Abstract
Under an ideal implementation of Model for End-stage Liver Disease (MELD)-based liver allocation, the only factors that would predict DDLT rates are MELD score, blood type, and donation service area (DSA). We aimed to determine whether additional factors are associated with DDLT rates in actual practice. Methods: Data from the Scientific Registry of Transplant Recipients on all adult candidates waitlisted between 03/01/2002 and 12/31/2008 (n=57,503) were analyzed. Status-1 candidates were excluded. Cox regression was used to model covariate-adjusted DDLT rates, stratified by DSA, blood type, liver-intestine policy and allocation MELD. Inactive time on the waitlist was not modeled, such that the computed DDLT hazard ratios (HR) are interpreted as “among actively listed candidates”. Results: Many factors, including candidate age, gender, prior DDLT, diagnosis, hospitalization status, height, and combined listing for liver-kidney and liver-intestine were significantly associated with DDLT rates. Factors associated with significantly lower covariate-adjusted DDLT rates were higher serum creatinine (HR=0.92; p<0.0001); higher bilirubin (HR=0.996; p=0.001), and receipt of dialysis (HR=0.83; p<0.0001). Mild ascites (HR=1.15; p<0.0001) and hepatic encephalopathy (Grade 1–2, HR=1.05; p=0.0236; grade 2–3, HR=1.10, p=0.0103) were associated with significantly higher adjusted DDLT rates. Conclusions: Adjusted DDLT rates among actively listed candidates are affected by many factors aside from those integral to the allocation system; including the components of the MELD score itself, as well as candidate factors that were considered but deliberately omitted from the MELD score in order to keep it objective. These results raise the question of whether additional candidate characteristics should be explicitly incorporated into the prioritization of wait list candidates; since such factors are already systematically affecting DDLT rates under the current allocation system.
Introduction
The Model for End-stage Liver Disease (MELD) score is the unit of allocation and donation service area (DSA) is the unit of distribution for deceased donor livers among chronic liver disease candidates listed for deceased donor liver transplantation (DDLT) in the United States.1
The MELD score was adopted in February 2002 as the basis for prioritizing end stage liver disease candidates awaiting DDLT. The intention was to reduce waitlist mortality by offering DDLT based upon the severity of liver disease, minimizing geographic disparities, and deemphasizing waiting time. 2, 3 The MELD score is a well-validated measure of waitlist mortality risk (severity of liver disease). 4, 5 In contrast to the Child-Turcotte-Pugh (CTP) score, which includes two subjective components (ascites and hepatic encephalopathy), MELD is calculated quantitatively using laboratory values of total bilirubin, international normalized ratio (INR) of prothrombin time, and serum creatinine.5 Candidates are offered deceased donor livers only if they are listed as “active” on the waitlist.
DSA is the distinct, non-overlapping geographic area served by each of 58 federally certified organ procurement organizations (OPO). DSAs may include one or more transplant programs of a given organ and one or more donor hospitals. DSA is currently the primary unit of distribution for deceased donor livers to candidates with chronic liver disease.
In a frictionless system, the DSA, active status, and MELD score are the only factors that should predict DDLT rates within blood group types. However, recent studies have shown that female gender is associated with a lower DDLT.6, 7, 9,12 Hispanics have an 8% lower DDLT rate compared to Whites, and Asians with MELD scores 30 to 40 have a 46% lower DDLT rate than Whites with comparable disease severity.8, 9, 12 The objective of the current study was to identify other candidate factors that influence DDLT rates among waitlisted candidates in the United States.
