Abstract
BACKGROUND
Leukemia is a common cancer among United States adults but there are few established risk factors. If leukemia risks are substantially influenced by exposures that vary in prevalence across generations, then population incidence rates should vary significantly by birth cohort. However, prior studies have not examined leukemia birth cohort effects using contemporary data and methods.
METHODS
We used incidence data from the National Cancer Institute's Surveillance, Epidemiology and End Results Program from 1992 through 2009 for adults 25 – 84 years old and age-period-cohort models to estimate incidence rate ratios according to birth cohort for acute myeloid leukemia (AML), acute lymphoid leukemia (ALL), chronic myeloid leukemia (CML), and chronic lymphoid leukemia (CLL).
RESULTS
Leukemia incidence varied significantly between birth cohorts for each major leukemia type in men and women except female AML; changes on the order of 1% per birth year or 20% per generation were observed. The most significant birth cohort signatures were observed for CLL and AML in men, which were decreasing and increasing, respectively, in cohorts born since 1946.
CONCLUSIONS
Our results support the hypothesis that adult leukemia risks are significantly modulated by environmental and lifestyle exposures.
IMPACT
A number of well-established (smoking, certain chemicals, radiation) and newly-recognized (obesity) leukemia risk factors are modifiable; ultimately, efforts to promote healthy lifestyles might also help reduce incidence rates of adult leukemia.
Keywords: Leukemia, Incidence, Age Factors, Sex Factors, United States/Epidemiology
INTRODUCTION
Leukemia (all types) is a common cancer among adults in the United States (US) with more than 40,000 new cases expected in 2012 (seer.cancer.gov). Adult leukemia incidence rates have significant international variation (1), secular trends (1–3), and characteristic patterns according to age, gender, and ethnic group (1–2, 4–6). Nonetheless, whereas the risks of secondary leukemias attributable to cancer chemotherapy and radiotherapy have been well characterized (7–8), there are currently only a few established primary risk factors for any major leukemia type (7–10). Therefore, it remains unclear if adult leukemias are largely a stochastic consequence of biological senescence (11–13), or alternatively if a substantial fraction might potentially be preventable through modification of environmental and lifestyle risk factors.
Assessment of birth cohort effects provides a potentially informative approach to shed light on this question (14). If cumulative exposures or exposures during sensitive ages to known or unknown leukemia risk factors vary in prevalence from one generation to the next, then incidence rates in the population should also vary significantly by birth cohort (15). Cohort patterns for childhood leukemias have been described (16–17); however, associations of birth cohort and adult leukemia rates have not been examined using contemporary data and methods. Therefore, we used nationally representative leukemia incidence data from the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) Program (18) and age-period-cohort models (19) to assess incidence trends and patterns according to birth cohort (20).
METHODS
Data
We obtained leukemia incidence data from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program for the 18 year period 1992 –2009 (seer.cancer.gov). As previously described (21–22), case and population data were combined from SEER's 13 (23) and 18 (24) Registries Databases; collectively, SEER's 18 Tumor Registries cover up to 28% of the US population.
We conducted separate analyses for each of the four major leukemia types: acute myeloid leukemia (AML), acute lymphoid leukemia (ALL), chronic myeloid leukemia (CML), and chronic lymphoid leukemia (CLL), as recorded in the SEER*Stat Database version 7.0.9 (25). We analyzed incidence rates for males and females separately to account for gender-related differences in the natural history. We limited our analysis to adult cases ages 25 – 84 years given the distinct etiologies of infant and childhood leukemias (26–30) and the small numbers of cases ages 15 – 24. We extracted single-year rates for all races combined (primary analyses), and for persons of non-Hispanic white race/ethnicity (sensitivity analyses). To obtain stable estimates using age-period-cohort analyses (see below), we grouped the single-year data into twenty 3-year age groups (25–27, …, 82 – 84) and six 3-year calendar periods (1992–1994, …, 2007–2009) spanning twenty-five partially overlapping 6-year birth cohorts (1910, 1913, …, 1982; referred to by mid-year of birth).
Statistical Analysis
The age-period-cohort model provides a flexible framework for characterizing cancer trends and patterns according to age at diagnosis, year of diagnosis (period), and year of birth (cohort) (31–33). Importantly, period effects in the APC model adjust for the influences of new treatments or diagnostic routines that can lead to changes in a specific diagnosis amongst all age groups at a certain point in time. Using age-period-cohort models, we examined the net drift, which estimates the average annual percentage change in incidence per calendar year or per year of birth (32, 34), the longitudinal age-at-onset curve, which summarizes the age-associated natural history for birth cohorts (14, 35), and the cohort rate-ratio curve (CRR) (20) , a recently introduced measure that describes age-specific incidence rates in each cohort relative to an arbitrary reference cohort, adjusted for any calendar period effects influencing all age groups simultaneously. We used the central 1946 cohort as the reference. The log-linear component of the CRR equals the net drift, and the non-linear component equals the Holford cohort deviations (15). We considered two likelihood ratio tests of the CRR values, a log-linear trend test and a test of non-linear birth cohort effects, equivalent to the Holford tests (15) of drift and cohort deviations, respectively. In exploratory analyses, we used the Tarone-Chu method (36) to examine local changes in incidence trends around the 1946 reference cohort. For all of our analyses P≤0.05 was considered statistically significant and CI denoted confidence interval.
