Abstract
Polychlorinated biphenyls (PCBs) are developmental neurotoxicants that produce cognitive and behavioral changes in children exposed during gestation and lactation. Coplanar PCBs bind the aryl hydrocarbon receptor (AHR) and can be sequestered in liver by cytochrome P450 1A2 (CYP1A2). The AHR is a ligand-activated transcription factor which increases expression of the CYP1 family, including CYP1A2. Our previous work examining genetic susceptibility to developmental PCB neurotoxicity showed that AhrbCyp1a2(−/−) mice with the high-affinity Ahrb allele and lacking CYP1A2 were most susceptible while AhrbCyp1a2(+/+) and poor-affinity AhrdCyp1a2(+/+) mice were resistant. To follow up, a fourth line of mice was generated with the AhrdCyp1a2(−/−) genotype and compared with the background strain AhrbCyp1a2(+/+). Dams received a PCB mixture or the corn oil vehicle at gestational day 10 (GD10) and postnatal day 5 (PND5). Offspring were tested at PND60 in open field locomotor, acoustic startle with pre-pulse inhibition (PPI), novel object recognition and Morris water maze. Locomotor activity was increased in PCB-treated AhrbCyp1a2(+/+) mice, but no differences were seen in control v. PCB-treated AhrdCyp1a2(−/−) mice. PCB-treated AhrdCyp1a2(−/−) mice had a higher baseline startle response and significantly reduced pre-pulse inhibition at the 74dB level compared with corn oil-treated controls (P<0.05). PCB-treated AhrdCyp1a2(−/−) mice had impairments in novel objective recognition (P<0.05) and during all three hidden platform phases of Morris water maze (P<0.01). Combined with our previous findings, these results indicate Cyp1a2 genotype is more important in susceptibility to PCB-induced deficits in learning and memory, but Ahr genotype appears more important when assessing acoustic startle-PPI and locomotor activity.
Keywords: polychlorinated biphenyls, PCBs, CYP1A2, aryl hydrocarbon receptor, developmental neurotoxicity
1. Introduction
Polychlorinated biphenyls are well known developmental neurotoxicants which occur in the environment in complex mixtures. Coplanar PCBs bind the aryl hydrocarbon receptor (AHR) (Denison and Nagy, 2003) whereas non-coplanar PCB toxicity is mediated primarily through the ryanodine receptor (reviewed in Pessah et al., 2010). Children exposed during early brain development are at higher risk of cognitive and behavioral changes which can persist into school age (Grandjean et al., 2001; Jacobson and Jacobson, 2003; Schantz et al., 2003). Although PCBs have been banned for decades, they resist degradation and bioaccumulate and biomagnify in food chains. The highest human exposures are through consumption of contaminated seafood and breast milk (Cok et al., 2012; Gomara et al., 2005; Someya et al., 2011; Todaka et al., 2011). Exposures have decreased in recent years (WHO, 2003); however, recent projections indicate PCBs will remain a problem for generations because highly exposed cohorts are now reaching reproductive age (Quinn et al., 2011).
Genetic differences in xenobiotic metabolizing enzymes could also leave a subset of the population at risk despite lower environmental PCB levels. Human studies clearly show differential responses to PCBs and other pollutants that bind the AHR (Novotna et al., 2007; Tsuchiya et al., 2003; van Duursen et al., 2005). In humans, there is a greater than 12-fold difference in AHR inducibility, although the polymorphism responsible has not been identified (Nebert et al., 2004).The level of CYP1A2 found in human livers varies about 60-fold (Nebert et al., 2006). In mice, maternal CYP1A2 can sequester planar pollutants, preventing their transfer to offspring and protecting against adverse health effects (Curran et al., 2011a; Dragin et al., 2006).
We modeled human genetic differences using mice with allelic variations at the Ahr and Cyp1a2 loci to better understand genetic susceptibility to PCB developmental neurotoxicity. We found AhrbCyp1a2(−/−) mice with high-affinity for binding co-planar PCBs but lacking CYP1A2 enzyme were more susceptible to learning and memory deficits compared with PCB-treated AhrbCyp1a2(+/+) wild type mice and poor-affinity AhrdCyp1a2(+/+) mice (Curran et al., 2011b).
Our prior pharmacokinetic studies using an environmentally relevant mixture of PCBs demonstrated that fetuses and pups exposed during gestation and lactation were differentially affected, depending on their Ahr and Cyp genotypes (Curran et al., 2011a). The highest exposures were found in AhrbCyp1a2(−/−) and AhrdCyp1a2(−/−) mice lacking a functioning CYP1A2 enzyme. These mice also had significantly lower birth weights, but only AhrbCyp1a2(−/−) had significantly retarded growth rates through weaning, and only high-affinity AhrbCyp1a2(−/−) mice had upregulation of CYP1A1 in pup brains. These data suggest that Ahr and Cyp genotypes both impact genetic susceptibility to developmental PCB exposures, but the effect of AHR activation in the developing brain remains unknown. To understand the relative importance of the two genes in developmental neurotoxicity, we conducted behavioral phenotyping on AhrdCyp1a2(−/−) mice with the poor affinity AHR, comparing them to the background strain C57Bl/6J (B6) mice with the AhrbCyp1a2(+/+) genotype.
2. Materials and Methods
2.1 Animals
C57BL/6J mice (B6) with the AhrbCyp1a2(+/+) genotype were purchased from The Jackson Laboratory (Bar Harbor, ME). AhrdCyp1a2(−/−) mice were generated at the University of Cincinnati by crossing AhrbCyp1a2(−/−) mice (Liang et al., 1996) with B6.D2-Ahrd mice (The Jackson Laboratory). This B6.D2-Ahrd line is congenic to the B6 strain, but carries the poor affinity Ahrd allele from the DBA2/J strain, and the AhrbCyp1a2(−/−) mice were back-crossed at least 8 generations onto a B6 background. This means the mice being compared in this experiment were more than 99% genetically identical, allowing us to assess the relative importance of the Ahr and Cyp1a2 genotypes in developmental PCB neurotoxicity.
Mice were housed in standard polysulfone shoebox cages with lab chow (Purina 5015) and water provided ad libitum. Virgin females between 3–4 months of age were mated with males of the same genotype and removed from the breeding cage the morning when a vaginal plug was found (GD0.5). Females were housed singly for delivery. Litters were culled or cross-fostered to keep litter size constant at 6 pups per dam. One male and one female pup were randomly selected for behavior studies and weaned at PND28. The littermates were used in other experiments. Offspring selected for behavioral experiments were housed by sex, genotype and treatment group. Behavior experiments began at PND60. All experiments were conducted under protocols approved by the NKU Institutional Animal Care and Use Committee and in full accordance with the Guide for the Care and Use of Laboratory Animals.
2.2 Treatment
Pregnant females were treated with either an environmentally relevant PCB mixture or the corn oil vehicle by gavage (15 ml/kg body wt.) at GD10 and PND5 using methods identical to our previous studies (Curran et al., 2011a, 2011b). The PCB mixture contained 10 mg/kg each of non-coplanar PCB 105, 118, 138, 153 and 180 and a ratio of coplanar PCB 77 (5 mg/kg), 126 (25 µg/kg) and 169 (250 µg/kg) based on their reported concentrations in the human food supply, cord blood and breast milk (De Koning and Karmaus, 2000; Gomara et al., 2005; Needham et al., 2005). Additional details regarding the dosing mixture can be found in Curran et al. (2011a).
