Abstract
Attention-deficit/hyperactivity disorder (ADHD) is characterized by widespread structural and functional abnormalities in the cortico-striato-thalmo-cortical (CSTC) loops that subserve attention and executive functions. In this study, we analyzed thalamic shape and its white matter connections using structural MRI and diffusion tensor imaging (DTI) data acquired from children with ADHD (n=19) and controls (n=19). Shape morphology of the thalamus was assessed using shape-based analysis, while connectivity between the thalamus and other brain regions was determined using probabilistic diffusion tractography. Shape-based analysis indicated significant regional atrophy in the left thalamus in children with ADHD compared to controls. Group analyses of white matter connectivity measures showed significantly decreased mean fractional anisotropy (FA) and volume of the tracts between thalamus and striatum, hippocampus, and prefrontal lobe in children with ADHD compared to controls. The structural abnormalities within the thalamus and the reduced integrity of the white matter tracks between thalamus and other brain regions, as shown from the results of this study, may be the anatomical bases of the impaired cognitive performances in the attention and executive function domains in ADHD.
Keywords: ADHD, thalamus, shape analysis, DTI tractography
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD), one of the most commonly diagnosed childhood neurodevelopmental disorders, is characterized by inattention, impulsivity, and/or hyperactivity, which interfere with various aspects of the academic, home, and/or social lives of these children (American Psychiatric Association, 1994). Studies report that children with ADHD have deficits in executive function (Sergeant et al., 2002), working memory (Castellanos and Tannock, 2002), response inhibition (Barkley, 1997), selective attention (Booth et al., 2005), and delay of reward (Sonuga-Barke et al., 1992). Although the etiology of these conditions are not clear, converging evidence indicates that cortico-striato-thalamo-cortical (CSTC) loops likely subserve the functions of attention and cognition, and disturbances of CSTC loops may cause abnormal information processing leading to the hallmark of ADHD (Rowe et al., 2005; Bush et al., 2005; Dickstein et al., 2006; Schneider et al., 2006; Ivanov et al., 2010; Cubillo et al., 2011).
Structural and functional imaging studies in ADHD have predominantly reported abnormalities of fronto-striatal/fronto-subcortical circuitry, which is a part of the CSTC loops, linking prefrontal cortex and the striatum (Schneider et al., 2006; Paloyelis et al., 2007). Structural and functional abnormalities in other cortical regions, including anterior cingulate, temporal, and posterior parietal cortices, have also been reported in ADHD (Cherkasova and Hechtman, 2009). These regions may contain efferents to and/or from fronto-striatal circuitry, and are partly mediated via the thalamus during attention and other cognitive performances. Disturbances of these regions may contribute to difficulty with attention in ADHD (Fan et al., 2005; Schneider et al., 2006).
The thalamus is an important component of the CSTC loops. It forms a crucial link between the basal ganglia (striatum) and cerebral cortices by relaying output to specific cortices and mediating the flow of information between cortical networks (McFarland and Haber, 2002; Smith et al., 2008). Studies have provided evidence of functional and structural abnormalities in the thalamus in individuals with ADHD. Biological studies have revealed an energy deficiency in the thalamus in children and adolescents with ADHD (Ferreira et al., 2009; Cortese et al., 2011). Task-based fMRI studies have demonstrated reduced blood-oxygen-level dependent (BOLD) activation in the thalamus during tasks of motor inhibition and cognitive switching in adults with ADHD, which are persistent from childhood (Cubillo et al., 2010), and reduced BOLD activation in the thalamus during task switching in adults with ADHD (Dibbets et al., 2011). Resting-state fMRI studies have reported abnormal functional connectivity between thalamus and striatum, hippocampus and amygdala within CSTC loops in children with ADHD (Cao et al., 2009; Qiu et al., 2011). Diffusion tensor imaging (DTI) studies have reported white matter abnormalities within the thalamus in children and adolescents with ADHD (Silk et al., 2009). Shape analyses of subcortical structures have been increasingly utilized to closely assess the locations and patterns of structural abnormalities within the subcortical structures in neurological and psychiatric disorders such as Schizophrenia, Parkinson’s or Alzheimer’s diseases (McKeown et al., 2008; Kang et al., 2008; Shaw and Rabin, 2009; Coscia et al., 2009; Zarei et al., 2010; Smith et al., 2010). Rarely have shape analyses been applied to the studies of ADHD, but one such study examined the thalamic morphology and reported regional deficits, especially at the pulvinar nuclei from both hemispheres in the youth with ADHD, but there was no change in overall thalamic volume, compared to controls (Ivanov et al., 2010).
As reviewed above, imaging studies have indicated thalamus-related functional and structural abnormalities in patients with ADHD. Based on these findings, we hypothesized that regional structural anomalies of the thalamus and disrupted white matter connections between thalamus and other brain regions could exist and may contribute to the pathophysiology of ADHD. In this study, we analyzed the structural MRI and DTI data acquired from children with combined-type ADHD and group-matched controls, to examine the thalamic shape features and its white matter connections with other brain regions. Vertex-based method was applied to the structural MRI data to analyze the thalamic shape features. Fractional anisotropy (FA), which is an index of the directional selectivity of water diffusion, and probabilistic diffusion tensor tractography were applied to the DTI data, to evaluate the connectivity patterns of the white matter pathways between thalamus and other brain areas.
