Abstract
Purpose
The purposes of this study were to confirm the prognostic value of an optimal morphologic response to preoperative chemotherapy in patients undergoing chemotherapy with or without bevacizumab before resection of colorectal liver metastases (CLM) and to identify predictors of the optimal morphologic response.
Patients and Methods
The study included 209 patients who underwent resection of CLM after preoperative chemotherapy with oxaliplatin- or irinotecan-based regimens with or without bevacizumab. Radiologic responses were classified as optimal or suboptimal according to the morphologic response criteria. Overall survival (OS) was determined, and prognostic factors associated with an optimal response were identified in multivariate analysis.
Results
An optimal morphologic response was observed in 47% of patients treated with bevacizumab and 12% of patients treated without bevacizumab (P < .001). The 3- and 5-year OS rates were higher in the optimal response group (82% and 74%, respectively) compared with the suboptimal response group (60% and 45%, respectively; P < .001). On multivariate analysis, suboptimal morphologic response was an independent predictor of worse OS (hazard ratio, 2.09; P = .007). Receipt of bevacizumab (odds ratio, 6.71; P < .001) and largest metastasis before chemotherapy of ≤ 3 cm (odds ratio, 2.12; P = .025) were significantly associated with optimal morphologic response. The morphologic response showed no specific correlation with conventional size-based RECIST criteria, and it was superior to RECIST in predicting major pathologic response.
Conclusion
Independent of preoperative chemotherapy regimen, optimal morphologic response is sufficiently correlated with OS to be considered a surrogate therapeutic end point for patients with CLM.
INTRODUCTION
For patients with colorectal liver metastases (CLM), hepatic resection combined with systemic therapy is the most effective strategy, achieving long-term survivals in the majority of patients with liver-only disease. Recent studies from high-volume centers have reported 5-year survival rates of 58% after potentially curative resection of CLM.1–3 These favorable surgical outcomes in CLM are attributed to improvements in multidisciplinary protocols, surgical technique, and perioperative management.4
Preoperative chemotherapy plays a pivotal role in the multidisciplinary management of CLM. Systemic chemotherapy can downsize metastases and increase their resectability5,6 and may also be helpful in the selection of patients most likely to benefit from surgery by allowing assessment of tumor response to chemotherapy.7,8 However, the conventional tumor size–based radiologic criteria of RECIST may be inadequate in assessing response to chemotherapy, especially in patients treated with a regimen including bevacizumab.9–11
We previously reported that novel criteria based on morphologic changes observed on computed tomography (CT) in patients with CLM undergoing preoperative chemotherapy predicted both pathologic response to chemotherapy and long-term outcomes.12 However, that analysis was limited by the size of the study population and inclusion of only patients treated with regimens containing bevacizumab. As a validation of the clinical relevance of the morphologic response criteria, this study was designed to assess a larger patient population including patients treated with and without bevacizumab.
In the current study, we investigated the prognostic impact of an optimal CT morphologic response in patients who were treated with preoperative oxaliplatin- or irinotecan-based chemotherapy with or without bevacizumab. Also, we analyzed clinical factors associated with an optimal morphologic response in this patient population.
PATIENTS AND METHODS
The Institutional Review Board of The University of Texas MD Anderson Cancer Center approved this retrospective study (PA12-0177). By searching a database of prospectively collected data, we identified 521 consecutive patients who underwent macroscopically curative resection (R0 or R1 resection) for CLM after single-line fluorouracil-based chemotherapy including oxaliplatin or irinotecan with or without bevacizumab between the period of January 2001 and December 2011. Among these patients, 260 patients in whom both pre- and postchemotherapy CT images were available were included in the current study. Thirty-six of these patients were included in our initial report.12
Imaging Analysis