2.3 Behavioral phenotyping
One male and one female per litter were used for behavioral testing beginning at PND60. The same animals were used for all experiments described herein with 15–20 litters per group.
2.3.1 Open field locomotor activity
Mice were placed individually into square plexiglass chambers (41 × 41cm) with a 16×16 photobeam array in the X–Y axes and a third row of photobeams (Z) to detect rearing. Movements were recorded on computer by a Photobeam Activity System (San Diego Instruments, San Diego, CA) in 5 min intervals for a 60 min test.
2.3.2 Acoustic startle with pre-pulse inhibition (PPI)
The SR-LAB apparatus (San Diego Instruments, San Diego, CA) was used to assess baseline acoustic startle response to a 120db sound burst (20 msec) and sensorimotor gating using pre-pulses of 74 db or 76 db given 70 msec prior to the 120db startle stimulus. A Latin Squares design with four trial types was repeated three times. The trials were no stimulus, startle only, and the 74db or 76db pre-pulse + startle. The peak response recorded by a piezoelectric sensor was analyzed as well as the percent attenuation under the pre-pulse conditions.
2.3.3 Novel object recognition
Mice were acclimated to white plastic circular arenas (61cm diameter) for 10 min a day for two days followed by two additional days of acclimation with practice objects distinct from those used during the later phases to reduce neophobia. On the fifth day, mice were placed in the arena with two identical familiarization objects and given up to 10 min to explore. The familiarization phase ended when 30 sec of exploration of objects occurred. Exploration was defined as mice oriented toward the object and within 1 cm of the object. One hour later, the mice were presented with a copy of the familiar object and a novel object. The percent time exploring the novel object out of 30 sec total exploration time was compared. All objects were made of sturdy material (glass or ceramic) and heavy enough so that they could not be moved by the mice. Objects were counter-balanced to avoid confounding by object preference.
2.3.4 Morris water maze (MWM)
Four phases of Morris water maze were used, beginning with cued platform (Vorhees and Williams, 2006). Mice were placed in a 122cm diameter pool filled with non-toxic white tempera paint and given 60 sec to find a 10cm diameter escape platform with an orange ball (cue) placed on a 10cm tall pole in the middle of the platform. Mice received 6 trials per day on Day One from fixed start and platform locations and 2 trials per day with random start and platform locations on Days Two-Six. Curtains surrounding the maze were closed for the cued platform phase, but opened for the hidden platform phases. During the hidden phases (acquisition, reversal and shift), mice used distal visual cues to navigate to the platform which was submerged 1 cm under the water. The platform was progressively smaller in diameter (10 cm, 7 cm and 5 cm) and moved to a new location for each week of testing. Each mouse received 4 trials per day (60 sec maximum with a 15sec inter-trial interval) for 6 days followed by a 30 sec probe trial on Day Seven. ANY-maze™ software (Stoelting, Inc.) was used to record the path traveled and the latency to reach the escape platform. The number of times each mouse failed to find the platform was also recorded. More detailed methods were reported previously (Curran et al., 2011b).
2.4 Hazards
PCBs are carcinogenic and highly toxic. All lab personnel were required to use lab coats, gloves and masks when handling chemicals or animals. All toxic chemicals and hazardous wastes were stored and disposed of under the guidance of the NKU Department of Environmental Health and Safety.
2.5 Statistical analysis
Data were analyzed using SAS 9.2 (SAS Institute Inc., Cary, NC) mixed model analyses of variance (ANOVA) with repeated measures for open field locomotor activity and Morris water maze. Swim speed was a covariate for Morris water maze. Data for acoustic startle-PPI and novel object recognition were analyzed using General Linear Model ANOVA and SPSS 17.0 (IBM, Armonk, NY). The main effects of treatment, genotype and sex and their interactions were assessed. When significant differences were found, post-hoc analyses were done using tests that correct for multiple comparisons. Statistical significance was set at P<0.05. All data are presented as means ± the standard error of the mean (SEM) with corrections for speed as a covariate in Morris water maze.
3. Results
3.1 Open field locomotor activity
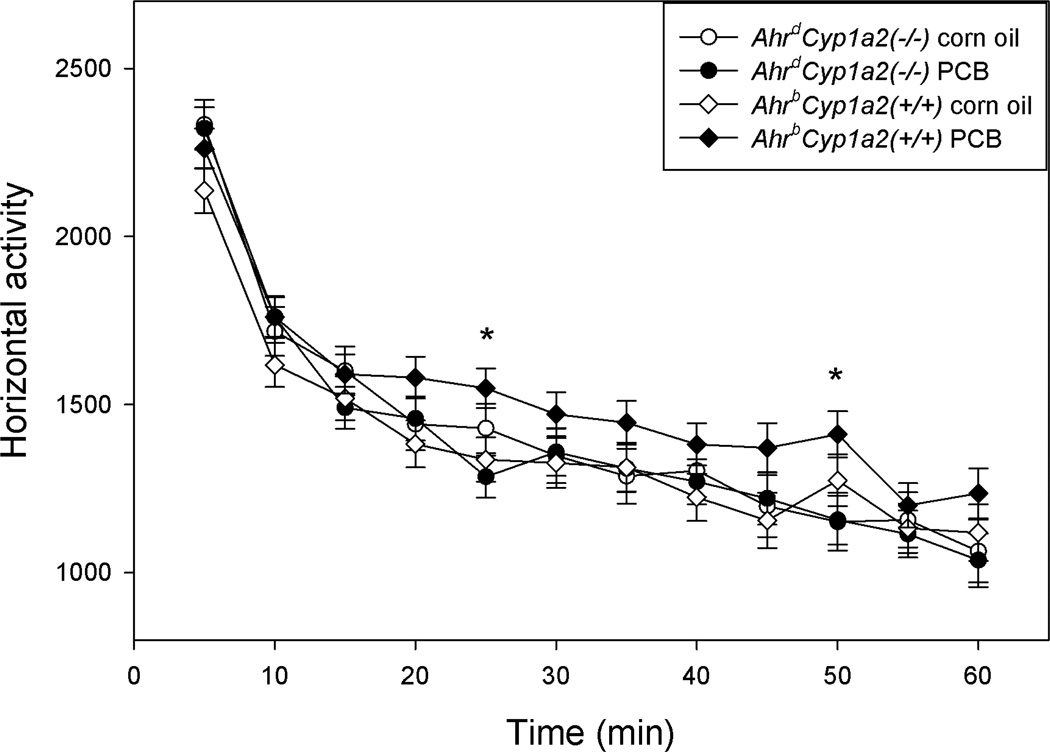
Locomotor activity was increased in PCB-treated AhrbCyp1a2(+/+) mice compared with corn oil-treated control mice of the same strain (F(1,185)=6.41). The differences were only statistically significant (P<0.05) at two time points (Fig. 1). No significant differences were seen in control v. PCB-treated AhrdCyp1a2(−/−) mice; however, male mice were significantly more active than female AhrdCyp1a2(−/−) mice (F(1,185) = 1.82; P<0.05). There were no significant differences in the number of rears (P>0.05). All mice showed the expected habituation with a significant decline in activity over the 60 min test (F(11,185)=119.80; P<0.0001).