RESULTS
Our study included a total of 91,448 leukemias (four major types diagnosed in males and females ages 25 through 84 years) and 7.01×108 person-years of follow-up (Table 1). A majority of cases, 58%, were diagnosed in men. The overall age-standardized rate was 17.8 and 10.5 per 100,000 person-years in men and women, respectively. Age-period-cohort models were successfully fitted. There was no significant over-dispersion or under-dispersion, residual plots did not reveal any systematic lack of fit, and fitted rates closely tracked observed rates.
Table 1.
Male and Female Leukemia Cases in the SEER 13 and 18 Registries Databases, 1992 – 2009.
| Sex | Leukemia Type* | No. of Cases** | Age-Standardized Rate† per 100,000 Person-Years | (SD) | Net Drift, percent per year‡ | 95% Confidence Interval (low, high) |
|---|---|---|---|---|---|---|
| Male | AML | 17,394 | 5.85 | (0.04) | +0.04 | (−0.36, +0.43) |
| ALL | 3,185 | 0.96 | (0.02) | +0.65 | (−0.16, +1.48) | |
| CML | 8,922 | 2.93 | (0.03) | −1.03 | (−1.52, −0.52) | |
| CLL | 23,819 | 10.05 | (0.07) | −0.98 | (−1.35, −0.61) | |
| Combined | 53,320 | 17.8 | (0.09) | −0.36 | (−0.59, −0.14) | |
| Female | AML | 14,296 | 3.95 | (0.03) | +0.14 | (−0.29, +0.58) |
| ALL | 2,627 | 0.73 | (0.01) | +2.54 | (+1.53, +3.56) | |
| CML | 6,115 | 1.69 | (0.02) | −0.95 | (−1.59, −0.31) | |
| CLL | 15,090 | 5.13 | (0.04) | −0.55 | (−1.05, −0.05) | |
| Combined | 38,128 | 10.5 | (0.06) | +0.05 | (−0.22, +0.32) |
Leukemia types based on ICD-O-3 codes. AML: 9840, 9861, 9866, 9867, 9871–9874, 9895–9897, 9910, 9920; ALL: 9826, 9835–9837; CML: 9863, 9875, 9876, 9945, 9946; CLL: 9823
Leukemia as first primary cancer. Patients diagnosed at ages 25 – 84 years old during 1992 – 2009 in SEER catchment areas with 3.42×108 and 3.59×108 person-years of follow-up among males and females, respectively.
2000 US Standard Population [Census P25-1130]. Age-standardized rate and its standard deviation (SD) estimated using weighted least squares.
Calculated from age-period-cohort model.
Net drifts for the four major leukemia types were significantly heterogeneous. CML decreased significantly over time among men and women, by around 1% per year (Table 1). CLL also declined significantly, by around 1% per year among men and 0.6% per year among women. In contrast, ALL rates increased significantly by around 2.5% per year among women and non-significantly by 0.7% per year among men. AML trends were not significant in either sex.
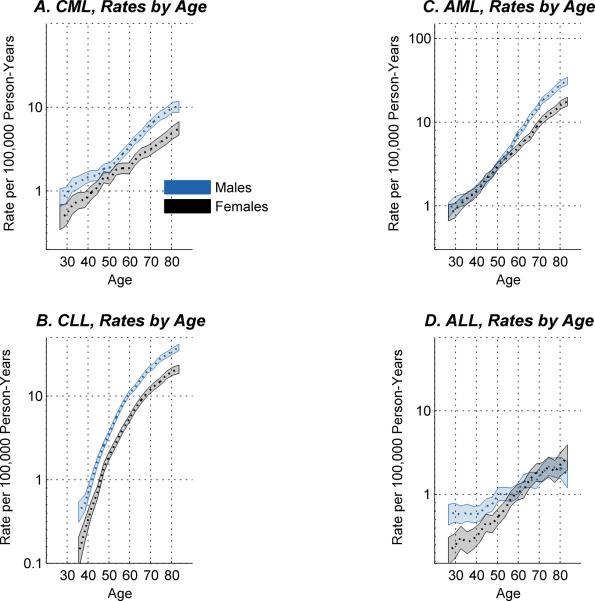
The longitudinal age incidence curves for the four major types (Figure 1) were broadly consistent with prior studies examining cross-sectional age incidence curves (1, 4); however, the longitudinal curves shown here are adjusted for period and cohort effects. On the logarithmic scale shown, age incidence curves for the chronic leukemias CML (Figure 1A) and CLL (Figure 1B) appear essentially parallel in males and females, with males consistently higher except perhaps for the male excess of CML, which appears slightly less prominent around the age of 50 years. In contrast, a pronounced male excess of AML emerges after age 50 years (Figure 1C), and a pronounced male excess of ALL diminishes after age 65 years (Figure 1D). We used the Wald test for parallelism (35) to compare the shape of the age-incidence curve in males versus females. P-values were P = 0.19 and 0.40, respectively, for CML and CLL, indicating little difference in shape for each type in males versus females, versus P = 6×10−4 and 0.03, respectively, for AML and ALL, indicating significant age-sex interaction.
