Abstract
Background
We inquired if fluorescence-guided surgery (FGS) could improve surgical outcomes in fluorescent orthotopic nude mouse models of human colon cancer.
Methods
We established fluorescent orthotopic mouse models of human colon cancer expressing a fluorescent protein. Tumors were resected under bright light surgery (BLS) or FGS. Pre- and post-operative images with the OV-100 Small Animal Imaging System were obtained to assess extent of surgical resection.
Results
All mice with primary tumor which had undergone FGS had complete resection as compared to 58% of mice in the BLS group (p=0.001). FGS resulted in decreased recurrence compared to BLS (33% vs 62%, p=0.049), and lengthened disease-free median survival from 9 weeks to >36 weeks. The median overall survival increased from 16 weeks in the BLS group to 31 weeks in the FGS group. FGS resulted in a cure in 67% of mice (alive without evidence of tumor at >6 months post-surgery) compared to only 37% of mice which underwent BLS (p=0.049).
Conclusions
Surgical outcomes in orthotopic nude mouse models of human colon cancer were signficantly improved with FGS. The current study can be translated to the clinic by various effective methods of fluorescently labeling tumors.
Keywords: colorectal cancer, orthotopic mouse models, fluorescence, resection, survival Short title: Fluorescence guided surgery for colon cancer
Introduction
The single most important prognostic factor for overall survival in patients with colorectal cancer (CRC) is not only the microscopic status of the resected margin but the complete resection of tumor at all sites (R0) (1, 2). Achieving an R0 resection can significantly improve 5-year survival rates of patients with colo-rectal cancer (3). Longterm survival after resection of CRC depends on the radicality of the surgical procedure (4). The complete detection of primary and metastatic lesions at the time of surgery is therefore vital in optimizing surgical resection. There is a clear survival advantage to complete resection of primary and metastatic CRC when clinically appropriate (5).
We have previously demonstrated the enhanced visualization and detection of primary and metastatic lesions with the use of fluorescent probes (6-9). In this study, we report the effectiveness of using fluorescence guided surgical resection to improve outcomes by optimizing surgical resection in mouse models of human colon cancer. Improved outcomes include reducing recurrence rates and thus improving overall survival.
Materials and Methods
Cell culture
Human colon cancer cell lines HCT-116-GFP, expressing green fluorescent protein (GFP) and HT-29-Ds Red, expressing red fluorescent protein (RFP), were maintained in DMEM (Gibco-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT). The cell culture media was supplemented with penicillin/streptomycin (Gibco-BRL), sodium pyruvate (Gibco-BRL), sodium bicarbonate (Cellgro, Manassas, VA), L-glutamine (Gibco-BRL), and minimal essential medium nonessential amino acids (Gibco-BRL). Cells were incubated at 37°C with 5% carbon dioxide.
Animal care
Female athymic nu/nu nude mice were maintained in a barrier facility on high-efficiency particulate air filtered racks. The animals were fed with autoclaved laboratory rodent diet (Teckland LM-485; Western Research Products, Orange, CA). All surgical procedures were performed under anesthesia with an intramuscular injection of 100 μL of a mixture of 100 mg/kg ketamine and 10 mg/kg xylazine. For each procedure, 20 μL of 1 mg/kg buprenorphine was administered for pain control. Euthanasia was achieved by 100% carbon dioxide inhalation, followed by cervical dislocation. All animal studies were approved by the UCSD Institutional Animal Care and Use Committee (IACUC) and conducted in accordance with the principles and procedures outlined in the National Institutes of Health (NTH) Guide for the Care and Use of Animals.
Subcutaneous Tumor Cell Implantation
Human HCT-116-GFP colon cancer cells were harvested by trypsinization and washed twice with serum-free medium. Cells (2×l06 in 100 μL serum-free media) were injected subcutaneously within 30 min of harvesting over the right and left flanks in female nu/nu mice between 4 and 6 weeks of age. Subcutaneous tumors were allowed to grow for 2-4 weeks until large enough to supply adequate tumor for orthotopic implantation.
Orthotopic Tumor Implantation
For cellular orthotopic implantation (COI), human colon cancer HT-29-Ds-Red cells were harvested by trypsinization and washed twice with serum-free medium. Viability was verified to be greater than 95% using the Vi-Cell XR automated cell viability analyzer (Beckman Coulter, Brea, CA). The HT-29-Ds-Red cells were resuspended at concentrations of 2 million cells per 10 μl of serum-free media. A midline abdominal incision was made and the cecum was delivered through the incision. Orthotopic human colon cancer models were established in nude mice by direct injection of fluorescent HT-29-Ds Red tumor cells into the wall of the cecum using a Hamilton syringe (Hamilton Co, Reno NV). For surgical orthotopic implantation (SOI), a single 1 mm3 tumor fragment from the HCT-116-GFP subcutaneous tumors was sutured to the mesenteric border of the cecal wall using 8-0 nylon surgical sutures. Upon completion, the cecum was returned to the abdomen and the incision was closed in two layers using 6.0 Ethibond non-absorbable sutures (Ethicon Inc., Somerville, NJ).
