Abstract
Background
Schistosomiasis mansoni is a major cause of portal fibrosis and portal hypertension. The Hedgehog pathway regulates fibrogenic repair in some types of liver injury.
Aims
Determine if Hedgehog-pathway activation occurs during fibrosis progression in schistosomiasis and to determine if macrophage-related mechanisms are involved.
Methods
Immunohistochemistry was used to characterize the cells that generate and respond to Hedgehog ligands in 28 liver biopsies from patients with different grades of schistosomiasis fibrosis staged by ultrasound. Cultured macrophages (RAW264.7 and primary rat Kupffer cells) and primary rat liver sinusoidal endothelial cells (LSEC) were treated with schistosome egg antigen (SEA) and evaluated by qRT-PCR. Inhibition of the Hedgehog-pathway was used to investigate its role in alternative activation of macrophages (M2) and vascular tube formation.
Results
Patients with schistosomiasis expressed more ligands (Shh and Ihh) and target genes (Patched and Gli2) than healthy individuals. Activated LSEC and myofibroblasts were Hedgehog-responsive (Gli2(+)) and accumulated in parallel with fibrosis stage (p<0.05). Double IHC for Ihh/CD68 showed that Ihh(+) cells were macrophages. In vitro studies demonstrated that SEA stimulated macrophages to express Ihh and Shh mRNA (p<0.05). Conditioned media from such macrophages induced luciferase production by Shh-LightII cells (p<0.001) and Hedgehog inhibitors blocked this effect (p<0.001). SEA-treated macrophages also up-regulated their own expression of M2 markers, and Hh-pathway inhibitors abrogated this response (p<0.01). Inhibition of the Hedgehog pathway in LSEC blocked SEA-induced migration and tube formation.
Conclusion
SEA stimulates liver macrophages to produce Hh-ligands, which promote alternative activation of macrophages, fibrogenesis, and vascular remodeling in schistosomiasis.
Keywords: schistosomiasis mansoni, hedgehog pathway, fibrosis, vascular remodeling, alternative activation of macrophages
Introduction
Schistosomiasis is one of the most prevalent tropical diseases, affecting more than 200 million people in over 74 different countries (1,2). Nearly 10% of patients develop hepatosplenic schistosomiasis which is characterized by periportal fibrosis and portal hypertension (3-7). Schistosoma mansoni is the only human schistosome species endemic in the Americas and schistosomiasis mansoni is a major public health problem in Latin America, especially in Brazil (8). In hepatic schistosomiasis mansoni, eggs deposited in the mesenteric veins are carried into the microvasculature of the liver and induce a granulomatous reaction that can evolve to portal fibrosis (8). There is no distortion of the architecture of the liver parenchyma and liver biosynthetic and excretory functions are usually normal (6). Although the severe pathologic manifestations of the disease were described in 1904 by Symmers (9) and have been a highly studied topic ever since, the mechanisms driving schistosomiais-associated fibrosis are not fully elucidated.
The early stages of schistosome infection are characterized by a vigorous immune response (10). During this process, lymphocytes and tissue macrophages produce abundant pro-inflammatory/anti-fibrogenic cytokines, such as interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) (10,11). However, egg deposition generates factors that suppress this pro-inflammatory state by eliciting production of other mediators, such as interleukin (IL)4 and IL13 (10,11). Recently, this egg-driven shift in immune response profile has been associated with alternative activation of macrophages (12,13). Macrophages that undergo this alternative type of activation have been dubbed M2 macrophages (14). Schistosomal granulomas are enriched with M2 macrophages, and such cells are believed to modulate the sequelae of chronic schistosome infection (12,13). M2 macrophages promote fibrosis both by suppressing production of IFNγ and other anti-fibrogenic cytokines, and by actively generating a repertoire of pro-fibrogenic factors, such as the enzyme Arginase-1 (Arg1) that is involved in collagen synthesis and other factors that have not yet been fully characterized (13,14). Some of these mediators are also presumed to promote angiogenesis because one of the most remarkable characteristics of schistosomiasis fibrosis is the related vascular alterations (6). The latter culminate in severe reduction and distortion of the portal venous system, and hyperplasia and hypertrophy of the arterial system, without appreciably changing the hepatic venous system (6).
The present study evaluates the hypothesis that both granuloma-associated angiogenesis and fibrogenesis during schistosome infection result from pathogen-mediated increases in macrophage production of Hedgehog (Hh) ligands. This concept was spurred by the acknowledged importance of macrophages in granulomatous inflammation, coupled with recent evidence that peripheral blood monocytes (which give rise to tissue macrophages) are capable of responding to Hh ligands, which stimulate their migration into tissues (15). Moreover, Hh ligands promote fibrogenic repair during other types of liver injury, and modulate endothelial cell activation and angiogenesis (16).
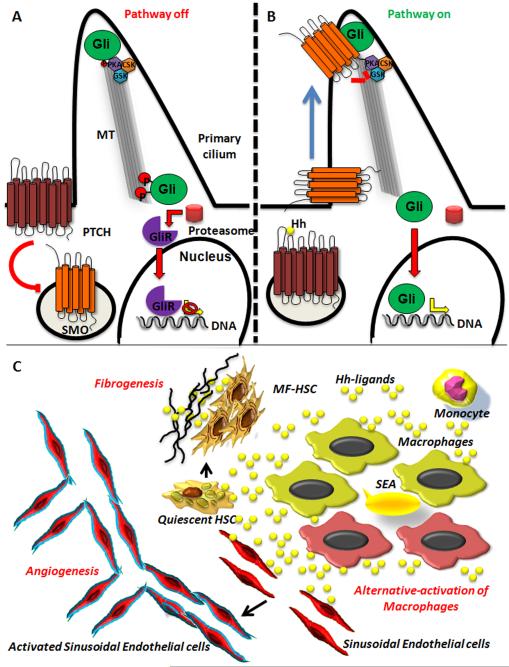
Hedgehog ligands (Sonic Shh, Indian Ihh and Desert Dhh) are a family of highly conserved morphogens that regulate tissue construction and remodeling (16). They are essential during embryogenesis, especially for limb and neural tube development (17). As in embryos, in many adult tissues, the Hh pathway modulates cell migration and proliferation, and functions as a viability factor for stem/progenitor cells (16). Recent studies demonstrate that the components of the Hh pathway are enriched in primary cilia (18). In the absence of Hh ligands, Patched (Ptch, the receptor for Hh ligands) inhibits its co-receptor Smoothened (Smo), restricting Smo to the base of the primary cilium where it is unable to activate the Glioma (Gli) family transcription factors, Gli1, Gli2 and Gli3, which are enriched in the top of this organelle (17) (Figure 7A). In this state, Gli3 is targeted to the proteasome where it is processed to a repressor, which then migrates to the nucleus and represses Hh-regulated genes (17) (Figure 7A). Binding of Hh ligands to Ptch permits Smo to migrate to the tips of primary cilia, where it promotes Gli1, 2 and 3 activation; the activated Glis then enter the nucleus and induce transcription of Hh-target genes (17) (Figure 7B).