Methods
Patient Population and Cohort
The study was based on data obtained from the Scientific Registry of Transplant Recipients (SRTR) and the Social Security Death Master File (SSDMF).10 The SRTR maintains a database of all candidates for and recipients of solid-organ transplants in the United States. Candidates on waiting lists for organ transplantation and those who receive organ transplants are tracked on a periodic basis with the use of data collection forms completed by organ transplant programs and submitted to the Organ Procurement and Transplantation Network (OPTN). The SRTR database has a uniform structure based upon mandatory transplant candidate registration information provided by the transplant program at the time of placement on the waitlist (transplant candidate registration form [TCR]). Online status updates are required for MELD scores and other clinical measures while a candidate is waitlisted, and information is provided by the transplant program at the time of DDLT (transplant recipient registration [TRR]). Transplant follow-up information is required at 6-months post-transplant, 1-year post-transplant, and yearly thereafter (transplant follow-up form [TRF]). These data, in addition to data from the OPTN regarding candidates on the waiting list and the allocation of organs, are included in the SRTR database.
The SRTR supplements information on vital status with data on deaths from the SSDMF. Data collection by the SRTR is exempt from oversight under the “public benefit or service program” provisions of the Code of Federal Regulations (45 CFR 46.101[b][5]), as approved by the institutional review board of the Health Resources and Services Administration of the Department of Health and Human Services. The SSDMF includes updated information on all participants in the Social Security system. Information on deaths reported to the Social Security system for the administration of the death, disability, and retirement benefit programs is kept in the SSDMF database.
The study population included all candidates with age ≥ 18 years who were listed for DDLT between March 1, 2002 and December 31, 2008 (n=57,503). Candidates listed as Status-1 were excluded. Data on each candidate were collected from TCR and TRR forms, as well as from status update files. Data from the SSDMF was used to ascertain death while on or after removal from the waiting list.
Statistical Approach
For descriptive analysis, continuous variables were expressed as median and inter-quartile range; categorical variables were expressed as percentages. Ascites was reported as none, mild, and moderate to severe; hepatic encephalopathy was reported as none, grade 1–2, and grade 3–4 on the TCR and updated on the TRR form for each candidate. The waitlist data on all the variables was complete except for hospitalization status (6% missing data) and serum sodium (38% missing data). The mandatory submission of serum sodium along with MELD covariates went into effect in November 1st 2004. Candidates listed and transplanted before November 1st 2004 accounted for the missing serum sodium data.
The primary outcome of interest was receipt of a DDLT. Unadjusted DDLT rates were calculated by dividing the number of DDLT by the aggregate patient-years at risk (active on the waiting list), expressed as transplants per 1,000 patient years (PY). For each candidate, time at risk began on the date of wait-listing and ended at the earliest of the receipt of a DDLT, the end of the study’s observation period (12/31/08), the granting of an exception score, or death. Since dead candidates cannot receive a DDLT, pertinent follow-up for such candidates ceased at the time of death. Our primary objective was to model DDLT rates and candidates can only receive a DDLT while they are listed in an active status, therefore, only active waitlist time was modeled, such that the covariate-adjusted DDLT hazard ratios (HR) are interpreted as “among active waitlisted candidates”, all-other-factors-being-equal.
The regression modeling was split into separate stages, each requiring different Cox models. First, to delineate the effect of candidate factors on DDLT rates at a given MELD, in a given DSA, and among active candidates, we fitted a time-dependent Cox regression model of covariate-adjusted DDLT rates. This model was stratified by DSA, blood type, liver-intestine policy and allocation MELD score, with the latter two coded as time-dependent covariates. Allocation MELD refers to the MELD score at which an actively listed candidate is allocated with a deceased donor liver. The liver-intestine policy refers to the additional 10 points given to candidates who were on both the liver and intestine waitlists, starting in March 2005.1 The model included terms for serum creatinine, bilirubin, dialysis, and serum sodium, candidate age, gender, race/ethnicity, diagnosis, height, weight, history of diabetes, ascites, hepatic encephalopathy, hospitalization status, albumin, listing for combined liver-kidney, combined liver-intestine and history of previous DDLT as well as the interaction between age and hospitalization status, female and height, female and creatinine, and creatinine and height.