2. Methods
2.1. Participants
Thirty-eight children (9–15 years old) were included in this study. The 19 children with ADHD were recruited from the Children’s Evaluation and Rehabilitation Center at Albert Einstein College of Medicine, and the Max and Celia Parnes Family Psychological and Psychoeducational Services Clinic of the Ferkauf Graduate School of Psychology. The 19 controls were recruited from an established pool of volunteers at Einstein and from local schools by newspaper advertisements. Inclusion criteria for ADHD group were meeting current DSM-IV criteria for combined type ADHD. We included the new K-SADS screening questions and supplements as appropriate to rule out pervasive developmental disorders, substance use and abuse, and posttraumatic stress (Kaufman et al., 1997). Comorbidity with Oppositional Defiant Disorder (ODD) with physical aggression (using DSM-IV diagnostic criteria) was exclusionary, as this type of ODD is likely to progress to CD, whereas ODD without physical aggression does not usually progress (Biederman et al., 1996). Similarly, all other current Axis I disorders (except for Specific Phobia, e.g., fear of the dark, which is extremely common and can represent a normal variant in young children), and lifetime history of pervasive developmental disorders were exclusionary. Healthy controls included children who had T-scores < 60 (< 1 SD) on all Conners Parent ADHD subscales (ADHD Index, Hyperactivity, Cognitive Problems/Inattention and all 3 DSM-IV subscales). Presence of any current Axis I disorders (except for Specific Phobia), Learning Disorders, or a lifetime history of any pervasive developmental disorders or substance abuse were exclusionary in healthy controls. Additional exclusion criteria for both groups included head trauma or seizures with loss of consciousness, MR safety and artifact risk factors (e.g. pacemakers, teeth braces), or IQ below 80, as measured by the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) to minimize neurobiological heterogeneity. Eight children in the ADHD group had been treated by short-term-effect stimulant medication (Ritalin). A 48 hours’ wash-out period was administrated to each of these patients before the day of the MRI scanning.
This study received Institutional Review Board Approval for human subjects’ research at Albert Einstein College of Medicine of Yeshiva University. After the study and its procedures were carefully explained, written informed consent was provided by all participants and their parents.
2.2. MRI acquisition protocol
High-resolution 3-dimensional T1-weighted structural MRI and DTI data were acquired from each subject on a 3T Philips Achiva TX MR system with a 32-channel phased array head coil (Invivo, Gainsville, Fl). T1-weighted data was acquired using MPRAGE sequences: TR = 9.8ms, TE = 4.6 ms, flip angle = 8°, voxel size = 1mm × 1mm × 1mm, FOV = 240mm × 188mm × 220mm, SENSE reduction factor =2.5. DTI data was acquired using an echo planar imaging (EPI) sequence with a b-value = 800 s/mm2 along 32 independent, non-collinear orientations. TR=7367ms, TE = 56ms, flip angle = 90°, voxel size: 1.67mm × 1.67mm × 2mm, field of view (FOV) = 240mm × 249mm, imaging matrix = 144 × 144, number of slices = 70, and SENSE reduction factor = 2.5. We also collected one additional image with no diffusion weighting (b = 0).
2.3. T1-weighted data processing
T1-weighted data were initially skull-stripped using the Brain Extraction Tool (BET) (Smith, 2002). Any non-brain tissues were then manually removed. Analyses of thalamic shape and volume were performed using the FSL/FIRST tool (Patenaude et al., 2008). FIRST tool provided the template of the thalamus from each side of the brain, which was generated by averaging a set of manually segmented thalami from the FSL training data. The surface parameterization of each thalamus template was then used to create a target mesh with a fixed number of vertices (Patenaude, 2007; Patenaude et al., 2011). For each subject involved in this study, the thalamus in each hemisphere was segmented and parameterized automatically aided by the training data from FIRST. The target mesh from the FIRST models (in MNI152 space) was used to which surfaces from the individual subjects were aligned. Pose (rotation and translation) was removed by minimizing the sum-of-squares difference between the corresponding vertices of a subject’s surface and the target. Then, the three-dimensional coordinates of corresponding vertices of each subject were compared to that of the target, representing the local shape changes of each subject. The result of each step in the FIRST procedure was visually inspected.
To calculate the volume of each thalamus from each subject while simultaneously reducing head-size related variability between subjects, we first normalized the brain size of each subject to the template provided by the FSL/SIENA tool, thus obtaining a scaling factor for that brain (FSL/SIENA tools (Smith.S.M. et al., 2002)). The normalized thalamic volume was defined as the FIRST obtained thalamic volume multiplied by the estimated scaling factor for that subject.
2.4. DTI data processing
DTI data was processed using FMRIB’s Diffusion Toolbox (FDT Version 2.0) (Behrens et al., 2007). For each subject, the diffusion-weight images were registered to the additionally acquired non-diffusion-weighted reference image (b0 image) using an affine, 12 degrees of freedom registration (Jenkinson and Smith, 2001). Then the fractional anisotropy (FA) value at each brain voxel was computed. The FSL/BEDPOSTX tool was used to generate the probability distributions of diffusion parameters within each voxel, including modeling for diffusion of crossing fibers along two directions (Behrens et al., 2007).