Figure 7. Schematic representation of the Hedgehog (Hh) pathway and the effects of its activation in Hh-responsive cells in schistosomiasis mansoni fibrosis.
In the absence of Hh-ligands, Patched (Ptch, the receptor for Hh ligands) inhibits its co-receptor Smoothened (Smo), restricting Smo in endocytic vesicles at the base of the primary cilium where it is unable to activate the Glioma (Gli) family transcription factors, Gli1, Gli2 and Gli3, which are enriched in the top of this organelle. In this state, Gli proteins undergo phosphorylation by several kinases (PKA, GSK3, CSK), become ubiquitinated and are targeted to the proteasome where Gli1 and 2 are degraded and Gli3 is processed to a repressor (GliR), which then migrates to the nucleus and represses Hh-regulated genes. B) Binding of Hh ligands to Ptch induce its endocytation and permits Smo to migrate to the tips of primary cilia, where it inhibits the kinases (PKA, GSK3, CSK) and promotes Gli1, 2 and 3 activation; the activated Glis then enter the nucleus and induce transcription of Hh-target genes. C) When Schistosoma mansoni eggs are trapted in small portal tracts in the liver, they elicit a inflammatory response. The live myracid inside the egg produce a series of substances (SEA) that induce the production of Hh-ligands (Ihh and Shh) by marcophages. Those ligands act autocrinally inducing the alternative activation of macrophages and act as chemoattractants for monocytes. Hh-ligands derived from macrophages acts paracrinally on quiescent stellate cells (HSC) activating them to became myofibroblastic HSC that produce extracellular matrix and also secrete Hh-ligands. Hh also plays a important role in the vascular remodeling characteristic of schistosomiais, inducing activation of liver sinusoidal endothelial cells and angiogenesis.
Cholangiocytes and dying hepatocytes, as well as several types of cells that accumulate in injured livers (e.g., myofibroblastic stellate cells, hepatic progenitors, and some types of lymphocytes) are capable of producing Hh ligands (16). The Hh ligands, in turn, promote the growth and viability of the myofibroblastic stellate cells (19,20), stimulate immature ductular cells to undergo epithelial to mesenchymal transition (21), and up-regulate local production of chemokines and chemokine receptors that recruit pro-fibrogenic immune cells to the liver (22). Because all of these responses expand hepatic myofibroblast populations, the Hh pathway is presumed to be a major driver of hepatic fibrogenesis. This concept is supported by evidence that the level of Hh pathway activity correlates with fibrosis stage in many liver diseases, including alcoholic (23) and nonalcoholic fatty liver disease (24), hepatitis B and C (25), autoimmune biliary tract disease in adults (21,26) and various types of congenital cholestatic liver diseases (27). To our knowledge, however, whether or not the Hh pathway has a role in the fibrogenesis that accompanies formation of periovular schistosome granulomas has not been examined.
As mentioned earlier, vascular remodeling is the other key outcome of the granulomatous reaction to hepatic schistosomiasis. It is tempting to speculate that Hh ligands may also have roles in this process because exposure of isolated endothelial cells to Hh ligands up-regulates their expression of adhesion molecules and activation markers (28). Moreover, inhibiting Hh signaling blocks endothelial cell activation and vascular tube formation (25). However, whether or not the granuloma-related angiogenesis that occurs during hepatic schistosomiasis is mediated by Hh signaling has not been assessed.
Herein, we used immunohistochemistry to characterize the types of cells that generate and respond to Hh ligands in the livers of patients with various stages of fibrosis due to chronic S. mansoni infection. To clarify the significance of the histologic findings for schistosomal liver disease pathogenesis, cultured macrophages were treated with schistosome soluble egg antigen (SEA) to determine direct effects of the pathogen antigens on Hh signaling. The ability of macrophage-conditioned medium to stimulate Hh signaling in other types of Hh-responsive cells was also examined. The results identify novel mechanisms by which pathogen-generated factors influence immune cells to modulate liver tissue remodeling.
Patients and Methods
Ethics statement
Wedge liver biopsies were obtained from patients with schistosomiasis mansoni that had an indication of surgical intervention (splenectomy and/or suturing of esophagus-gastric varices) under a protocol (ETIC 204/06) approved by the Ethical Board of the Federal University of Minas Gerais, Brazil and the Duke University institutional review board. Written informed consent was obtained from all patients.
Human samples
A total of 28 wedge liver biopsies from patients with different grades of schistosomiasis fibrosis staged by ultrasound (WHO protocol - Niamey Working Group 1996; pattern A=3 patients, D=5, Dc=2, Ec=17, F=1) were used in this study. Briefly, abdomens of schistosomiasis patients were examined by ultrasound using a real-time ALOKA SSD device 1700 with an electronic convex 3.5 MHz transducer. Hepatic fibrosis was classified as absent (pattern A), mild (pattern B, C, D and Dc) or moderate to intense (pattern E, Ec and F). All patients were referred to the Tropical Diseases Outpatient Clinic of the Federal University of Minas Gerais Hospital (Belo Horizonte, Brazil) with a clinical diagnosis of hepatosplenic schistosomiasis mansoni, portal hypertension and indication of surgical intervention (splenectomy and/or suturing of esophagus-gastric varices). The diagnosis was based on the presence of eggs in stool or in rectal biopsy and a history of contact with stream waters of an endemic area. Wedge liver biopsies (~3cm3) were collected from the left lobe during surgical procedures and were formalin fixed, paraffin-embedded (FFPE) and sections were prepared for histological analysis. FFPE sections were also obtained from residual healthy liver tissues of two donor livers that were utilized for split liver transplantation at Duke University Hospital. Subjects included in this study did not have other causes of chronic liver diseases.
Histology and Immunohistochemistry
Serial sections were stained with H&E for general histology and Picro-sirius red for fibrosis assessment. Immunohistochemistry analysis was performed to evaluate the hedgehog pathway (Hedgehog ligands Ihh and Shh, receptor and target gene Patched, Ptch, and the transcription factor Gli2), presence of monocytes/macrophages (CD68), activated sinusoidal endothelial cells (CD31) and activated stellate cells/myofibroblasts (alpha smooth muscle actin, αSMA). Antigen retrieval techniques were used as needed (25). Each assay was performed with a control human section without schistosomiasis and positive and negative controls. Antibodies used are listed in Supplemental Table 1. Antigens were demonstrated by diaminobenzidine (DAB, DAKO) or Ferangi Blue (FB8125, Biocare Medical).
Quantification analysis was performed for Ptch and Gli2 mono staining and also for Gli2/CD31 and Gli2/αSMA double staining. For Ptch, 5 representative ×200 fields per section containing similar size portal tracks were evaluated (Normal=2, Pattern A=3, D- Dc=6, Ec-F=10) by morphometry using MetaMorph (Universal Imaging Corp, Downington, PA). The numbers of Gli2 positive nuclei were counted in 5 representative ×200 fields per section from each subject (Normal=2, Pattern A=3, D-Dc=3, Ec-F=8) by 2 independent observers. The numbers of Gli2/αSMA and Gli2/CD31 double-positive cells were counted in 10 representative ×400 fields from each subject (Normal=2, Pattern A=2, Ec-F=5 (Gli2/αSMA) or 8 (Gli2/CD31)).