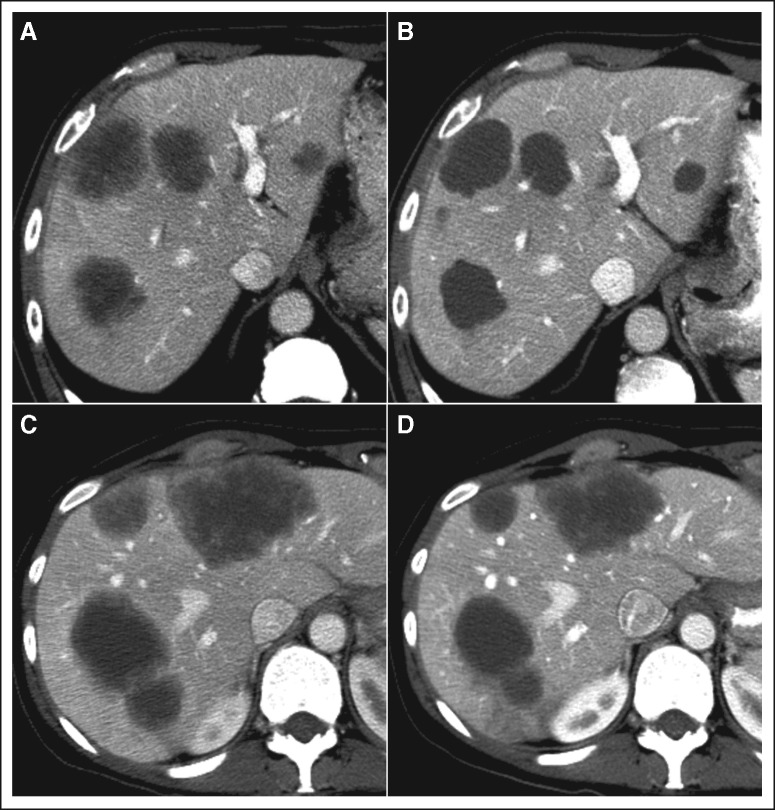
Enhanced CT scans were performed with a multidetector row CT, four, 16, or 64 slice (Light-Speed; GE Healthcare, Piscataway, NJ), using a triphasic liver protocol or single-phase technique as we described previously.12 Parameters used for CT varied with patient size and were, on average, 120 kv with mAs 200 to 350. CT images were reviewed by three radiologists (P.B., C.C., and E.M.L.) blinded to clinical data, and the morphology was assessed according to the following morphologic criteria: group 1, homogeneous low attenuation with a thin, sharply defined tumor-liver interface; group 3, heterogeneous attenuation with a thick, poorly defined tumor-liver interface; and group 2, intermediate morphology that cannot be rated as group 1 or 3.12 Optimal morphologic response to chemotherapy was defined as a change in morphology from group 3 or 2 to group 1 (Fig 1A). Change in morphology from group 3 to group 2 and absence of remarkable changes in morphology were defined as suboptimal morphologic response (Fig 1B). In patients with multiple tumors, morphologic response was assigned based on the response seen in the majority of tumors. Response to chemotherapy was also determined according to RECIST.13
Fig 1.
Optimal and suboptimal morphologic response after chemotherapy. (A and B) Optimal morphologic response and RECIST stable disease. (C and D) Suboptimal response and RECIST stable disease.
Statistical Analysis
Continuous variables were compared using the Mann-Whitney U test, and categorical variables were compared using the χ2 test or Fisher's exact test, where appropriate. Overall survival (OS) was measured from the date of hepatic resection until the date of death or last follow-up. Disease-free survival (DFS) was measured from the date of hepatic resection until the date of radiographic detection of recurrence or last follow-up. Survival curves were generated using the Kaplan-Meier method, and differences between curves were evaluated with the log-rank test. To identify prognostic factors for survival, a multivariate regression analysis was performed using the Cox proportional hazards model with backward elimination for variables with P < .1 in univariate analysis. To identify factors associated with an optimal morphologic response, a multivariate analysis was performed using the logistic regression model for clinical variables with P < .1 in univariate analysis. Analyses were performed with IBM SPSS software (ver 19.0. SPSS, Chicago, IL). All statistical tests were two-sided, and significance was set at P < .05.
RESULTS
Patient Characteristics
Of the 260 patients included in the study, 51 patients (20%) were unsuitable for radiologic evaluation because of low-quality CT images (n = 26), lesions too small to characterize (< 1 cm before or after chemotherapy; n = 24), or both reasons (n = 1) and were excluded from the analysis. Baseline characteristics of the final cohort (n = 209) are listed in Table 1. The cohort included 124 men and 85 women with a median age of 58 years (range, 25 to 87 years). Seventy-two percent of the patients had CLM detected within 1 year of diagnosis of their colorectal primary tumors. Seventeen patients (8%) had extrahepatic lesions (lung nodules; n = 10; peritoneal nodules, n = 5; or distant lymph node metastases, n = 2), and all of them were curatively resected simultaneously or subsequently to hepatectomy. Eighteen patients (9%) had history of adjuvant chemotherapy after resection of the primary tumor within 1 year of diagnosis of liver metastases. All patients received fluorouracil-based chemotherapy before liver resection, including oxaliplatin and bevacizumab (47% of patients), irinotecan and bevacizumab (29%), oxaliplatin without bevacizumab (20%), and irinotecan without bevacizumab (4%). The median number of chemotherapy cycles before hepatectomy was six (range, one to 24 cycles). Portal vein embolization was performed in 29 patients (14%), and major hepatectomy (≥ three segments) was performed in 140 patients (67%). Microscopically negative surgical margins were obtained in 192 patients (92%). Postoperative adjuvant chemotherapy was administered to 160 patients (77%). The median follow-up duration was 38 months (range, 1 to 128 months). Baseline characteristics were comparable between the initial population (n = 521) and the studied population (n = 209) except for minor differences in chemotherapy regimens and size of liver metastases (Appendix Table A1, online only).
