Abstract
BACKGROUND
Temporal increases in the consumption of sugar-sweetened beverages have paralleled the rise in obesity prevalence, but whether the intake of such beverages interacts with the genetic predisposition to adiposity is unknown.
METHODS
We analyzed the interaction between genetic predisposition and the intake of sugar-sweetened beverages in relation to body-mass index (BMI; the weight in kilograms divided by the square of the height in meters) and obesity risk in 6934 women from the Nurses’ Health Study (NHS) and in 4423 men from the Health Professionals Follow-up Study (HPFS) and also in a replication cohort of 21,740 women from the Women’s Genome Health Study (WGHS). The genetic-predisposition score was calculated on the basis of 32 BMI-associated loci. The intake of sugar-sweetened beverages was examined prospectively in relation to BMI.
RESULTS
In the NHS and HPFS cohorts, the genetic association with BMI was stronger among participants with higher intake of sugar-sweetened beverages than among those with lower intake. In the combined cohorts, the increases in BMI per increment of 10 risk alleles were 1.00 for an intake of less than one serving per month, 1.12 for one to four servings per month, 1.38 for two to six servings per week, and 1.78 for one or more servings per day (P<0.001 for interaction). For the same categories of intake, the relative risks of incident obesity per increment of 10 risk alleles were 1.19 (95% confidence interval [CI], 0.90 to 1.59), 1.67 (95% CI, 1.28 to 2.16), 1.58 (95% CI, 1.01 to 2.47), and 5.06 (95% CI, 1.66 to 15.5) (P = 0.02 for interaction). In the WGHS cohort, the increases in BMI per increment of 10 risk alleles were 1.39, 1.64, 1.90, and 2.53 across the four categories of intake (P = 0.001 for interaction); the relative risks for incident obesity were 1.40 (95% CI, 1.19 to 1.64), 1.50 (95% CI, 1.16 to 1.93), 1.54 (95% CI, 1.21 to 1.94), and 3.16 (95% CI, 2.03 to 4.92), respectively (P = 0.007 for interaction).
CONCLUSIONS
The genetic association with adiposity appeared to be more pronounced with greater intake of sugar-sweetened beverages.
Obesity has become a major threat to public health throughout the world.1 The dramatic changes in diet and lifestyle during the past three decades are believed to have played a key role in triggering the obesity epidemic.2 In the past several years, large-scale genomewide association studies have successfully identified multiple loci associated with the body-mass index (BMI); these loci consist of commonly distributed variants that determine the overall susceptibility to obesity.3 A meta-analysis of genomewide association studies has established that 32 loci are associated with BMI at a genomewide significance level.4,5 However, few studies have examined the interaction between environmental factors and the genetic predisposition to adiposity.2
During the past 30 years, the consumption of sugar-sweetened beverages has increased dramatically.6 Compelling evidence supports a positive link between the consumption of sugar-sweetened beverages and the risk of obesity.6–11 The temporal patterns in the increasing consumption of these beverages have paralleled the rise in the prevalence of obesity6; in the United States, both the intake of sugar-sweetened beverages and the prevalence of obesity have more than doubled since the late 1970s.12 Therefore, we hypothesized that a high intake of sugar-sweetened beverages would influence the association between the genetic predisposition and adiposity.
To test this hypothesis, we used longitudinal data to examine the interaction between the intake of sugar-sweetened beverages and a genetic-predisposition score, which was calculated on the basis of the 32 loci that have an established association with BMI,5 in relation to BMI and incident obesity in women and men from two prospective cohorts: the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). We replicated the analysis in a large, independent, prospective cohort from the Women’s Genome Health Study (WGHS).
METHODS
STUDY POPULATION
The NHS is a prospective cohort study of 121,700 female registered nurses who were 30 to 55 years old at the inception of the study in 1976.13 The HPFS is a prospective cohort study of 51,529 male health professionals in the United States who were 40 to 75 years old at the inception of the study in 1986.14 The participants completed food-frequency questionnaires every 4 years. For this analysis, we used 1980 as the baseline for the NHS and 1986 as the baseline for the HPFS, when the first dietary data were collected. The current analysis included 6934 initially healthy women and 4423 initially healthy men of European ancestry for whom genotype data based on genomewide association studies were available.15–19
The WGHS is a prospective cohort study of initially healthy U.S. women.20 Study participants were health professionals who were 45 years of age or older and did not have any major chronic diseases during the period from 1992 through 1995. A total of 21,740 women who had confirmed self-reported European ancestry, for whom genotype data and responses to food-frequency questionnaires were available, and who did not have diabetes at baseline were included in the current analysis.