Tumor Resection
A total of 51 mice were used in the experiments; 21 of them underwent fluorescence-guided surgery (FGS) and the other 24 mice underwent bright light surgery (BLS). Once tumors reached a size of 2-4 mm in diameter, tumor-bearing mice were randomly assigned to the bright light surgery (BLS) group or to the fluorescence-guided surgery (FGS) group. Prior to resection of the colon tumor, mice were anesthetized as described, and their abdomens were sterilized. The cecum was delivered through a midline incision and the exposed colon tumor was imaged preoperatively with the Olympus OV-100 Small Animal Imaging System (Olympus Corp, Tokyo Japan) under both standard bright field and fluorescence illumination. Resection of the primary colon tumor was performed using the MVX-10 fluorescence-dissecting microscope (Olympus) under bright light illumination for the BLS group and under fluorescence illumination through an RFP (excitation HQ 545/30×, emission 620/60m) or GFP filter (excitation HQ 470/10m, emission 525/50m) for the FGS group. For primary tumor resection, the cecal stump was sutured closed in a running fashion with 8-0 nylon surgical sutures. For the carcinomatosis model, a total of 6 mice harboring human HCT-116-GFP colon cancer were allowed to progress to advanced, metastatic disease. These mice were then randomly assigned to the FGS or BLS group. Prior to surgical resection, the mice were terminated and a large midline incision was made to expose the entire abdomen and preoperative images were taken as described above. Resection of primary and metastatic lesions was performed under bright light illumination for the BLS group and under fluorescence illumination through a GFP filter for the FGS group. Postoperatively, the surgical resection bed in all mice was imaged with the OV-100 Small Animal Imaging System under both standard bright field and fluorescence illumination to assess completeness of surgical resection.
Animal Imaging
To assess for recurrence and to follow tumor progression postoperatively, weekly whole body imaging of the mice was performed with the Olympus OV-100 Small Animal Imaging System (Olympus Corp, Tokyo Japan), containing an MT-20 light source (Olympus Biosystems, Planegg Germany) and DP70 CCD camera (Olympus Corp.). All images were analyzed with Image J v1.440 (National Institutes of Health, Bethesda, MD).
Tissue histology
For correlation of surgical findings with histology, tumor samples were removed with surrounding normal tissues at the time of resection. Fresh tissue samples were fixed in Bouins solution and regions of interest embedded in paraffin prior to sectioning and staining with H&E for standard light microscopy. H&E-stained permanent sections were examined using an Olympus BX41 microscope equipped with a Micropublisher 3.3 RTV camera (QImaging, Surrey, B.C., Canada). All images were acquired using QCapture software (QImaging) without post-acquisition processing.
Data processing & statistical analysis
PASWStatistics 18.0 (SPSS, Inc.) was used for all statistical analyses. Tumor burdens are expressed as either median (range) or mean ± SEM. A Student's t-test was used to compare continuous variables between two groups; ANOVA models were used to compare multiple groups. Comparisons between categorical variables were analyzed using Fisher's exact tests. Survival outcomes were compared using log rank tests, and hazard ratios with their 95% confidence intervals were estimated using Cox proportional hazards models. A p-value of ≤0.05 was considered statistically significant for all comparisons.
Results
Fluorescence-Guided Surgery of CRC in an Orthotopic Mouse Mode
Our first aim was to improve surgical outcomes with FGS in orthotopic mouse models of human colon cancer. To achieve this, orthotopic mouse models were established by implanting brightly fluorescent HT-29-ds Red tumor cells or HCT-116-GFP tumor fragments into the cecal walls of nu/nu female mice. There was no significant difference in preoperative tumor burden among the randomized groups (p=0.728). The Olympus MVX-10 long working-distance fluorescence-dissecting microscope was used to perform surgeries in all mice. Resections in the BLS group were accomplished under bright light illumination. Fluorescent lighting was achieved through a GFP or RFP filter which was used to perform FGS.