Table 1.
Baseline Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | No. of Patients | % |
|---|---|---|
| Age, years | ||
| Median | 58 | |
| Range | 25-87 | |
| Sex | ||
| Male | 124 | 59 |
| Female | 85 | 41 |
| Primary tumor | ||
| Colon | 150 | 72 |
| Rectum | 59 | 28 |
| Primary tumor nodal status | ||
| Positive | 126 | 62 |
| Negative | 76 | 38 |
| Extrahepatic disease | ||
| Present | 17 | 8 |
| Absent | 192 | 92 |
| Adjuvant chemotherapy for primary tumor within 1 year of diagnosis of CLM | ||
| Yes | 18 | 9 |
| No | 191 | 91 |
| DFI from diagnosis of primary tumor to diagnosis of liver metastases, years | ||
| < 1 | 151 | 72 |
| ≥ 1 | 58 | 28 |
| Liver metastases | ||
| Solitary | 78 | 37 |
| Multiple | 131 | 63 |
| Tumor size before surgery, cm | ||
| Median | 2.7 | |
| Range | 0.5-15 | |
| Fluorouracil-based chemotherapy regimen | ||
| Oxaliplatin | 41 | 20 |
| Oxaliplatin + bevacizumab | 99 | 47 |
| Irinotecan | 60 | 29 |
| Irinotecan + bevacizumab | 9 | 4 |
| No. of chemotherapy cycles before hepatectomy | ||
| Median | 6 | |
| Range | 1-24 | |
| Portal vein embolization | 29 | 14 |
| Hepatectomy | ||
| Minor resection | 69 | 33 |
| Major resection | 140 | 67 |
| Operating time, minutes | ||
| Median | 195 | |
| Range | 55-680 | |
| Blood loss, mL | ||
| Median | 250 | |
| Range | 0-6,000 | |
| Transfusion | 22 | 11 |
| Pringle's maneuver | 148 | 71 |
| Surgical margin | ||
| R0 | 189 | 92 |
| R1 | 17 | 8 |
| Morbidity | ||
| Any | 85 | 41 |
| Major | 29 | 14 |
| Death within 90 days | 4 | 2 |
Abbreviations: CLM, colorectal liver metastases; DFI, disease-free interval; R0, microscopically negative surgical margin; R1, microscopically positive surgical margin.
Correlations Between Morphologic Response, RECIST, and Pathologic Response
Of the 559 CT scans reviewed, 371 were performed with triphasic liver protocol, and 188 were performed with the single-phase technique. Optimal morphologic response was observed in 63 patients (30%), and the remaining 146 patients (70%) were classified as having suboptimal response. Assessment of preoperative chemotherapy response using RECIST determined that 69 patients (33%) were classified as having partial response (PR), and the remaining 140 (67%) patients were classified as having stable disease (SD) or progressive disease (PD). Using morphologic response criteria, 27 (39%) of 69 patients with PR and 36 (26%) of 140 patients with SD or PD were classified as having an optimal morphologic response (P = .06).
Among 128 patients in whom pathologic responses were available, major pathologic response (1% to 49% residual cancer cells) was observed in 89 patients (70%), and the remaining 39 patients (30%) had minor pathologic responses (≥ 50% residual cancer cells) in histopathologic examinations. Major pathologic response was more sensitively predicted by morphologic response (rate of major pathologic response was 92% among patients with optimal morphologic response and 59% among patients with suboptimal morphologic response; P < .001) than by RECIST (rate of major pathologic response was 83% among patients with PR and 66% among patients with SD or PD; P = .04).
Association Between Treatment With Bevacizumab and Morphologic Response
An optimal morphologic response was observed in 51 (47%) of 108 patients treated with bevacizumab and 12 (12%) of 102 patients treated without bevacizumab. Multivariate analysis demonstrated that receipt of bevacizumab and largest metastasis before chemotherapy of ≤ 3 cm were strongly associated with an optimal morphologic response (Table 2).
Table 2.