The present study was approved by the local institutional review boards. All participants provided written informed consent for the NHS, HPFS, or WGHS; written informed consent for the present study was not additionally required. An expanded description of the methods is available in the Supplementary Appendix, available with the full text of this article at NEJM.org.
ASSESSMENT OF BEVERAGE INTAKE
We used similar semiquantitative food-frequency questionnaires to assess food and beverage intakes in the three cohorts.21,22 For sugar-sweetened beverages, we included caffeinated colas, caffeine-free colas, carbonated noncola soft drinks, and noncarbonated sugar-sweetened beverages (fruit punches, lemonades, or other fruit drinks). Artificially sweetened beverages included caffeinated, caffeine-free, and noncarbonated low-calorie beverages. The reproducibility and validity of the food-frequency questionnaires have been described elsewhere.21–23
ASSESSMENT OF BMI AND COVARIATES
In the NHS and HPFS, height and body weight were assessed by means of a questionnaire at baseline, and weight was requested on each follow-up questionnaire. Self-reported weights were highly correlated with measured weights (r = 0.97 for men and women).24 The BMI was calculated as the weight in kilograms divided by the square of the height in meters. Participants with a BMI of 30 or higher were classified as obese. Information about lifestyle factors was derived from biennial questionnaires.13,14 The validity of the self-reported height, body weight, and physical-activity data has been described previously.24–26 Diet quality was assessed with the use of the Alternative Healthy Eating Index (with scores ranging from 2.5 to 87.5 and higher scores indicating a healthier diet).27 In the WGHS, weight and physical activity were assessed by means of the baseline and follow-up questionnaires.28,29 Information about medical history and other dietary and lifestyle factors was collected from questionnaires at baseline.28,29
GENOTYPING
We selected 32 single-nucleotide polymorphisms (SNPs) that represent all 32 loci that are known to be associated with BMI.5 SNP genotyping and imputation have been described in detail elsewhere.15–20,28 Most of the SNPs were genotyped or had a high imputation quality score (r2 ≥0.8), as assessed with the use of MACH software, version 1.0.16 (Center for Statistical Genetics, University of Michigan) (Table S1 in the Supplementary Appendix).
GENETIC-PREDISPOSITION SCORE
The genetic-predisposition score was calculated on the basis of the 32 SNPs with the use of a previously reported weighting method; scores range from 0 to 64, with higher scores indicating a higher genetic predisposition to obesity.5 Each SNP was weighted according to its relative effect size (β coefficient). To obtain a more accurate and precise effect size of each SNP on BMI, we used β coefficients derived from a meta-analysis of studies involving a total of approximately 126,000 persons.5 We rescaled the weighted score to reflect the number of risk alleles: each point of the genetic-predisposition score corresponded to one risk allele.
STATISTICAL ANALYSIS
To minimize reverse causality in the NHS and HPFS, we analyzed the data prospectively with the assessment of beverage intake 4 years before the assessment of BMI. Generalized linear models with repeated-measures analysis were applied to estimate the difference in BMI for each increment of 10 risk alleles, stratified according to the four categories of beverage intake (less than one serving per month, one to four servings per month, two to six servings per week, and one or more servings per day). Cox proportional-hazards models were used to estimate the relative risk, per increment of 10 risk alleles, that obesity would develop during follow-up (in 4-year intervals), stratified according to the four categories of intake of sugar-sweetened beverages. Participants who were obese at baseline were excluded from this analysis. The effects of interactions between the genetic-predisposition score and beverage intake on BMI or the risk of obesity were tested by including the respective interaction terms in the models (e.g., sugar-sweetened beverage intake × genetic-predisposition score). The analyses were repeated in the WGHS cohort to replicate the results in the NHS and HPFS cohorts. We used a principal component analysis to calculate the eigenvectors indicating population stratification in the WGHS cohort.20 We also used general linear models to estimate differences in BMI associated with one serving per day of a sugar-sweetened beverage according to the quartiles of the genetic-predisposition score in each cohort. The findings across cohorts were pooled by means of inverse-variance–weighted, fixed-effects meta-analyses. All reported P values are nominal and two-sided. Statistical analyses were performed with the use of SAS software, version 9.1 (SAS Institute), or R software, version 2.13.0 (R Foundation).