Figure 1. Open field locomotor activity.
High-affinity AhrbCyp1a2(+/+) mice exposed to an environmentally relevant mixture of coplanar and non-coplanar congeners during early brain development showed significantly higher activity levels at the 25 and 50 min intervals (P<0.05). All mice showed some level of habituation with significantly reduced activity levels over the course of the 60 min test (P<0.0001). Means ± SEM.
3.2 Acoustic startle with pre-pulse inhibition (PPI)
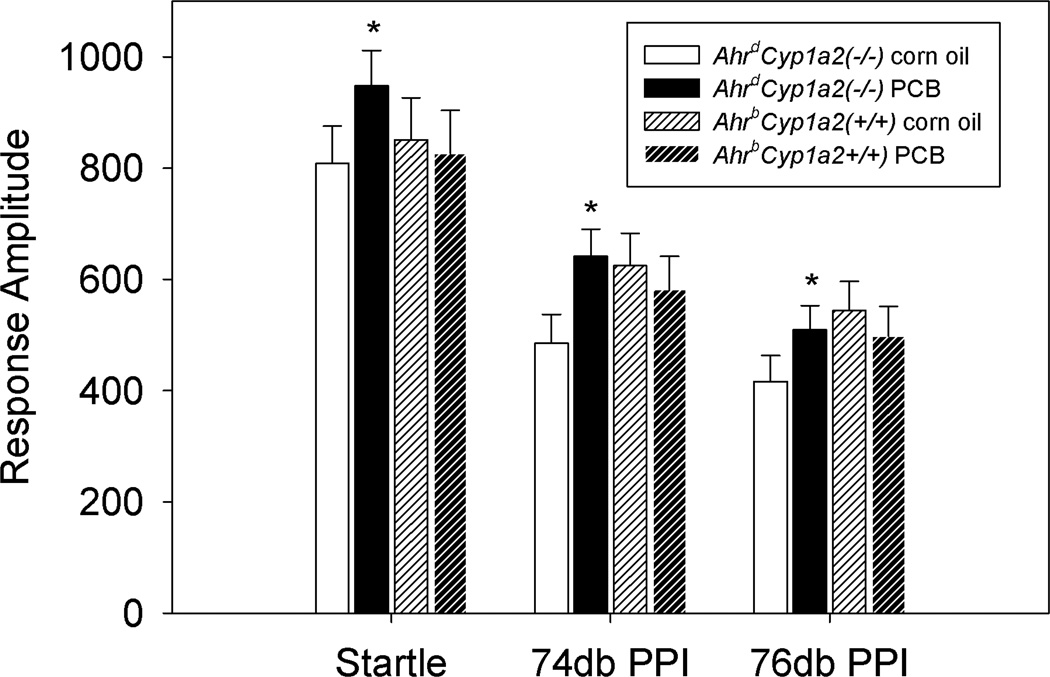
All groups showed normal sensorimotor gating with an attenuated startle response following either the 74db or 76db pre-pulse (Fig.2). PCB-treated AhrdCyp1a2(−/−) mice showed a significantly higher startle response under all three conditions (startle, 74db pre-pulse + startle and 76db pre-pulse + startle) compared with their corn oil-treated controls (P<0.05). There was a trend toward a gene x treatment interaction (P=0.073). There were no significant differences between PCB-treated AhrbCyp1a2(+/+) mice and their corn oil-treated controls.
Figure 2. Acoustic startle with pre-pulse inhibition (PPI).
Poor-affinity AhrdCyp1a2(−/−) mice exposed to PCBs during development had a significantly higher response to the startle response compared to their corn oil-treated controls under all three conditions tested (startle, 74db pre-pulse + startle, and 76db pre-pulse + startle). The decreased response amplitude under the pre-pulse conditions indicates normal sensorimotor gating. Means ± SEM. * P<0.05.
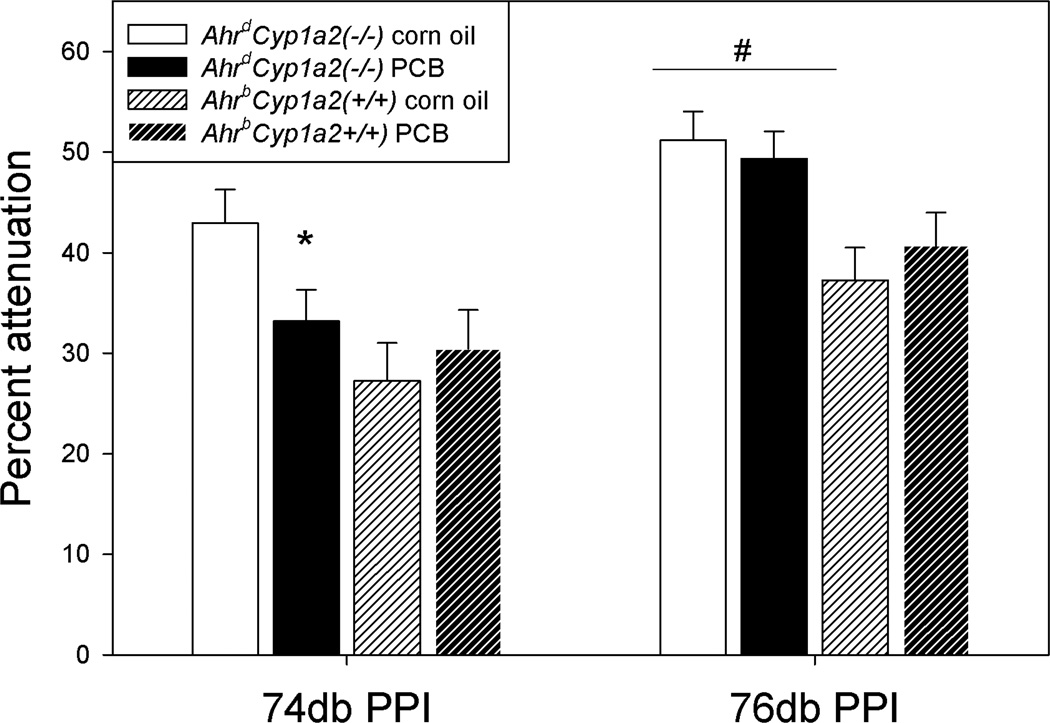
PCB-treated AhrdCyp1a2(−/−) mice had significantly reduced startle attenuation under the 74db pre-pulse condition (P<0.05) compared to their corn oil-treated controls (Fig. 3). There was a significant main effect of genotype under the 76db pre-pulse condition with AhrdCyp1a2(−/−) mice showing greater attenuation compared with AhrbCyp1a2(+/+) mice (P<0.001). There were no significant differences in startle attenuation when comparing PCB-treated AhrbCyp1a2(+/+) mice and their corn oil-treated controls.
Figure 3. Startle attenuation.