Univariate and Multivariate Analysis of Predictors of Optimal Radiographic Response
| Factor | No. of Patients | Patients With Optimal Response |
Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | P | OR | 95% CI | P | OR | 95% CI | ||
| Age, years | |||||||||
| > 60 | 80 | 24 | 30.0 | .972 | 0.99 | 0.53 to 1.81 | |||
| ≤ 60 | 129 | 39 | 30.2 | ||||||
| Sex | |||||||||
| Male | 124 | 32 | 25.8 | .100 | 0.61 | 0.33 to 1.10 | |||
| Female | 85 | 31 | 36.5 | ||||||
| No. of liver metastases | |||||||||
| Solitary | 78 | 23 | 29.5 | .873 | 0.95 | 0.51 to 1.76 | |||
| Multiple | 131 | 40 | 30.5 | ||||||
| Size of largest metastasis before chemotherapy, cm* | |||||||||
| ≤ 3 | 115 | 42 | 36.5 | .025 | 2.00 | 1.09 to 3.75 | .025 | 2.12 | 1.10 to 4.19 |
| > 3 | 94 | 21 | 22.3 | ||||||
| Differentiation of tumor | |||||||||
| Well/moderate | 177 | 55 | 31.1 | .484 | 1.35 | 0.59 to 3.39 | |||
| Poor | 32 | 8 | 25.0 | ||||||
| Fluorouracil-based chemotherapy regimen | |||||||||
| Irinotecan | 69 | 11 | 17.5 | .001 | 0.32 | 0.15 to 0.65 | .950 | ||
| Oxaliplatin | 140 | 52 | 37.1 | ||||||
| Bevacizumab | |||||||||
| Yes | 108 | 51 | 47.2 | < .001 | 6.64 | 3.35 to 14.0 | < .001 | 6.71 | 2.97 to 16.6 |
| No | 101 | 12 | 11.9 | ||||||
Abbreviation: OR, odds ratio.
Cutoff value was decided by receiver operating characteristic curve analysis for optimal response.
Clinical Outcomes
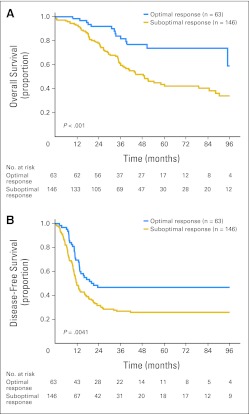
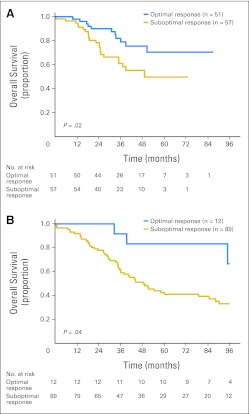
Within 90 days of hepatectomy, the rates of any morbidity, major morbidity (defined as ≥ grade 3 using a standard classification system14), and mortality were 41%, 14%, and 2%, respectively. For the entire cohort, the 1-, 3-, and 5-year OS rates were 95%, 66%, and 52%, respectively. The 1-, 3-, and 5-year OS rates were higher in the optimal response group (98%, 82%, and 74%, respectively) compared with the suboptimal response group (93%, 60%, and 45%, respectively; P < .001; Fig 2A). Likewise, the 1-, 3-, and 5-year DFS rates were 70%, 47%, and 47%, respectively, in the optimal response group and 49%, 27%, and 26%, respectively, in the suboptimal response group (P = .0041; Fig 2B). In subset comparison, patients with optimal morphologic response showed better outcome regardless of the use of bevacizumab (Fig 3).
Fig 2.
(A) Overall survival and (B) disease-free survival by morphologic response in 209 patients undergoing resection of colorectal liver metastases after preoperative chemotherapy.
Fig 3.
Overall survival and morphologic response according to preoperative chemotherapy: (A) with bevacizumab and (B) without bevacizumab.
Prognostic Factors for OS and DFS
On multivariate analysis of factors associated with OS after hepatectomy, node-positive primary tumor (hazard ratio [HR], 2.04), positive surgical margin (HR, 2.21), and suboptimal morphologic response (HR, 2.09) were independent predictors of poor outcome (Table 3). Response according to RECIST (PR v SD or PD) was not a significant predictor (P = .148). On multivariate analysis of factors associated with DFS after hepatectomy, node-positive primary tumor (HR, 2.23), multiple metastases (HR, 1.73), largest tumor size more than 5 cm (HR, 2.13), positive surgical margin (HR, 1.92), PR in RECIST (HR, 1.49), and suboptimal response (HR, 1.82) were identified as significant factors (Appendix Table A2, online only).
Table 3.
