Abstract
There is considerable evidence that stressful early life events influence a variety of physical health problems later in life. Childhood adversity has been linked to elevated rates of morbidity and mortality from a number of chronic diseases. Immune dysregulation may be one potential pathway that explains this link. In this mini-review, we summarize human studies demonstrating that severe early life stressors have lasting immune consequences. We propose a model outlining potential biobehavioral pathways that explain how early life stressors leave people vulnerable to these maladaptive outcomes. Finally, we suggest ideas for future work to test different aspects of this model.
1. Introduction
There is mounting evidence that stressful early life events influence a variety of physical health problems later in life. Indeed, childhood adversity has been linked to elevated rates of morbidity and mortality from a number of chronic diseases (Miller, Chen, & Parker, 2011). For example, people who experienced severe life stressors as children (e.g., abuse, neglect, family conflict, low socioeconomic conditions) are at greater risk factor for cardiovascular disease, type II diabetes, cancer, and a variety of somatic difficulties than those who did not have these early life experiences (Miller et al., 2011). Although adults who experienced early life adversity often have poor health practices, the connection between severe psychological stress early in life and adult health exists even after accounting for these factors (Miller et al., 2011).
The pathways linking early adversity to adult health are clearly multifaceted. They include both behavioral and physiological components. Emerging evidence suggests that immune dysregulation may be one potential pathway linking stressful early life events to physical health problems.
In this mini-review, we summarize work demonstrating that severe early life stressors explain some of the variation in immune responses seen in adults. Specifically, we review work showing that early adversity is associated with elevated inflammation, telomere shortening, latent herpes virus reactivation, and a poorer response to an immunogenic tumor. We focus our review on these four markers because of their links to early adversity. We then propose a model outlining potential biobehavioral pathways that explains how early life stressors leave people vulnerable to these maladaptive outcomes. Finally, we suggest ideas for future work to test different aspects of this model. Because of space constraints, the focus of our review is entirely on human studies.
2. Early adversity and immune dysregulation
2.1 Inflammation
Inflammation has well-documented associations with early life stress. Inflammation is a risk factor for cardiovascular disease, type II diabetes, osteoporosis, periodontal disease, and rheumatoid arthritis (Ershler & Keller, 2000; Libby, 2007). Children who were maltreated have elevated inflammation compared with those who were not maltreated (Danese et al., 2010). This association persists in adulthood. For example, in a large-scale longitudinal prospective study, those who were neglected during the first decade of their life had higher CRP levels at age 32 compared with those who were not neglected. Indeed, more than 10% of adult low-grade inflammation was attributable to child maltreatment (Danese, Pariante, Caspi, Taylor, & Poulton, 2007). Middle-aged adults who had harsh family environments as children had higher CRP levels than those from healthy family environments (Taylor, Lehman, Kiefe, & Seeman, 2006). African Americans, but not whites, who experienced early life adversities had higher IL-6, fibrinogen, E-selectin, and sICAM-1 levels than those who did not experience early life adversities (Slopen et al., 2010). Compared to controls, women with childhood abuse-related posttraumatic stress disorder (PTSD) had increased nuclear factor κB (NF-κB) activity (Pace et al., 2012), an intracellular signaling molecule that regulates proinflammatory cytokine gene expression.
The association between early life experiences and elevated inflammation persists among older adults. In a study of healthy older adult family dementia caregivers and noncaregivers (average age of 70), those who had experienced emotional, physical, or sexual abuse as children were more likely to have higher IL-6 and TNF-α levels compared to those who were not abused (Kiecolt-Glaser et al., 2011). Importantly, this association was detectable even among distressed dementia caregivers.
Early adversity is also associated with larger acute stress-induced increases in inflammation. In a study comparing males with a history of early life stress and major depression with controls, major depression patients who experienced early life stress had a more pronounced IL-6 response to an experimental laboratory stress task than controls (Pace et al., 2006). In another study of healthy adults without depression or post-traumatic stress disorder, plasma IL-6 levels were higher in response to an experimental laboratory stressor among those who reported childhood trauma compared with those who did not report childhood trauma (Carpenter et al., 2010). Likewise, in a study of older adult family dementia caregivers and noncaregivers, those with a history of childhood abuse had greater IL-6 responses to naturally occurring daily stressors compared with those without a history of childhood abuse (Gouin, Glaser, Malarkey, Beversdorf, & Kiecolt-Glaser, in press).