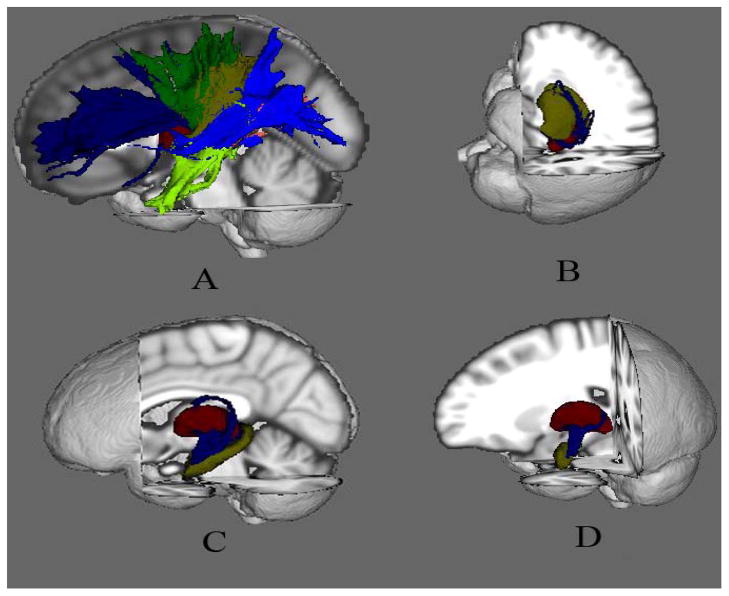
The seed and target region-of-interest (ROI) clusters for probabilistic tractography were created based on the Harvard-Oxford Cortical Atlases (Desikan R.S. et al., 2006) and the Julich Histological Atlas (Eickhoff et al., 2005) from the MNI standard space, and mapped to the DTI data. These ROI clusters included the thalamus, striatum (including putamen, caudate nuclei and globus pallidum), hippocampus, amygdala, and cerebral cortices (prefrontal, primary motor, premotor, somatosensory, posterior parietal, temporal, and occipital cortices), from each hemisphere. We used the multi-fibre probabilistic connectivity-based method to determine the number of pathways between the seed (thalamus) and each of the target clusters (Johansen-Berg et al., 2004). We utilized the default setting of parameters for the Markov Chain MonteCarlo estimation of the probabilistic tractography: 5000 individual pathways were drawn on the principle fibre direction of each voxel within the seed ROI using the probability distributions; curvature threshold of 80° to exclude implausible pathways; a maximum number of 2000 travel steps of each sample pathway and a 0.5mm step length. This step also provided an estimation of the distribution of the sample pathways, which had been sent out from the seed ROI, in the remainder of the whole brain. The number of pathways that existed through each voxel from the remainder of the brain was labeled. The non-zero labeling voxels were taken as the initial elements of the tracks between the seed and target. In order to remove the brain voxels with low probability of connecting the seed and target, the final tracks excluded the voxels, if one had a number of pathways that was less than the average of the pathway numbers from all the non-zero labeling voxels. Figure 1 demonstrated the resulting tracts from each thalamus for one subject randomly selected from the control group. The mean FA value and volume of each tract for each subject were extracted for group comparisons.
Figure 1.
The visual demonstration of the white matter tracks in the left hemisphere constructed from one subject randomly selected in the control group. Item A showed the tracks between thalamus and prefrontal cortex (T-PFC, blue), motor cortex (T-MC, green), somatosensory cortex (T-SSC, yellow), posterior parietal cortex (T-PPC, pink), temporal cortex (T-TC, light blue), and occipital cortex (T-OC, light green); B showed the track between thalamus and striatum (T-S); C showed the track between thalamus and hippocampus (T-H); and D showed the track between thalamus and amygdale (T-A). (Thalamus is labeled in red.)
2.5. Statistical analysis
In the thalamic shape analysis, group comparisons of vertex measures of each thalamus between children with ADHD and normal controls were carried out using an F-statistic and corrected for multiple comparisons using FDR corrections. Group comparisons of the connectivity-based DTI data analysis were performed using SPSS18 (SPSS Inc, Somers, NY), where volumes and mean FA of the fiber tracts were compared between children with ADHD and normal controls using multiple analysis of covariance analysis. Clusters from left and right hemispheres were analyzed separately. The Bonferroni correction for multiple comparisons was applied. For both thalamic shape and white matter connectivity analyses, age was added as random effect covariate and gender was added as fixed effect covariate, during group comparisons. A significance threshold of P < 0.05 was used.
3. Results
3.1. Demographic information
Demographic information for the participants was provided in Table 1. ADHD and controls group did not differ significantly in age (t = 1.78, df = 36, p = 0.084) and IQ (t = 1.57, df = 36, p = 0.125), while they differed significantly in gender (χ2 = 4.34, df=1, p = 0.037). Participants were dominated by right handedness in both groups (ADHD: 17/19; controls: 18/19).
Table 1.
Demographic Characteristics of children with ADHD and controls
| Controls (n=19) (Mean ± SD) | ADHD (n=19) (Mean ± SD) | Test statistic | P | |
|---|---|---|---|---|
| Age | 12.2 ± 2.3 | 10.9 ± 2.3 | t=1.78 | 0.083 |
| Male/Female | 10/9 | 14/5 | χ2 = 4.34 | 0.037 |
| Left/Right handedness | 1/19 | 2/17 | -- | -- |
| Full scale IQ | 110.5 ± 12.6 | 102.8 ± 17.0 | t=1.57 | 0.125 |
3.2. Thalamic volume
Compared to controls, children with ADHD exhibited significantly smaller thalamic volume in the right hemisphere (ADHD, 4917.6± 560.9 mm3; Controls, 5309.0 ± 654 mm3, F = 5.332, df = 1, 34, P= 0.027) after controlling for age and gender. A smaller thalamic volume in the left hemisphere (ADHD, 4876.5± 592.8 mm3; Controls, 5199.4 ± 657.5 mm3) was also found, although the finding was below the significance level (F = 0.815, df = 1, 34, P=0.373) after controlling for age and gender.