The difference in response under pre-pulse conditions was calculated as the percent attenuation from the baseline startle response. PCB-treated AhrdCyp1a2(−/−) mice showed significantly reduced attenuation under the 74db pre-pulse condition compared to their corn-oil treated controls. There was a significant main effect of genotype under the 76db pre-pulse condition, but no treatment effect. Means ± SEM. * P<0.05, # P<0.001.
3.3 Novel object recognition
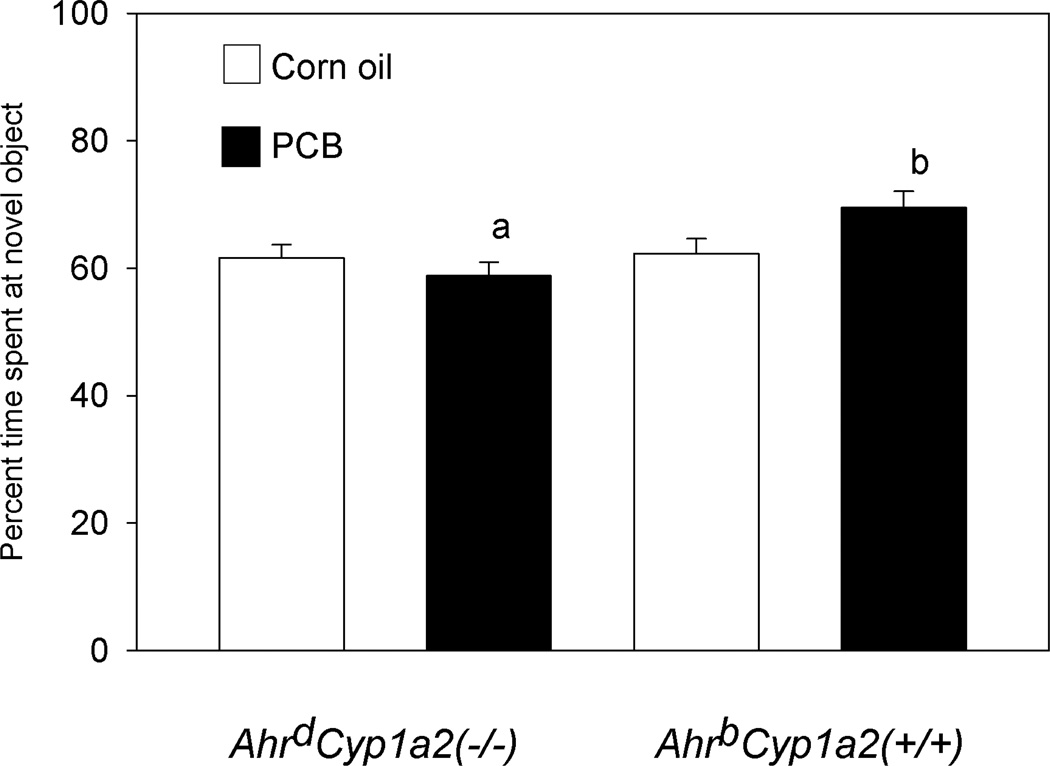
There was a significant gene x treatment interaction in novel object recognition (F(1,94)=4.91; P<0.05) with PCB-treated AhrdCyp1a2(−/−) mice spending less time exploring the novel object while PCB-treated AhrbCyp1a2(+/+) mice spent a greater percentage of time exploring the novel object (Fig. 4). There was also a significant main effect of genotype (F(1,94)=6.26; P<0.05). AhrdCyp1a2(−/−) mice spent 60.2% of the time exploring the novel object compared with 65.9% novel object exploration time by AhrbCyp1a2(+/+) mice. Despite these differences, all groups of mice did spend more time on average exploring the novel object compared with the familiar object.
Figure 4. Novel object recognition.
PCB-treated AhrdCyp1a2(−/−) spent significantly less time exploring the novel object during the test phase compared with PCB-treated AhrbCyp1a2(+/+) mice. There was a significant gene x treatment interaction (P<0.05) and significant main effect of genotype (P<0.05). Means ± SEM. a = different from PCB-treated animals of the other genotype. b = different from all other groups.
3.4 Morris water maze
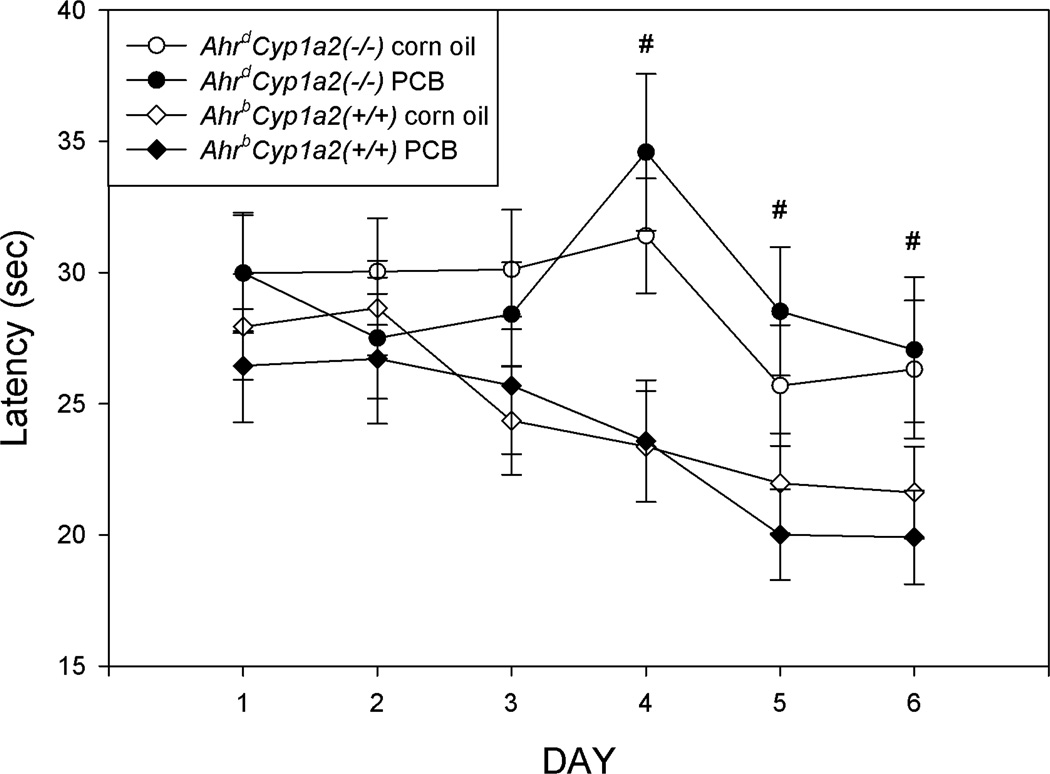
There were no significant differences in the cued platform phase (P>0.05) with all mice successfully learning to navigate to the visibly marked escape platform (data not shown). Therefore, all animals were used in the three hidden platform phases. Data were analyzed for both latency to find the platform and distance traveled. Graphs are only presented for latency (Figs. 5–7), because the trends and significance values were similar for both endpoints. There was a significant main effect of genotype in the acquisition phase for both distance traveled (F(1,118)=8.86; P<0.01) and latency (F(1,118)=11.48; P<0.01) to find the platform. AhrdCyp1a2(−/−) had significantly longer latencies and swam a longer distance to find the platform on the final three days of testing compared to wild-type AhrbCyp1a2(+/+) mice (Fig. 5).
