]
- 6.Smith ER, Adams SA, Das IP, et al. Breast cancer survival among economically disadvantaged women: The influences of delayed diagnosis and treatment on mortality. Cancer Epidemiol Biomarkers Prev. 2008;17:2882–2890. doi: 10.1158/1055-9965.EPI-08-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganry O, Peng J, Dubreuil A. Influence of abnormal screens on delays and prognostic indicators of screen-detected breast carcinoma. J Med Screen. 2004;11:28–31. doi: 10.1177/096914130301100107. [DOI] [PubMed] [Google Scholar]
- 8.Olivotto IA, Gomi A, Bancej C, et al. Influence of delay to diagnosis on prognostic indicators of screen-detected breast carcinoma. Cancer. 2002;94:2143–2150. doi: 10.1002/cncr.10453. [DOI] [PubMed] [Google Scholar]
- 9.Alderson MR, Hamlin I, Staunton MD. The relative significance of prognostic factors in breast carcinoma. Br J Cancer. 1971;25:646–656. doi: 10.1038/bjc.1971.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloom HJ. The influence of delay on the natural history and prognosis of breast cancer: A study of cases followed for five to twenty years. Br J Cancer. 1965;19:228–262. doi: 10.1038/bjc.1965.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkley D, Haybittle JL. A 15-year follow-up study of patients treated for carcinoma of the breast. Br J Radiol. 1968;41:215–221. doi: 10.1259/0007-1285-41-483-215. [DOI] [PubMed] [Google Scholar]
- 12.Dennis CR, Gardner B, Lim B. Analysis of survival and recurrence vs. patient and doctor delay in treatment of breast cancer. Cancer. 1975;35:714–720. doi: 10.1002/1097-0142(197503)35:3<714::aid-cncr2820350326>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Devitt JE. The clinical stages of breast cancer: What do they mean? Can Med Assoc J. 1967;97:1257–1262. [PMC free article] [PubMed] [Google Scholar]
- 14.Elwood JM, Moorehead WP. Delay in diagnosis and long-term survival in breast cancer. BMJ. 1980;280:1291–1294. doi: 10.1136/bmj.280.6227.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphrey LJ, Swerdlow M. Factors influencing the survival of patients with carcinoma of the breast. Am J Surg. 1963;106:440–444. doi: 10.1016/0002-9610(63)90127-2. [DOI] [PubMed] [Google Scholar]
- 16.Robbins GF, Bross I. The significance of delay in relation to prognosis of patients with primary operable breast cancer. Cancer. 1957;10:338–344. doi: 10.1002/1097-0142(195703/04)10:2<338::aid-cncr2820100214>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Sheridan B, Fleming J, Atkinson L, et al. The effects of delay in treatment of survival rates in carcinoma of the breast. Med J Aust. 1971;1:262–267. [PubMed] [Google Scholar]
- 18.Blackman DJ, Masi CM. Racial and ethnic disparities in breast cancer mortality: Are we doing enough to address the root causes? J Clin Oncol. 2006;24:2170–2178. doi: 10.1200/JCO.2005.05.4734. [DOI] [PubMed] [Google Scholar]
- 19.Gregorio DI, Cummings KM, Michalek A. Delay, stage of disease, and survival among white and black women with breast cancer. Am J Public Health. 1983;73:590–593. doi: 10.2105/ajph.73.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hébert-Croteau N, Freeman CR, Latreille J, et al. A population-based study of the impact of delaying radiotherapy after conservative surgery for breast cancer. Breast Cancer Res Treat. 2004;88:187–196. doi: 10.1007/s10549-004-0594-7. [DOI] [PubMed] [Google Scholar]
- 21.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 22.Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: Retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol. 2009;27:2157–2162. doi: 10.1200/JCO.2008.19.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hershman DL, Wang X, McBride R, et al. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys. 2006;65:1353–1360. doi: 10.1016/j.ijrobp.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 24.Hershman DL, Wang X, McBride R, et al. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat. 2006;99:313–321. doi: 10.1007/s10549-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 25.Lohrisch C, Paltiel C, Gelmon K, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2006;24:4888–4894. doi: 10.1200/JCO.2005.01.6089. [DOI] [PubMed] [Google Scholar]
- 26.Neave LM, Mason BH, Kay RG. Does delay in diagnosis of breast cancer affect survival? Breast Cancer Res Treat. 1990;15:103–108. doi: 10.1007/BF01810782. [DOI] [PubMed] [Google Scholar]
- 27.Porta M, Gallén M, Malats N, et al. Influence of “diagnostic delay” upon cancer survival: An analysis of five tumour sites. J Epidemiol Community Health. 1991;45:225–230. doi: 10.1136/jech.45.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards MA, Smith P, Ramirez AJ, et al. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer. 1999;79:858–864. doi: 10.1038/sj.bjc.6690137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tartter PI, Pace D, Frost M, et al. Delay in diagnosis of breast cancer. Ann Surg. 1999;229:91–96. doi: 10.1097/00000658-199901000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vujovic O, Yu E, Cherian A, et al. Effect of interval to definitive breast surgery on clinical presentation and survival in early-stage invasive breast cancer. Int J Radiat Oncol Biol Phys. 2009;75:771–774. doi: 10.1016/j.ijrobp.2008.11.049. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson GS, Edgerton F, Wallace HJ, Jr, et al. Delay, stage of disease and survival from breast cancer. J Chronic Dis. 1979;32:365–373. doi: 10.1016/0021-9681(79)90078-x. [DOI] [PubMed] [Google Scholar]