3.3. Shape analysis using vertex-based comparison
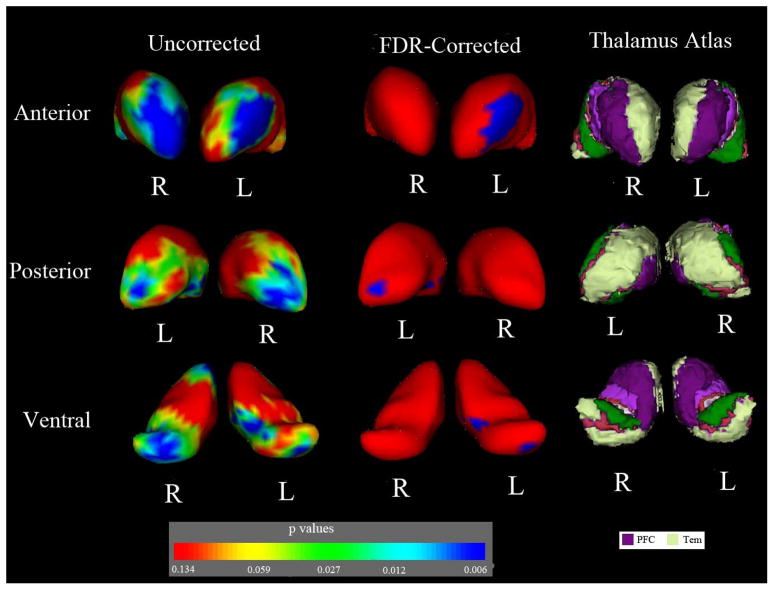
Group comparisons of vertex displacement in the thalamus revealed significant regional atrophy in the anterior and posterior regions of the thalami from both hemispheres in ADHD group. After FDR correction for multiple comparisons, children with ADHD showed significant Regional atrophy in the ventral anterior nuclei, medial dorsal nuclei and pulvinar nuclei of the left thalamus. According to the Oxford Thalamus Connectivity Probability Atlas (Behrens et al., 2003), these areas in the thalamic nuclei should have connections with the prefrontal and temporal cortices. FDR-corrected analysis of the right thalamus showed that there was no significant difference of regional morphology between two groups. Figure 2 provided a visual demonstration of group comparisons of the bilateral thalamic shapes.
Figure 2.
Group comparison of the regional thalamic shape in each hemisphere. The first and second columns illustrated the statistical maps using vertex-based comparison of each thalamus between children with ADHD and controls, with and without FDR corrections. The third column showed that according to the thalamus atlas, the regions, which had regional shape abnormalities in ADHD group, were connected with the prefrontal and temporal cortices. PFC = prefrontal cortex; Tem = temporal cortex. In the color-coded bar of p values, p=0.134 (F =2), p=0.059 (F =2.75), p=0.027 (F=3.5), p=0.012 (F=4.25), p=0.006(F=5) were given (freedom of degree: 3, 32).
3.4. DTI connectivity-based analysis
The results of between-group comparisons of the volumes of white matter fiber tracts between the thalamus and cortical regions were provided in Table 2. Compared to the controls, children with ADHD showed significantly smaller volume of the white matter fiber tracts between thalamus and striatum (left T-S, F=5.67, df=1, 34, p=0.023), and between thalamus and motor cortex (left T-MC, F=4.66, df=1, 34, p=0.038) in the left hemisphere. A significantly smaller volume of the white matter fiber tracts between thalamus and striatum (right T-S, F=4.24, df =1, 34, p=0.047) in the right hemisphere was also shown. There were no significant differences in other tracts. Group comparisons of the mean FA within the white matter fiber tracts showed significant FA reductions in the white matter bundles between the thalamus and striatum bilaterally (T-S, left: F=4.82, df=1, 34, p=0.035; right: F=4.37, df=1, 34, p=0.044), between the thalamus and hippocampus (left T-H, F=5.34, df=1, 34, p=0.027) in the left hemisphere, and between the thalamus and prefrontal cortex (right T-PFC, F=4.21, df=1, 34, p=0.048) in the right hemisphere in children with ADHD compared to controls (Table 3).
Table 2.
Group comparisons in the volumes (mm3) of the white matter fiber tracts
| ROI | Control (Mean ± SD) | ADHD (Mean ± SD) | p-value |
|---|---|---|---|
| Left T-S | 5438 ± 1785 | 5088 ± 1177 | 0.023 |
| Left T-H | 2292 ± 716 | 1828 ± 859 | 0.516 |
| Left T-A | 1962 ± 768 | 1917 ± 682 | 0.194 |
| Left T-PFC | 14677 ± 5045 | 13782 ± 4119 | 0.240 |
| Left T-MC | 9343 ± 2271 | 7705 ± 2903 | 0.038 |
| Left T-SSC | 4253 ± 1334 | 4139 ± 1593 | 0.307 |
| Left T-PPC | 7143 ± 2118 | 6613 ± 3216 | 0.646 |
| Left T-TC | 7160 ± 2829 | 6305 ± 2654 | 0.538 |
| Left T-OC | 10514 ± 3960 | 9639 ± 4410 | 0.518 |
| Right T-S | 5995 ± 1718 | 5513 ± 1392 | 0.047 |
| Right T-H | 1619 ± 565 | 1419 ± 584 | 0.507 |
| Right T-A | 2112 ± 1082 | 2075 ± 1526 | 0.445 |
| Right T-PFC | 15829 ± 4048 | 14977 ± 4494 | 0.663 |
| Right T-MC | 8008 ± 2112 | 6982 ± 2898 | 0.914 |
| Right T-SSC | 4337 ± 1543 | 3852 ± 1193 | 0.810 |
| Right T-PPC | 7503 ± 1865 | 7122 ± 2887 | 0.478 |
| Right T-TC | 7019 ± 3080 | 5383 ± 2793 | 0.085 |
| Right T-OC | 12169 ± 2850 | 10630 ± 3409 | 0.693 |
T-S: the tract between thalamus and striatum; T-H: the tract between thalamus and hippocampus; T-A: the tract between thalamus and amygdale; T-PFC: the tract between thalamus and prefrontal cortex; T-MC: the tract between thalamus and motor cortex; T-SSC: the tract between thalamus and somatosensory cortex; T-PPC: the tract between thalamus and posterior parietal cortex; T-TC: the tract between thalamus and temporal cortex; T-OC: the tract between thalamus and occipital cortex.