RESULTS
BASELINE CHARACTERISTICS OF THE THREE COHORTS
The baseline characteristics of the participants from the three cohorts are shown in Table 1. At baseline, the mean (±SD) intakes of sugar-sweetened beverages were 0.33±0.70, 0.32±0.56, and 0.26±0.57 servings per day in the NHS cohort (in 1980), the HPFS cohort (in 1986), and the WGHS cohort (in 1992), respectively. The baseline intake was positively associated with BMI in all three cohorts (P<0.001, P = 0.007, and P<0.001 in the NHS, HPFS, and WGHS cohorts, respectively). In addition, as compared with participants with a lower intake of sugar-sweetened beverages, those with a higher intake were younger and tended to have lower levels of alcohol consumption, physical activity, and intake of artificially sweetened beverages and a lower score on the Alternative Healthy Eating Index; they also had a higher total energy intake.
Table 1.
Baseline Characteristics of the Participants in the Three Cohorts, According to Intake of Sugar-Sweetened Beverages.*
| Characteristic | Servings of Sugar-Sweetened Beverages | P Value† | |||
|---|---|---|---|---|---|
| <1 per Month | 1–4 per Month | 2–6 per Week | ≥1 per Day | ||
| NHS cohort | |||||
|
| |||||
| Participants — no. | 2843 | 1776 | 1496 | 819 | |
|
| |||||
| Age — yr | 48.7±6.5 | 48.0±6.7 | 46.9±6.9 | 45.8±6.8 | <0.001 |
|
| |||||
| BMI‡ | 24.5±4.6 | 24.5±4.7 | 24.9±4.8 | 25.2±5.7 | <0.001 |
|
| |||||
| Alcohol consumption — g/day | 7.7±11.1 | 5.8±9.9 | 5.5±8.9 | 5.3±9.7 | <0.001 |
|
| |||||
| Current smoking — no. (%) | 722 (25.4) | 361 (20.3) | 316 (21.1) | 248 (30.3) | 0.58 |
|
| |||||
| Physical activity — MET-hr/wk§ | 15.5±18.3 | 13.4±17.8 | 13.1±16.2 | 12.6±17.0 | <0.001 |
|
| |||||
| Time spent watching television — hr/wk | 13.4±12.0 | 13.4±11.5 | 13.5±11.2 | 13.6±12.0 | 0.67 |
|
| |||||
| Total energy intake — kcal/day | 1460±457 | 1580±479 | 1690±475 | 1840±524 | <0.001 |
|
| |||||
| Alternative Healthy Eating Index score¶ | 30.7±9.1 | 28.6±8.7 | 28.2±8.4 | 27.5±8.0 | <0.001 |
|
| |||||
| Artificially sweetened beverages — servings/day | 0.61±0.99 | 0.32±0.71 | 0.26±0.55 | 0.35±0.74 | <0.001 |
|
| |||||
| Genetic-predisposition score|| | 29.3±4.0 | 29.1±4.0 | 29.0±4.0 | 29.1±4.0 | 0.15 |
|
| |||||
| HPFS cohort | |||||
|
| |||||
| Participants — no. | 1627 | 1058 | 1338 | 400 | |
|
| |||||
| Age — yr | 56.4±8.3 | 55.4±8.6 | 53.0±8.6 | 51.4±8.9 | <0.001 |
|
| |||||
| BMI‡ | 25.7±3.4 | 25.7±3.3 | 25.9±3.3 | 26.0±3.3 | 0.07 |
|
| |||||
| Alcohol consumption — g/day | 13.0±16.2 | 11.4±14.7 | 11.3±14.9 | 10.7±18.3 | 0.02 |
|
| |||||
| Current smoking — no. (%) | 132 (8.1) | 97 (9.2) | 124 (9.3) | 51 (12.8) | 0.02 |
|
| |||||
| Physical activity — MET-hr/wk§ | 20.6±28.6 | 19.2±23.8 | 18.8±27.0 | 19.7±27.7 | 0.62 |
|
| |||||
| Time spent watching television — hr/wk | 12.0±9.0 | 11.4±8.5 | 11.3±8.4 | 13.0±9.0 | 0.03 |
|
| |||||
| Total energy intake — kcal/day | 1853±566 | 1967±570 | 2172±600 | 2458±633 | <0.001 |
|
| |||||
| Alternative Healthy Eating Index score¶ | 46.2±11.7 | 44.9±10.7 | 43.4±10.1 | 41.2±10.6 | <0.001 |
|
| |||||