Univariate and Multivariate Analysis of Predictors of Overall Survival
| Factor | No. of Patients | 5-Year Overall Survival (%) | Median Overall Survival (months) | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | ||||
| Age, years | |||||||||
| > 60 | 80 | 49.7 | 51.2 | .528 | 1.15 | 0.74 to 1.75 | |||
| ≤ 60 | 129 | 54.3 | 84.7 | ||||||
| Primary tumor nodal status | |||||||||
| Positive | 126 | 44.6 | 49.0 | .003 | 2.01 | 1.27 to 3.28 | .009 | 2.04 | 1.25 to 3.46 |
| Negative | 76 | 65.1 | 114.2 | ||||||
| Extrahepatic disease | |||||||||
| Present | 17 | 49.3 | 30.1 | .196 | 1.68 | 0.74 to 3.27 | |||
| Absent | 192 | 53.2 | 84.7 | ||||||
| Adjuvant chemotherapy for primary tumor within 1 year of diagnosis of CLM | |||||||||
| Yes | 18 | 37.3 | 35.4 | .079 | 1.97 | 0.92 to 3.73 | .529 | ||
| No | 191 | 53.8 | 84.7 | ||||||
| DFI, years | |||||||||
| < 1 | 151 | 50.7 | 60.1 | .556 | 1.15 | 0.73 to 1.89 | |||
| ≥ 1 | 58 | 56.1 | 90.3 | ||||||
| Preoperative chemotherapy regimen | |||||||||
| Oxaliplatin | 140 | 52.6 | 90.3 | .896 | 0.97 | 0.63 to 1.52 | |||
| Irinotecan | 69 | 51.5 | 60.1 | ||||||
| Bevacizumab | |||||||||
| No | 101 | 48.2 | 54.3 | .232 | 1.31 | 0.84 to 2.05 | |||
| Yes | 108 | 59.9 | NA | ||||||
| No. of tumors | |||||||||
| Multiple | 131 | 37.9 | 58.1 | .208 | 1.32 | 0.86 to 2.07 | |||
| Solitary | 78 | 58.4 | 90.3 | ||||||
| Size of largest metastasis before surgery, cm | |||||||||
| > 5 | 38 | 41.1 | 44.3 | .116 | 1.51 | 0.89 to 2.44 | |||
| ≤ 5 | 171 | 55.6 | 88.3 | ||||||
| Surgical margin | |||||||||
| Positive | 17 | 19.2 | 44.0 | .060 | 1.93 | 0.97 to 3.49 | .032 | 2.21 | 1.08 to 4.14 |
| Negative | 189 | 55.8 | 88.3 | ||||||
| Adjuvant chemotherapy | |||||||||
| No | 49 | 38.3 | 44.3 | .035 | 1.65 | 1.04 to 2.54 | .060 | ||
| Yes | 160 | 57.1 | 90.3 | ||||||
| RECIST response | |||||||||
| SD or PD | 140 | 47.7 | 51.0 | .055 | 1.57 | 0.99 to 2.58 | .148 | ||
| PR | 69 | 61.7 | 88.3 | ||||||
| Morphologic response | |||||||||
| Suboptimal | 146 | 43.7 | 49.0 | < .001 | 2.48 | 1.46 to 4.49 | .007 | 2.09 | 1.22 to 3.83 |
| Optimal | 63 | 73.8 | 114.2 | ||||||
Abbreviations: CLM, colorectal liver metastases; DFI, disease-free interval from diagnosis of primary tumor to diagnosis of liver metastases; HR, hazard ratio; NA, not available; PD, progressive disease; PR, partial response; SD, stable disease.
DISCUSSION
In this study, we analyzed preoperative prognostic factors in 209 patients who underwent hepatic resection of CLM. This analysis determined that CT morphologic response to preoperative chemotherapy is a strong predictor of long-term outcomes after surgery in patients treated with or without bevacizumab. An optimal morphologic response was associated with high 5-year OS and DFS rates of 74% and 47%, respectively. The study also demonstrated that suboptimal morphologic response, node-positive primary tumor, and positive surgical margins were most strongly associated with poor prognosis after resection of CLM.
To date, several clinical predictive models have been proposed in patients undergoing hepatic resection for CLM.15–20 However, many of these clinical risk scores were developed using patient data that predated modern chemotherapy, making their application to patients treated with a contemporary multidisciplinary approach controversial.21,22 Previously, our group and others have reported that improved survival correlates with pathologic response to preoperative chemotherapy, which we proposed as a new outcome end point after resection of CLM.23–25 Subsequently, we reported novel CT morphologic response criteria for predicting pathologic response to chemotherapy and survival in small cohorts of patients with resectable and unresectable CLM treated with chemotherapy regimens containing bevacizumab.12 Comparison of the accuracy of the morphologic response criteria with traditional RECIST criteria showed that the morphologic response is superior to RECIST for prediction of pathologic response and survival.
The current study expands on our previous work by examining a larger patient population enriched with those treated without bevacizumab. This allowed comparison of radiologic and pathologic response with and without bevacizumab. This analysis indicated that the rate of optimal morphologic response was relatively low (12%) in patients treated without bevacizumab compared with patients treated with bevacizumab (47%). On multivariate analysis, bevacizumab was strongly associated with an optimal morphologic response (odds ratio, 6.71). Multivariate analysis performed in the entire cohort confirmed that suboptimal morphologic response was an independent factor associated with a two-fold increase in the risk of death. Furthermore, the morphologic response was superior to RECIST in predicting major pathologic response, confirming results of our previous study.12 On the basis of these results, morphologic response can be used as an alternate end point that predicts long-term outcome in patients receiving preoperative chemotherapy for CLM.