Table 3.
Group comparisons of the FA values of the white matter fiber tracts
| ROI | Control (mean ± SD) | ADHD (mean ± SD) | p-value |
|---|---|---|---|
| Left T-S | 0.43 ± 0.03 | 0.41 ± 0.03 | 0.035 |
| Left T-H | 0.31 ± 0.04 | 0.30 ± 0.03 | 0.027 |
| Left T-A | 0.35 ± 0.02 | 0.34 ± 0.03 | 0.482 |
| Left T-PFC | 0.44 ± 0.03 | 0.43 ± 0.04 | 0.850 |
| Left T-MC | 0.50 ± 0.02 | 0.50 ± 0.03 | 0.718 |
| Left T-SSC | 0.49 ± 0.02 | 0.50 ± 0.02 | 0.752 |
| Left T-PPC | 0.45 ± 0.03 | 0.46 ± 0.03 | 0.278 |
| Left T-TC | 0.40 ± 0.03 | 0.40 ± 0.04 | 0.784 |
| Left T-OC | 0.47 ± 0.02 | 0.47 ± 0.03 | 0.683 |
| Right T-S | 0.42 ± 0.02 | 0.40 ± 0.03 | 0.044 |
| Right T-H | 0.31 ± 0.05 | 0.31 ± 0.02 | 0.770 |
| Right T-A | 0.34 ± 0.04 | 0.33 ± 0.04 | 0.252 |
| Right T-PFC | 0.44 ± 0.03 | 0.43 ± 0.03 | 0.048 |
| Right T-MC | 0.49 ± 0.02 | 0.50 ± 0.03 | 0.133 |
| Right T-SSC | 0.49 ± 0.02 | 0.50 ± 0.03 | 0.117 |
| Right T-PPC | 0.45 ± 0.03 | 0.46 ± 0.03 | 0.408 |
| Right T-TC | 0.42 ± 0.04 | 0.41 ± 0.04 | 0.634 |
| Right T-OC | 0.45 ± 0.02 | 0.47 ± 0.03 | 0.061 |
T-S: the tract between thalamus and striatum; T-H: the tract between thalamus and hippocampus; T-A: the tract between thalamus and amygdale; T-PFC: the tract between thalamus and prefrontal cortex; T-MC: the tract between thalamus and motor cortex; T-SSC: the tract between thalamus and somatosensory cortex; T-PPC: the tract between thalamus and posterior parietal cortex; T-TC: the tract between thalamus and temporal cortex; T-OC: the tract between thalamus and occipital cortex.
4. Discussion
This study examined the thalamic volume, surface shape, and the integrity of the white matter connections between the thalamus and the striatum, hippocampus, amygdala, and cortical regions from the prefrontal, motor, somatosensory, parietal, temporal, and occipital cortices in children with ADHD and controls, using shape-based analysis and connectivity-based analysis on structural and DTI data. Shape-based analysis demonstrated the significantly reduced right thalamic volume in children with ADHD. Regions with local surface atrophy were located in the ventral anterior nuclei, medial dorsal nuclei and pulvinar of the left thalamus. Connectivity-based analysis demonstrated that the connectivity bundles between the thalamus and the striatum were disturbed in children with ADHD.
Our study demonstrated structural anomalies associated with the thalamus in children with ADHD. The thalamus has been shown to be an important component of the CSTC loops that are of critical importance for the modulation of higher cognitive, emotional and attentional processes (Mega and Cummings, 1994). The thalamus has been found to involve in the selective control of the flow of sensory-motor information during different states of sleep-wake cycle and arousal through the thalamo-cortical connections and regulate the states of consciousness, sleep and alertness (McCormick and Bal, 1994; Guillery and Sherman, 2002). A portion of children with ADHD had reported sleep difficulties, such as inadequate sleep duration, excessive daytime sleepiness, et al (Owwns, 2005). The excessive motor activity could be a cause for these children with ADHD to stay awake and alert (Weinberg and Brumback, 1990). These evidences showed that children with ADHD may have a deficit in alertness or hypoarousal functioning (Miano et al., 2006; Konofal et al., 2010). The structural anomalies of the thalamus and the reduced integrity of its white matter connections may contribute to these symptoms.