Figure 1.
Age-period-cohort longitudinal age-at-onset curves for CML (A), CLL (B), AML (C), and ALL (D), for men (blue curves) and women (grey curves) ages 25 – 84 years. Shaded regions show point-wise 95% confidence limits. The levels of the curves reflect the rates for the 1946 reference birth cohort, as described in Methods.
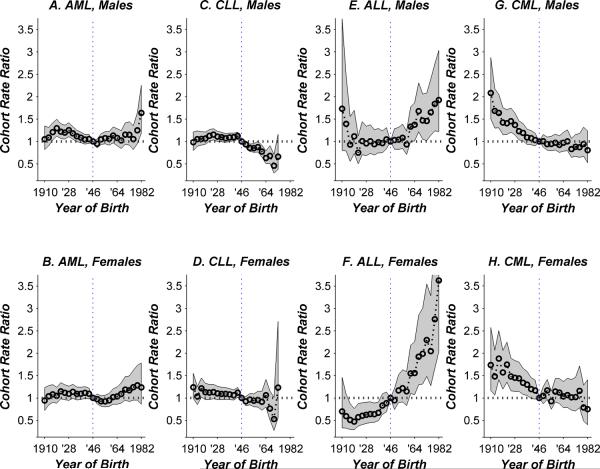
The longitudinal age incidence curves in Figure 1 are set to the level that best matches absolute rates in the reference cohort of persons born circa 1946. Under the age-period-cohort model, the adjusted age-incidence curve for any other cohort is proportionally higher or lower according to the corresponding value of the cohort rate ratio (CRR) curve (Figure 2). CRR values varied significantly for each major leukemia type except AML in females (Table 2), on account of significant log-linear trend, significant non-linear birth cohort effects, or both. Specifically, among women, log-linear trend was significant for ALL, CML, and CLL. Among men, the log-linear trend was also significant for ALL, CML, and CLL, and in addition, the non-linear birth cohort effects were significant for AML and CLL and trending to significance for ALL.
Figure 2.
Cohort rate ratio curves by type of leukemia and sex, relative to the experience of the 1946 reference birth cohort, as described in Methods. Shaded regions show point-wise 95% confidence limits. Horizontal dotted line shows rate ratio of 1 (no difference between given cohort and reference cohort). Vertical dotted line marks the 1946 reference cohort.
Table 2.
Statistical Significance of Cohort Rate Ratios
| Sex | Leukemia Type | Log-Linear Trend per Birth Year P-Value† | Non-Linear Birth Cohort Effects, P-Value† |
|---|---|---|---|
| All Males | AML | 0.66 | 0.004 |
| ALL | 0.05 | 0.08 | |
| CML | 7×10−8 | 0.71 | |
| CLL | 0.01 | 8×10−6 | |
| All Females | AML | 0.24 | 0.30 |
| ALL | 2×10−7 | 0.84 | |
| CML | 4×10−4 | 0.24 | |
| CLL | 0.05 | 0.30 |
See Methods for details.
CRR values for AML in males (Figure 2A) increased then decreased among successive cohorts born prior to 1946, peaking around 1.25-fold higher for cohorts born from 1916 through 1931. Thereafter, the log-linear trend was stable or consistently increasing. In exploratory analysis, a nominally significant local acceleration was detected for men (P = 0.01) when the 7 adjacent birth cohorts on either side of the reference cohort (1949 – 1967 versus 1925 – 1943) were contrasted using the Tarone-Chu method. AML birth cohort patterns appeared qualitatively similar in women compared to men (Figure 2B versus 2A; P = 0.29 for differences between males and females). However, among women, neither the log-linear nor the non-linear birth cohort effects were statistically significant (Table 2).
For AML the distribution of the underlying 13 ICD-O-3 codes changed markedly over time. The frequency of the most common code (9861) decreased abruptly around the middle of our study period, from 78% of cases in 1992 to 54% of cases in 2009, and a number of less common codes (including 9871–9874) increased substantially. Therefore, we did not attempt to fit age-period-cohort models for specific codes.
CRR values for CLL in males declined significantly (Figure 2C); furthermore, the rate of decline was significantly faster in cohorts born after 1946 than before (Table 2 and Figure 2C). Compared to the 1946 cohort, the CLL rate in the 1964 cohort was 0.63-fold lower (95% CI: 0.50 to 0.79-fold lower). CRR values for CLL in females also declined significantly (Figure 2D); however, there was no acceleration of the decline in younger cohorts.