| Artificially sweetened beverages — servings/day | 0.67±1.11 | 0.40±0.78 | 0.35±0.67 | 0.30±0.71 | <0.001 |
|
| |||||
| Genetic-predisposition score|| | 29.3±4.0 | 29.0±4.0 | 29.2±4.0 | 29.0±4.0 | 0.57 |
|
| |||||
| WGHS cohort | |||||
|
| |||||
| Participants — no. | 9759 | 4780 | 5531 | 1670 | |
|
| |||||
| Age — yr | 54.9±7.0 | 55.1±7.4 | 54.3±7.1 | 52.9±6.2 | <0.001 |
|
| |||||
| BMI‡ | 25.8±4.8 | 25.7±4.7 | 25.7±4.9 | 26.1±5.4 | 0.03 |
|
| |||||
| Alcohol consumption — g/day | 5.1±9.5 | 4.0±7.8 | 3.6±7.1 | 3.2±7.5 | <0.001 |
|
| |||||
| Current smoking — no. (%) | 985 (10.1) | 508 (10.6) | 678 (12.3) | 326 (19.5) | <0.001 |
|
| |||||
| Physical activity — MET-hr/wk§ | 16.2±19.4 | 14.4±17.5 | 13.5±17.3 | 12.1±17.1 | <0.001 |
|
| |||||
| Total energy intake — kcal/day | 1613±488 | 1737±505 | 1847±527 | 2050±559 | <0.001 |
|
| |||||
| Artificially sweetened beverages — servings/day | 0.97±1.28 | 0.68±1.02 | 0.55±0.99 | 0.41±1.03 | <0.001 |
|
| |||||
| Genetic-predisposition score|| | 29.3±3.8 | 29.0±3.7 | 28.9±3.7 | 28.6±3.8 | 0.001 |
Plus–minus values are means ±SD. Baseline data were from 6934 women in the Nurses’ Health Study (NHS, 1980), 4423 men in the Health Professionals Follow-up Study (HPFS, 1986), and 21,740 women in the Women’s Genome Health Study (WGHS, 1992). Physical activity was assessed in 1986 for the NHS cohort. Television watching was assessed in 1992 for the NHS cohort and in 1988 for the HPFS cohort.
P values are for the trend across the four categories of intake of sugar-sweetened beverages.
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters.
MET denotes metabolic equivalents.
Scores on the Alternative Healthy Eating Index range from 2.5 to 87.5, with higher scores indicating a healthier diet.27
The genetic-predisposition score ranges from 0 to 64, with higher scores indicating a higher genetic predisposition to obesity.
The genetic-predisposition score ranged from 13 to 43, and the mean scores in the NHS, HPFS, and WGHS cohorts were 29.1±3.9, 29.2±3.9, and 29.1±3.8, respectively. The genetic-predisposition scores showed similar normal distributions across the three cohorts (Fig. S1 in the Supplementary Appendix). As expected, in all cohorts, participants with a higher genetic-predisposition score had a higher BMI (P<0.001 for all cohorts) (Fig. S1 in the Supplementary Appendix).
GENETIC PREDISPOSITION AND BMI ACCORDING TO BEVERAGE INTAKE
In the NHS and HPFS cohorts, the genetic association with BMI was stronger among participants with a higher intake of sugar-sweetened beverages than among those with a lower intake (Table 2). No significant heterogeneity in the interaction effect was observed between the two cohorts (P = 0.75 for heterogeneity). In the pooled results for women and men, the increases in BMI for an increment of 10 risk alleles were 1.00 for an intake of less than one serving per month, 1.20 for one to four servings per month, 1.37 for two to six servings per week, and 1.85 for one or more servings per day (P<0.001 for interaction). Further adjustment for dietary and lifestyle factors and total energy intake did not change the results.
Table 2.