This study demonstrated a prominent local atrophy in the ventral anterior thalamic nuclei and significant volume reduction in the white matter fiber tracts between this subregion of the thalamus and the motor cortex in the left hemisphere in children with ADHD. The ventral anterior thalamic nuclei receive prominent cortico-striatum inputs from more rostral motor and prefrontal cortical areas, and project information to more caudal motor areas, affecting motor output or behavior (McFarland and Haber, 2002). Animal studies using electroencephalography techniques have also shown that the failure of dopaminergic transmission disinhibited the indirect pathway and reduced the inhibitory effects of the direct pathway in the cortico-striatal-thalamic loop, both of which release inhibitory neurons that project to ventral lateral, ventral anterior and centromedian nuclei of the thalamus, and the abnormal inhibition of these thalamic nuclei led to disturbances of both motor and emotional control (Sterman, 2000). These findings together suggest that the structural abnormalities of the ventral anterior nuclei and the disturbance of the white matter connections between this sub-region and other brain areas may contribute to the deficits of inhibitory and higher order motor control in children with ADHD.
Our study also demonstrated the disturbed white matter fiber pathways between the thalamus and striatum in children with ADHD. Modern anatomical and physiological studies have shown that the ventral anterior and ventral lateral nuclei of the thalamus provide substantial connections with the striatum, and mediate basal ganglia output to the frontal cortex (McFarland and Haber, 2001; Matsumoto et al., 2001; Smith et al., 2004; Kuramoto et al., 2009). The ventral anterior nuclei of the thalamus have been found to be one of the major sources of the thalamo-striatal projections, and associated with arousal, attention, orienting and action selection (Kimura et al., 2004; McHaffie et al., 2005). The disrupted white matter fiber connections between the thalamus and striatum may play a vital role in the pathophysiology of the inattention component of ADHD.
Our findings of the regional atrophy in the pulvinar complex are consistent with a previous study in youth with ADHD (Ivanov et al., 2010). DTI analyses of our study also reported FA reduction of the white matter connections between the pulvinar complex and hippocampus in children with ADHD compared to controls. Efferents of the pulvinar complex have been found to terminate in cortical regions in the prefrontal, parietal, occipital, and temporal lobes as well as in the limbic regions, including hippocampus (Berson and Graybiel, 1978; Romanski et al., 1997). These pulvinar-cortical and pulvinar-limbic connections have been found to subserve arousal, selective attention, learning and memory function and orientation to visual and auditory stimuli (Grieve et al., 2000; Casanova et al., 2001). Pulvinar nuclei have also been found to generate signal related to the salience of visual objects and be involved in the selection of salient targets and filtering of non-salient distracters(Robinson and Petersen, 1992). Thus, structural abnormalities associated with the pulvinar complex in children with ADHD could contribute to disrupted attention (Michael and Desmedt, 2004).
Our study reported significantly reduced volume of the thalamus on the right hemisphere. A previous study reported that there was no thalamic volume difference between youth with ADHD and group matched controls (Ivanov et al., 2010). One possible reason for this inconsistency is that the majority of patients with ADHD (31 from the 46), who were involved in Ivanov’s study, had been treated by medications before and at the time of scanning. Ivanov et al. reported that these treated patients had larger thalamic volumes than those who had not been treated (Ivanov et al., 2010). In our study, 8 of the 19 children with ADHD had been taking short-term-effect stimulant, Ritalin. The others were never medicated. We scheduled a wash-out period of 48 hours for the 8 medicated patients before the scanning date. Another possible reason for this inconsistency is the difference of age ranges in both studies. We included children from 9 to 15 years old, whereas Ivanov et al. included a larger age range (8 to 18 years old).
Our study has several limitations. This study found regional thalamic shape abnormality and decreased integrity in the white matter tracts between thalamus and other brain regions in children with ADHD. However, MRI-based measures themselves could not provide further insight of these structural anomalies. A study using 1H-magnetic resonance spectroscopy (MRS) reported reduced neuronal energetic metabolism in the pulvinar region of the thalamus (Ferreira et al., 2009), which provided support of a theory of the ‘energetic deficiency’ in fronto-striatal-thalamic structures in ADHD (Todd and Botteron, 2001). Deficient energy may result in the reductions of the both target region size and number of catecholaminergic synapses by interfering with activity-dependent synapse formation and stabilization (Todd et al., 1995; Todd and Botteron, 2001). Second, we included both male and female subjects in both groups. The gender difference between the two groups was significant. We acknowledge possible gender-related differences of the subcortical and white matter maturation patterns in typical developing children (Reiss et al., 1996; Elize et al., 2001), and possible sex-related heterogeneity in ADHD (Ramtekkar et al., 2010). However, the sample size of our study was not large enough to run the between-gender comparisons in each group. Although we added the gender as a fixed effect covariate for group comparisons, a future research can focus specifically on examining the gender effects upon abnormalities of the thalamic shape and its white matter connections. One more future work should also focus on the examination of the associations between these thalamic-related structural abnormalities and the clinical symptoms in ADHD.