We experienced significant improvement in visualization of primary colon cancer as well as separate sub-millimeter metastatic deposits of tumor on the cecum allowing enhanced distinction of tumor from surrounding normal bowel and mesenteric tissue in the FGS group (Figure 1). This enhanced ability to identify tumor margins permitted a more complete resection of the cancer resulting in a significant difference in rates of R0 resections among the surgical groups (Table 1). An R0 resection was achieved in all mice undergoing FGS based on postoperative imaging. However, BLS afforded an R0 resection rate of only 58%, thereby leaving significantly more mice with residual disease postoperatively compared to FGS (p=0.001).
Figure 1.
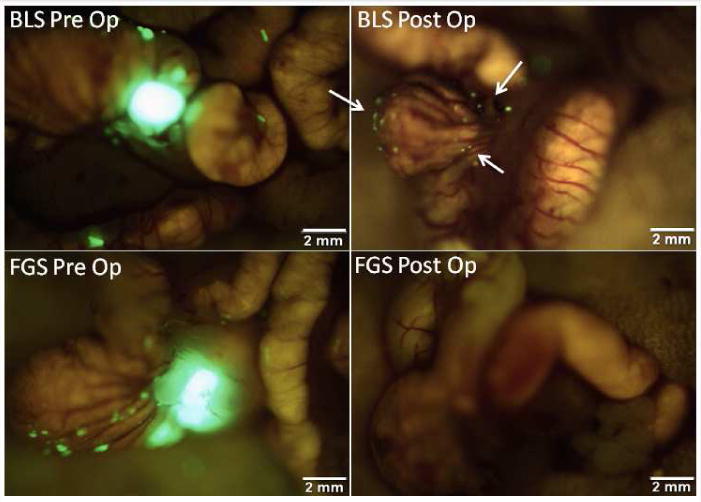
Pre- and postoperative images under FGS and BLS in an orthotopic mouse model. These are representative pre- and postoperative images of a mouse from the BLS group (top panel) and the FGS group (bottom panel). The enhanced ability to visualize and identify tumor margins under fluorescence-guidance permitted a more complete resection. All mice in the FGS group underwent an R0 resection while only 58% of mice in the BLS group had no evidence of residual fluorescent tumor on postoperative images (arrows in right upper panel) (p=0.001).
Table 1.
Rate of R0 resection by surgical method.
| Surgical Method | R0 resection | R1/R2 resection |
|---|---|---|
| Fluorescence-guided surgery | 21/21 (100%) | 0/21 (0%) |
| Bright light surgery | 11/19 (57.9%) | 8/19 (42.1%) |
P=0.001, Fisher's exact test.
The improved resection at the time of the initial operation resulted in a decreased rate of recurrence seen in the FGS group compared to the BLS group. While 33% of the mice in the FGS group had evidence of disease recurrence, the BLS group had recurrence rates of 62.5% (p=0.049) (Table 2). FGS afforded a disease-free survival (DFS) advantage over BLS of 12.5 weeks (mean DFS for BLS of 23.2 weeks vs mean DFS for FGS of 35.7 weeks; p=0.107). The median disease-free survival for the BLS group was 9 weeks compared to over 52 weeks in the FGS group (p=0.107). More than half of mice in the FGS group had no evidence of tumor recurrence at the conclusion of the study, one year postoperatively (Figure 2).
Table 2.
Rate of recurrence, disease-free survival, and overall survival by surgical method.
| Surgical Method | Rate of recurrence | Disease-free survival | Overall survival |
|---|---|---|---|
| Fluorescence-guided surgery | 7/21 (33.3%)* | >36 weeks | 31 weeks |
| Bright Light Surgery | 15/24 (62.5%)* | 9 weeks | 16 weeks |
p=0.049, Fisher's exact test.
Disease-free and overall survival are median values.
Figure 2.
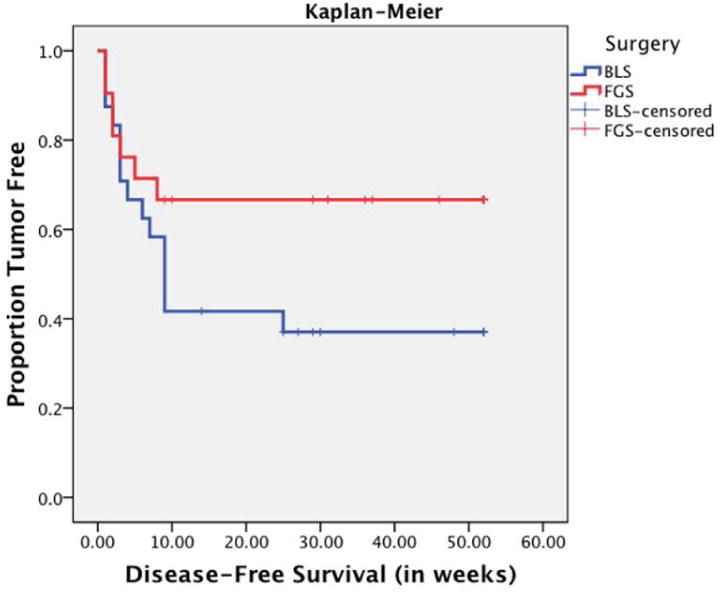
Kaplan Meier curve for disease-free survival. Mice undergoing fluorescence-guided surgery had longer mean disease-free survival by 8 weeks (from 17 weeks in BLS to 25 weeks in FGS). The median disease-free survival in the BLS group was 9 weeks. As evident by the FGS interpolation line, more than 50% of the mice in the FGS group were free of tumor at 36 weeks and were considered cures.