Figure 5. Morris water maze acquisition phase (10cm platform).
There was a significant main effect of genotype on Days 4–6 with poor-affinity AhrdCyp1a2(−/−) mice having longer latencies to reach the escape platform. Means adjusted for speed as a covariate ± SEM. # P<0.01.
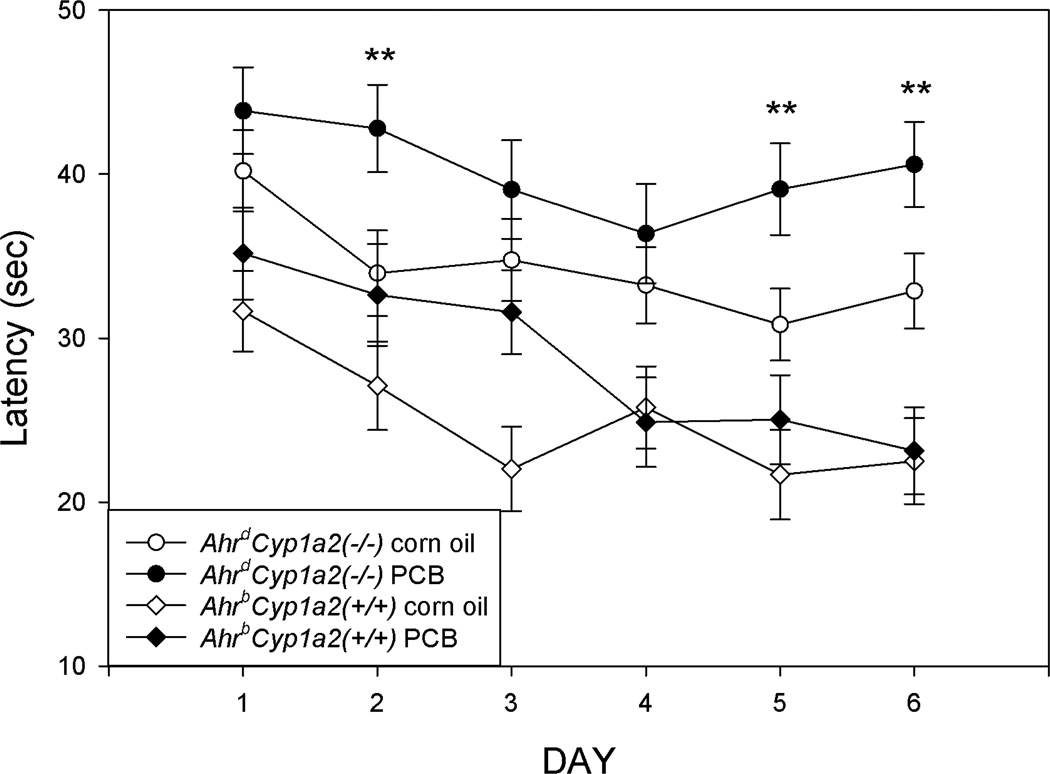
Figure 7. Morris water maze shift phase (5cm platform).
PCB-treated poor-affinity AhrdCyp1a2(−/−) mice had significantly longer latencies to reach the escape platform on Days 2, 5 and 6. There was a significant main effect of genotype with AhrdCyp1a2(−/−) mice having longer latencies compared with AhrbCyp1a2(+/+) wild type mice. Means adjusted for speed as a covariate ± SEM. ** P<0.0001.
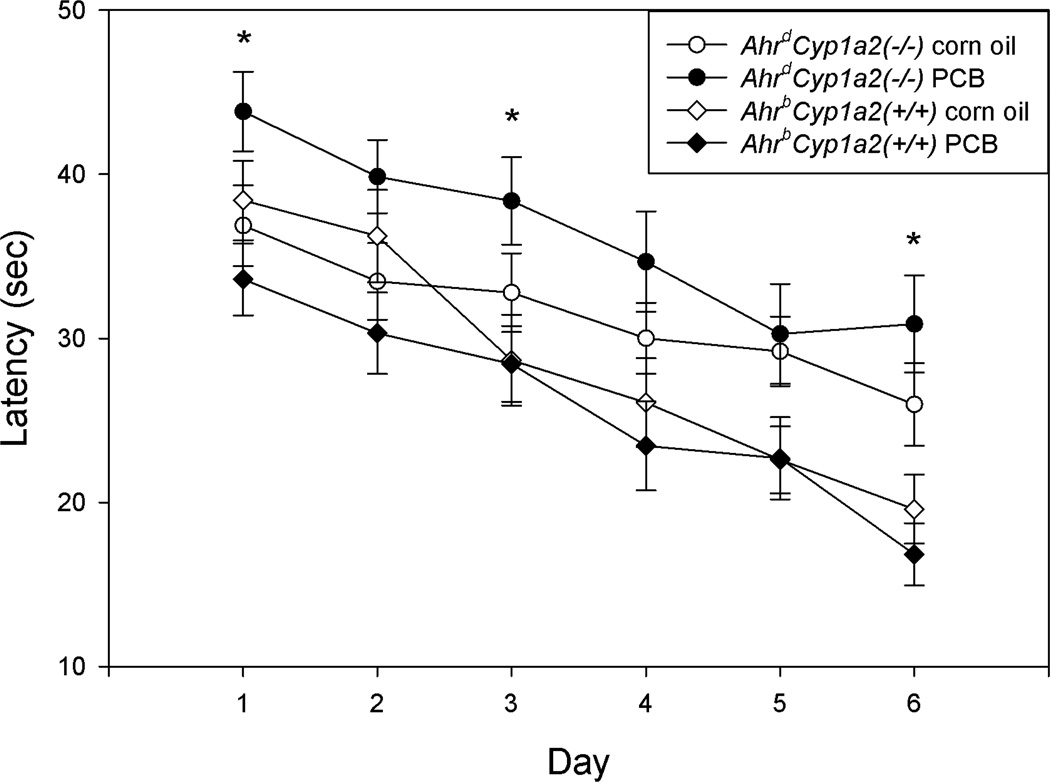
There was a significant gene x treatment interaction during the reversal phase with PCB-treated AhrdCyp1a2(−/−) mice having the longest latencies to reach the platform (F(1,118)=4.59; P<0.05) and a trend for significant gene x treatment interaction (F(1,118)=3.38; P=0.068) for distance traveled (Fig. 6). PCB-treated AhrdCyp1a2(−/−) males performed significantly worse than PCB-treated AhrdCyp1a2(−/−) females (P<0.01). PCB-treated AhrbCyp1a2(+/+) mice had the shortest latencies to find the escape platform on four of the six days of testing, although the differences were not significant compared with corn oil-treated controls.
Figure 6. Morris water maze reversal phase (7cm platform).