In addition to having higher levels of systemic inflammation, chronic stress in childhood may also program a proinflammatory phenotype in monocytes and macrophages. Indeed, newborns exposed to maternal stress in utero had larger ex vivo inflammatory responses to microbial challenge compared with those not exposed to maternal stress (Wright, 2010). Teenagers who were raised in harsh family environments as children had progressively larger ex vivo inflammatory cytokine responses to lipopolysaccharide (LPS) stimulation than those from supportive family environments (Miller & Chen, 2010). In adulthood, those who were raised in lower SES environments as children also displayed larger ex vivo cytokine responses to microbial challenges (Miller et al., 2009).
Maternal warmth may buffer some of the negative consequences of growing up in a low SES environment. Adults who were disadvantaged (low SES) early in life but had mothers who were more warm and caring exhibited less Toll-like receptor-stimulated production of IL-6 compared with those who were in lower SES environments as children but did not experience maternal warmth (Chen, Miller, Kobor, & Cole, 2010).
2.2 Telomeres
Early adversity may also facilitate cell aging by reducing telomere length. Elevated inflammation can activate T-cell proliferation, which shortens telomeres (Aviv, 2004; Kiecolt-Glaser & Glaser, 2010). Shorter telomeres have been linked to mortality (Epel et al., 2004).
Those who were institutionalized for a greater length of time as young children had significantly shorter telomere length in middle childhood compared with those who were institutionalized for less time (Drury et al., 2011). Likewise, young adults who were maltreated as children had shorter telomeres in peripheral blood mononuclear cells than those who reported no maltreatment (Tyrka et al., 2010). Furthermore, adults who reported more childhood adverse life events had shorter telomeres than those who reported fewer childhood adversities (Kananen et al., 2010). Reported exposure to childhood trauma was also associated with shortened telomeres among those with and without posttraumatic stress disorder (PTSD) (O’Donovan et al., 2011). Indeed, childhood trauma accounted for the PTSD group difference such that only participants with PTSD and exposure to multiple categories of childhood trauma had significantly shorter telomeres than control subjects.
This association between early adversity and telomere length can even be detected among older adults who are experiencing considerable current stress. In a study of healthy older adult family dementia caregivers and noncaregivers, those who experienced emotional, physical, or sexual abuse as children had shorter telomeres compared with those who were not abused (Kiecolt-Glaser et al., 2011). Accordingly, early life stress has been linked to shorter telomeres in a several different populations.
2.3 Latent herpesvirus reactivation
Research on latent herpesviruses has provided evidence that stressful early life events can dysregulate cellular immune function. Herpesviruses are able to evade destruction by the immune system; after people are infected with a herpesvirus, they carry the virus(es) with them in a latent state for the rest of their lives. Under normal circumstances, these viruses produce asymptomatic infections. However, reactivation of the latent virus may occur when cellular immunity is compromised. With a less competent cellular immune response, herpesviruses can replicate more readily, as reflected by increased antibody titers to the virus. Psychological stress and depression can also dysregulate cellular immunity, and enhance herpesvirus reactivation (Stowe et al., 2010).
Stressful events early in life can promote herpesvirus reactivation in children and adolescents. Adolescents who were abused or institutionalized had higher antibody titers to herpes simplex virus type-1 (HSV-1), reflecting poorer cellular immune system control over the latent virus, compared to individuals who were not abused or institutionalized (Shirtcliff, Coe, & Pollak, 2009). Likewise, adolescent girls who experienced traumatic life events had elevated Epstein-Barr virus (EBV) antibody titers compared with girls who did not experience trauma (McDade et al., 2000). Furthermore, children and adolescents growing up in poverty had elevated cytomegalovirus (CMV) antibody titers compared with those from higher income families (Dowd, Palermo, & Aiello, 2011).
Early vulnerabilities also have lasting consequences for latent herpesvirus reactivation. In a study of 108 breast cancer survivors, those who experienced more childhood adversities had higher EBV and CMV antibody titers than those with fewer childhood adversities (Fagundes, Glaser, Malarkey, & Kiecolt-Glaser, in press). Indeed, these associations were not explained by health behaviors, suggesting that those who experienced major stressors early in life are more vulnerable to immune dysregulation in adulthood independent of their subsequent health behaviors. Accordingly, stressful early life events leave both children and adults more vulnerable to latent herpesvirus reactivation.