Increase in BMI per Increment of 10 Risk Alleles, According to Intake of Sugar-Sweetened and Artificially Sweetened Beverages in the NHS and HPFS Cohorts.*
| Analysis | Increase in BMI | P Value for Interaction | |||
|---|---|---|---|---|---|
| <1 Serving per Month | 1–4 Servings per Month | 2–6 Servings per Week | ≥1 Serving per Day | ||
| Sugar-sweetened beverages | |||||
|
| |||||
| NHS cohort | |||||
|
| |||||
| Model 1† | 1.17±0.18 | 1.66±0.16 | 1.84±0.23 | 2.12±0.39 | 0.004 |
|
| |||||
| Model 2‡ | 1.18±0.17 | 1.56±0.16 | 1.78±0.22 | 2.03±0.38 | 0.008 |
|
| |||||
| HPFS cohort | |||||
|
| |||||
| Model 1† | 0.80±0.20 | 0.42±0.21 | 1.05±0.19 | 1.59±0.37 | 0.06 |
|
| |||||
| Model 2‡ | 0.77±0.19 | 0.43±0.20 | 1.08±0.19 | 1.54±0.37 | 0.02 |
|
| |||||
| Pooled cohorts§ | |||||
|
| |||||
| Model 1† | 1.00±0.13 | 1.20±0.13 | 1.37±0.15 | 1.85±0.27 | <0.001 |
|
| |||||
| Model 2‡ | 1.00±0.13 | 1.12±0.12 | 1.38±0.14 | 1.78±0.27 | <0.001 |
|
| |||||
| Artificially sweetened beverages | |||||
|
| |||||
| NHS cohort | |||||
|
| |||||
| Model 1† | 1.43±0.20 | 1.37±0.21 | 1.43±0.20 | 1.42±0.29 | 0.88 |
|
| |||||
| Model 2‡ | 1.42±0.18 | 1.25±0.21 | 1.39±0.20 | 1.36±0.27 | 0.91 |
|
| |||||
| HPFS cohort | |||||
|
| |||||
| Model 1† | 0.94±0.18 | 0.78±0.24 | 0.40±0.20 | 1.04±0.31 | 0.63 |
|
| |||||
| Model 2‡ | 0.95±0.18 | 0.76±0.23 | 0.45±0.20 | 0.92±0.29 | 0.46 |
|
| |||||
| Pooled cohorts§ | |||||
|
| |||||
| Model 1† | 1.16±0.13 | 1.11±0.16 | 0.92±0.14 | 1.24±0.21 | 0.83 |
|
| |||||
| Model 2‡ | 1.19±0.13 | 1.03±0.16 | 0.92±0.14 | 1.16±0.20 | 0.64 |
Plus–minus values are β coefficients ±SE. Data were derived from repeated-measures analysis for women in the NHS (five measures during the period from 1980 to 1998) and for men in the HPFS (three measures during the period from 1986 to 1998). Data on beverage intake were assessed 4 years before the assessment of BMI.
Data were adjusted for age and source of genotyping data.
Data were further adjusted for level of physical activity, time spent watching television, status with respect to current smoking, alcohol intake, Alternative Healthy Eating Index score, and total energy intake.
Results for the two cohorts were pooled by means of inverse-variance–weighted, fixed-effects meta-analyses.
Considering the strong effect of the FTO (the fat-mass and obesity–related gene) loci on BMI, we performed a sensitivity analysis that excluded the FTO genetic variant in the calculation of the genetic-predisposition score, and the results were similar (P<0.001 for interaction in the pooled data). In addition, a sensitivity analysis was conducted with the use of follow-up data up to 2006 from the NHS and HPFS cohorts, which yielded a similar but weaker interaction pattern (P = 0.006 for interaction in the pooled data). In contrast, we did not find a significant interaction between the intake of artificially sweetened beverages and the genetic-predisposition score in relation to BMI (P = 0.83 for interaction).
We then performed similar analyses with the data from 21,740 women from the WGHS. In those analyses, the association for each increment of 10 risk alleles was an increase in BMI of 1.39, 1.64, 1.90, and 2.53 across the four categories of intake (P = 0.001 for interaction), with adjustment for age, geographic region, eigenvectors (derived from genomewide association studies20), lifestyle factors, and total energy intake (Table 3).
Table 3.
Increase in BMI per Increment of 10 Risk Alleles, According to Intake of Sugar-Sweetened and Artificially Sweetened Beverages in the WGHS Cohort.*
| Analysis | Increase in BMI | P Value for Interaction | |||
|---|---|---|---|---|---|
| <1 Serving per Month | 1–4 Servings per Month | 2–6 Servings per Week | ≥1 Serving per Day | ||
| Sugar-sweetened beverages | |||||
|
| |||||
| Model 1† | 1.46±0.13 | 1.65±0.19 | 1.97±0.18 | 2.43±0.36 | 0.002 |
|
| |||||
| Model 2‡ | 1.39±0.13 | 1.64±0.19 | 1.90±0.18 | 2.53±0.36 | 0.001 |
|
| |||||
| Artificially sweetened beverages | |||||
|
| |||||
| Model 1† | 1.54±0.15 | 1.38±0.25 | 1.50±0.16 | 1.74±0.18 | 0.33 |
|
| |||||
| Model 2‡ | 1.60±0.15 | 1.43±0.25 | 1.46±0.16 | 1.63±0.18 | 0.81 |
Plus–minus values are β coefficients ±SE. Data are for 21,740 women in the WGHS. Data on beverage intake were assessed at baseline, which was 3 years before the assessment of BMI.