Cell culture studies
RAW264.7 (ATCC, Manassas, VA) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) until they reached 70% confluency. Primary rat Kupffer cells (prKC) (Life Technologies, Carlsbad, CA) were plated in RPMI 1640 containing 2% FBS and cultured for 56h. After pilot experiments were completed to establish dose and time of incubation, RAW264.7 and prKC were treated with 10μg/mL of Soluble Egg Antigen (SEA, prepared at the Centers for Disease Control and Prevention, Atlanta, GA) or vehicle (phosphate buffered saline, PBS) for 6h. RNA and conditioned media were collected for analysis. RAW264.7 cells were incubated in parallel with lipopylysaccharide (LPS, 1μg/mL) or vehicle (PBS) for 3, 6 and 9h with total RNA collected for analysis to control for the effects of contamination. Shh-LightII cells (ATCC) were cultured in 96-well plates and then treated with the conditioned medium from prKC (± SEA) or control media (DMEM/10% FBS) ± SEA (10μg/mL). To verify that induction of luciferase was due to Hh signaling initiated by macrophage-derived Hh ligands, reporter cells were treated with macrophage-conditioned media ± cyclopamine (Toronto Research Chemicals, Toronto, Canada) or an inactive analog, tomatidine (Toronto Research Chemicals). Treated cells were then cultured for 2 days under standard conditions, and firefly and Renilla spp. luciferase activities were determined using a dual luciferase kit (Promega) according to the manufacture’s protocols. Fold increase of Gli1 induction was then normalized to the control medium.
To determine if the Hh pathway influenced the alternative activation of macrophages, RAW264.7 cells were stimulated with of SEA (10μg/mL) in the presence of either Cyclopamine (1μM) or GDC-0449 (1μM, Selleck, Houston, TX) both of which are specific antagonists of Smo or vehicle control (dimethyl sulfoxide, DMSO) for 6h. Total RNA was collected to assess changes in the expression of alternative activation markers (Chi3l3, Arg1 and Fizz1) and Hh pathway activity (Gli1, Ptch).
Primary rat liver sinusoidal endothelial cells (LSEC) were isolated by collagenase perfusion, iodixanol density gradient centrifugation and centrifugal elutriation as previously described (29). LSEC were seeded on collage-coated inserts and treated with DMSO ± SEA (10 μg/mL) or SEA (10μg/mL) with GDC-0449 (1μM) for 20h. Effects on migration were assessed by counting the number of cells in the bottom of the inserts (29). To evaluate if Hh plays a role in angiogenesis mediated by SEA, primary rat SEC were seeded on growth factor reduced Matrigel and treated with DMSO ± SEA (10 μg/mL) or SEA (10μg/mL) with GDC-0449 (1μM) for 6h. Effects on vascular tube formation were assessed by quantifying the length of capillary like-tube as previously described (29).
RNA analysis
RNA was extracted with TRizol® (Invitrogen) according to the manufacturer’s protocol. Reverse transcription was performed with the First Strand Superscript™ II kit (Invitrogen) using the random hexamers protocol. The samples were analyzed by Real-time PCR (qRT-PCR) and the primers sequence used are described in Supplemental Table 2. Each sample was analyzed in duplicate and target gene levels in treated cells are shown as a ratio to levels detected in corresponding control samples, according to the ΔΔCt method. S9 was used as an internal control.
Statistical analysis
Results are expressed as means ± Standard Error of the Mean (SEM). Comparisons between groups were performed using Student’s t-test. The p-values are two tailed and significance was accepted at the 5% level.
Results
Patient populations
All patients came from endemic areas for schistosomiasis in the state of Minas Gerais, Brazil. Twenty eight consecutive patients with schistosomiasis and no other known cause of liver disease who had a clinical indication for splenectomy to alleviate portal hypertension, and consented to have a wedge liver biopsy performed intra-operatively, were enrolled from 2008-2009. Although all patients had hepatosplenic schistosomiasis, they had a diverse range of fibrosis stage by ultrasonography (ranging from absent to intense) as reported earlier in a study using a subgroup of our cohort (30). Representative Picro-sirius red histochemisty from patients with different degrees of fibrosis are shown on Supplemental Figure 1. Most of the study cohort were males (78.5%) with an average age of 40.6 (±10) years. There was little difference between the ages of patients across fibrosis stages, except that the individuals from the Dc pattern group were about one decade younger than the other patients (Table 1).
Table 1.
The demographic data of the selected patients with schistosomiasis mansoni.
| Fibrosis stage |
N° of patients |
Average age |
Gender |
|---|---|---|---|
| A | 3 | 39 (±16) | 2M/1F |
| D | 5 | 40.8 (±10) | 2M/3F |
| Dc | 2 | 27.5 (±5) | 2M |
| Ec | 17 | 42.3 (±10) | 15M/2F |
| F | 1 | 39 | 1M |
|
| |||
| Summary | 28 | 40.6 (±10) | 22M/6F |
Liver cell production of Hedgehog ligands (Ihh and Shh) increases in chronic schistosomiasis mansoni
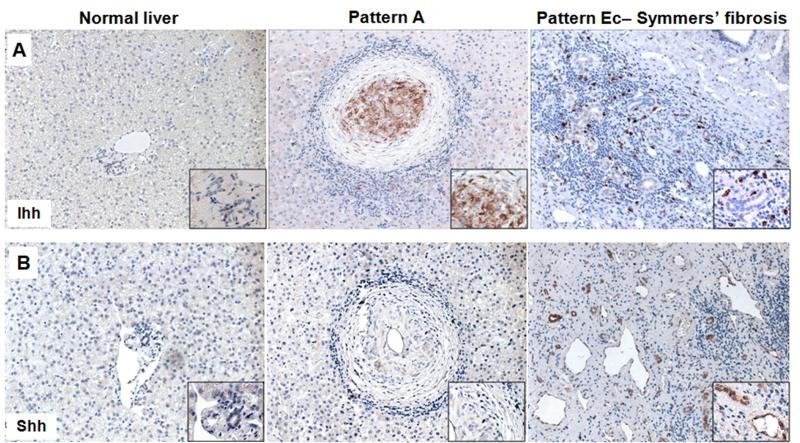
Very few cells expressing Hh ligands were observed in the livers of the two control subjects without schistosomiasis (Figure 1A,B). In contrast, Hh ligand expression was easily demonstrated in all schistosomiasis patients. Livers from patients with pattern A (no detectable fibrosis by US examination) expressed Ihh, but not Shh (Figure 1A,B). In patients with the advanced stages of schistosomiasis fibrosis (pattern Ec), Ihh and Shh expression were noted in the fibrous septa, with Ihh being expressed by mesenchymal cells and Shh by some ductular cells and endothelial cells. Some of the cases with early disease had active granulomas that were positive for Ihh. The Ihh(+) cells in the granulomas were buried in the stroma surrounding the egg, an area that is enriched with macrophages and other immune cells.