2.4 Tumor environment
Work from our lab has provided evidence that early life stress may impact the immunogenic tumor environment. A minority of cancers are immunogenic, meaning the immune system plays a prominent role in their appearance and progression (Fagundes, Glaser, Johnson, et al., in press). Basal cell carcinoma (BCC), the most common form of skin cancer, is highly immunogenic (Fagundes, Glaser, Johnson, et al., in press). Among BCC patients who experienced a severe stressor in the past year, those who were emotionally maltreated by their mothers or fathers as children were more likely to have poorer immune responses as reflected by lower levels of mRNA for CD25, CD3e, ICAM-1, and CD68 to their BCC tumors (Fagundes, Glaser, Johnson, et al., in press). Because few tumor types are immunogenic, caution is warranted when considering these results in the context of cancer more broadly. There are many different causes and types of cancer, and some are more affected by developmental processes, environmental exposure, and immune dysregulation than others.
3. Mechanisms
In sum, severe early life stress may have lasting long-term consequences for immune dysregulation. There are likely many different mechanisms that underlie these associations. Adults who experienced severe stressors as children are more likely to develop poorer health habits, including poorer sleep patterns and nutrition, less exercise, and greater alcohol and cigarette use (Miller et al., 2011). The effects appear to remain significant even after controlling for health behaviors (Miller et al., 2011).
According to biological embedding models of childhood adversity, early life stress during sensitive periods may dysregulate the development of certain physiological systems (Miller et al., 2011). Therefore, those who experienced major stressors early in life may be more vulnerable to immune dysregulation in adulthood regardless of their subsequent health behaviors. Drawing upon insights from this perspective, Miller and colleagues (Miller et al., 2011) developed an excellent model to help explain why those who experienced early life stress have an exaggerated cytokine response to microbial challenge. They argue that early life stress (a) programs monocytes/macrophages to mount an excessive inflammatory response to microbial stimulation, and (b) promotes resistance to inhibitory mechanisms designed to dampen inflammation (primarily by making immune cells insensitive to the anti-inflammatory effects of cortisol). This model considerably advances our understanding of how early life stress leads to a heightened inflammatory state, especially as a result of microbial challenge. Nevertheless, it remains unclear how early adversity impacts other aspects of immune dysregulation.
Building upon theoretical work from developmental psychology, stress physiology, and psychoneuroimmunology, we propose that early adversity leads to lasting changes in immune function that are enhanced in the context of more recent life stressors because of greater stress sensitivity. Those who experience severe early life stress show enhanced emotional and physiological stress sensitivity to subsequent stress (Dougherty, Klein, & Davila, 2004). They also have fewer social and psychological resources to manage stress (Fagundes, Bennett, Derry, & Kiecolt-Glaser, 2011). As a result, those who experienced early life stressors may exhibit greater immune dysregulation when a stressor occurs in adulthood (see figure 1). We outline evidence for this model below.
Figure 1.
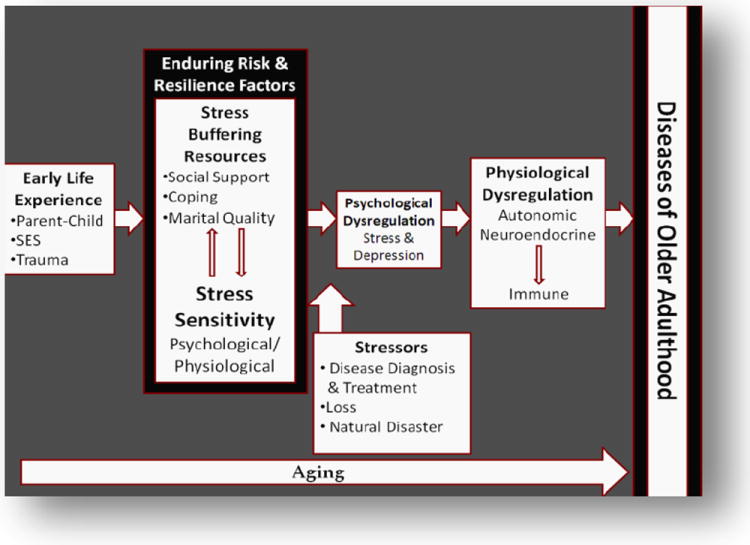
Model depicting how early adversity leads to adult immune dysregulation. The basic premise of this model is that those who experienced early adversities show enhanced stress sensitivity, and have fewer social and psychological resources available to help them cope with stress in adulthood.