The improved resection rates and decreased incidence of tumor recurrence among mice in the FGS group translated into an overall survival advantage. On average, FGS mice experienced a 7.6-week survival advantage over BLS mice (mean OS for BLS of 21.3 weeks vs mean OS for FGS of 28.9 weeks, p=0.210). The median overall survival increased from 16 weeks in the BLS group to 31 weeks in the FGS group (p=0.210) (Figure 3). At the conclusion of the study, 67% of mice in the FGS group had undergone curative resection and were alive 6 months postoperatively with no evidence of tumor recurrence. A curative resection rate was achieved in only 37.5% of mice in the BLS group (p=0.049)
Figure 3.
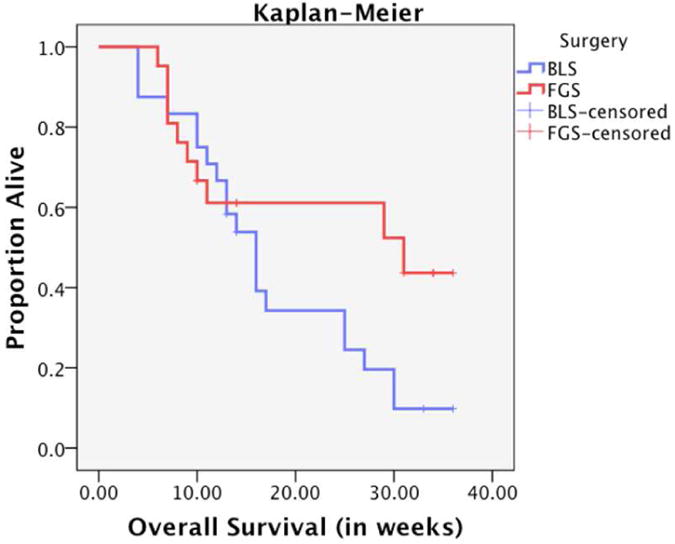
Kaplan Meier curve for overall survival. Fluorescence-guided surgery afforded mice with an increased overall survival by over 6 weeks. The median overall survival was increased from 16 weeks to 31 weeks.
Fluorescence Guided Surgery in a Carcinomatosis Mouse Model
Secondly, we set forth to evaluate the effectiveness of fluorescence-guided surgery in order to achieve improved outcomes of metastatic disease in a carcinomatosis mouse model of human colon cancer. Orthotopic mouse models were established as previously described. Tumor was allowed to progress to an advanced, metastatic stage in which the entire abdominal cavity was burdened by green fluorescent tumor. Advanced tumor stage was confirmed with whole body imaging with the OV-100 Small Animal Imaging System. Mice were randomized to undergo bright light surgery (n=3) or fluorescence-guided surgery (n=3). Prior to resection, the mice were terminated and their abdomens were exposed via a midline incision. Preoperative bright field and fluorescence images were taken with the OV-100 and tumor burden was assessed using ImageJ.
There was no significant difference in preoperative tumor burden among the surgical groups (p=0.737). The enhanced visualization of fluorescent disseminated tumor deposits in the mice permitted a significantly greater extent of tumor reduction using FGS compared to BLS (99.9% vs. 76.9%, p = 0.006) (Figure 4a). While achieving a mean postoperative tumor burden of 0.07 ± 0.04 mm2 in the FGS group, tumor reduction capabilities in the BLS group were not as successful, achieving a mean postoperative tumor burden of 18.1 ± 1.9 mm2 (p=0.001) (Figure 4b).
Figure 4.