PCB-treated poor-affinity AhrdCyp1a2(−/−) mice had significantly longer latencies to reach the escape platform on Days 1, 3 and 6. There was a significant gene x treatment interaction. Means adjusted for speed as a covariate ± SEM. * P<0.05.
The greatest differences were seen in the final phase of hidden platform testing with a 5cm diameter platform (Fig. 7). There was a highly significant main effect of genotype for both latency to find the platform (F(1,118)=29.16; P<0.0001) and distance traveled (F(1,118)=19.32; P<0.0001). There was also a significant main effect of treatment for latency (F(1,118)=6.26; P<0.05) and distance traveled (F(1,118)=4.21; P<0.05). PCB-treated AhrdCyp1a2(−/−) mice took significantly longer to find the escape platform on three of the six test days.
In all three hidden platform phases, PCB-treated AhrdCyp1a2(−/−) mice had the highest number of failures to find the platform in the 60sec time limit (Fig. 8). There was a significant main effect of genotype in the acquisition phase (F(1,118)=10.30; P<0.01) with higher failure rates in both corn oil and PCB-treated AhrdCyp1a2(−/−) mice. In the reversal phase, there was a significant main effect of genotype (F(1,118)=10.99; P<0.01) and a significant gene x treatment interaction (F(1,118)=7.21; P<0.01). Interestingly, PCB-treated AhrdCyp1a2(−/−) mice made more errors than their corn oil-treated controls whereas PCB-treated AhrbCyp1a2(+/+) mice made fewer errors than all other groups. In the most difficult shift phase, both PCB-treated groups had more failures than their respective corn oil-treated controls. There was a significant main effect of genotype (F(1,118)=17.25; P<0.001) and treatment (F(1,118)=7.61; P<0.01), but no significant gene x treatment interaction in the shift phase.
Figure 8. Morris water maze failures.
PCB-treated poor-affinity AhrdCyp1a2(−/−) mice had more failures to find the platform in all three phases of Morris water maze testing.; There was a significant main effect of genotype for the acquisition and shift phases. There was a significant genotype x treatment in the reversal phase with the highest failure rate in PCB-treated AhrdCyp1a2(−/−) mice and the lowest failure rate in PCB-treated AhrbCyp1a2(+/+) wild type mice. Means ± SEM. # P<0.01,** P<0.0001.
4. Discussion
Polychlorinated biphenyls (PCBs) are ubiquitous persistent organic pollutants that produce a multitude of adverse health effects, including learning, memory and behavioral deficits in children exposed during gestation and lactation (Schantz et al., 2003; WHO 2003). Coplanar PCBs bind to the aryl hydrocarbon receptor and initiate transcription of all three members of the CYP1 family, including CYP1A2 which can sequester planar pollutants in liver (Curran et al., 2011a; Diliberto et al., 1997; Dragin et al., 2006). Studies of highly exposed human populations consistently find differences in the response given similar exposures (Novotna et al., 2007; Tsuchiya et al., 2003; van Duursen et al., 2005). This suggests that genetic differences can alter an individual’s susceptibility to PCB toxicity. We developed a mouse model to mimic known human variation in AHR inducibility and basal CYP1A2 to explore genetic susceptibility to developmental neurotoxicity. We used an environmentally relevant mixture of PCBs, because individual PCB congeners produce widely varying results and single-congener studies are less likely to be informative for public health (Ulbrich & Stahlmann, 2004).
In our previous studies using three lines of mice with genetic variation at the Ahr and Cyp1a2(−/−) loci, we found PCB-treated AhrbCyp1a2(−/−) mice with high affinity for coplanar PCBs had significant deficits in learning and memory compared with corn oil-treated controls and PCB-treated AhrbCyp1a2(+/+) and poor-affinity AhrdCyp1a2(+/+) mice (Curran et al., 2011b). Deficits were correlated with increased levels of PCBs in fetal and pup tissues (Curran et al., 2011a). AhrdCyp1a2(−/−) offspring also had higher PCB levels in fetal and pup tissues and experienced significantly higher rates of neonatal loss. Only PCB-treated AhrbCyp1a2(+/+) pups, however, had persistent AHR activation through weaning as evidenced by CYP1A1 upregulation in liver and brain.
We have now extended our behavioral studies to include a fourth line of mice with the poor-affinity AhrdCyp1a2(−/−) genotype. Because all mice had been back-crossed onto a C57Bl/6J (B6) background and are more than 99% genetically identical, we used high-affinity AhrbCyp1a2(+/+) mice as the comparison group. PCB-treated AhrbCyp1a2(+/+) mice showed increased activity in locomotor activity. This is consistent with our previous findings (Curran et al., 2011b) and reports of others testing rodents with a high-affinity AHR (Eriksson & Frederiksson, 1998; Eriksson, 1997).
Although developmental PCB exposure is known to cause auditory deficits in rats (Meerts et al., 2004; Powers et al., 2006), we found no evidence of auditory impairments using acoustic startle with pre-pulse inhibition in any group of mice. All mice responded to the 120db startle stimulus and showed normal sensorimotor gating with an attenuated response to the startle stimulus under both the 74db and 76db pre-pulse conditions. PCB-treated AhrdCyp1a2(−/−) mice did show an enhanced baseline startle response and significantly reduced attenuation compared with their corn oil-treated controls (Figs. 2–3). This is consistent with the pattern reported previously in PCB-treated AhrdCyp1a2(+/+) mice (Curran et al., 2011b).
Human studies have found dose-dependent impairments in visual recognition by infants exposed to PCBs prenatally (Jacobson et al., 1985). We used novel object recognition to look for similar deficits in our mouse lines. PCB-treated AhrdCyp1a2(−/−) mice showed modest impairments in novel object recognition while PCB-treated AhrbCyp1a2(+/+) mice spent more time exploring the novel object than all other groups. Although there was a statistically significant gene x treatment interaction, caution must be used when interpreting these results. The differences were modest, and all groups spent the majority of exploration time at the novel object rather than the familiar object. This suggests all mice successfully remembered the familiar object, regardless of genotype or treatment. In contrast, our previous study showed a sharp reduction in novel object exploration PCB-treated AhrbCyp1a2(−/−) compared to all other groups (Curran et al., 2011b). In sum, the data indicate high-affinity AhrbCyp1a2(−/−) mice are most susceptible to deficits in visual recognition memory.
The Morris water maze is a widely used test of spatial learning and memory with multiple variations (Vorhees and Williams, 2006). Studies using the commercial PCB mixture Aroclor 1254 have produced mixed results in rat models. While Gilbert et al. (2000) reported no deficits in Morris water maze despite deficits in long-term potentiation in the dentate gyrus, others have reported significant deficits in rats exposed prenatally and lactationally to the same Aroclor mixture (Provost et al. 1999; Yang et al. 2009). There were differences in treatment and testing paradigms across these studies which might explain some of the observed differences in results.