Second, to quantify the effect of MELD on DDLT rates, we fitted a separate covariate-adjusted time-dependent Cox regression model stratified by DSA, blood type and liver-intestine policy. Instead of stratification by MELD, the MELD score was included in the model as a set of categorical covariates, one per MELD score.11 The goal of this model was to assess the degree to which DDLT rates are monotone with respect to MELD score.
The third Cox regression model was stratified by individual MELD score (i.e., in addition to DSA, blood type, and intestine-listing policy). Since the model was stratified on MELD, we could not directly estimate its effect on DDLT rates. However, the purpose of this component of the analysis was to estimate the impact of the remaining covariates. After fitting this third model, we calculated each candidate’s HR (using the model covariates; which excluded those adjusted for through stratification). We then divided each candidate-specific HR by its respective MELD score-specific mean, such that the scaled candidate-specific HRs would average to 1 within each MELD score. We then examined box-whisker plots within each MELD score. The variability within each MELD score-specific distribution of HRs reflects the systematic heterogeneity in DDLT rates (due to the impact of age, gender, race/ethnicity, diagnosis, height, weight, history of diabetes, ascites, encephalopathy, diabetes, hospitalization status, serum sodium, albumin, combined liver-kidney listing, combined liver-intestine listing and history of previous DDLT). Results were interpreted as among candidates with the same MELD score, adjusted for DSA, blood type and liver-intestine policy.
All statistical analyses were conducted using SAS v9.2 (SAS Institute; Cary, NC, USA).
Results
Baseline Characteristics of the Cohort at Listing
Characteristics of the 57,503 candidates at listing are shown in Table 1. The median age of the cohort was 53 years; 66% were males; 72% were Caucasian, 8% were African-American, 14% were Hispanic, 5% were Asian American, and less than 1% were of other race/ethnicity. Twenty-two percent of the candidates did not have ascites, 53% had mild, and 25% had moderate to severe ascites at listing. Sixty-two percent had hepatic encephalopathy, of which 5% were grade 3–4. The median serum bilirubin, serum creatinine, INR, and serum sodium levels were 2.2 mg/dL, 1.0 mg/dL, 1.4, and 137 mMol/L, respectively. At listing, 4% of candidates were on dialysis and 9% had serum sodium ≤ 131 mMol/L.
Table 1.
Baseline Characteristics of Cohort at Listing
| Variables | Median (IQR) or % (N=57,503) |
|---|---|
| Age (years) | 53 years (48–59) |
| Male | 66% |
| Females | 34% |
| White | 72% |
| Hispanic | 14% |
| Black | 8% |
| Asian | 5% |
| Other race | 1% |
| Hepatitis C | 33% |
| Hepatocellular Carcinoma | 17% |
| Weight (kg) | 82 (70–95) |
| Height (cm) | 173 (165–180) |
| Serum Creatinine (mg/dL) | 1.0 (0.8–1.3) |
| On dialysis | 4% |
| Serum Bilirubin (mg/dL) | 2.2 (1.3–4.4) |
| INR | 1.4 (1.2–1.6) |
| Serum Sodium ≤ 131 mMol/L | 9% |
| Ascites | 78% |
| Hepatic Encephalopathy | |
| - None | 38% |
| - Grade 1–2 | 57% |
| - Grade3–4 | 5% |
| Previous LT | 6% |
| Hospitalized in ICU | 4% |
| Hospitalized not in ICU | 10% |
| Blood type A | 38% |
| Blood type AB | 4% |
| Blood type B | 12% |
| Blood type O | 46% |
| Combined liver-intestine listing | 0.5% |
| Combined liver-kidney listing | 6% |
Unadjusted DDLT Rates among Active Candidates
There were 21,730 DDLTs performed among 57,503 active waitlist candidates during the study period. The characteristics of the DDLT recipients were listed in Table 2. The median time to DDLT among recipients was 67 days. The overall unadjusted DDLT incidence rate was 415 transplants per 1,000 patient-years.
Table 2.