Figure 1. Liver cell production of Hedgehog ligands (Ihh and Shh) increases in chronic schistosomiasis mansoni.
A) Ihh immunostaining on liver sections from a representative control without schistosomiasis (left), from a representative schistosomiais patient with granulomas but no ultrasound detectable fibrosis (middle) and from a representative subject with severe schistosomiasis fibrosis (right), showing an increased number of stromal cells expressing Ihh in schistosomiasis. B) Immunohistochemical staining for Shh in representative liver sections from a healthy individual without schistosomiasis (left), from an infected individual without severe pathology (middle) and from a patient with Symmers’ fibrosis (right), showing an enrichment of ductular and endothelial cells positive for Shh in the fibrous septa. Final magnification ×200; insert ×600.
Macrophages produce Hedgehog ligands in schistosomiasis
To determine if any of the Ihh producing cells in patients’ liver tissues might be macrophages, we performed double immunohistochemistry for Ihh and the macrophage marker, CD68. The granulomas of patients with pattern A, and the fibrous septa of patients with pattern Ec, were enriched with cells that expressed both markers (Figure 2A). In vitro studies with a macrophage cell line (RAW264.7) and primary rat liver macrophages (prKC) confirmed the in vivo data. For example, after 6h of incubation with 10μg/mL of SEA, RAW264.7 cells up-regulated Ihh mRNA (Figure 2B) and prKC cells showed induced expression of Ihh and Shh (Figure 2C). Expression of these ligands remained low in cells that were incubated in the same media that contained vehicle alone (Figure 2B,C). To verify that the Hh ligand production was due to SEA components solely and not attributable to LPS contamination, RAW264.7 cells were treated with 1μg of LPS for 3, 6 and 9h or vehicle. Compared to the vehicle-treated cells, there was no significant change in the mRNA leves of Ihh, Gli1 and Ptch1 (Supplemental Figure 2). The conditioned media from SEA-exposed macrophages was also able to stimulate the production of luciferase by Shh Light II cells (Figure 2D). These cells have been stably transfected with Gli1-luciferase reporter constructs, and are routinely used to demonstrate Hh signaling activity. Hence, our finding suggests that the SEA-exposed macrophages release Hh ligands that are biologically active. To verify that the stimulation of the Gli1 reporter system was dependent upon activation of canonical Hh signaling, these experiments were repeated with addition of cyclopamine (an antagonist of Smoothened) or its inactive analog, tomatidine. Tomatidine had no effect on the ability of macrophage-conditioned medium to activate the Gli-luciferase reporter, whereas induction of reporter activity was abrogated by cyclopamine (Figure 2D). The aggregate data, therefore, demonstrate that schistosome antigens trigger macrophages to produce and release biologically active Hh ligands.
Figure 2. Macrophages produce Hedgehog ligands in schistosomiasis.
A) Dual immunostaining for Ihh (brown) and CD68 (blue), a marker of monocytes and macrophages, in a representative liver section from a schistosomiasis patient without severe pathology (left) and from a patient with Symmers’ fibrosis (right), showing an increased number of macrophages expressing Ihh. Final magnification ×200 (Pattern A), ×400 (Pattern Ec); insert ×600. Hh ligand mRNA expression in RAW264.7 cells, a mouse macrophage cell line (B), and in primary rat Kupffer cells (C) incubated with 10μg of SEA or PBS for 6 hours. D) Gli1-reporter assay. Shh-lightII cells were treated with conditioned media (CM) from KC previously treated for 6 hours with 10μg of SEA or with PBS and control media ± 10μg of SEA. Luciferase production was measured after 48 hours (left). To verify that luciferase production was due to Hh ligands derived from KC, Shh-LightII cells were incubated with CM from KC treated with SEA or PBS ± Cyclopamine or its inactive analog Tomatidine (right). Results were normalized to luciferase induction by normal media; Means ± S.E.M. are displayed. *p<0.05; **p<0.005.
Hh ligands stimulate alternative macrophage activation
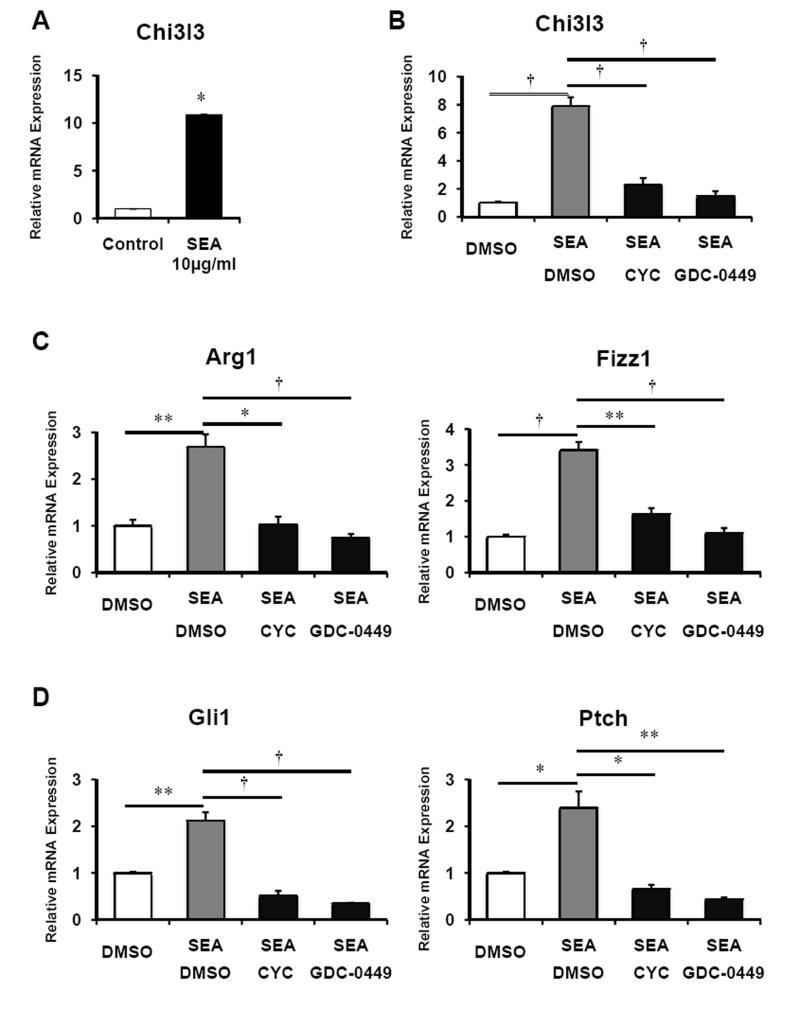
Peripheral blood monocytes, the precursors of macrophages, express Patched and activate Hh signaling in response to Hh ligands (15). Those findings suggested that macrophage production of Hh ligands might trigger changes in their phenotype. Indeed, exposing prKC or RAW264.7 cells to SEA up-regulated mRNA for Chitinase 3-like 3 (Chi3l3) (Figure 3A,B), a marker of alternatively activated (M2) macrophages (14). This response was abrogated by treating RAW264.7 with cyclopamine or GDC-0449, two different pharmacologic antagonists of Smoothened (Figure 3B). SEA-induced expression of two other M2 markers: Arg1 and Fizz1 (Figure 3C) (also known as resistin like alpha, Retnlα) was also inhibited, and abrogation of M2 marker expression occurred in parallel with repression of SEA-induction of Gli1 and Ptch (Figure 3D), target genes of the Hh pathway. Therefore, SEA up-regulates Hh-dependent mechanisms in macrophages that promote autocrine induction of the alternative activation program.