Data were adjusted for age, geographic region, and eigenvectors (derived from genomewide association studies20).
Data were further adjusted for level of physical activity, status with respect to current smoking, alcohol intake, and total energy intake.
GENETIC PREDISPOSITION AND RISK OF OBESITY ACCORDING TO BEVERAGE INTAKE
In the NHS, there were 1107 incident cases of obesity among 6402 initially nonobese women during 18 years of follow-up (1980 to 1998), and in the HPFS, there were 297 incident cases of obesity among 3889 initially nonobese men during 12 years of follow-up (1986 to 1998). There was a significant interaction between the intake of sugar-sweetened beverages and the genetic-predisposition score in relation to the risk of incident obesity among women in the NHS (P = 0.03 for interaction). We observed a nonsignificant interaction pattern among men in the HPFS (P = 0.20 for interaction), but there was no significant heterogeneity in the results from these two cohorts (P = 0.84 for heterogeneity).
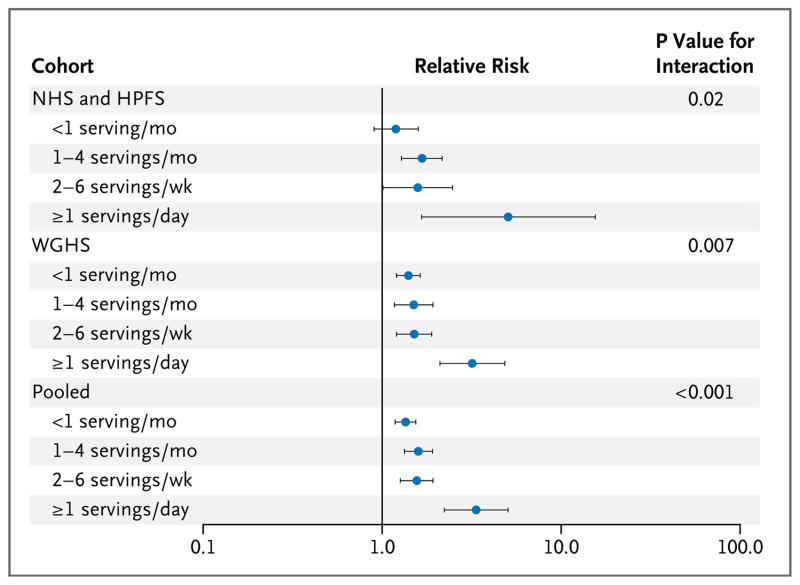
In the combined results from the NHS and HPFS cohorts (Fig. 1), the relative risks of incident obesity for each increment of 10 risk alleles were 1.19 (95% confidence interval [CI], 0.90 to 1.59) for an intake of sugar-sweetened beverages of less than one serving per month, 1.67 (95% CI, 1.28 to 2.16) for one to four servings per month, 1.58 (95% CI, 1.01 to 2.47) for two to six servings per week, and 5.06 (95% CI, 1.66 to 15.5) for one or more servings per day (P = 0.02 for interaction). The results were similar in the WGHS cohort (Fig. 1), in which 2280 women (of 18,127 women who were nonobese at baseline) became obese during 6 years of follow-up (1992 to 1998). Across the four categories of intake, the relative risks of incident obesity per increment of 10 risk alleles were 1.40 (95% CI, 1.19 to 1.64), 1.50 (95% CI, 1.16 to 1.93), 1.54 (95% CI, 1.21 to 1.94), and 3.16 (95% CI, 2.03 to 4.92) (P = 0.007 for interaction). No significant heterogeneity was observed among the three cohorts (P = 0.38 for heterogeneity). We then combined the results from all three cohorts, and the pooled relative risks of incident obesity for each increment of 10 risk alleles were 1.35 (95% CI, 1.18 to 1.54), 1.59 (95% CI, 1.33 to 1.91), 1.56 (95% CI, 1.26 to 1.92), and 3.35 (95% CI, 2.22 to 5.05) across the four categories of intake (P<0.001 for interaction).
Figure 1. Relative Risk of the Development of Obesity per Increment of 10 Risk Alleles, According to Intake of Sugar-Sweetened Beverages.