Candidate Factors Significantly Associated with DDLT Rates
| Candidate Factors Associated with Higher DDLT Rates | Hazard Ratio | p-value | Median or % among transplanted |
|---|---|---|---|
|
| |||
| Age at LT (per 5-year increase) | 1.02 | 0.0011 | 53 years |
| Height (per 10 cm increase) | 1.03 | 0.0048 | 173 cm |
| Hepatitis C (vs. all other) | 1.07 | 0.0004 | 39% |
| Hepatocellular Carcinoma (vs. all other) | 1.26 | <0.0001 | 5% |
| Grade 1–2 Hepatic Encephalopathy (vs. none) | 1.05 | 0.0236 | 64% |
| Grade 3–4 Hepatic Encephalopathy (vs. none) | 1.10 | 0.0103 | 12% |
| Mild Ascites (vs. none)+ | 1.15 | <0.0001 | 54% |
| Hospitalization (vs. not hospitalized) | |||
| - In the ICU | 1.19 | <0.0001 | 6% |
| - Not in the ICU | 1.15 | <0.0001 | 16% |
| Combined liver-intestine listing | 1.79 | <0.0001 | 0.7% |
| Combined liver-kidney listing | 1.90 | <0.0001 | 9% |
|
| |||
| Candidate Factors Associated with Lower DDLT Rates | Hazard Ratio | p-value | Median or % among transplanted |
|
| |||
| Female (vs. male) | 0.83 | <0.0001 | 32% |
| Serum Creatinine (per mg/dL increase) | 0.92 | <0.0001 | 1.3 mg/dl |
| Dialysis (vs. not) | 0.83 | <0.0001 | 11% |
| Bilirubin (per mg/dL increase) | 0.996 | 0.0010 | 5.1mg/dl |
| History of Prior Liver Transplant (vs. not) | 0.69 | <0.0001 | 8% |
Moderate to severe ascites was not significant.
Factors Other Than MELD, DSA And Blood Type Associated with Significantly Higher or Lower DDLT Rates
The top half of Table 2 shows main effects associated with higher DDLT rates. Every five-year increase in candidate age was associated with a 2% higher DDLT rate. Candidate characteristics including hepatitis C, hepatocellular carcinoma, hepatic encephalopathy, and mild ascites were each independently associated with significantly higher DDLT rates. Candidates who were hospitalized (whether in the ICU or not) had a higher DDLT rate than non-hospitalized candidates. Each 10 cm increase in height was associated with a 3% higher DDLT rate (HR=1.03, P=0.0048). Listing for combined liver-kidney and combined liver-intestine were associated with 79% and 90% increased DDLT rates, respectively.
Candidate factors associated with lower DDLT rates are shown in the bottom half of Table 2. DDLT rates were 17% lower among females compared to males. Prior DDLT and higher bilirubin were also associated with significantly lower DDLT rates. Each unit increase in serum creatinine and receipt of dialysis were associated with 8% and 17% lower DDLT rates, respectively.
In a supplementary analysis, the interaction between height and creatinine was also found to be significant (HR = 1.001, p = 0.0294). This suggests that the DDLT rate for a shorter candidate was lower than a taller candidate at a given creatinine and at a given MELD score. Interactions between age*hospitalized in the ICU (p=0.23), age*hospitalized not in the ICU (p=0.78), female*creatinine (p=0.90) and female*height (p=0.23) were not significant. Race/ ethnicity did not affect the DDLT rates.