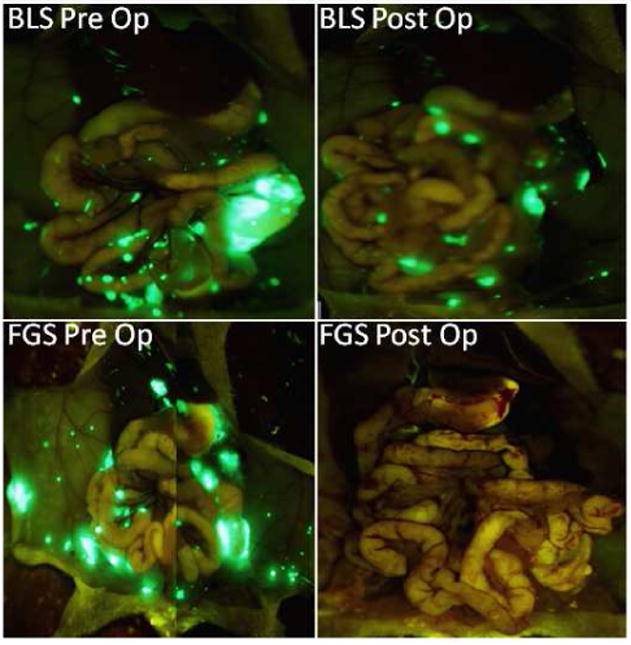
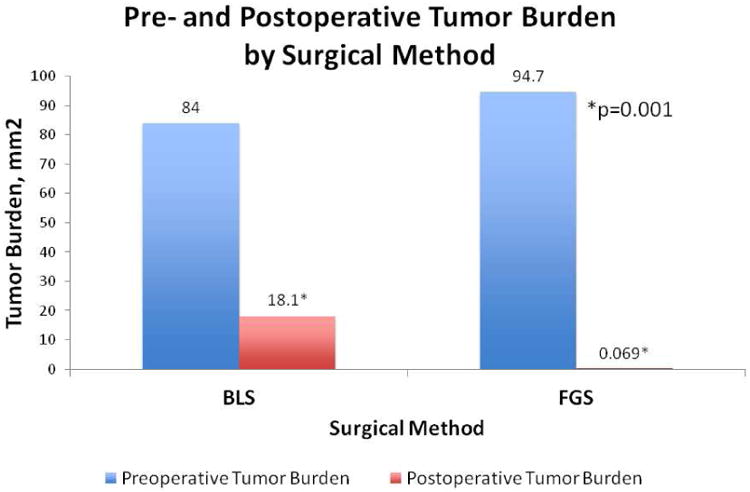
a) Pre- and postoperative images in a carcinomatosis mouse model of human colon cancer. With FGS, 99.9% of the tumor burden was resected as evident by the lack of fluorescent tumor deposits in the postoperative FGS picture. In contrast, only 76.9% of the tumor was resected, with BLS leaving a 23% tumor burden (p=0.006). b) Pre- and postoperative tumor burden by surgical method. FGS afforded an enhanced ability to detect tumor from surrounding normal bowel and mesenteric tissue. As a result, a significantly smaller postoperative tumor burden was achieved with FGS (0.069 ± 0.04 mm2) compared to BLS (18.1 ± 1.9 mm2); p=0.001. There was no statistically significant difference in preoperative tumor burden between the two groups (p=0.737).
BLS% Tumor Reduction: 76.9%, FGS% Tumor Reduction: 99.9%, p=0.006
For correlation of surgical and gross findings with tissue histology in this orthotopic model of colon cancer, representative resected tissue samples were processed for microscopic examination. Histology demonstrated the growth pattern of HT-29 human colon carcinoma cells in the mouse cecum (Figure 5). The tumor cells form infiltrative nests that spread within the submucosa and extend through the full thickness of the bowel wall.
Figure 5.
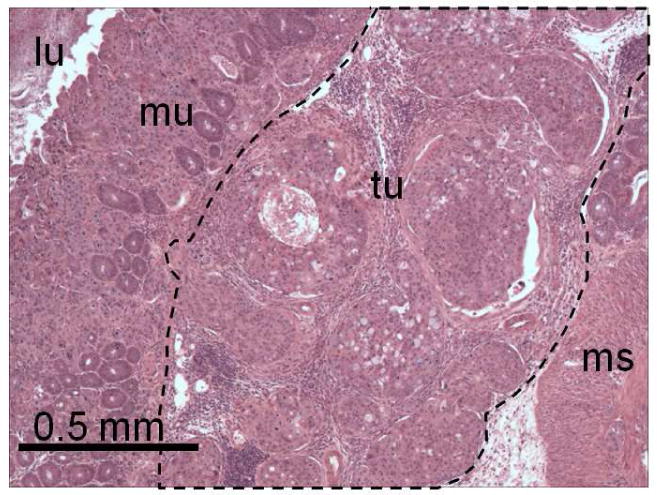
Histology of representative surgically resected tumor sample. H&E-stained tissue section demonstrating orthotopically implanted HT-29 human colon carcinoma cells growing within the mouse cecum (lu, lumen of bowel; mu, mucosa; tu, tumor; ms, muscularis).