Figure 3. Hedgehog ligands stimulate alternative macrophage activation.
A) mRNA expression of the alternative activation marker of macrophages Chitinase 3 like 3 (Chi3l3) in primary rat Kupffer cells treated with 10μg/ml of SEA for 6 hours. Results were normalized by mRNA expression in PBS-treated cells. B-D) mRNA expression of the alternative activation makers of macrophages (B) Chi3l3, (C) Arginase 1 (Arg1) and Fizz1 (also known as Rentlα, resistin like alpha), and (D) Hedgehog pathway target genes, Gli1 and Patched (Ptch) in RAW264.7 cells treated with DMSO ± 10 μg/ml SEA or 10μg/ml SEA with either 1μM Cyclopamine or 1μM GDC-0449 (specific antagonists of Smoothened, co-receptor of the Hedgehog pathway) for 6 hours. Results were normalized by mRNA expression in DMSO-treated cells. Means ± S.E.M. are displayed. *p<0.05; **p<0.01; †p<0.005.
Hedgehog responsive cells accumulate during schistosomiasis fibrosis progression
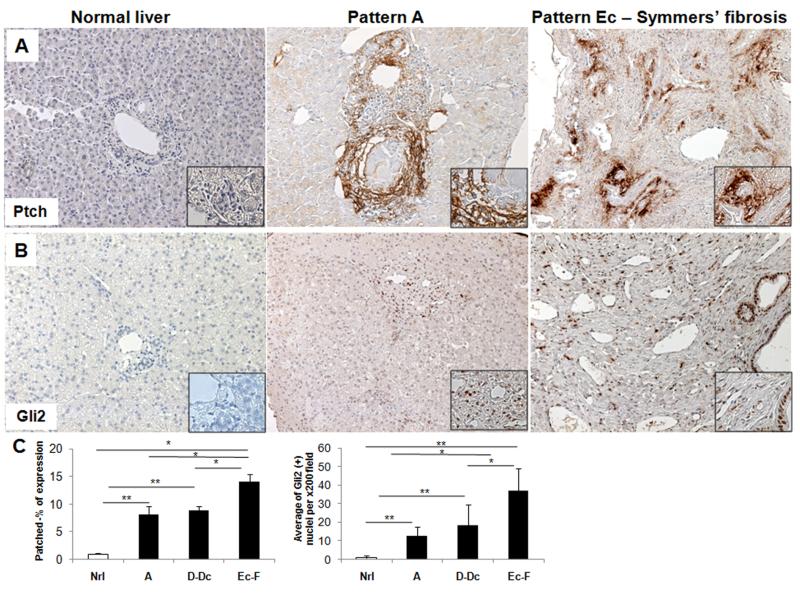
Alternative activation of macrophages occurs during many types of chronic wound healing responses and is typically associated with fibrogenesis and angiogenesis. Fibroblastic and angiogenic cells are generally Hh-responsive and Hh ligands stimulate the outgrowth of such cells. Therefore, we examined our tissue samples to determine if hepatic accumulation of Hh-responsive cells varied in accordance with levels of Hh ligand production. As reported previously, our non-diseased livers harbored very few cells that expressed the Hh-target genes, Ptch and Gli2 (25). In contrast, Ptch- and Gli2-expressing cells were abundant in liver samples from all patients with schistosomiasis (Figure 4). Moreover, as with the level of Hh ligand production (Figure 1), levels of Ptch and Gli2 expression correlated strongly with disease stage (Figure 4C). In subjects with low pathology (pattern A) hepatic schistosomiasis, Ptch(+) cells localized in fibrotic portal areas, as well as in areas of fibrosis surrounding granulomas (Figure 4A). In the livers of patients with advanced (pattern Ec) disease, cells that expressed Ptch and Gli2 were particularly enriched in the fibrous septa (Figure 4A). In infected individuals with both low (pattern A) and advanced (pattern Ec) pathology, some of the Hh-responsive cells also appeared to line vascular spaces (Figure 4B). In all stages of the disease, Gli2(+) cells were evident in ductular structures and in the stroma, where they often lined vascular spaces (Figure 4B).
Figure 4. Hedgehog responsive cells accumulate during schistosomiasis fibrosis progression.
A) Immunohistochemical staining for Patched (Ptch), the Hh receptor and also a target gene of the pathway, in representative healthy control liver section without schistosomiasis (left), in a representative subject with pattern A (middle) and in a subject with pattern Ec fibrosis (right), showing an increase of Hh responsive cells in the fibrous septa. In the liver of the patient with pattern A, Ptch(+) stromal cells are in the granulomatous reaction to the S. mansoni egg. B) Immunostaining for the Hedgehog transcription factor Gli2 in representative liver section from normal liver (left), and in representative patients with pattern A (middle) or pattern Ec fibrosis showing an enrichment of Gli2(+) cells in the fibrous septa. Note that many stromal, ductal, and endothelial cells express the Hh transcription factor. Final magnification ×200, inserts ×600. C) Quantification of the Ptch and Gli2 immunostaining. Ptch morphometry (Normal (Nrl)=2 subjects; Pattern A=3; D-Dc=6 and Ec-F=10) (left) and number of Gli2(+) nuclei per ×200 field (Normal (Nrl)=2; Pattern A=3; D-Dc=3; Ec-F=8)) (right). Results were normalized by percentage of expression of Ptch or number of Gli2(+) nuclei in normal individuals; mean ± S.E.M. are displayed.*p<0.05; **p<0.001.