Association of MELD and Other Factors with DDLT Rates
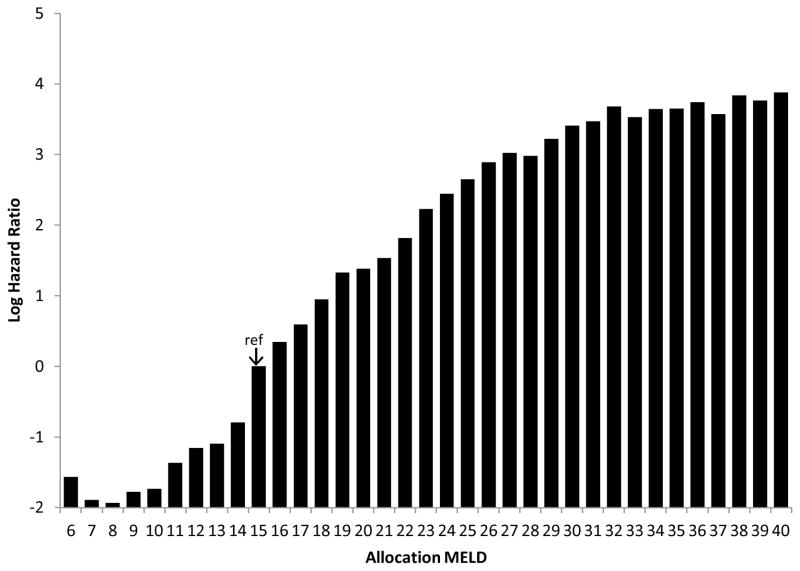
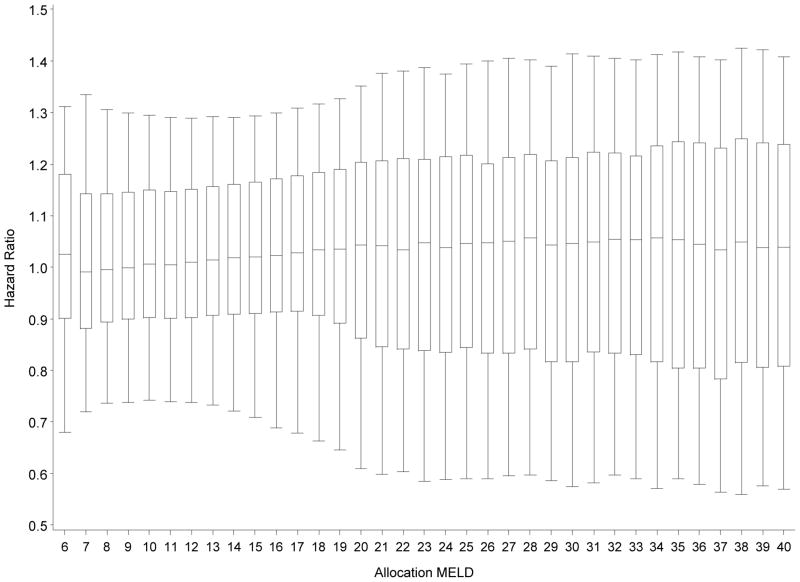
Figure 1 depicts the effect of MELD (i.e., current allocation MELD score) on DDLT rates. MELD score was used as a time-dependent covariate such that the fitted model incorporates changes in each candidate’s MELD score over the follow-up period. MELD=15 served as the reference score and, hence, had a HR set equal to 1 (log HR=0). As expected, higher MELD scores tended to be associated with higher covariate-adjusted DDLT rates. Figure 1 confirms that DDLT rates increase strongly with increasing MELD score, as would be expected. However, certain traits of this plot are noteworthy. First, candidates with MELD=6 had a higher transplant rate than those with MELD=7, 8, 9, or 10. In fact, DDLT rates actually decreased across the MELD 6 to 8 range. Between MELD=9 and MELD=27, the DDLT rates increased nearly monotonically, as expected, up to MELD=32. Although, the DDLT rate was highest among MELD=40 candidates, DDLT rates were fairly similar for MELD scores between 32 and 40. Figure 2 shows the variability in DDLT rates within each given allocation MELD score, adjusted for DSA, blood type and liver/intestine policy. Within each MELD score, the box represents the 75th and 25th percentiles; the whiskers denote the 95th and 5th percentiles. The horizontal line within each box denotes the median, which is very close to 1, in each case; a consequence of scaling the HRs within each MELD to have a mean of 1.0. Approximately 50% of the distribution of HRs was within the 0.9 to 1.1 interval, meaning that at a given MELD score, half of the candidates were within 10% of the average predicted DDLT rate. However, examination of the whiskers reveals that, at most MELD scores, about 10% of candidates were ≥30% away from the MELD-score specific average (i.e., the HR being either <0.7 or >1.3).