Discussion
The importance of achieving an R0 resection is so critical to the outcome of colorectal cancer surgery that a grading system for the quality of colon cancer surgery was developed. Initially, the grading system was used to compare the quality of laparoscopic assisted surgery with open resection for colorectal cancer (10). This grading system however was based on the mesorectal grading system used in the Medical Research Council (MRC) CR07 trial which demonstrated a strong correlation between decreased local recurrence after rectal cancer resection and obtaining a mesorectal plane of surgical dissection (11). West et al. used the grading system to analyze how the quality of colon cancer surgery (removing an intact colonic mesentery or mesocolon) can affect long-term survival and locoregional recurrence (12). A 15% overall survival advantage at 5 years was reported with mesocolic-plane surgery compared with surgery in the muscularis-propria plane (HR 0.57, 95% CI: 0.38-0.85, p=0.006). The strongest survival advantage, however, was seen in patients with stage III disease (27% survival advantage at 5 years; HR 0.39 [0.21-0.72], p<0.0001).
The concept that a mesocolon or mesorectal resection decreases locoregional recurrence and improves 5-year survival rates highlights the importance of removing the mesentery intact once the tumor has spread outside of the muscle layer and into the surrounding lymph nodes. Total mesorectal excision (TME) became a standardized approach and has since significantly improved outcomes in rectal cancer surgery (4).
Campos and colleagues further emphasized the importance of achieving an R0 resection in their cohort study of 679 patients by achieving a 5-year survival rate of 60% compared to no survivors at 5 years among R1 and R2 resections (3). This group further argued the importance of removing all tumor-bearing tissue to achieve a radical resection under acceptable operative risks to give the patient the best opportunity for cure and prolonged survival.
Fluorescent imaging techniques take advantage of unique characteristics of the tumor cell to enhance delineation between tumor and normal surrounding tissue. With such in vivo modalities, the surgeon can objectively visualize tumor margins at the time of surgery further enhancing tumor resection. To enhance intraoperative detection of tumor margins, researchers have engineered different targeting strategies to fluorescently label native tumors, such as activatable probes that rely on tumor-specific enzymatic activity (13-15), replication-competent viruses engineered to express GFP in the presence of activated telomerase (16-19); and antigen-specific antibodies that can be directly conjugated to a fluorescent probe (6, 7, 20). Bouvet and Hoffman recently summarized the state of the art of making tumors glow for FGS (21).
Fluorescently labeling tumors has many clinical applications. Flourescent labeling allows visualizing, and potentially resecting, tumor that is invisible under bright light. In our study, the main body of the primary tumor could be visualized under normal bright light. However, what tended to be “invisible” were the small satellite tumors near the surgical margins. Because of failure to detect these micro-lesions at the surgical margins, cancer cells were unknowingly left behind, which could ultimately result in recurrence. When tumor was visualized under fluorescence, minor adjustments to our resection approach permitted more precise excision of such lesions without compromising the lumen or bowel function. Fluorescence guidance could prove to be invaluable in cancers such as rectal cancer where the status of the radial margin plays a significant factor in prognosis.
Fluorescence guided surgery can also be applied to minimally invasive techniques. We have previously described a method for fluorescence laparoscopy and demonstrated its improved diagnostic abilities that allow the surgeon to accurately visualize and detect tumor without compromising background illumination necessary for surgical navigation (8, 9, 22, 23). Robotic applications of fluorescence laparoscopy under controlled lighting, including near ultraviolet laparoscopy, have recently been described and have important clinical potential (24-26). Therefore, fluorescence guided minimally invasive surgical techniques could be applied to colon and rectal cancer which are increasingly being removed with laparoscopic or robotic assistance.
Although this study is unique in its design for survival and disease-free results after resection with fluorescence-guidance, there are some limitations. Due to the small animal model, safe resections of the cecum were technically challenging limiting the overall number of mice available for this experiment. As a result, the study was not adequately powered to demonstrate statistical significance in disease free survival, although there was a strong trend for improved survival with FGS. In addition, the follow-up time was limited due to the short life span of a healthy nude mouse. In the end, the improved overall survival and disease-free survival experienced by the mice in the FGS group (as illustrated in the Kaplan-Meier curves) trended to statistical significance. Overall, this proof of principle study demonstrates the improved surgical outcomes that can be achieved with FGS and suggests the importance of repeating such studies with more clinically-applicable methods of fluorescently labeling human cancers, such as with fluorophore-conjugated antibodies directed to unique tumor antigens.