For ALL in males (Figure 2E) non-linear birth cohort effects were trending to significance (P=0.08, Table 2), and there was a nominally significant local acceleration around the 1946 reference cohort (P = 0.03), such that CRR values were consistently increasing in younger cohorts born after 1946. For example, compared to the 1946 reference cohort, the ALL rate in the 1964 cohort was 1.37-fold higher (95% CI: 1.01 to 1.87 fold higher). Among females, the CRR values for ALL (Figure 2F) also increased consistently and significantly by cohort, but there was no evidence for any changes around the 1946 reference cohort. As for AML, the frequency of specific ALL codes also changed abruptly over time, therefore separate models were not considered.
Birth cohort patterns for CML appear very similar in men and women (Figures 2G and 2H, respectively). In both sexes, rates were highest in the oldest cohorts born circa 1910. The rates clearly declined by around 1% per birth year through the cohorts born circa 1946. The CRR values appear to have remained stable from that cohort on. However, the apparent leveling off of the CRR curves does not result in a statistically significant test of non-linear birth cohort effects in males or females (Table 2). Interestingly, in exploratory analysis, we detected a nominally significant local deceleration for men (P = 0.004 for 1949 – 1967 versus 1925 – 1943). The corresponding difference in slopes was similar for women, but the slope contrast was not statistically significant (P = 0.11). As a sensitivity analysis, we excluded the 22 percent of CML cases with ICD-O-3 code 9945 corresponding to the clinically and cytogenetically distinct entity of CMML, and obtained very similar results.
As a sensitivity analysis, we estimated CRR curves specifically for non-Hispanic whites. The values were very similar to those shown for all races combined. However, for a number of the curves the levels of statistical significance were reduced, as expected given the diminished numbers of cases.
DISCUSSION
The four major leukemia types are established endpoints for descriptive (37) and analytical (38–41) epidemiologic studies; however, it is increasingly clear from molecular studies that these categories do not represent homogeneous groups of similar diseases but instead constitute heterogeneous groups of related diseases. Within the major types the etiologies of the component diseases remain largely unclear and are very difficult to study because of the small numbers. For this reason, we and others have focused on the broad-based major types, recognizing that the mixing of subgroups might weaken or obscure any underlying association signals.
Therefore, it is quite striking to report that leukemia incidence varied significantly between birth cohorts for each major leukemia type in men and women except female AML; changes on the order of 1% per birth year or 20% per generation were observed. Our results are consistent with the hypothesis that many leukemia risks among adults are substantially affected by known, suspected, and perhaps unrecognized environmental and lifestyle exposures that change in prevalence from one birth cohort to the next (32, 34). Compared to the patterns found for some other malignancies, notably testicular (42–44) and breast (45–46) cancers, associations of leukemia risks and birth cohort are comparatively subtle. Of course, the comparatively weak signal strengths might reflect the broad mixture of diseases under study.
A key strength of our study is we analyzed the sexes separately. Indeed, our longitudinal age incidence curves revealed significant age-sex interactions for AML and ALL after accounting for period and cohort effects. This finding has repeatedly been noted in prior cross-sectional studies in which cohort artifacts cannot entirely be ruled out. Therefore, our analysis strongly supports the hypothesis that acute leukemia risks are modified by hormonal changes over the lifespan in males or females. Importantly, our separate estimates of birth cohort effects for males and females are adjusted for these differences in the natural histories between the sexes.
The strongest specific (i.e. non-linear) birth cohort signatures were observed for CLL and AML in men. The significant birth cohort signature for AML in males is intriguing given there are a number of established AML risk factors include ionizing radiation, certain chemicals, including benzene and formaldehyde, and tobacco (7, 47–48). Therefore, the significant rise and fall of AML incidence in cohorts of men born prior to 1946 might reflect, at least in part, increases and decreases in these well-established carcinogens.
There is increasing evidence that obesity should be recognized as an important established risk factor for leukemia, particularly AML, based on diverse single-population studies (38, 40, 49–52) and in meta-analyses (53–54). In the US obesity has become prevalent in all age groups (55). In exploratory analysis, we also detected a significant acceleration in AML risk (consistent with either a genuine increase in risk or a slowing of a decreasing risk) beginning with the baby boomer cohorts. Importantly, these cohorts of men are not yet elderly. Therefore, if the significant acceleration in AML risk in successive cohorts born since 1946 is in part a consequence of obesity, our findings suggest that obesity, and any other putative risk factor increasing in these cohorts, must modulate the risks beginning in early adulthood or middle age.
Non-linear birth cohort effects for CLL were highly significant in men but not in women, even though the shape of the age incidence curve for CLL was remarkably similar in women compared to men. This discrepancy might reflect smaller numbers of CLL cases in women compared to men. Alternatively, if CLL is increasingly diagnosed and managed outside of the hospital setting especially in younger men, the cohort patterns reported here might reflect decreasing completeness of hospital-based CLL reporting to SEER rather than genuine declines in incidence (56). Existing (small) adjustments for reporting delay apply to all ages and therefore modify period effects but not cohort effects in age-period-cohort models. Development of reporting delay adjustments that vary by age, sex, and calendar year might help determine whether cohort patterns for CLL reflect genuine changes in risk or reporting artifacts.