We used a four-week water maze paradigm that increased the difficulty of the task over three weeks of hidden platform testing. The advantage of this protocol is its ability to tease out subtle differences in cognitive function (Vorhees and Williams, 2006). We found significant differences in all three hidden platform phases with the greatest differences in the final week of testing. Intriguingly, PCB-treated AhrdCyp1a2(−/−) males showed the greatest impairments in Morris water maze whereas female PCB-treated AhrbCyp1a2(−/−) knockouts showed the greatest deficits in our previous studies (Curran et al. 2011b). Sex differences in exposed human populations and rodent studies have been reported previously, but the most susceptible sex varies by study. Males exposed during development are generally considered at higher risk of adverse health effects (Hertz-Picciotto et al., 2005; Widholdm et al., 2001), but females exposed occupationally appear at higher risk of neurological disorders (Seegal et al., 2011, 2010). Recent rodent studies highlight the importance of testing both male and female offspring for sex-specific effects after developmental PCB exposure (Miller et al., 2010).
PCB-treated AhrbCyp1a2(+/+) mice showed the greatest improvements in acquisition and reversal learning compared to corn oil-treated controls and had significantly fewer failures in the reversal phase. The trends are consistent with our previous findings and studies in rats exposed to AHR agonists during gestation and lactation (Curran et al., 2011b; Seo et al.,1999; Widholm et al. 2003). Across all studies, the data indicate that enhanced AHR activation during early brain development could be beneficial. However, high-affinity Ahr rodents often show higher locomotor activity levels after exposure to coplanar PCBs (Curran et al., 2011b; Erikkson, 1997), so the possibility remains that these effects are more closely related to activity levels rather than actual improvements in spatial learning and memory. This interpretation is supported by the higher failure rate for both PCB-treated groups in the most difficult shift phase (Fig. 8).
Pollutants that bind the AHR have been linked with cognitive deficits in exposed offspring (Dutta et al., 2010; Hermann et al., 2008; McAllister et al., 2008,). Human studies clearly show differential responses to pollutants that bind the AHR (Novotna et al., 2007; Tsuchiya et al., 2003; van Duursen et al., 2005). Simple tests exist to measure induction of the AHR-regulated CYP1A genes (van Duursen et al. 2005) and CYP1A2 enzyme activity (Lambert et al. 2006) in humans. If either the AHR or CYP1A2 gene turns out to be critical in developmental neurotoxicity in humans, the appropriate test could be used to screen populations in the most highly contaminated areas.
5. Conclusion
Our studies represent the first attempt to examine genetic differences in the AHR pathway and developmental PCB neurotoxicity. PCB-treated AhrbCyp1a2(−/−) mice had significant deficits in learning and memory in both novel object recognition and Morris water maze (Curran et al., 2011b). Here, we report similar deficits in AhrdCyp1a2(−/−) mice with the greatest deficits in PCB-treated male knockouts in Morris water maze. Our findings confirm that PCB-treated high-affinity Ahrb mice are more susceptible to behavioral deficits as seen in open field locomotor activity. We also confirmed our previous findings that PCB-treated Ahrd mice have an increased startle response compared with corn oil-treated controls. Therefore, Cyp1a2(−/−) genotype appears most important for endpoints related to learning and memory while Ahr genotype appears most relevant when assessing locomotor activity and startle response. Behavioral and cognitive differences in corn oil-treated mice with allelic differences at the Ahr and Cyp1a2 loci suggest further studies are needed to understand the function of the AHR and CYP1A2 in normal brain development and function. Our studies strongly support ongoing efforts to understand genetic susceptibility to persistent organic pollutants including PCBs. Considering the findings reported here and elsewhere, sex differences in susceptibility should also remain an area of high interest for future studies.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the excellent animal care provided by Amanda Greene, Cory Pfefferman, and Dresden Salatin. The AhrdCyp1a2(−/−) mice were a generous gift from Dr. Daniel W. Nebert, University of Cincinnati Medical Center (Cincinnati, OH, USA).
This project was supported by grants from the National Center for Research Resources (5P20RR016481-12) and the National Institute of General Medical Sciences (8P20GM103436-12) from the National Institutes of Health, the Kentucky NSF Experimental Program to Stimulate Competitive Research (EPSCoR) Research Startup Fund (RSF-034-07), the Northern Kentucky University (NKU) Faculty Development Project Grant program, and the NKU Center for Integrative Natural Science and Mathematics (CINSAM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts to report.
References
- Cok I, Mazmanci B, Mazmanci MA, Turgut C, Henkelmann B, Schramm KW. Analysis of human milk to assess exposure to PAHs, PCBs and organochlorine pesticides in the vicinity Mediterranean city Mersin, Turkey. Environ Int. 2012;40:63–69. doi: 10.1016/j.envint.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Curran CP, Nebert DW, Genter MB, et al. In utero and lactational exposure to PCBs in mice: adult offspring show altered learning and memory depending on Cyp1a2 and Ahr genotypes. Environ Health Perspect. 2011;119(9):1286–1293. doi: 10.1289/ehp.1002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran CP, Vorhees CV, Williams MT, Genter MB, Miller ML, Nebert DW. In utero and lactational exposure to a complex mixture of polychlorinated biphenyls: toxicity in pups dependent on the Cyp1a2 and Ahr genotypes. Toxicol Sci. 2011;119(1):189–208. doi: 10.1093/toxsci/kfq314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKoning EP, Karmaus W. PCB exposure in utero and via breast milk. A review. J Expo Anal Environ Epidemiol. 2000;10(3):285–293. doi: 10.1038/sj.jea.7500090. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Diliberto JJ, Burgin D, Birnbaum LS. Role of CYP1A2 in hepatic sequestration of dioxin: studies using CYP1A2 knock-out mice. Biochem Biophys Res Commun. 1997;236(2):431–433. doi: 10.1006/bbrc.1997.6973. [DOI] [PubMed] [Google Scholar]
- Dragin N, Dalton TP, Miller ML, Shertzer HG, Nebert DW. For dioxin-induced birth defects, mouse or human CYP1A2 in maternal liver protects whereas mouse CYP1A1 and CYP1B1 are inconsequential. J Biol Chem. 2006;281(27):18591–18600. doi: 10.1074/jbc.M601159200. [DOI] [PubMed] [Google Scholar]
- Dutta K, Ghosh D, Nazmi A, Kumawat KL, Basu A. A common carcinogen benzo[a]pyrene causes neuronal death in mouse via microglial activation. PLoS One. 2010;5(4):e9984. doi: 10.1371/journal.pone.0009984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P. Developmental neurotoxicity of environmental agents in the neonate. Neurotoxicology. 1997;18(3):719–726. [PubMed] [Google Scholar]
- Eriksson P, Fredriksson A. Neurotoxic effects in adult mice neonatally exposed to 3,3'4,4'5-pentachlorobiphenyl or 2,3,3'4,4'-pentachlorobiphenyl. Changes in brain nicotinic receptors and behaviour. Environ Toxicol Pharmacol. 1998;5(1):17–27. doi: 10.1016/s1382-6689(97)10002-3. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mundy WR, Crofton KM. Spatial learning and long-term potentiation in the dentate gyrus of the hippocampus in animals developmentally exposed to Aroclor 1254. Toxicol Sci. 2000;57(1):102–111. doi: 10.1093/toxsci/57.1.102. [DOI] [PubMed] [Google Scholar]