Acknowledgments
This work partially supported by the Rose F. Kennedy IDD Research Center program grant (HD071593) from the National Institute of Health, and was conducted at and sponsored by the Gruss Magnetic Resonance Research Center of the Albert Einstein College of Medicine. The authors would also like to present their gratitude to Mr. Sada Guzman and Ms. Yrany Alvarado for their contributions to the MR data acquisition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. AmericanPsychiatric Press; 1994. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Mattjews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Berson DM, Graybiel AM. Parallel thalamic zones in the LP-pulvinar complex of the cat identified by their afferent and efferent connections. Brain Research. 1978;147:139–148. doi: 10.1016/0006-8993(78)90778-3. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Milberger S, Jetton JG, Chen L, Mick E, Greene RW, Russell RL. Is childhood oppositional defiant disorder a precursor to adolescent conduct disorder? Findings from a four-year follow-up study of children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1193–1204. doi: 10.1097/00004583-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Venport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) The Journal of Child Psychology and Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attentiondeficit/hyperactivity disorder: a review and suggested future directions. Biological Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Cao X, Cao Q, Long X, Sun L, Sui M, Zhu C, Zuo X, Zang Y, Wang Y. Abnormal resting-state functional connectivity patterns of the putamen in medication-naïve children with attention deficit hyperactivity disorder. Brain Research. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Casanova C, Merabet L, Desautels A, Minville K. Higher-order motion processing in the pulvinar. Progress in Brain Research. 2001;134:71–82. doi: 10.1016/s0079-6123(01)34006-2. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Cherkasova MV, Hechtman L. Neuroimaging in attention-deficit hyperactivity disorder: beyond the frontostriatal circuitry. Canadian Journal of Psychiatry. 2009;54:651–664. doi: 10.1177/070674370905401002. [DOI] [PubMed] [Google Scholar]
- Cortese S, Azoulay B, Castellanos FX, Chalard F, lecendreus M, Chechin D, Delorme R, Sebag G, Sbarbati A, Mouren M, Bernardina BD, Konofal E. Brain iron levels in attention-deficit/hyperactivity disorder: A pilot MRI study. The World Journal of Biological Psychiatry. 2011 doi: 10.3109/15622975.2011.570376. In press. [DOI] [PubMed] [Google Scholar]
- Coscia DM, Narr KL, Robinson DG, Hamilton LS, Sevy S, Burdick KE, Gunduz-Bruce H, McCormack J, Bilder RM, Szeszko PR. Volumetric and shape analysis of the thalamus in first-episode schizophrenia. Human Brain Mapping. 2009;30:1236–1245. doi: 10.1002/hbm.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. Journal of Psychiatric Research. 2010;44:629–639. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2011;18:209–213. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Buckner RL, Dale AM, Maguire BP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dibbets P, Evers ATE, Hurks PMP, Bakker K. Differential brain activation patterns in adult attention-deficit hyperactivity disorder (ADHD) associated with task switching. Neuropsychology. 2011;24:413–423. doi: 10.1037/a0018997. [DOI] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. The Journal of Child Psychology and Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroreport. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Elize S, Blasey MC, Freund SL, Hastie T, Reiss LA. Brain anatomy, gender and IQ in children and adolescents with fragile X syndrome. Brain. 2001;124:1610–1618. doi: 10.1093/brain/124.8.1610. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Ferreira PE, Palmini A, Grevet EH, Hoefel JR, Rohde LA, Anes M, Ferreira EE, Belmonte-de-Abreu P. Differentiating attention-deficit/hyperactivity disorder inattentive and combined types: a 1H-magnetic resonance spectroscopy study of fronto-striato-thalamic regions. Journal of Neural Transmission. 2009;116:623–629. doi: 10.1007/s00702-009-0191-3. [DOI] [PubMed] [Google Scholar]
- Grieve KL, Acuna C, Cudeiro J. The primate pulvinar nuclei: vision and action. Trends in Neurosciences. 2000;23:35–39. doi: 10.1016/s0166-2236(99)01482-4. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L, Sanchez-Pena J, Miller A, Chalravarty MM, Klahr K, Durkin K, Greenhill L, Peterson BS. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. The American Journal of Psychiatry. 2010;167:397–408. doi: 10.1176/appi.ajp.2009.09030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith MJ. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, Matthews PM. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15:31–39. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- Kang D, Kin S, Kin C, Choi J, Jang J, Jung M, Lee J, Kim S, Kwon J. Thalamus surface shape deformity in obsessive-compulsive disorder and schizophrenia. Neuroreport. 2008;19:609–613. doi: 10.1097/WNR.0b013e3282fa6db9. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. The schedule for affective disorders and schizophrenia for school aged children: present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kimura M, Minamimoto T, Matsumoto N, Hori Y. Monitoring and switching of cortico-basal ganglia loop functions by the thalamo-striatal system. Neuroscience Research. 2004;48:355–360. doi: 10.1016/j.neures.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Konofal E, Lecendreux M, Cortese S. Sleep and ADHD. Sleep Medicine. 2010;11:652–658. doi: 10.1016/j.sleep.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Kuramoto E, Furuta T, Nakamura CK, Unzai T, Hioki H, Kaneko T. Two types of thalamocortical projections from the motor thalamic nuclei of the rat: A single neuron-tracing study using viral vectors. Cerebral Cortex. 2009;19:2065–2077. doi: 10.1093/cercor/bhn231. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Minamimoto T, Graybiel AM, Kimura M. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. Journal of Neurophysiology. 2001;85:960–976. doi: 10.1152/jn.2001.85.2.960. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sensory gating mechanisms of the thalamus. Current Opinion in Neurobiology. 