Figure 1. Effect of allocation MELD, per single level change, on DDLT rate.
MELD score-specific hazard ratios (HR) are estimated from a model which stratified by DSA, blood type and intestine-listing policy. Each score is compared to the reference, MELD=15 (HR=1).
Figure 2. MELD score-specific box-whisker plots of the DDLT hazard ratios (HR).
The HR’s are estimated using a model stratified by MELD, DSA, blood type and intestine-listing policy. Candidate-specific HRs estimated using all remaining covariates and scaled by their MELD score specific means such that they average to 1. Within each MELD score, the box represents the 75th and 25th percentiles, while the whiskers denote the 95th and 5th percentile. The line in the middle of the box denotes the median, which tends to be very close to 1 since the HRs within each MELD score were scaled such that they average to 1. If MELD score was the only factor (other than DSA, blood type and liver/intestine policy) to affect DDLT rates, then there would be no distribution of HRs; within a given MELD score, each candidate would have the same predicted DDLT rate which, after scaling, would equal 1. The whiskers within each MELD score-specific distribution of HRs reflects the systematic heterogeneity in DDLT rates due to various candidate factors.
Re-examining Figure 2, if MELD score was actually the only factor (other than DSA, blood type and liver/intestine policy) to affect DDLT rates, then there would be no distribution of HRs; within a given MELD score, each candidate would have the same predicted DDLT rate which, after scaling, would equal 1. The whiskers within each MELD score-specific distribution of HRs reflects the systematic heterogeneity in DDLT rates (due to the impact of age, gender, diagnosis, height, weight, history of diabetes, ascites, encephalopathy, diabetes, hospitalization status, serum sodium, albumin, interaction between height and creatinine, listing for combined liver-kidney, combined liver-intestine and history of previous DDLT). The variation in Figure 2 reveals, for example, that candidates may have a predicted DDLT rate of 30% greater, or 30% less than their respective MELD score-specific average, due to the combination of their adjustment covariate profile as described above (i.e., age, gender, diagnosis, height, weight, history of diabetes, ascites, encephalopathy, hospitalization status, serum sodium, albumin, listing for combined liver-kidney, combined liver-intestine and history of previous DDLT).
Discussion
Our study identified the important candidate factors – poor renal function, dialysis, poor liver function, and history of previous LT – that negatively affect DDLT rates in actual clinical practice among waitlisted candidates. We also validated previously identified factor, female gender, as important determinant of DDLT rates. In addition, our study found some new candidate factors such as age, height, hospitalization status, hepatitis C, HCC, complications of portal hypertension -such as hepatic encephalopathy and mild ascites and listing for combined liver-kidney transplant and listing for combined liver-intestine transplant were associated with high DDLT rates.
Organ acceptance and actual receipt of each DDLT offer is a complex process. It involves the collective decision making that depends upon many measured and unmeasured candidate and donor-related factors, including but not limited to the candidate’s clinical condition at the time of offer, donor availability, donor type, donor-recipient size match, etc. It is plausible that physician judgment, donor factors and unmeasured candidate factors resulted in higher DDLT rates for those listed for combined liver-kidney transplant, combined liver-intestine transplant, and hospitalized candidates had higher DDLT rates despite stratification by allocation MELD score.
Although the allocation MELD (urgency) was still the major determinant of DDLT rates in United States, there was great deal of variation in the distribution of DDLT rates at a given MELD score. This variation reflects the significant contribution of additional candidate factors that results in actual offer acceptance and receipt of DDLT and consistent with physician judgment. We found that DDLT rates did not increase monotonically with allocation MELD score, after adjusting for DSA, blood type, and intestine policy. There was a steady increase in the DDLT rates from MELD=15 to MELD=32. However, there appeared to be little increase in DDLT rates from MELD=32 to MELD=40, even though the risk of waitlist death has been shown previously to increase dramatically across this range.