3.1 Early adversity and psychological stress sensitivity
There is a clear link between early adversity and increased psychological vulnerability to stressful life events in adulthood. For example, women who had a history of childhood sexual abuse were more likely to suffer from depression after experiencing a severe stressful life event in adulthood (Dougherty et al., 2004). Furthermore, those who were abused as children or come from low SES backgrounds are more likely to perceive ambiguous situations as more threatening (Miller et al., 2011). As a result, these individuals experience stressors more frequently and intensely than others.
3.2 Early adversity and physiological stress sensitivity
Severe early life stress has also been associated with physiological stress sensitivity. Childhood maltreatment disrupts neurobiological development and thus alters the brain’s response to stress (Heim et al., 2000). As a result, childhood abuse has been linked to more pronounced stress-induced HPA activity in adulthood (Heim et al., 2000). Although cortisol acutely inhibits inflammation, chronically high cortisol levels can sometimes lead to glucocorticoid insensitivity. Glucocorticoid insensitivity allows immune cells to produce proinflammatory cytokines in an unregulated environment, thus raising inflammation (Miller et al., 2011).
Childhood abuse has also been linked to more pronounced stress-induced autonomic activity (Heim et al., 2000). The autonomic stress response is typically characterized by enhanced sympathetic activity and dampened parasympathetic activity, consistent with alterations in inflammation. Norepinephrine enhances the production of proinflammatory cytokines (Bierhaus et al., 2003). On the other hand, higher parasympathetic activity results in lower levels of inflammation via the cholinergic anti-inflammatory pathway that facilitates acetylcholine release (Tracey, 2009). Catecholamines also have other effects on a variety of immune responses (Glaser & Kiecolt-Glaser, 2005).
3.3 Early adversity and stress-buffering resources
In addition to being more psychologically and physiologically sensitive to adult stress, those who experienced early adversities also have fewer social and psychological resources to rely upon in order to help them cope with stress (Fagundes et al., 2011). Considerable evidence suggests that social support can help reduce stress-induced immune dysregulation. People who believe others will provide necessary resources for them are better able to cope with stressful situations compared with their counterparts who do not hold these beliefs (Fagundes et al., 2011). However, those who were abused or neglected as children report receiving less social support as adults (Fagundes et al., 2011). Children who come from highly distressed households are less likely to develop social and emotional skills that are crucial for establishing supportive close relationships in adulthood (Fagundes et al., 2011). Compared to people with positive early relationship experiences, those who were abused or neglected are more likely to report receiving less social support later in life (Fagundes et al., 2011).
Early adversity also shapes adults’ characteristic style of coping with stress (Fagundes et al., 2011); those who were maltreated as children are more likely to use maladaptive strategies such as avoidance, wishful thinking, and social withdrawal (Hyman, Paliwal, & Sinha, 2007). These strategies have all been linked to elevated stress (Hyman et al., 2007). Accordingly, when a stressor occurs, those who experienced early life adversities use fewer adaptive coping strategies to minimize the negative psychological and physiological consequences of the event.
3.4 Epigenetics
Epigenetics offers another possible mechanism linking early adversity to immune dysregulation. Epigenetics refers to modifications in DNA and its associated histone proteins that help regulate gene transcription (Jones & Takai, 2001). DNA methylation is the most likely epigenetic mechanism underlying gene-environment interactions, and may underlie many of the associations between adverse early life events and subsequent mental and physical health problems (Crews, 2010; McGowan et al., 2009).
Early adversity may alter the expression and DNA methylation of certain genes linked to adult immune dysregulation. For example, methylation of the exon 1F NR3C1 promoter was higher, and hippocampal NR3C1 gene expression was lower in male suicide victims with troubled childhood relationships compared to controls (McGowan et al., 2009). Lower NR3C1 gene expression has been linked to more pronounced HPA axis activity (Ridder et al., 2005). Adolescents who were raised in lower SES environments as children had higher levels of Toll-like receptor 4 (TLR4) mRNA compared to their higher SES counterparts (Miller & Chen, 2007). Those with higher levels of TLR4 mRNA are more likely to have excessive inflammation compared to those with lower levels.