CML is characterized by a signature lesion, The BCR-ABL translocation (57). Since a necessary cause of CML is so specific and the disease is so rare in all populations, one might hypothesize that the incidence of CML might be more driven by stochastic events than specific environmental risk factors. We and others (1, 58) have reported significant overall declines in CML incidence; in this study we also observed apparently stable CML rates in cohorts born since 1946. This new observation is consistent with the hypothesis that CML is less environmentally sensitive than AML which is increasing in the same cohorts, but needs to be replicated in other populations.
ALL is a highly heterogeneous disease and little is known about its causes (59). We observed significant gender differences in natural history (Figure 1), time trends (Table 1), and birth cohort effects (Figure 2). ALL does appear to be increasing in both younger men and women.
A major limitation of our study is the primary analysis included persons of all race/ethnic groups, because even using SEER-18 there were relatively limited numbers of cases to estimate birth cohort effects with sufficient precision within distinct racial/ethnic groups. Therefore, our primary results could potentially be biased by changes in population structure. However, we did have sufficient numbers of cases to restrict our analyses to the largest subgroup of non-Hispanic whites, and obtained nearly identical estimates, although the levels of statistical significance were reduced in some cases. Therefore, it appears unlikely that the cohort effects we observed are simply an artifact of changes in the racial/ethnic composition of the population covered by SEER.
A second major limitation is we analyzed the four major types in their entirety even though only CLL is based on a single diagnostic entity. The SEER database includes 13 distinct ICD-O-3 codes for AML and 4 each for CML and ALL. However, exploratory analysis found abrupt secular changes in the distribution of these codes over time that might reflect changes in coding practices as much as any genuine shift in disease spectrum. Therefore, we did not pursue separate analyses for the various subtypes defined by ICD-O-3 codes.
In summary, our results are consistent with the hypothesis that risks of adult leukemia are significantly modulated by environmental and lifestyle exposures and therefore may be modifiable. However, we did not identify notably sharp or specific changes in risk associated with birth cohort, except for increases in AML and decreases in CLL in younger men born since 1946. In light of the limitations noted above, our results also suggest that identification of stronger signals may require that future studies split each major type into a few consistently-defined and biologically-related families of subtypes (60–61). This strategy has proven very informative in studies using estrogen receptor status of breast tumors (62–63). For population-based studies of the leukemias, such subtypes might reflect advances in cytogenetics (13, 64).
ACKNOWLEDGMENTS
The authors are grateful to Ju-Hyun Park for his thorough statistical review. We would also like to thank the reviewers for their helpful comments that greatly improved the content of our manuscript.
Financial support: This research was supported entirely by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Footnotes
CONTRIBUTIONS PSR and WFA designed the study, guided the analysis, interpreted the data, and drafted the manuscript. LKW assembled data for analysis, analyzed and interpreted the data, and edited the manuscript.
CONFLICT OF INTEREST The authors declare no conflicts of interest.
References
- 1.Groves FD, Linet MS, Devesa SS. Patterns of occurrence of the leukaemias. EurJCancer. 1995;31A(6):941–9. doi: 10.1016/0959-8049(95)00024-0. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen LC, Nielsen OJ, Johansen C. Trends in adult leukemia incidence and survival in Denmark, 1943–2003. Cancer Causes Control. 2009;20(9):1671–80. doi: 10.1007/s10552-009-9417-9. Epub 2009/08/13. [DOI] [PubMed] [Google Scholar]
- 3.Xie Y, Davies SM, Xiang Y, Robison LL, Ross JA. Trends in leukemia incidence and survival in the United States (1973–1998) Cancer. 2003;97(9):2229–35. doi: 10.1002/cncr.11316. Epub 2003/04/25. [DOI] [PubMed] [Google Scholar]