Activated SECs and myofibroblastic cells are hedgehog responsive
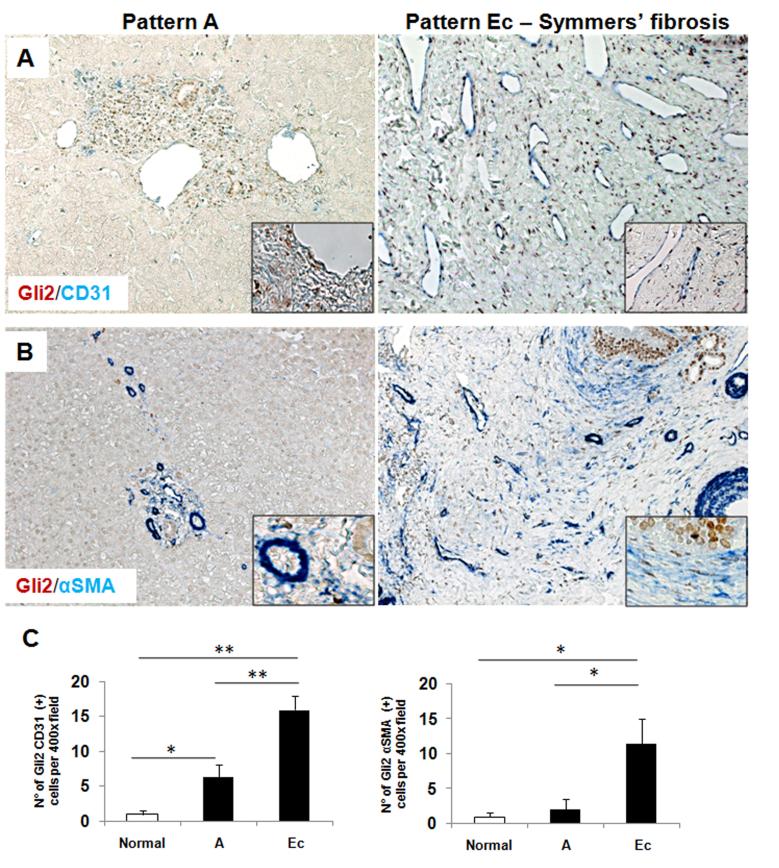
Schistosome infection causes a portal-based vascular remodeling and fibrosis, and Hh signaling modulates the growth of endothelial cell and myofibroblasts, promoting both vascular remodeling and fibrogenesis. Therefore, evidence that Hh-responsive cells resided in close proximity to vascular spaces in livers of infected individuals (Figure 4) suggested that the accumulation of Hh ligands might activate neighboring endothelial cells and thereby contribute to schistosomiasis-associated vascular abnormalities. To address this issue, double IHC was performed to identify cells that co-expressed CD31, a marker of activated sinusoidal endothelial cells (SEC), and the Hh-target gene, Gli2. Compared to control livers, livers from patients with schistosomiasis demonstrated accumulation of activated SEC (Figure 5A,C). The net hepatic content of CD31(+) cells paralleled the stage of the liver disease, increasing as pathology advanced. Moreover, the great majority of CD31(+) cells had Gli2(+) nuclei, indicative of active Hh signaling (Figure 5A,C). Because Hh ligands also promote the growth of fibroblastic cells, double-staining for Gli2 and the myofibroblast marker, αSMA, was also performed. Increases in the numbers of double(+) fibroblastic cells also mirrored disease progression, with the greatest numbers of Hh-responsive myofibroblastic cells occurring in livers that had the highest levels of Hh ligand production (Figure 1) and the most fibrosis (Figure 5B,C).
Figure 5. Activated Sinusoidal Endothelial cells and Myofibroblasts are Hedgehog responsive in schistosomiasis mansoni.
Double immunohistochemistry for Gli2 (brown) and CD31 (blue) (A) and for Gli2 (brown) and αSMA (blue) (B) in representative liver sections from subjects with pattern A (left) or with pattern Ec fibrosis (middle). Final magnification ×400, insert ×600. C) Quantification of the immunostaining for Gli2/CD31 (left) and for Gli2/αSMA (right) from selected patients. (Normal=2 subjects, Pattern A=2, Ec=8 (Gli2/CD31) or 5 (Gli2/αSMA)). Results were normalized to the number of double positive cells in normal individuals; means ± S.E.M. are displayed. *p<0.05; **p<0.001.
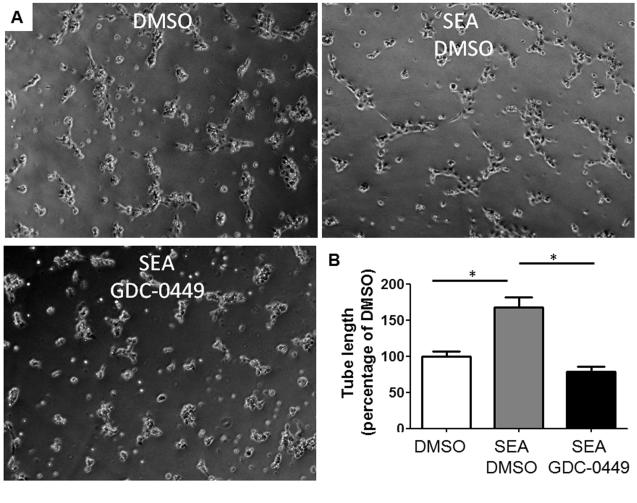
To further demonstrate that Hh signaling regulates the vascular remodeling that occurs in schistosomiasis, we incubated primary rat SEC seeded in matrigel with DMSO ± 10 μg/mL SEA or 10μg/mL SEA with 1μM GDC-0449 for 6h and quantified the length of capillary like-tube (vascular tube formation assay) (Figure 6). We observed that SEA, as described by others (31), induce tube formation but the pharmacologic inhibition of the Hh pathway abrogates this effect (p<0.01) (Figure 6). Analysis of the migration assay also indicate a pivotal role of the Hh pathway in the migration of SEC induced by SEA since the inhibition of this pathway with GDC-0449 blocked this effect (p<0.05) (Supplemental Figure 3). The results presented here propose a novel mechanism that explains the angiogenesis and vascular remodeling mediated by SEA in schistosomiasis mansoni.
Figure 6. Hedgehog pathway regulates vascular remodeling in schistosomiasis mansoni.
A) Primary rat SEC were seeded on growth factor reduced Matrigel and treated with DMSO ± 10 μg/ml SEA or 10μg/ml SEA with 1μM GDC-0449 (specific antagonists of Smoothened, co-receptor of the Hedgehog pathway) for 6 hours. B) Effects on vascular tube formation were assessed by quantifying the length of capillary like-tube. Representative micrographs and statistical summary are shown. *p<0.01. Final magnification x200.
Discussion
Our findings demonstrate that soluble factors from S. mansoni eggs (SEA) stimulate macrophages to produce and release biologically-active Hh ligands. This information suggests novel mechanisms that may contribute to the pathogenesis of hepatic schistosomiasis because Hh ligands are pleiotropic morphogens that regulate immune responses, angiogenesis, and fibrogenesis (Figure 7C).