Assessment of morphologic response is not difficult, and its high reproducibility with good interobserver agreement has been previously shown.12 In our experience, the morphologic response can be used by medical oncologists, radiologists, and surgeons involved in multidisciplinary decision making. Morphologic response can be used to predict prognosis after resection or in patients who are not candidates for surgery, because our prior study demonstrated that in patients with unresectable disease, optimal response to chemotherapy with bevacizumab was associated with significantly longer median survival compared with suboptimal response (31 months v 19 months, respectively).12
We acknowledge that determination of the morphologic response requires several components. Determination of the morphologic response relies on high-quality CT imaging with an adequate enhancement protocol. Also, morphologic response may be difficult to assess when a tumor is small (usually < 1 to 1.5 cm). In fact, in the current study, the morphologic response was difficult to determine in 51 of 260 patients because of low quality of CT images and/or small size of metastases. Although response by RECIST was not a significant prognostic factor in the multivariate analysis, size change is a widely accepted indicator of response to chemotherapy. Recent studies have suggested that a change in cutoff value for change in tumor diameter may improve the discriminatory ability of the RECIST in CLM.26,27 Therefore, integration of morphologic criteria and size criteria may be needed to improve the accuracy of radiologic response evaluation.
The limitations of this study include the retrospective analysis of data and the selected population. However, the study was based on prospectively collected data, and the patients were treated using a similar approach to imaging evaluation28,29 after preoperative chemotherapy.4,30 Another limitation is that patients who were treated with biologic agents other than bevacizumab were not included. Because a recently reported randomized trial showed that chemotherapy with cetuximab may yield high response rates and improve resectability,31 further validation of the morphologic response criteria in patients treated with cetuximab or panitumumab is warranted.
In conclusion, the current study confirms that the CT morphologic response to preoperative chemotherapy is a dominant predictor of both OS and DFS in patients undergoing hepatic resection for CLM. On the basis of these data, CT morphologic response may serve as an alternate outcome end point before hepatic resection of CLM.
Appendix
Table A1.
Comparison of Background Demographics and Clinical Characteristics Between Study Population and Excluded Patients
| Demographic or Clinical Characteristic | Study Population (n = 209) |
Initial Population (n = 521) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | .805 | ||||
| Median | 58 | 57 | |||
| Range | 25-87 | 23-87 | |||
| Sex | |||||
| Male | 124 | 59 | 269 | 52 | .059 |
| Female | 85 | 41 | 252 | 48 | |
| Primary tumor | |||||
| Colon | 150 | 72 | 392 | 75 | .333 |
| Rectum | 59 | 28 | 129 | 25 | |
| Primary tumor nodal status | |||||
| Positive | 126 | 62 | 171 | 35 | .581 |
| Negative | 76 | 38 | 312 | 65 | |
| Extrahepatic disease | |||||
| Present | 17 | 8 | 38 | 7 | .697 |
| Absent | 192 | 92 | 483 | 93 | |
| Adjuvant chemotherapy for primary tumor within 1 year of diagnosis of CLM | |||||
| Yes | 18 | 9 | 62 | 12 | .199 |
| No | 191 | 91 | 459 | 88 | |
| DFI from diagnosis of primary tumor to diagnosis of liver metastases, years | |||||
| < 1 | 151 | 72 | 390 | 75 | .467 |
| ≥ 1 | 58 | 28 | 131 | 25 | |
| Liver metastases | |||||
| Solitary | 78 | 37 | 125 | 36 | .705 |
| Multiple | 131 | 63 | 219 | 64 | |
| Tumor size before surgery, cm | .005 | ||||
| Median | 2.7 | 2.2 | |||
| Range | 0.5-15 | 0.5-16 | |||
| Fluorouracil-based chemotherapy regimen | |||||
| Oxaliplatin | 41 | 20 | 96 | 18 | < .001 |
| Oxaliplatin + bevacizumab | 99 | 47 | 266 | 51 | |
| Irinotecan | 60 | 29 | 95 | 19 | |
| Irinotecan + bevacizumab | 9 | 4 | 64 | 12 | |
| No. of chemotherapy cycles before hepatectomy | .146 | ||||
| Median | 6 | 6 | |||
| Range | 1-24 | 1-30 | |||
| Portal vein embolization | 29 | 14 | 66 | 13 | .661 |
| Hepatectomy | |||||
| Minor resection | 69 | 33 | 191 | 37 | .352 |
| Major resection | 140 | 67 | 330 | 63 | |
| Operating time, minutes | .958 | ||||
| Median | 195 | 195 | |||
| Range | 55-680 | 55-714 | |||
| Blood loss, mL | .634 | ||||
| Median | 250 | 250 | |||
| Range | 0-6,000 | 0-6,000 | |||
| Transfusion | 22 | 11 | 53 | 10 | .626 |
| Pringle's maneuver | 148 | 71 | 362 | 70 | .682 |
| Surgical margin | |||||
| R0 | 189 | 92 | 466 | 92 | .812 |
| R1 | 17 | 8 | 39 | 8 | |
| Morbidity | |||||
| Any | 85 | 41 | 207 | 40 | .815 |
| Major | 29 | 14 | 40 | 14 | .984 |
| Death within 90 days | 4 | 2 | 13 | 3 | .638 |
Abbreviations: CLM, colorectal liver metastases; DFI, disease-free interval; R0, microscopically negative surgical margin; R1, microscopically positive surgical margin.