For the discovery phase, with data from the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS) cohorts, the analyses were based on 18 years of follow-up for 6402 initially nonobese women (1980 to 1998, 1107 incident cases of obesity) and 12 years of follow-up for 3889 initially nonobese men (1986 to 1998, 297 incident cases of obesity). Shown are the pooled relative risks of incident obesity, with adjustment for age, source of genotyping data, level of physical activity, status with respect to current smoking, alcohol intake, time spent watching television, Alternative Healthy Eating Index score, and total energy intake. For the replication phase, with data from the Women’s Genome Health Study (WGHS) cohort, the analyses were based on a median of 6 years of follow-up for 18,127 initially nonobese women (1992 to 1998, 2280 incident cases of obesity). Shown are the relative risks of incident obesity, with adjustment for age, geographic region, eigenvectors, level of physical activity, status with respect to current smoking, alcohol intake, and total energy intake. Horizontal bars indicate 95% confidence intervals.
BEVERAGE INTAKE AND BMI ACCORDING TO GENETIC PREDISPOSITION
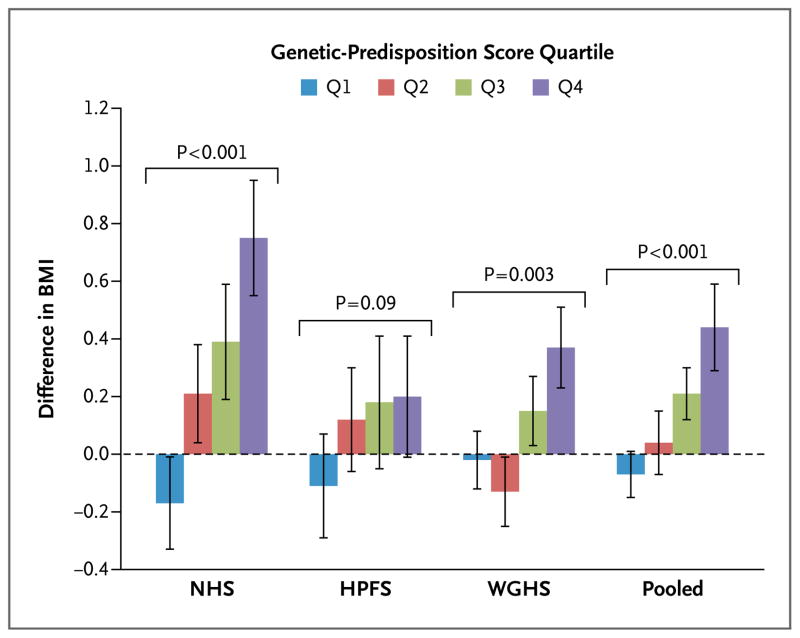
We also examined the association between intake of sugar-sweetened beverages and BMI according to the quartiles of the genetic-predisposition score. As shown in Figure 2, the increment in BMI associated with one serving of a sugar-sweetened beverage per day consistently increased across the quartiles of the genetic-predisposition score in the three cohorts. No significant heterogeneity was observed among the three cohorts (P = 0.12 for heterogeneity). In the pooled results from the three cohorts, the association between intake (one serving of a sugar-sweetened beverage per day) and BMI was stronger among persons with a genetic-predisposition score in the highest quartile (β coefficient [±SE], 0.44±0.15; P = 0.003) than among those with a genetic-predisposition score in the lowest quartile (β coefficient, −0.07±0.08; P = 0.36) (P<0.001 for interaction).
Figure 2. Difference in BMI Associated with One Serving of a Sugar-Sweetened Beverage per Day, According to the Quartile of the Genetic-Predisposition Score.
Data are effect sizes (β coefficients [±SE]) of sugar-sweetened beverage intake (one serving per day) on body-mass index (BMI; the weight in kilograms divided by the square of the height in meters), stratified according to the quartile of the genetic-predisposition score. In the NHS cohort, the median scores across the quartiles were 24.5 (range, 13.1 to 26.3), 27.8 (range, 26.4 to 29.0), 30.3 (range, 29.1 to 31.7), and 33.6 (range, 31.8 to 43.4); in the HPFS cohort, 24.9 (range, 16.0 to 26.5), 27.9 (range, 26.6 to 29.1), 30.4 (range, 29.2 to 31.7), and 33.6 (range, 31.8 to 41.9); and in the WGHS cohort, 24.7 (range, 15.3 to 26.5), 27.8 (range, 26.6 to 29.1), 30.3 (range, 29.2 to 31.6), and 33.4 (range, 31.7 to 43.4). In the NHS and HPFS cohorts, the analyses were based on data from the first 4 years in women (1980 to 1984) and men (1986 to 1990), respectively, with adjustment for age, source of genotyping data, level of physical activity, time spent watching television, status with respect to current smoking, alcohol intake, and Alternative Healthy Eating Index score. In the WGHS cohort, the analyses were based on data from the first 3 years, with adjustment for age, geographic region, eigenvectors, level of physical activity, status with respect to current smoking, and alcohol intake. P values are for interaction. I bars indicate standard errors.