Alternative activation of macrophages is believed to play a key role in remodeling responses that lead to hepatic fibrosis and angiogenesis. We demonstrated that macrophages embedded within egg-associated granulomas in the livers of patients with hepatic schistosomiasis expressed Hh ligands, and found that SEA directly triggered cultured macrophages to produce these factors which then acted in an autocrine fashion to induce the expression of genes associated with the alternatively activated macrophage phenotype (i.e., M2). Evidence that Hh ligands promote alternative activation of macrophages extends current understanding of the Hh pathway’s immunomodulatory actions. Hh signaling has already been shown to regulate thymic development, modulate activation of adult CD4(+) and CD8(+) T cells (32), influence the viability and cytokine profiles of hepatic NKT cells (33), and stimulate macrophage chemotaxis (15). Interestingly, the pro-inflammatory cytokine IFNγ induces expression of Hh ligands in certain types of neural progenitor cells (34). Schistosomes triggers macrophage IFNγ production as part of the initial response to infection (10). Thus, hepatic macrophages are exposed to at least two factors (i.e., SEA and IFNγ) that might stimulate them to produce Hh ligands. Macrophages were recently found to express cell surface receptors and intracellular signaling components of the Hh pathway, indicating that they are capable of transducing Hh ligand-initiated signals (15). In our study, SEA-treated macrophages released biologically-active Hh ligands that induced Hh signaling and activated the transcription of Hh-regulated genes. Moreover, various manipulations that inhibited Hh signaling in SEA-treated macrophages prevented them from expressing Chi3l3, a well-accepted marker of the alternatively activated (M2) phenotype (35). An earlier report demonstrated that Hh ligands stimulated NKT cells to produce IL4 and IL13, two key mediators of granulomatous (type 2) inflammation (33). Thus, the aggregate data support the concept that Hh pathway activation helps to mediate the switch between M1 and M2 immune responses that typically occurs during chronic infection with S. mansoni.
Accumulation of M2 macrophages is thought to be involved in the aberrant tissue remodeling responses that accompany chronic granulomatous inflammation during hepatic schistosomiasis (13). Our discovery that M2 macrophages generate soluble Hh ligands provides a novel explanation for these previous observations because Hh ligands are paracrine mediators of fibrogenesis and angiogenesis (16). Hh ligands promote the viability and growth of myofibroblastic hepatic stellate cells (MF-HSC) and stimulate such cells to produce collagen, as well as factors that remodel the extracellular matrix (19,20). Hh ligands also enhance hepatic recruitment of circulating fibrocytes and promote certain types of immature liver epithelial cells to acquire a more fibroblastic phenotype (21). Various types of liver injury cause hepatic accumulation of Hh ligands and mice with genetic defects that permit excessive Hh pathway activation develop overly exuberant hepatic fibrosis following liver injury (16). Hence, it is conceivable that the increased production of Hh ligands that we noted in the livers of patients with chronic schistosomiasis contributed to their hepatic fibrosis. This concept is supported by at least two lines of evidence. First, immunohistochemistry localized the Hh-regulated transcription factor, Gli2, to nuclei of liver cells that expressed the myofibroblast marker αSMA. Second, the hepatic content of Hh-responsive myofibroblasts increased in parallel with hepatic expression of Hh ligands and fibrosis stage in patients with schistosomiasis. These findings do not preclude a role for other known pro-fibrogenic factors, such as IL13, in schistosoma-related fibrogenesis. Rather, they identify Hh ligands as potential proximal mediators of both M2 cytokine production and the fibrotic liver remodeling that often accompanies M2-predominant immune responses during chronic S. mansoni infection.
Finally, the discovery that SEA triggers immune responses that enrich livers from schistosomiasis patients with Hh ligands suggests a novel mechanism for the pathogenesis of schistosomiasis-related hepatic angiogenesis, namely Hh-mediated vascular remodeling. Support for this concept is provided by the immunohistochemistry data which demonstrate a striking localization of Gli2 in nuclei of CD31(+) hepatic endothelial cells and by in vitro assays with primary rat LSEC incubated with SEA in which the inhibition of the Hh pathway abrogated migration and vascular tube formation in those cells. Moreover, the numbers of these Hh-responsive, activated endothelial cells increased in parallel with the hepatic content of Hh ligands and was greatest in livers of patients with the most advanced fibrosis. It is well accepted that endothelial cells and their progenitors are Hh-responsive and that Hh signaling regulates vasculogenesis and angiogenesis (25,28,36). In addition, membrane microparticles from activated T lymphocytes (37) and myofibroblastic liver cells (28) induce Hh signaling and activate transcription of Hh-regulated genes in endothelial cells from adults. Therefore, it is reasonable to conclude that chronic exposure to pathogen-derived factors that enrich the hepatic microenvironment with Hh ligands not only promotes liver fibrosis, but also causes fibrosis-associated angiogenesis.
Coupled with the new evidence that Hh ligands may also mediate the switch from M1 to M2 immune responses, this concept has important therapeutic implications for patients with chronic schistosomiasis. In persons with advanced hepatosplenic disease, treatment of infection with praziquantel, which kills the adult worms, is not sufficient to reverse the accumulated tissue damage. It is possible, for example, that treatment with pharmacologic inhibitors of Smoothened would constrain, or even ameliorate, pathologic tissue remodeling. These benefits would be most helpful to patients who had developed fibro-vascular complications of hepatic schistosomiasis and may be a non-surgical intervention to reduce the morbidity and mortality associated with the resultant portal hypertension. Further research is justified to examine this issue.
Supplementary Material
Supplemental Figure 1 – Distribution of collagen I and III in schistosomiasis mansoni fibrosis. Picro Sirius red histochemistry of the liver of representative normal individuals (A) and from patients with pattern A (B), pattern D (C) and pattern Ec fibrosis (D). Final magnification ×100.
Supplemental Figure 2 – LPS treatment does not induce hedgehog ligand production or pathway activation in macrophages. A) Hh ligand (Ihh) and target genes (Ptch1 and Gli1) mRNA expression in a mouse macrophage cell line (RAW264.7) incubated with 1μg of LPS or PBS for 3,6 and 9 hours. The results were not statistical significant when compared to PBS-treated cells.
Supplemental Figure 3 - Hedgehog pathway regulates migration of sinusoidal endothelial cells in schistosomiasis mansoni. A) Primary rat SEC were seeded on collagen coated insert and treated with DMSO ± 10 μg/ml SEA or 10μg/ml SEA with 1μM GDC-0449 (specific antagonists of Smoothened, co-receptor of the Hedgehog pathway) for 20 hours. B) Effects on migration were assessed by counting the number of cells in the bottom of the inserts. Representative micrographs and statistical summary are shown. *p<0.05, **p<0.01. Final magnification x200.
Acknowledgements
This research was funded by NIH grant R01 DK077794 awarded to AMD, 5K08 DK080980 awarded to SSC, CNPq grant awarded to FEP and Capes and CNPq fellowship from the Brazilian government awarded to TAP. We would like to thank Professor Virginia Hora Rios Leite from the Pathology Department of the Medical School of the Federal University of Minas Gerais for sending us some of the samples here analyzed.