- Gomara B, Bordajandi LR, Fernandez MA, et al. Levels and trends of polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (PCBs) in Spanish commercial fish and shellfish products, 1995–2003. J Agric Food Chem. 2005;53(21):8406–8413. doi: 10.1021/jf050835z. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Burse VW, et al. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol Teratol. 2001;23(4):305–317. doi: 10.1016/s0892-0362(01)00155-6. [DOI] [PubMed] [Google Scholar]
- Herrmann M, King K, Weitzman M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Curr Opin Pediatr. 2008;20(2):184–190. doi: 10.1097/MOP.0b013e3282f56165. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Charles MJ, James RA, Keller JA, Willman E, Teplin S. In utero polychlorinated biphenyl exposures in relation to fetal and early childhood growth. Epidemiology. 2005;16(5):648–656. doi: 10.1097/01.ede.0000173043.85834.f3. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Evidence for PCBs as neurodevelopmental toxicants in humans. Neurotoxicology. 1997;18(2):415–424. [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J Pediatr. 2003;143(6):780–788. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, Dowler JK. The effect of intrauterine PCB exposure on visual recognition memory. Child Dev. 1985;56(4):853–860. [PubMed] [Google Scholar]
- Lambert GH, Needham LL, Turner W, Lai TJ, Patterson DG, Jr., Guo YL. Induced CYP1A2 activity as a phenotypic biomarker in humans highly exposed to certain PCBs/PCDFs. Environ Sci Technol. 2006;40(19):6176–6180. doi: 10.1021/es0608646. [DOI] [PubMed] [Google Scholar]
- Liang HC, Li H, McKinnon RA, et al. Cyp1a2(−/−) null mutant mice develop normally but show deficient drug metabolism. Proc Natl Acad Sci U S A. 1996;93(4):1671–1676. doi: 10.1073/pnas.93.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallister MM, Maguire M, Ramesh A, et al. Prenatal exposure to benzo(a)pyrene impairs later-life cortical neuronal function. Neurotoxicology. 2008;29(5):846–854. doi: 10.1016/j.neuro.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts IA, Lilienthal H, Hoving S, et al. Developmental exposure to 4-hydroxy-2,3,3',4',5-pentachlorobiphenyl (4-OH-CB107): long-term effects on brain development, behavior, and brain stem auditory evoked potentials in rats. Toxicol Sci. 2004;82(1):207–218. doi: 10.1093/toxsci/kfh252. [DOI] [PubMed] [Google Scholar]
- Miller VM, Kahnke T, Neu N, et al. Developmental PCB exposure induces hypothyroxinemia and sex-specific effects on cerebellum glial protein levels in rats. Int J Dev Neurosci. 2010;28(7):553–560. doi: 10.1016/j.ijdevneu.2010.07.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signaling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6(12):947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279(23):23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- Needham LL, Barr DB, Caudill SP, et al. Concentrations of environmental chemicals associated with neurodevelopmental effects in U.S. population. Neurotoxicology. 2005;26(4):531–545. doi: 10.1016/j.neuro.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Novotna B, Topinka J, Solansky I, Chvatalova I, Lnenickova Z, Sram RJ. Impact of air pollution and genotype variability on DNA damage in Prague policemen. Toxicol Lett. 2007;172(1–2):37–47. doi: 10.1016/j.toxlet.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Powers BE, Widholm JJ, Lasky RE, Schantz SL. Auditory deficits in rats exposed to an environmental PCB mixture during development. Toxicol Sci. 2006;89(2):415–422. doi: 10.1093/toxsci/kfj051. [DOI] [PubMed] [Google Scholar]
- Provost TL, Juarez de Ku LM, Zender C, Meserve LA. Dose- and age-dependent alterations in choline acetyltransferase (ChAT) activity, learning and memory, and thyroid hormones in 15- and 30-day old rats exposed to 1.25 or 12.5 PPM polychlorinated biphenyl (PCB) beginning at conception. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(5):915–928. doi: 10.1016/s0278-5846(99)00035-4. [DOI] [PubMed] [Google Scholar]
- Quinn CL, Wania F, Czub G, Breivik K. Investigating intergenerational differences in human PCB exposure due to variable emissions and reproductive behaviors. Environ Health Perspect. 2011;119(5):641–646. doi: 10.1289/ehp.1002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect. 2003;111(3):357–376. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, Fitzgerald EF, Hills EA, et al. Estimating the half-lives of PCB congeners in former capacitor workers measured over a 28-year interval. J Expo Sci Environ Epidemiol. 2011;21(3):234–246. doi: 10.1038/jes.2010.3. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Marek KL, Seibyl JP, et al. Occupational exposure to PCBs reduces striatal dopamine transporter densities only in women: a beta-CIT imaging study. Neurobiol Dis. 2010;38(2):219–225. doi: 10.1016/j.nbd.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BW, Sparks AJ, Medora K, Amin S, Schantz SL. Learning and memory in rats gestationally and lactationally exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Neurotoxicol Teratol. 1999;21(3):231–239. doi: 10.1016/s0892-0362(98)00049-x. [DOI] [PubMed] [Google Scholar]
- Someya M, Ohtake M, Kunisue T, et al. Persistent organic pollutants in breast milk of mothers residing around an open dumping site in Kolkata, India: specific dioxin-like PCB levels and fish as a potential source. Environ Int. 2010;36(1):27–35. doi: 10.1016/j.envint.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Todaka T, Hirakawa H, Kajiwara J, et al. Concentrations of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and polychlorinated biphenyls in blood and breast milk collected from pregnant women in Sapporo City, Japan. Chemosphere. 2011;85(11):1694–1700. doi: 10.1016/j.chemosphere.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y, Nakai S, Nakamura K, Hayashi K, Nakanishi J, Yamamoto M. Effects of dietary habits and CYP1A1 polymorphisms on blood dioxin concentrations in Japanese men. Chemosphere. 2003;52(1):213–219. doi: 10.1016/S0045-6535(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Ulbrich B, Stahlmann R. Developmental toxicity of polychlorinated biphenyls (PCBs): a systematic review of experimental data. Arch Toxicol. 2004;78(5):252–268. doi: 10.1007/s00204-003-0519-y. [DOI] [PubMed] [Google Scholar]
- van Duursen MB, Sanderson JT, Van den BM. Cytochrome P450 1A1 and 1B1 in human blood lymphocytes are not suitable as biomarkers of exposure to dioxin-like compounds: polymorphisms and interindividual variation in expression and inducibility. Toxicol Sci. 2005;85(1):703–712. doi: 10.1093/toxsci/kfi089. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL. Spatial reversal learning in Aroclor 1254-exposed rats: sex-specific deficits in associative ability and inhibitory control. Toxicol Appl Pharmacol. 2001;174(2):188–198. doi: 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Seo BW, Strupp BJ, Seegal RF, Schantz SL. Effects of perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on spatial and visual reversal learning in rats. Neurotoxicol Teratol. 2003;25(4):459–471. doi: 10.1016/s0892-0362(03)00014-x. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Polychlorinated Biphenyls: Human Health Aspects. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- Yang D, Kim KH, Phimister A, et al. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect. 2009;117(3):426–435. doi: 10.1289/ehp.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]