1994;4:550–556. doi: 10.1016/0959-4388(94)90056-6. [DOI] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Organization of thalamostriatal terminals from the ventral motor nuclei in the macaque. The Journal of Comparative Neurology. 2001;429:321–336. doi: 10.1002/1096-9861(20000108)429:2<321::aid-cne11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. The Journal of Neuroscience. 2002;15:8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P. Subcortical loops through the basal ganglia. Trends in Neurosciences. 2005;28:401–407. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Uthama A, Abugharbieh R, Palmer S, Lewis M, Huang X. Shape (but not volume) changes in the thalami in Parkinson disease. BMC Neurology. 2008;8:8. doi: 10.1186/1471-2377-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega MS, Cummings JL. Frontal-subcortical circuits and neuropsychiatric disorders. The journal of Neuropsychiatry and Clinical Neurosciences. 1994;6:358–370. doi: 10.1176/jnp.6.4.358. [DOI] [PubMed] [Google Scholar]
- Miano S, Donfrancesco R, Bruni O, Ferri R, Galiffa S, Pagani J, Montemitro E, Kheirandish L, Gozsal D, Oia VM. NREM sleep instability is reduced in children with attention-deficit/hyperactivity disorder. Sleep. 2006;29:797–803. [PubMed] [Google Scholar]
- Michael GA, Desmedt S. The human pulvinar and attentional processing of visual distractors. Neuroscience Letters. 2004;362:176–181. doi: 10.1016/j.neulet.2004.01.062. [DOI] [PubMed] [Google Scholar]
- Owwns AJ. The ADHD and sleep conundrum: A review. Journal of Developmental & Behavioral Pediatrics. 2005;26:312–322. doi: 10.1097/00004703-200508000-00011. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Kuntsi J, Asherson P. Functional MRI in ADHD: a systematic literature review. Expert Review of Neurotherapeutics. 2007;7:1337–1356. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B. DPhil Thesis. University of Oxford; 2007. Bayesian statistical models of shape and appearance for subcortical brain segmentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith MJ, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith S, Kennedy D. FIRST-FMRIB’s integrated registration and segmentation tool. Human Brain Mapping Conference.2008. [Google Scholar]
- Qiu M, Ye Z, Li Q, Liu G, Xie B, Wang J. Changes of brain structure and function in ADHD children. Brain Topography. 2011;24:243–252. doi: 10.1007/s10548-010-0168-4. [DOI] [PubMed] [Google Scholar]
- Ramtekkar UP, Reisersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:217–228. [PMC free article] [PubMed] [Google Scholar]
- Reiss LA, Abrams TM, Singer SH, Ross LJ, Denckla BM. Brain development, gender and IQ in children - A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Petersen SE. The pulvinar and visual salience. Trends in Neurosciences. 1992;15:127–132. doi: 10.1016/0166-2236(92)90354-b. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Giguere M, Bates JF, Goldman-Rakic SP. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. The Journal of Comparative Neurology. 1997;379:313–332. [PubMed] [Google Scholar]
- Rowe DL, Robinson PA, Lazzaro IL, Powles RC, Gordon E, Williams LM. Biophysical modeling of tonic cortical electrical activity in attention deficit hyperactivity disorder. International Journal of Neuroscience. 2005;115:1273–1305. doi: 10.1080/00207450590934499. [DOI] [PubMed] [Google Scholar]
- Schneider M, Retz W, Coogan A, Thome J, Rosler M. Anatomical and functional brain imaging in adult attention-deficit/hyperactivity disorder (ADHD) - A neurological view. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:32–41. doi: 10.1007/s00406-006-1005-3. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Oosterlaan J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behavioural Brain Research. 2002;130:3–28. doi: 10.1016/s0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- Shaw P, Rabin C. New insights into attention-deficit/hyperactivity disorder using structural neuroimaging. Current Psychiatry Reports. 2009;11:393–398. doi: 10.1007/s11920-009-0059-0. [DOI] [PubMed] [Google Scholar]
- Silk JT, Vance A, Rinehart N, Bradshaw LJ, Cunnington R. Structural development of the basal ganglia in attention deficit hyperactivity disorder: A diffusion tensor imaging study. Psychiatry Research: Neuroimaging. 2009;172:220–225. doi: 10.1016/j.pscychresns.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Wang L, Cronenwett W, Mamah D, Barch DM, Csernansky JG. Thalamic morphology in schizophrenia and schizoaffective disorder. Journal of Psychiatric Research. 2010;45:378–385. doi: 10.1016/j.jpsychires.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: Anatomical and functional organization in normal and parkinsonian states. Brain Research Bulletin. 2008;78:60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends in Neurosciences. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N. Accurate, robust and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion--I. the effect of delay on choice. The Journal of Child Psychology and Psychiatry. 1992;33:387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Sterman MB. EEG markers for attention deficit disorder: Pharmacological and neurofeedback applications. Child Study Journal. 2000;30:1–23. [Google Scholar]
- Todd RD, Botteron KN. Is attention-deficit/hyperactivity disorder an energy deficit syndrome? Biological Psychiatry. 2001;50:151–158. doi: 10.1016/s0006-3223(01)01173-8. [DOI] [PubMed] [Google Scholar]
- Todd RD, Swarzenski B, Visconti P, Giovanardi-Rossi P. Structural and functional development of the human brain. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology, Vol. 1: Theory and Methods. New York: John Wiley & Sons; 1995. pp. 161–194. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Weinberg WA, Brumback RA. Primary disorder of vigilance: A novel explanation of inattentiveness, daydreaming, boredom, restlessness, and sleepiness. The Journal of Pediatrics. 1990;116:720–725. doi: 10.1016/s0022-3476(05)82654-x. [DOI] [PubMed] [Google Scholar]
- Zarei M, Patenaude B, Damoiseaux J, Morgese C, Smith S, Matthews PM, Barkhof F, Rombouts SA, Sanz-Arigita E, Jenkinson M. Combining shape and connectivity analysis: an MRI study of thalamic degeneration in Alzheimer’s disease. Neuroimage. 2010;49:1–8. doi: 10.1016/j.neuroimage.2009.09.001. [DOI] [PubMed] [Google Scholar]