Table A2.
Univariate and Multivariate Analysis of Predictors of DFS
| Factor | No. of Patients | 5-Year DFS (%) | Median DFS (months) | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | ||||
| Age, years | |||||||||
| > 60 | 80 | 38.2 | 16.1 | .182 | 0.79 | 0.55 to 1.12 | |||
| ≤ 60 | 129 | 29.2 | 12.9 | ||||||
| Primary tumor nodal status | |||||||||
| Positive | 126 | 23.6 | 11.8 | < .001 | 1.89 | 1.30 to 2.81 | < .001 | 2.23 | 1.48 to 3.43 |
| Negative | 76 | 48.1 | 26.0 | ||||||
| Extrahepatic disease | |||||||||
| Present | 17 | NA | 12.4 | .326 | 1.35 | 0.72 to 2.30 | |||
| Absent | 192 | 33.7 | 13.5 | ||||||
| Adjuvant chemotherapy for primary tumor within 1 year of diagnosis of CLM | |||||||||
| Yes | 18 | 7.8 | 11.6 | .105 | 1.63 | 0.89 to 2.74 | |||
| No | 191 | 34.4 | 13.6 | ||||||
| DFI, years | |||||||||
| < 1 | 151 | 30.3 | 13.1 | .294 | 1.23 | 0.84 to 1.85 | |||
| ≥ 1 | 58 | 37.9 | 16.8 | ||||||
| Preoperative chemotherapy regimen | |||||||||
| Oxaliplatin | 140 | 33.1 | 13.4 | .743 | 0.94 | 0.66 to 1.35 | |||
| Irinotecan | 69 | 31.6 | 13.8 | ||||||
| Bevacizumab | |||||||||
| No | 101 | 32.0 | 12.9 | .716 | 1.07 | 0.76 to 1.50 | |||
| Yes | 108 | 33.3 | 13.6 | ||||||
| No. of liver metastases | |||||||||
| Multiple | 131 | 25.4 | 11.8 | .004 | 1.69 | 1.19 to 2.46 | .009 | 1.73 | 1.15 to 2.65 |
| Solitary | 78 | 43.3 | 21.7 | ||||||
| Size of largest metastasis before surgery, cm | |||||||||
| > 5 | 38 | 16.7 | 9.5 | .002 | 1.99 | 1.30 to 2.95 | .003 | 2.13 | 1.31 to 3.34 |
| ≤ 5 | 171 | 35.9 | 16.1 | ||||||
| Surgical margin | |||||||||
| Positive | 17 | 12.5 | 9.5 | .011 | 2.24 | 1.23 to 3.79 | .031 | 1.92 | 1.02 to 3.38 |
| Negative | 189 | 33.6 | 15.4 | ||||||
| Adjuvant chemotherapy | |||||||||
| No | 49 | 26.4 | 11.8 | .197 | 1.31 | 0.86 to 1.93 | |||
| Yes | 160 | 34.1 | 13.6 | ||||||
| RECIST response | |||||||||
| SD or PD | 140 | 28.4 | 12.8 | .050 | 1.44 | 1.00 to 2.13 | .046 | 1.49 | 1.01 to 2.24 |
| PR | 69 | 41.1 | 17.1 | ||||||
| Morphologic response | |||||||||
| Suboptimal | 146 | 26.1 | 11.8 | .003 | 1.76 | 1.20 to 2.65 | .004 | 1.82 | 1.15 to 2.83 |
| Optimal | 63 | 46.9 | 21.1 | ||||||
Abbreviations: CLM, colorectal liver metastases; DFI, disease-free interval from diagnosis of primary tumor to diagnosis of liver metastases; DFS, disease-free survival; HR, hazard ratio; NA, not available; PD, progressive disease; PR, partial response; SD, stable disease.