4. Future Directions
In sum, our model suggests that those who experienced severe early life events are at greater risk for immune dysregulation compared with those who did not experience such adversities because they (a) are more psychologically and physiological sensitive to stress, and (b) have fewer social and psychological resources available to help them cope with stress. Accordingly, they exhibit greater immune dysregulation. Although evidence supports different aspects of this model, more research is clearly needed.
Our model’s primary premise is that early adversity leads to greater stress sensitivity, which in turn puts people at greater risk for immune dysregulation. There are two ways to test this component of the model. First, researchers can present individuals with an acute stressor, and evaluate differential stress-induced physiological changes. Those who experienced early adversity show greater increases in inflammation and greater stress-induced autonomic and HPA activation to laboratory stressors (Carpenter et al., 2010; Heim et al., 2000). Second, researchers can evaluate whether those who experienced early adversity show more pronounced immune dysregulation when confronted with a stressful life event. This is particularly important to understand if this model applies to more chronic stressors. Because severe chronic stressors have long-lasting influences on people’s physiology, this approach has particular promise for understanding how early adversity impacts physical health (Miller et al., 2011).
Presumably, early adversity is associated with more pronounced immune dysregulation because of greater stress-induced autonomic and neuroendocrine activation. However, there are no studies in humans to show that the association between early adversity and any marker of immune dysregulation exists because of more pronounced autonomic stress and/or neuroendocrine activity. Evaluating this pathway by assessing autonomic, neuroendocrine, and immune markers simultaneously is an important direction for future research. Although this review is focused on human work, we note that animal models allow for more fine grained mechanistic explanations of these processes.
The model also suggests that those who experienced early adversities are less likely to benefit from supportive relationships in adulthood. However, there is not substantial data to support this aspect of the model. There is clearly an association between early adversity and less self-reported social support (Fagundes et al., 2011). However, there are no studies to date that have actually demonstrated that those who experienced early adversities show less physiological benefits from supportive individuals compared with those who did not experience early adversities.
Up to this point, researchers have characterized all adult stressors similarly. However, those who experienced early adversities may find certain events more stressful than others. For example, relational conflicts in adulthood may be particularly stressful for those who experienced more relational adversities as children. In future work, it would be interesting to see if different types of stressors elicit different physiological responses.
Research linking early adversity to immune dysregulation has not substantively considered whether the timing of adverse early events moderates the immunological implications of these experiences. It stands to reason that the body’s stress-regulatory systems are more vulnerable to permanent dysregulation during certain developmental periods than others. Future work should investigate if there are specific developmental periods where adversities have the greatest consequence for subsequent long-term immune dysregulation.
Finally, future work should explore novel treatment options for those exposed to early life adversity. Selective serotonin reuptake inhibitors, a class of drugs typically used to treat depression and anxiety, can inhibit the production of IL-6 (Vogelzangs et al., 2012). Cognitive behavioral therapy, a well-established treatment for depression, may also reduce inflammation. These treatments may be particularly beneficial for those who experienced early adversity.
In sum, there is strong evidence that adverse early experiences make people more vulnerable to immune dysregulation in adulthood. However, we are only beginning to understand the pathways by which this phenomenon occurs in humans. More research is needed to understand why the consequences of early adversity persist across the lifespan.
Acknowledgments
Work on this paper was supported in part by NIH grants CA131029, CA126857, the Kathryn & Gilbert Mitchell endowment, the S. Robert Davis endowment, and an American Cancer Society Postdoctoral Fellowship Grant PF-11-007-01-CPPB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimersthat apply to the journal pertain.
Contributor Information
Christopher P. Fagundes, The Institute for Behavioral Medicine Research, The Ohio State University College of Medicine, Comprehensive Cancer Center
Ronald Glaser, The Institute for Behavioral Medicine Research and Department of Molecular Virology, Immunology, and Medical Genetics, The Ohio State University College of Medicine, Comprehensive Cancer Center.