Our study showed that high serum creatinine and receipt of dialysis (components of MELD) were associated with lower DDLT rates in the MELD era. We have previously demonstrated that at a given MELD score, every unit increase in serum creatinine is associated with lower waitlist mortality. Association of lower DDLT rates with high creatinine at a given MELD score in the current study demonstrate evidence-based clinical practice of allocating scarce resources to the sickest first. However, negative effect of creatinine on DDLT rates was ameliorated with the increase in height. Candidates on dialysis at the time of transplant have overall significant but lower survival benefit from DDLT compared to their counterparts not on dialysis, at a given MELD score.12, 13 This perception of lower but significant survival benefit associated with dialysis at a given MELD score, may affect clinical practice, resulting in lower DDLT rates for such candidates.
The current study confirmed lower DDLT rates associated with female gender.7–9, 17, 18 It is speculated that lower DDLT rates among females compared to males could be due to lower creatinine (MELD covariate) and/or recipient size mismatch. To tease out why females had lower DDLT rates, we looked at the interaction of female and creatinine as well as female and height, however, none of these interactions were significant suggesting that lower DDLT rates among females, at a given MELD, is not affected by creatinine or height.
Despite the time at risk ended at granting of exception MELD score, candidates with HCC had higher DDLT rates compared to others. Since MELD exceptions were applied to stage T1 and T2 HCC before the July 1st, 2005 and to candidates with stage T2 HCC after July 1st 2005, HCC candidates examined in our study were either stage T1 HCC (after July 2005) or Stage T3 HCC (beyond Milan criteria) who did not get the exception and were transplanted with their laboratory MELD score. We speculate that these candidates may get DDLT from marginal and high risk donor organs and that might be driving the higher DDLT rates among HCC candidates.
One of the goals of MELD-based allocation and distribution policy was to provide transplantation to the sickest candidates first. The higher DDLT rates among hospitalized candidates in the ICU setting and non-ICU setting compared to non-hospitalized candidates suggest that MELD-based allocation has done fairly well in this aspect. Our current study as well as previous studies,11, 20, 21 evaluating the effectiveness of MELD-based allocation policy have provided evidence that current allocation policy is meeting its goal of balancing the needs of the most medically urgent candidates against the practical limitations of extensive sharing across large geographic areas.
The main limitation of the study is its retrospective observational design and associated problems such as selection bias, inability to assess the effect of unmeasured candidate characteristics, and missing data on some candidate level variables that may have affected the results. The grading of ascites and hepatic encephalopathy was based upon program reporting using subjective definitions and may be subject to misclassification. Despite these limitations, our results show that DDLT rates among active candidates are affected by many candidate factors other than DSA and allocation MELD. Further, had our covariates been measured more precisely and subject to less error, we would have had greater statistical power and perhaps additional significant findings would have emerged.
In conclusion, under the current allocation rules, MELD and DSA are the most important determinants of deceased-donor liver transplant rates, but other measured candidate characteristics are systematically affecting which candidates do and do not receive a DDLT; within the same DSA, with the blood type and at the same MELD score.
Acknowledgments
Funding Sources: This research was presented, in part, as a poster of excellence at the American Transplant Congress, 2010, held in San Diego, California. Dr. Sharma is supported by National Institutes of Health (NIH) grant KO8 DK-088946 and American College of Gastroenterology Research Award. The statistical methodology development and analysis for this investigation was supported in part by National Institutes of Health (NIH) grant 5R01 DK-70869 to Dr. Schaubel. The Scientific Registry of Transplant Recipients is funded by contract number 231-00-0116 from the Health Resources and Services Administration (HRSA), US Department of Health and Human Services. The views expressed herein are those of the authors and not necessarily those of the US Government. This study was approved by HRSA’s SRTR project officer. HRSA has determined that this study satisfies the criteria for the IRB exemption described in the “Public Benefit and Service Program” provisions of 45 CFR 46.101(b)(5) and HRSA Circular 03.