- 4.McNally RJ, Rowland D, Roman E, Cartwright RA. Age and sex distributions of hematological malignancies in the U.K. HematolOncol. 1997;15(4):173–89. doi: 10.1002/(sici)1099-1069(199711)15:4<173::aid-hon610>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Matasar MJ, Ritchie EK, Consedine N, Magai C, Neugut AI. Incidence rates of the major leukemia subtypes among US Hispanics, Blacks, and non-Hispanic Whites. Leuk Lymphoma. 2006;47(11):2365–70. doi: 10.1080/10428190600799888. Epub 2006/11/17. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto JF, Goodman MT. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997–2002. Cancer Causes Control. 2008;19(4):379–90. doi: 10.1007/s10552-007-9097-2. Epub 2007/12/08. [DOI] [PubMed] [Google Scholar]
- 7.Zeeb H, Blettner M. Adult leukaemia: what is the role of currently known risk factors? Radiat Environ Biophys. 1998;36(4):217–28. doi: 10.1007/s004110050075. Epub 1998/04/02. [DOI] [PubMed] [Google Scholar]
- 8.Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107(9):2099–107. doi: 10.1002/cncr.22233. Epub 2006/10/05. [DOI] [PubMed] [Google Scholar]
- 9.Greaves MF. Aetiology of acute leukaemia. Lancet. 1997;349(9048):344–9. doi: 10.1016/s0140-6736(96)09412-3. Epub 1997/02/01. [DOI] [PubMed] [Google Scholar]
- 10.Linet MS, Schubauer-Berigan MK, Weisenburger DD, Richardson DB, Landgren O, Blair A, et al. Chronic lymphocytic leukaemia: an overview of aetiology in light of recent developments in classification and pathogenesis. British journal of haematology. 2007;139(5):672–86. doi: 10.1111/j.1365-2141.2007.06847.x. Epub 2007/11/21. [DOI] [PubMed] [Google Scholar]
- 11.Lipschitz DA, Mitchell CO, Thompson C. The anemia of senescence. Am J Hematol. 1981;11(1):47–54. doi: 10.1002/ajh.2830110106. Epub 1981/01/01. [DOI] [PubMed] [Google Scholar]
- 12.Harding C, Pompei F, Lee EE, Wilson R. Cancer suppression at old age. Cancer Res. 2008;68(11):4465–78. doi: 10.1158/0008-5472.CAN-07-1670. Epub 2008/06/04. [DOI] [PubMed] [Google Scholar]
- 13.Appelbaum FR, Gundacker HM, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006 doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mbulaiteye SM, Anderson WF, Ferlay J, Bhatia K, Chang C, Rosenberg PS, et al. Pediatric, elderly, and emerging adult-onset peaks in Burkitt's lymphoma incidence diagnosed in four continents, excluding Africa. Am J Hematol. 2012;87(6):573–8. doi: 10.1002/ajh.23187. Epub 2012/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. AnnuRevPublic Health. 1991;12:425–57. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
- 16.McNally RJ, Cairns DP, Eden OB, Kelsey AM, Taylor GM, Birch JM. Examination of temporal trends in the incidence of childhood leukaemias and lymphomas provides aetiological clues. Leukemia. 2001;15(10):1612–8. doi: 10.1038/sj.leu.2402252. [DOI] [PubMed] [Google Scholar]
- 17.Spix C, Eletr D, Blettner M, Kaatsch P. Temporal trends in the incidence rate of childhood cancer in Germany 1987–2004. Int J Cancer. 2008;122(8):1859–67. doi: 10.1002/ijc.23281. Epub 2007/12/14. [DOI] [PubMed] [Google Scholar]
- 18.Health USDoHaHSNIo. SEER - Surveillance Epidemiology and End Results Program. Available from: http://seer.cancer.gov.
- 19.Holford TR, Armitage P, Colton T. Encyclopedia of Biostatistics. John Wiley & Sons, Ltd.; 2005. Age-Period-Cohort Analysis; pp. 82–99. [Google Scholar]
- 20.Jemal A, Ma J, Rosenberg PS, Siegel R, Anderson WF. Increasing Lung Cancer Death Rates Among Young Women in Southern and Midwestern States. J Clin Oncol. 2012 doi: 10.1200/JCO.2012.42.6098. Epub 2012/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson WF, Camargo MC, Fraumeni JF, Jr, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303(17):1723–8. doi: 10.1001/jama.2010.496. Epub 2010/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mbulaiteye SM, Anderson WF, Bhatia K, Rosenberg PS, Linet MS, Devesa SS. Trimodal age-specific incidence patterns for Burkitt lymphoma in the United States, 1973–2005. Int J Cancer. 2010;126(7):1732–9. doi: 10.1002/ijc.24934. Epub 2009/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SEER-13 . Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence-SEER 13 Regs Limited-Use, Nov 2007 Sub (1973–2005) <Single Ages to 85+, Katrinia/Rita Population Adjustment> -Linked To County Attributes - Total U.S., 1969–2005 Counties. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2008. www.seer.cancer.gov released April 2008, based on November 2007 submission. [Google Scholar]
- 24.SEER-18 . Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2011 Sub, Vintage 2009 Pops (2000–2009) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2010 Counties. National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; 2012. www.seer.cancer.gov released April 2012, based on the November 2011 submission. [Google Scholar]