Janice K. Kiecolt-Glaser, The Institute for Behavioral Medicine Research, Comprehensive Cancer Center, and Department of Psychiatry, The Ohio State University College of Medicine
References
- Aviv A. Telomeres and human aging: Facts and fibs. Science of Aging, Knowledge, and Environment. 2004;51:43. doi: 10.1126/sageke.2004.51.pe43. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller G, Kobor M, Cole S. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2010;16(7):729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D. Epigenetics, brain, behavior, and the environment. Hormones. 2010;9(1):41–50. doi: 10.14310/horm.2002.1251. [DOI] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, et al. Biological embedding of stress through inflammation processes in childhood. Molecular Psychiatry. 2010;16(3):244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Davila J. A growth curve analysis of the course of dysthymic disorder: The effects of chronic stress and moderation by adverse parent-child relationships and family history. Journal of Consulting and Clinical Psychology. 2004;72(6):1012. doi: 10.1037/0022-006X.72.6.1012. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Palermo TM, Aiello AE. Family poverty is associated with cytomegalovirus antibody titers in US children. Health Psychology. 2011 doi: 10.1037/a0025337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury S, Theall K, Gleason M, Smyke A, De Vivo I, Wong J, et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Molecular Psychiatry. 2011 doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annual Review of Medicine. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Bennett JM, Derry HM, Kiecolt-Glaser JK. Relationships and inflammation across the lifespan: Social developmental pathways to disease. Social and Personality Psychology Compass. 2011;5(11):891–903. doi: 10.1111/j.1751-9004.2011.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Johnson SL, Andridge RR, Di Gregorio MP, Chen M, et al. Basal cell carcinoma: Stressful life events and the tumor environment. Archives of General Psychiatry. doi: 10.1001/archgenpsychiatry.2011.1535. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Child adversity and herpesvirus latency in breast cancer survivors. Health Psychology. doi: 10.1037/a0028595. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Glaser R, Malarkey WB, Beversdorf DQ, Kiecolt-Glaser JK. Childhood abuse and inflammatory responses to daily stressors. Annals of Behavioral Medicine. doi: 10.1007/s12160-012-9386-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Sinha R. Childhood maltreatment, perceived stress, and stress-related coping in recently abstinent cocaine dependent adults. Psychology of Addictive Behaviors. 2007;21(2):233. doi: 10.1037/0893-164X.21.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293(5532):1068. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- Kananen L, Surakka I, Pirkola S, Suvisaari J, Lönnqvist J, Peltonen L, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. Plos One. 2010;5(5):e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J, Gouin J, Weng N, Malarkey W, Beversdorf D, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine. 2011;73(1):16. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Psychological stress, telomeres, and telomerase. Brain, Behavior, and Immunity. 2010;24(4):529–530. doi: 10.1016/j.bbi.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutrition Reviews. 2007;65(12):S140–S146. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- McDade TW, Stallings JF, Angold A, Costello EJ, Burleson M, Cacioppo JT, et al. Epstein-Barr virus antibodies in whole blood spots: a minimally invasive method for assessing an aspect of cell-mediated immunity. Psychosomatic Medicine. 2000;62(4):560–568. doi: 10.1097/00006842-200007000-00015. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, CD’Alessio A, Dymov S, Labonté B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature neuroscience. 2009;12(3):342. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosomatic Medicine. 2007;69(5):402. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science. 2010;21(6):848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011 doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, et al. Childhood Trauma Associated with Short Leukocyte Telomere Length in Posttraumatic Stress Disorder. Biological Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry. 2006;163(9):1630–1632. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain, Behavior, and Immunity. 2012;26:13–17. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, et al. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. The Journal of Neuroscience. 2005;25(26):6243. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proceedings of the National Academy of Sciences. 2009;106(8):2963–2967. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, et al. Early life adversity and inflammation in African Americans and Whites in the midlife in the united states survey. Psychosomatic Medicine. 2010;72(7):694–701. doi: 10.1097/PSY.0b013e3181e9c16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe RP, Peek MK, Perez NA, Yetman DL, Cutchin MP, Goodwin JS. Herpesvirus reactivation and socioeconomic position: a community-based study. Journal of Epidemiology and Community Health. 2010;64(8):666–671. doi: 10.1136/jech.2008.078808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Lehman B, Kiefe C, Seeman T. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry. 2006;60(8):819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nature Reviews Immunology. 2009;9(6):418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biological Psychiatry. 2010;67(6):531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, Duivis H, Beekman A, Kluft C, Neuteboom J, Hoogendijk W, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Translational Psychiatry. 2012;2(2):e79. doi: 10.1038/tp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ. Perinatal stress and early life programming of lung structure and function. Biological Psychology. 2010;84(1):46–56. doi: 10.1016/j.biopsycho.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