Conclusion
The present study demonstrated improved surgical outcomes with fluorescence-guided surgery in patient-like mouse models of human colon cancer. Complete resections were more achievable with fluorescence-guided surgery compared to standard bright light surgery. The improved resection results led to significantly decreased recurrence rates and longer disease-free survival in the FGS group. More mice in the FGS group underwent curative resection and experienced longer overall survival compared to mice in the BLS group. Fluorescence guided surgery allowed identification and removal of sub-millimeter size tumor deposits along the cecum. By enhancing the surgeon's ability to delineate tumor margins, a more complete tumor resection was obtainable, resulting in improved disease-free survival and overall survival as well as cures. This novel approach has significant potential to improve outcomes in the surgical treatment of colon cancer. Our future goals are to utilize unique characteristics of colon cancer, such as fluorophore-conjugated antibodies to CEA, to fluorescently label tumors in the patient in order to more effectively delineate surgical margins to improve long-term outcomes.
Acknowledgments
Work supported in part by grants from the National Cancer Institute CA142669 and CA132971 (to M.B. and AntiCancer, Inc) and T32 training grant CA121938-5 (to C.A.M.).
Footnotes
Presented at the Academic Surgical Congress 2012 Annual Meeting, Feb. 14-16, 2012, Las Vegas, Nevada
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andreoni B, Chiappa A, Bertani E, Bellomi M, Orecchia R, Zampino M, Fazio N, Venturino M, Orsi F, Sonzogni A, Pace U, Monfardini L. Surgical outcomes for colon and rectal cancer over a decade: results from a consecutive monocentric experience in 902 unselected patients. World J Surg Oncol. 2007;5:73. doi: 10.1186/1477-7819-5-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruo L, Guillem JG. Surgical management of primary colorectal cancer. Surg Oncol. 1998;7:153–163. doi: 10.1016/s0960-7404(99)00033-x. [DOI] [PubMed] [Google Scholar]
- 3.Campos FG, Calijuri-Hamra MC, Imperiale AR, Kiss DR, Nahas SC, Cecconello I. Locally advanced colorectal cancer: results of surgical treatment and prognostic factors. Arq Gastroenterol. 2011;48:270–275. doi: 10.1590/s0004-28032011000400010. [DOI] [PubMed] [Google Scholar]
- 4.Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354–364. doi: 10.1111/j.1463-1318.2008.01735.x. discussion 364-355. [DOI] [PubMed] [Google Scholar]
- 5.Misiakos EP, Karidis NP, Kouraklis G. Current treatment for colorectal liver metastases. World J Gastroenterol. 2011;17:4067–4075. doi: 10.3748/wjg.v17.i36.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaushal S, McElroy MK, Luiken GA, Talamini MA, Moossa AR, Hoffman RM, Bouvet M. Fluorophore-conjugated anti-CEA antibody for the intraoperative imaging of pancreatic and colorectal cancer. J Gastrointest Surg. 2008;12:1938–1950. doi: 10.1007/s11605-008-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McElroy M, Kaushal S, Luiken GA, Talamini MA, Moossa AR, Hoffman RM, Bouvet M. Imaging of primary and metastatic pancreatic cancer using a fluorophore-conjugated anti-CA19-9 antibody for surgical navigation. World J Surg. 2008;32:1057–1066. doi: 10.1007/s00268-007-9452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metildi CA, Kaushal S, Lee C, Hardamon CR, Snyder CS, Luiken GA, Talamini MA, Hoffman RM, Bouvet M. An LED light source and novel fluorophore combinations improve fluorescence laparoscopic detection of metastatic pancreatic cancer in orthotopic mouse models. J Am Coll Surg. 2012;214:997–1007. e1002. doi: 10.1016/j.jamcollsurg.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran Cao HS, Kaushal S, Metildi CA, Menen RS, Lee C, Snyder CS, Messer K, Pu M, Luiken GA, Talamini MA, Hoffman RM, Bouvet M. Tumor-specific fluorescence antibody imaging enables accurate staging laparoscopy in an orthotopic model of pancreatic cancer. Hepatogastroenterology. 2012;59:1994–1999. doi: 10.5754/hge11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 11.Quirke P, Williams GT, Ectors N, Ensari A, Piard F, Nagtegaal I. The future of the TNM staging system in colorectal cancer: time for a debate? Lancet Oncol. 2007;8:651–657. doi: 10.1016/S1470-2045(07)70205-X. [DOI] [PubMed] [Google Scholar]