- 25.National Cancer I. SEER*Stat Statistical Software. 2007 [Google Scholar]
- 26.Doll R. The Epidemiology of Childhood Leukaemia. Journal of the Royal Statistical SocietySeries A. 1989;152(3):341–51. [Google Scholar]
- 27.Bhatia S, Neglia JP. Epidemiology of childhood acute myelogenous leukemia. JPediatrHematolOncol. 1995;17(2):94–100. doi: 10.1097/00043426-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Westergaard T, Andersen PK, Pedersen JB, Olsen JH, Frisch M, Sorensen HT, et al. Birth characteristics, sibling patterns, and acute leukemia risk in childhood: a population-based cohort study. JNatlCancer Inst. 1997;89(13):939–47. doi: 10.1093/jnci/89.13.939. [DOI] [PubMed] [Google Scholar]
- 29.Smith MA, Simon R, Strickler HD, McQuillan G, Ries LA, Linet MS. Evidence that childhood acute lymphoblastic leukemia is associated with an infectious agent linked to hygiene conditions. Cancer Causes Control. 1998;9(3):285–98. doi: 10.1023/a:1008873103921. Epub 1998/07/31. [DOI] [PubMed] [Google Scholar]
- 30.Greaves MF, Maia AT, Wiemels JL, Ford AM. Leukemia in twins: lessons in natural history. Blood. 2003;102(7):2321–33. doi: 10.1182/blood-2002-12-3817. Epub 2003/06/07. [DOI] [PubMed] [Google Scholar]
- 31.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39(2):311–24. [PubMed] [Google Scholar]
- 32.Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: Age-period-cohort models. StatMed. 1987;6(4):469–81. doi: 10.1002/sim.4780060406. [DOI] [PubMed] [Google Scholar]
- 33.Carstensen B. Age-period-cohort models for the Lexis diagram. Statistics in medicine. 2007;26(15):3018–45. doi: 10.1002/sim.2764. Epub 2006/12/21. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg PS, Anderson WF. Age-period-cohort models in cancer surveillance research: ready for prime time? Cancer Epidemiol Biomarkers Prev. 2011;20(7):1263–8. doi: 10.1158/1055-9965.EPI-11-0421. Epub 2011/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson WF, Rosenberg PS, Menashe I, Mitani A, Pfeiffer RM. Age-related crossover in breast cancer incidence rates between black and white ethnic groups. J Natl Cancer Inst. 2008;100(24):1804–14. doi: 10.1093/jnci/djn411. Epub 2008/12/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarone RE, Chu KC. Evaluation of birth cohort patterns in population disease rates. AmJEpidemiol. 1996;143(1):85–91. doi: 10.1093/oxfordjournals.aje.a008661. [DOI] [PubMed] [Google Scholar]
- 37.Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118(9):2338–66. doi: 10.1002/cncr.27514. Epub 2012/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasim K, Levallois P, Abdous B, Auger P, Johnson KC. Lifestyle factors and the risk of adult leukemia in Canada. Cancer Causes Control. 2005;16(5):489–500. doi: 10.1007/s10552-004-7115-1. Epub 2005/06/30. [DOI] [PubMed] [Google Scholar]
- 39.Fernberg P, Odenbro A, Bellocco R, Boffetta P, Pawitan Y, Zendehdel K, et al. Tobacco use, body mass index, and the risk of leukemia and multiple myeloma: a nationwide cohort study in Sweden. Cancer Res. 2007;67(12):5983–6. doi: 10.1158/0008-5472.CAN-07-0274. Epub 2007/06/19. [DOI] [PubMed] [Google Scholar]
- 40.Song YM, Sung J, Ha M. Obesity and risk of cancer in postmenopausal Korean women. J Clin Oncol. 2008;26(20):3395–402. doi: 10.1200/JCO.2007.15.7867. Epub 2008/07/10. [DOI] [PubMed] [Google Scholar]
- 41.Wong O, Harris F, Yiying W, Hua F. A hospital-based case-control study of acute myeloid leukemia in Shanghai: analysis of personal characteristics, lifestyle and environmental risk factors by subtypes of the WHO classification. Regul Toxicol Pharmacol. 2009;55(3):340–52. doi: 10.1016/j.yrtph.2009.08.007. Epub 2009/08/26. [DOI] [PubMed] [Google Scholar]
- 42.Bergstrom R, Adami HO, Mohner M, Zatonski W, Storm H, Ekbom A, et al. Increase in testicular cancer incidence in six European countries: a birth cohort phenomenon. Journal of the National Cancer Institute. 1996;88(11):727–33. doi: 10.1093/jnci/88.11.727. Epub 1996/06/05. [DOI] [PubMed] [Google Scholar]
- 43.Bray F, Richiardi L, Ekbom A, Forman D, Pukkala E, Cuninkova M, et al. Do testicular seminoma and nonseminoma share the same etiology? Evidence from an age-period-cohort analysis of incidence trends in eight European countries. Cancer Epidemiol Biomarkers Prev. 2006;15(4):652–8. doi: 10.1158/1055-9965.EPI-05-0565. Epub 2006/04/15. [DOI] [PubMed] [Google Scholar]