Reference List
- 1.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaral RS, Tauil PL, Lima DD, Engels D. An analysis of the impact of the Schistosomiasis Control Programme in Brazil. Mem Inst Oswaldo Cruz. 2006;101(Suppl 1):79–85. doi: 10.1590/s0074-02762006000900012. [DOI] [PubMed] [Google Scholar]
- 3.Prata A. Schistosomiasis mansoni in Brazil. Baillieres Clin Trop Med Comm Dis. 1987;2:349–369. [Google Scholar]
- 4.Pinto-Silva RA, Abrantes WL, Antunes CM, Lambertucci JR. Sonographic features of portal hypertension in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1994;36:355–61. doi: 10.1590/s0036-46651994000400008. [DOI] [PubMed] [Google Scholar]
- 5.Lambertucci JR, Serufo JC, Gerspacher-Lara R, Rayes AA, Teixeira R, Nobre V, et al. Schistosoma mansoni: assessment of morbidity before and after control. Acta Trop. 2000;77:101–9. doi: 10.1016/s0001-706x(00)00124-8. [DOI] [PubMed] [Google Scholar]
- 6.Andrade ZA. Schistosomal hepatopathy. Mem Inst Oswaldo Cruz. 2004;99(5 Suppl 1):51–7. doi: 10.1590/s0074-02762004000900009. [DOI] [PubMed] [Google Scholar]
- 7.Maia MD, Lopes EP, Ferraz AA, Barros FM, Domingues AL, Ferraz EM. Evaluation of splenomegaly in the hepatosplenic form of mansonic schistosomiasis. Acta Trop. 2007;101:183–6. doi: 10.1016/j.actatropica.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Andrade ZA. Schistosomiasis and liver fibrosis. Parasite Immunol. 2009;31:656–63. doi: 10.1111/j.1365-3024.2009.01157.x. [DOI] [PubMed] [Google Scholar]
- 9.Symmers WSC. Note on a new form of liver cirrhosis due to the presence of the ova of Bilharzia hæmatobia. J Path Bact. 1904;9:237–239. [Google Scholar]
- 10.Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31:163–76. doi: 10.1111/j.1365-3024.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 11.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 12.Hesse M, Cheever AW, Jankovic D, Wynn TA. NOS-2 mediates the protective anti-inflammatory and antifibrotic effects of the Th1-inducing adjuvant, IL-12, in a Th2 model of granulomatous disease. Am J Pathol. 2000;157:945–55. doi: 10.1016/S0002-9440(10)64607-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148–54. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Dunaeva M, Voo S, van Oosterhoud C, Waltenberger J. Sonic hedgehog is a potent chemoattractant for human monocytes: diabetes mellitus inhibits Sonic hedgehog-induced monocyte chemotaxis. Basic Res Cardiol. 2010;105:61–71. doi: 10.1007/s00395-009-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omenetti A, Choi S, Michelotti G, Diehl AM. Hedgehog signaling in the liver. J Hepatol. 2011;54:366–73. doi: 10.1016/j.jhep.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teglund S, Toftgård R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–21. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 19.Sicklick JK, Li YX, Choi SS, Qi Y, Chen W, Bustamante M, et al. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest. 2005;85:1368–80. doi: 10.1038/labinvest.3700349. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown K, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–42. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omenetti A, Syn WK, Jung Y, Francis H, Porrello A, Witek RP, et al. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology. 2009;50:518–27. doi: 10.1002/hep.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung Y, Brown KD, Witek RP, Omenetti A, Yang L, Vandongen M, et al. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology. 2008;134:1532–43. doi: 10.1053/j.gastro.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488.e8. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira Tde A, Witek RP, Syn WK, Choi SS, Bradrick S, Karaca GF, et al. Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab Invest. 2010;90:1690–703. doi: 10.1038/labinvest.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung Y, McCall SJ, Li YX, Diehl AM. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology. 2007;45:1091–6. doi: 10.1002/hep.21660. [DOI] [PubMed] [Google Scholar]
- 27.Omenetti A, Bass LM, Anders RA, Clemente MG, Francis H, Guy CD, et al. Hedgehog activity, epithelial-mesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology. 2011;53:1246–58. doi: 10.1002/hep.24156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320–330.e2. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie G, Choi SS, Syn WK, Michelotti GA, Swiderska M, Karaca G, et al. Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation. Gut. 2012 Feb 23; doi: 10.1136/gutjnl-2011-301494. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voieta I, de Queiroz LC, Andrade LM, Silva LC, Fontes VF, Barbosa A, Jr, et al. Imaging techniques and histology in the evaluation of liver fibrosis in hepatosplenic schistosomiasis mansoni in Brazil: a comparative study. Mem Inst Oswaldo Cruz. 2010;105:414–21. doi: 10.1590/s0074-02762010000400011. [DOI] [PubMed] [Google Scholar]
- 31.Loeffler DA, Lundy SK, Singh KP, Gerard HC, Hudson AP, Boros DL. Soluble egg antigens from Schistosoma mansoni induce angiogenesis-related processes by up-regulating vascular endothelial growth factor in human endothelial cells. J Infect Dis. 2002;185:1650–6. doi: 10.1086/340416. [DOI] [PubMed] [Google Scholar]
- 32.Crompton T, Outram SV, Hager-Theodorides AL. Sonic hedgehog signalling in T-cell development and activation. Nat Rev Immunol. 2007;7:726–35. doi: 10.1038/nri2151. [DOI] [PubMed] [Google Scholar]
- 33.Syn WK, Witek RP, Curbishley SM, Jung Y, Choi SS, Enrich B, et al. Role for hedgehog pathway in regulating growth and function of invariant NKT cells. Eur J Immunol. 2009;39:1879–92. doi: 10.1002/eji.200838890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun L, Tian Z, Wang J. A direct cross-talk between interferon-gamma and sonic hedgehog signaling that leads to the proliferation of neuronal precursor cells. Brain Behav Immun. 2010;24:220–8. doi: 10.1016/j.bbi.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vokes SA, Yatskievych TA, Heimark RL, McMahon J, McMahon AP, Antin PB, et al. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131:4371–80. doi: 10.1242/dev.01304. [DOI] [PubMed] [Google Scholar]
- 37.Soleti R, Benameur T, Porro C, Panaro MA, Andriantsitohaina R, Martínez MC. Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis. 2009;30:580–8. doi: 10.1093/carcin/bgp030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 – Distribution of collagen I and III in schistosomiasis mansoni fibrosis. Picro Sirius red histochemistry of the liver of representative normal individuals (A) and from patients with pattern A (B), pattern D (C) and pattern Ec fibrosis (D). Final magnification ×100.
Supplemental Figure 2 – LPS treatment does not induce hedgehog ligand production or pathway activation in macrophages. A) Hh ligand (Ihh) and target genes (Ptch1 and Gli1) mRNA expression in a mouse macrophage cell line (RAW264.7) incubated with 1μg of LPS or PBS for 3,6 and 9 hours. The results were not statistical significant when compared to PBS-treated cells.
Supplemental Figure 3 - Hedgehog pathway regulates migration of sinusoidal endothelial cells in schistosomiasis mansoni. A) Primary rat SEC were seeded on collagen coated insert and treated with DMSO ± 10 μg/ml SEA or 10μg/ml SEA with 1μM GDC-0449 (specific antagonists of Smoothened, co-receptor of the Hedgehog pathway) for 20 hours. B) Effects on migration were assessed by counting the number of cells in the bottom of the inserts. Representative micrographs and statistical summary are shown. *p<0.05, **p<0.01. Final magnification x200.