Abbreviations
- DDLT
Deceased Donor Liver Transplantation
- DSA
Donor Specific Area
- MELD
Model for End Stage Liver Disease
- OPO
Organ Procurement Organization
References
- 1.UNOS/OPTN. 3.6 Organ Distribution: Allocation of Livers. Policies. 2002 http://www.unos.org/PoliciesandBylaws2/policies/docs/policy_8.doc.
- 2.Institute of Medicine; DHHS. Committee on organ transplantation. Assessing current policies and the potential impact of DHHS final rule. Washington DC: National Academy of Press; 1999. Analysis of waiting time; pp. 57–88. [Google Scholar]
- 3.Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, Krom RA, Kim WR. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567–80. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 4.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 5.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–6. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 6.Mindikoglu AL, Regev A, Seliger SL, Magder LS. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver Transpl. 2010;16:1147–57. doi: 10.1002/lt.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300:2371–8. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathur AK, Schaubel DE, Gong Q, Guidinger MK, Merion RM. Racial and ethnic disparities in access to liver transplantation. Liver Transpl. 16:1033–40. doi: 10.1002/lt.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volk ML, Choi H, Warren GJ, Sonnenday CJ, Marrero JA, Heisler M. Geographic variation in organ availability is responsible for disparities in liver transplantation between Hispanics and Caucasians. Am J Transplant. 2009;9:2113–8. doi: 10.1111/j.1600-6143.2009.02744.x. [DOI] [PubMed] [Google Scholar]
- 10.Levine GN, McCullough KP, Rodgers AM, Dickinson DM, Ashby VB, Schaubel DE. Analytical methods and database design: implications for transplant researchers, 2005. Am J Transplant. 2006;6:1228–42. doi: 10.1111/j.1600-6143.2006.01277.x. [DOI] [PubMed] [Google Scholar]
- 11.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–13. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 12.Schaubel DE, Guidinger MK, Biggins SW, Kalbfleisch JD, Pomfret EA, Sharma P, Merion RM. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9:970–81. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma P, Schaubel DE, Guidinger MK, Merion RM. Effect of pretransplant serum creatinine on the survival benefit of liver transplantation. Liver Transpl. 2009;15:1808–13. doi: 10.1002/lt.21951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid AE, Resnick M, Chang Y, Buerstatte N, Weissman JS. Disparity in use of orthotopic liver transplantation among blacks and whites. Liver Transpl. 2004;10:834–41. doi: 10.1002/lt.20174. [DOI] [PubMed] [Google Scholar]
- 15.Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010;145:1158–63. doi: 10.1001/archsurg.2010.272. [DOI] [PubMed] [Google Scholar]
- 16.Asrani SK, Kim WR, Kamath PS. Race and receipt of liver transplantation: location matters. Liver Transpl. 2010;16:1009–12. doi: 10.1002/lt.22123. [DOI] [PubMed] [Google Scholar]
- 17.Mathur AK, Schaubel DE, Qi G, Guidinger MK, Merion RM. Racial and Ethnic Disparities in Transplant Rates Have Improved in the MELD Era. Am J Transplant. 2009;9:Abs# 584, 360. [Google Scholar]
- 18.Mindikoglu AL, Regev A, Seliger SL, Magder LS. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver Transpl. 16:1147–57. doi: 10.1002/lt.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma P, Balan V, Hernandez JL, Harper AM, Edwards EB, Rodriguez-Luna H, Byrne T, Vargas HE, Mulligan D, Rakela J, Wiesner RH. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004;10:36–41. doi: 10.1002/lt.20012. [DOI] [PubMed] [Google Scholar]
- 20.Freeman RB, Wiesner RH, Edwards E, Harper A, Merion R, Wolfe R. Results of the first year of the new liver allocation plan. Liver Transpl. 2004;10:7–15. doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 21.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]