Footnotes
The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant No. CA016672.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Scott Kopetz, Roche (C), sanofi-aventis (C); Chusilp Charnsangavej, Novartis Pharmaceuticals (C) Stock Ownership: None Honoraria: Evelyne M. Loyer, Roche; Jean-Nicolas Vauthey, Roche, sanofi-aventis Research Funding: Scott Kopetz, Roche; Dipen M. Maru, Taiho Pharma USA; Chusilp Charnsangavej, Roche; Jean-Nicolas Vauthey, Roche Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Junichi Shindoh, Evelyne M. Loyer, Jean-Nicolas Vauthey
Collection and assembly of data: All authors
Data analysis and interpretation: Junichi Shindoh, Evelyne M. Loyer, Scott Kopetz, Dipen M. Maru, Yun Shin Chun, Giuseppe Zimmitti, Thomas A. Aloia, Jean-Nicolas Vauthey
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopetz S, Vauthey JN. Perioperative chemotherapy for resectable hepatic metastases. Lancet. 2008;371:963–965. doi: 10.1016/S0140-6736(08)60429-8. [DOI] [PubMed] [Google Scholar]
- 5.Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh AA, Gentner B, Wu TT, et al. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg. 2003;7:1082–1088. doi: 10.1016/j.gassur.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Adam R, Pascal G, Castaing D, et al. Tumor progression while on chemotherapy: A contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen PJ, Kemeny N, Jarnagin W, et al. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg. 2003;7:109–115. doi: 10.1016/S1091-255X(02)00121-X. [DOI] [PubMed] [Google Scholar]
- 9.Grothey A, Hedrick EE, Mass RD, et al. Response-independent survival benefit in metastatic colorectal cancer: A comparative analysis of N9741 and AVF2107. J Clin Oncol. 2008;26:183–189. doi: 10.1200/JCO.2007.13.8099. [DOI] [PubMed] [Google Scholar]
- 10.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 11.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26:271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun YS, Vauthey JN, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwatsuki S, Dvorchik I, Madariaga JR, et al. Hepatic resection for metastatic colorectal adenocarcinoma: A proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malik HZ, Prasad KR, Halazun KJ, et al. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007;246:806–814. doi: 10.1097/SLA.0b013e318142d964. [DOI] [PubMed] [Google Scholar]
- 18.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver: A prognostic scoring system to improve case selection, based on 1568 patients—Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 19.Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 20.Zakaria S, Donohue JH, Que FG, et al. Hepatic resection for colorectal metastases: Value for risk scoring systems? Ann Surg. 2007;246:183–191. doi: 10.1097/SLA.0b013e3180603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayez N, Lalmahomed ZS, van der Pool AE, et al. Is the clinical risk score for patients with colorectal liver metastases still useable in the era of effective neoadjuvant chemotherapy? Ann Surg Oncol. 2011;18:2757–2763. doi: 10.1245/s10434-011-1819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarnagin WR. Clinical scoring systems for stratifying risk after resection of hepatic colorectal metastases: Still relevant? Ann Surg Oncol. 2011;18:2711–2713. doi: 10.1245/s10434-011-1821-1. [DOI] [PubMed] [Google Scholar]
- 23.Adam R, Wicherts DA, de Haas RJ, et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: Myth or reality? J Clin Oncol. 2008;26:1635–1641. doi: 10.1200/JCO.2007.13.7471. [DOI] [PubMed] [Google Scholar]
- 24.Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: A new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 25.Rubbia-Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 26.De Roock W, Piessevaux H, De Schutter J, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–515. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki C, Blomqvist L, Sundin A, et al. The initial change in tumor size predicts response and survival in patients with metastatic colorectal cancer treated with combination chemotherapy. Ann Oncol. 2012;23:948–954. doi: 10.1093/annonc/mdr350. [DOI] [PubMed] [Google Scholar]
- 28.Vauthey JN, Rousseau DL., Jr: Liver imaging: A surgeon's perspective. Clin Liver Dis. 2002;6:271–295. doi: 10.1016/s1089-3261(03)00076-x. [DOI] [PubMed] [Google Scholar]
- 29.Vauthey JN. Liver imaging: A surgeon's perspective. Radiol Clin North Am. 1998;36:445–457. doi: 10.1016/s0033-8389(05)70034-8. [DOI] [PubMed] [Google Scholar]
- 30.Vauthey JN, Nordlinger B, Kopetz S, et al. Sequenced chemotherapy and surgery for potentially resectable colorectal liver metastases: A debate over goals of research and an approach while the jury remains out. Ann Surg Oncol. 2010;17:1983–1986. doi: 10.1245/s10434-010-1007-2. [DOI] [PubMed] [Google Scholar]
- 31.Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]