- 44.Verhoeven R, Houterman S, Kiemeney B, Koldewijn E, Coebergh JW. Testicular cancer: marked birth cohort effects on incidence and a decline in mortality in southern Netherlands since 1970. Int J Cancer. 2008;122(3):639–42. doi: 10.1002/ijc.23061. Epub 2007/09/04. [DOI] [PubMed] [Google Scholar]
- 45.Tarone RE, Chu KC. Implications of birth cohort patterns in interpreting trends in breast cancer rates. Journal of the National Cancer Institute. 1992;84(18):1402–10. doi: 10.1093/jnci/84.18.1402. [DOI] [PubMed] [Google Scholar]
- 46.Holford TR, Cronin KA, Mariotto AB, Feuer EJ. Changing patterns in breast cancer incidence trends. JNatlCancer InstMonogr. 2006;(36):19–25. doi: 10.1093/jncimonographs/lgj016. [DOI] [PubMed] [Google Scholar]
- 47.Doll R. Cancers weakly related to smoking. Br Med Bull. 1996;52(1):35–49. doi: 10.1093/oxfordjournals.bmb.a011531. Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 48.Vineis P, Alavanja M, Buffler P, Fontham E, Franceschi S, Gao YT, et al. Tobacco and cancer: recent epidemiological evidence. Journal of the National Cancer Institute. 2004;96(2):99–106. doi: 10.1093/jnci/djh014. Epub 2004/01/22. [DOI] [PubMed] [Google Scholar]
- 49.Ross JA, Parker E, Blair CK, Cerhan JR, Folsom AR. Body mass index and risk of leukemia in older women. Cancer Epidemiol Biomarkers Prev. 2004;13(11 Pt 1):1810–3. Epub 2004/11/10. [PubMed] [Google Scholar]
- 50.Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF., Jr Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15(1):35–43. doi: 10.1023/B:CACO.0000016573.79453.ba. Epub 2004/02/19. [DOI] [PubMed] [Google Scholar]
- 51.Pan SY, Johnson KC, Ugnat AM, Wen SW, Mao Y. Association of obesity and cancer risk in Canada. American journal of epidemiology. 2004;159(3):259–68. doi: 10.1093/aje/kwh041. Epub 2004/01/27. [DOI] [PubMed] [Google Scholar]
- 52.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. Epub 2007/11/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. Epub 2008/02/19. [DOI] [PubMed] [Google Scholar]
- 54.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer. 2008;122(6):1418–21. doi: 10.1002/ijc.23176. Epub 2007/11/22. [DOI] [PubMed] [Google Scholar]
- 55.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22(1):39–47. doi: 10.1038/sj.ijo.0800541. Epub 1998/03/03. [DOI] [PubMed] [Google Scholar]
- 56.Dores GM, Anderson WF, Curtis RE, Landgren O, Ostroumova E, Bluhm EC, et al. Chronic lymphocytic leukaemia and small lymphocytic lymphoma: overview of the descriptive epidemiology. British journal of haematology. 2007;139(5):809–19. doi: 10.1111/j.1365-2141.2007.06856.x. Epub 2007/10/19. [DOI] [PubMed] [Google Scholar]
- 57.Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112(13):4808–17. doi: 10.1182/blood-2008-07-077958. Epub 2008/12/10. [DOI] [PubMed] [Google Scholar]
- 58.Xie Y, Davies SM, Xiang Y, Robison LL, Ross JA. Trends in leukemia incidence and survival in the United States (1973–1998) Cancer. 2003;97(9):2229–35. doi: 10.1002/cncr.11316. [DOI] [PubMed] [Google Scholar]
- 59.Faderl S, O'Brien S, Pui CH, Stock W, Wetzler M, Hoelzer D, et al. Adult acute lymphoblastic leukemia: concepts and strategies. Cancer. 2010;116(5):1165–76. doi: 10.1002/cncr.24862. Epub 2010/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoch C, Kern W, Krawitz P, Dugas M, Schnittger S, Haferlach T, et al. Dependence of age-specific incidence of acute myeloid leukemia on karyotype. Blood. 2001;98(12):3500. doi: 10.1182/blood.v98.12.3500. [DOI] [PubMed] [Google Scholar]
- 61.Bacher U, Kern W, Schnittger S, Hiddemann W, Haferlach T, Schoch C. Population-based age-specific incidences of cytogenetic subgroups of acute myeloid leukemia. Haematologica. 2005;90(11):1502–10. [PubMed] [Google Scholar]
- 62.Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, et al. The decrease in breast-cancer incidence in 2003 in the United States. The New England journal of medicine. 2007;356(16):1670–4. doi: 10.1056/NEJMsr070105. Epub 2007/04/20. [DOI] [PubMed] [Google Scholar]
- 63.Anderson WF, Katki HA, Rosenberg PS. Incidence of breast cancer in the United States: current and future trends. Journal of the National Cancer Institute. 2011;103(18):1397–402. doi: 10.1093/jnci/djr257. Epub 2011/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moorman AV, Chilton L, Wilkinson J, Ensor HM, Bown N, Proctor SJ. A population-based cytogenetic study of adults with acute lymphoblastic leukemia. Blood. 2010;115(2):206–14. doi: 10.1182/blood-2009-07-232124. Epub 2009/11/10. [DOI] [PubMed] [Google Scholar]