- 32.Feldman JG, Saunders M, Carter AC, et al. The effects of patient delay and symptoms other than a lump on survival in breast cancer. Cancer. 1983;51:1226–1229. doi: 10.1002/1097-0142(19830401)51:7<1226::aid-cncr2820510709>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 33.Charlson ME. Delay in the treatment of carcinoma of the breast. Surg Gynecol Obstet. 1985;160:393–399. [PubMed] [Google Scholar]
- 34.Vernon SW, Tilley BC, Neale AV, et al. Ethnicity, survival, and delay in seeking treatment for symptoms of breast cancer. Cancer. 1985;55:1563–1571. doi: 10.1002/1097-0142(19850401)55:7<1563::aid-cncr2820550726>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Huguley CM, Jr, Brown RL, Greenberg RS, et al. Breast self-examination and survival from breast cancer. Cancer. 1988;62:1389–1396. doi: 10.1002/1097-0142(19881001)62:7<1389::aid-cncr2820620725>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Machiavelli M, Leone B, Romero A, et al. Relation between delay and survival in 596 patients with breast cancer. Oncology. 1989;46:78–82. doi: 10.1159/000226689. [DOI] [PubMed] [Google Scholar]
- 37.Rossi S, Cinini C, Di Pietro C, et al. Diagnostic delay in breast cancer: Correlation with disease stage and prognosis. Tumori. 1990;76:559–562. doi: 10.1177/030089169007600609. [DOI] [PubMed] [Google Scholar]
- 38.Raabe NK, Fossaa SD. Primary invasive breast carcinoma in Oslo 1980-1989: Incidence and delay. Acta Oncol. 1996;35:9–15. doi: 10.3109/02841869609098473. [DOI] [PubMed] [Google Scholar]
- 39.Fisher ER, Redmond C, Fisher B. A perspective concerning the relation of duration of symptoms to treatment failure in patients with breast cancer. Cancer. 1977;40:3160–3167. doi: 10.1002/1097-0142(197712)40:6<3160::aid-cncr2820400661>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 40.Anderson RT, Camacho FT, Balkrishnan R, et al. Use of cancer registry data for research on patterns of breast cancer care of individuals with Medicaid insurance. J Clin Oncol. 2005;23:533s. (suppl; abstr 6021) [Google Scholar]
- 41.Raleigh, NC: North Carolina Division of Medical Assistance; 2008. North Carolina Department of Health and Human Services: Medicaid in North Carolina: Annual Report, State Fiscal Year 2007. [Google Scholar]
- 42.Abrams JA, Buono DL, Strauss J, et al. Esophagectomy compared with chemoradiation for early stage esophageal cancer in the elderly. Cancer. 2009;115:4924–4933. doi: 10.1002/cncr.24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Washington, DC: Executive Office of the President of the United States; 2003. Office of Management and Budget: Measuring Rurality: New Definitions in 2003. [Google Scholar]
- 44.Bethesda, MD: US Department of Health and Human Services; 2010. Health Resources and Services Administration: HPSA by State and County. [Google Scholar]
- 45.D'Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996;49:1429–1433. doi: 10.1016/s0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 46.Caplan LS, Helzlsouer KJ, Shapiro S, et al. Reasons for delay in breast cancer diagnosis. Prev Med. 1996;25:218–224. doi: 10.1006/pmed.1996.0049. [DOI] [PubMed] [Google Scholar]
- 47.Caplan LS, May DS, Richardson LC. Time to diagnosis and treatment of breast cancer: Results from the National Breast and Cervical Cancer Early Detection Program, 1991-1995. Am J Public Health. 2000;90:130–134. doi: 10.2105/ajph.90.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorin SS, Heck JE, Cheng B, et al. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166:2244–2252. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 49.Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100:1595–1604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 50.Lund MJ, Brawley OP, Ward KC, et al. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109:545–557. doi: 10.1007/s10549-007-9675-8. [DOI] [PubMed] [Google Scholar]
- 51.Maly RC, Leake B, Silliman RA. Breast cancer treatment in older women: Impact of the patient-physician interaction. J Am Geriatr Soc. 2004;52:1138–1145. doi: 10.1111/j.1532-5415.2004.52312.x. [DOI] [PubMed] [Google Scholar]
- 52.Ramirez AJ, Westcombe AM, Burgess CC, et al. Factors predicting delayed presentation of symptomatic breast cancer: A systematic review. Lancet. 1999;353:1127–1131. doi: 10.1016/s0140-6736(99)02142-x. [DOI] [PubMed] [Google Scholar]
- 53.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 54.Richardson LC, Royalty J, Howe W, et al. Timeliness of breast cancer diagnosis and initiation of treatment in the National Breast and Cervical Cancer Early Detection Program, 1996-2005. Am J Public Health. 2010;100:1769–1776. doi: 10.2105/AJPH.2009.160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryerson AB, Benard VB, Major AC. Atlanta, GA: Department of Health and Human Services; 2005. The National Breast and Cervical Cancer Early Detection Report: 1991-2002, National Report. [Google Scholar]
- 56.Washington, DC: US Department of Commerce; 2010. US Census Department: 2010 USA Quick Facts. [Google Scholar]
- 57.Altekruse SF, Kosary CL, Krapcho M, et al., editors. SEER cancer statistics review, 1975-2007, National Cancer Institute. http://seer.cancer.gov/csr/1975_2007/