- 12.West NP, Morris EJ, Rotimi O, Cairns A, Finan PJ, Quirke P. Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. Lancet Oncol. 2008;9:857–865. doi: 10.1016/S1470-2045(08)70181-5. [DOI] [PubMed] [Google Scholar]
- 13.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci U S A. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen QT, Olson ES, Aguilera TA, Jiang T, Scadeng M, Ellies LG, Tsien RY. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci U S A. 2010;107:4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson ES, Aguilera TA, Jiang T, Ellies LG, Nguyen QT, Wong EH, Gross LA, Tsien RY. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol (Camb) 2009;1:382–393. doi: 10.1039/b904890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishimoto H, Kojima T, Watanabe Y, Kagawa S, Fujiwara T, Uno F, Teraishi F, Kyo S, Mizuguchi H, Hashimoto Y, Urata Y, Tanaka N, Fujiwara T. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat Med. 2006;12:1213–1219. doi: 10.1038/nm1404. [DOI] [PubMed] [Google Scholar]
- 17.Kishimoto H, Zhao M, Hayashi K, Urata Y, Tanaka N, Fujiwara T, Penman S, Hoffman RM. In vivo internal tumor illumination by telomerase-dependent adenoviral GFP for precise surgical navigation. Proc Natl Acad Sci U S A. 2009;106:14514–14517. doi: 10.1073/pnas.0906388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishimoto H, Aki R, Urata Y, Bouvet M, Momiyama M, Tanaka N, Fujiwara T, Hoffman RM. Tumor-selective, adenoviral-mediated GFP genetic labeling of human cancer in the live mouse reports future recurrence after resection. Cell Cycle. 2011;10:2737–2741. doi: 10.4161/cc.10.16.16756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishimoto H, Urata Y, Tanaka N, Fujiwara T, Hoffman RM. Selective metastatic tumor labeling with green fluorescent protein and killing by systemic administration of telomerase-dependent adenoviruses. Mol Cancer Ther. 2009;8:3001–3008. doi: 10.1158/1535-7163.MCT-09-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy MD, Jallad KN, Thompson DH, Ben-Amotz D, Low PS. Optical imaging of metastatic tumors using a folate-targeted fluorescent probe. J Biomed Opt. 2003;8:636–641. doi: 10.1117/1.1609453. [DOI] [PubMed] [Google Scholar]
- 21.Bouvet M, Hoffman RM. Glowing tumors make for better detection and resection. Sci Transl Med. 2011;3:110fs110. doi: 10.1126/scitranslmed.3003375. [DOI] [PubMed] [Google Scholar]
- 22.Tran Cao HS, Kaushal S, Lee C, Snyder CS, Thompson KJ, Horgan S, Talamini MA, Hoffman RM, Bouvet M. Fluorescence laparoscopy imaging of pancreatic tumor progression in an orthotopic mouse model. Surg Endosc. 2011;25:48–54. doi: 10.1007/s00464-010-1127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran Cao HS, Kaushal S, Menen RS, Metildi CA, Lee C, Snyder CS, Talamini MA, Hoffman RM, Bouvet M. Submillimeter-resolution fluorescence laparoscopy of pancreatic cancer in a carcinomatosis mouse model visualizes metastases not seen with standard laparoscopy. J Laparoendosc Adv Surg Tech A. 2011;21:485–489. doi: 10.1089/lap.2011.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobis S, Knopf J, Silvers C, Yao J, Rashid H, Wu G, Golijanin D. Near infrared fluorescence imaging with robotic assisted laparoscopic partial nephrectomy: initial clinical experience for renal cortical tumors. J Urol. 2011;186:47–52. doi: 10.1016/j.juro.2011.02.2701. [DOI] [PubMed] [Google Scholar]
- 25.Rossi EC, Ivanova A, Boggess JF. Robotically assisted fluorescence-guided lymph node mapping with ICG for gynecologic malignancies: a feasibility study. Gynecol Oncol. 2012;124:78–82. doi: 10.1016/j.ygyno.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Wagner OJ, Louie BE, Vallieres E, Aye RW, Farivar AS. Near-infrared fluorescence imaging can help identify the contralateral phrenic nerve during robotic thymectomy. Ann Thorac Surg. 2012;94:622–625. doi: 10.1016/j.athoracsur.2012.04.119. [DOI] [PubMed] [Google Scholar]