DISCUSSION
In two prospective cohorts of U.S. women and men, we found that greater consumption of sugar-sweetened beverages was associated with a more pronounced genetic predisposition to an elevated BMI and an increased risk of obesity. The findings were further replicated in an independent large cohort of U.S. women. In all three cohorts, the combined genetic effects on BMI and obesity risk among persons consuming one or more servings of sugar-sweetened beverages per day were approximately twice as large as those among persons consuming less than one serving per month. These data suggest that persons with greater consumption of sugar-sweetened beverages may be more susceptible to genetic effects on adiposity. Viewed differently, persons with a greater genetic predisposition to obesity appeared to be more susceptible to the deleterious effects of sugar-sweetened beverages on BMI. Our findings further underscore the need to test interventions that reduce the intake of sugary drinks as a means of reducing the risk of obesity and related diseases.
Our study shows a significant interaction between an important dietary factor, the intake of sugar-sweetened beverages, and a genetic-predisposition score in relation to BMI and obesity risk. Most studies that have investigated gene–environment interactions in relation to obesity have focused on a single locus, FTO, and physical activity; and they have yielded inconsistent results.28,30–34 Major reasons for the inconsistent results include small samples in previous studies and the small effect of a single genetic variant. Recently, a meta-analysis with a large sample (218,166 persons) validated a modest interaction between physical activity and FTO variants in relation to obesity risk.35
In the current analysis, we calculated a genetic-predisposition score that was composed of multiple genetic variants, which has become the preferred method in analyses of gene–environment interactions.36 As expected, owing to the modest effect of each individual SNP on BMI, most of the individual SNPs did not show significant interactions with intake of sugar-sweetened beverages in relation to BMI (Table S2 in the Supplementary Appendix). Furthermore, our prospective analysis minimized the potential reverse causality that is more likely to occur in cross-sectional studies,37 and the repeated-measures analysis with the use of longitudinally collected data represented long-term dietary habits, reduced random measurement error, and enhanced the robustness of our findings. The highly consistent results were replicated in a large independent cohort, further verifying the reliability of our findings.
Numerous studies have shown a positive association between the intake of sugar-sweetened beverages, obesity, and related cardiometabolic diseases.6–11 As reported elsewhere in this issue of the Journal, two randomized intervention studies show that a reduction in the consumption of sugar-sweetened beverages and a replacement of sugar-sweetened beverages with noncaloric sweetened beverages reduced weight gain in children.38,39 Although further evidence is warranted, these data support a causal relationship among the consumption of sugar-sweetened beverages, weight gain, and the risk of obesity. The current finding that the genetic effects on adiposity are stronger in persons with higher intake than in those with lower intake provides useful information on the role of sugar-sweetened beverages in triggering obesity; increased consumption might contribute to the obesity epidemic by interacting with a genetic predisposition to elevated BMI. From another perspective, persons with a greater genetic predisposition may be more susceptible to obesity-inducing effects of sugar-sweetened beverages.
The intake of sugar-sweetened beverages contributes to obesity through several potential mechanisms, including a high caloric content with low satiety and incomplete compensation for these liquid calories, resulting in an increased total energy intake.40,41 In addition, because of the large amounts of rapidly absorbable carbohydrates in sugar-sweetened beverages, greater consumption may increase the risks of insulin resistance, beta-cell dysfunction, inflammation, visceral adiposity, and other metabolic disorders.42,43 However, it is unclear whether these factors linking the intake of sugar-sweetened beverages to obesity modify the genetic effect, accounting for the observed interactions. Because the biologic functions of most established BMI-associated loci are largely unknown,5 the underlying mechanisms of the interaction between the intake of sugar-sweetened beverages and a genetic predisposition to elevated adiposity or obesity need to be clarified in future studies.
The major strengths of this study include the prospective design, the large sample, use of repeated measures of sugar-sweetened beverage intake and BMI, comprehensive coverage of the established BMI-associated genetic factors, and replication of the results across three cohorts. Several limitations need to be acknowledged. Measurement errors in the intake of sugar-sweetened beverages and other dietary factors are inevitable, but the food-frequency questionnaires have been extensively validated.21–23 In addition, the self-reported weight and height in our cohorts were found to be highly reliable.24 Although we adjusted for several major lifestyle and dietary factors in the analysis, the potential for confounding by unmeasured or unknown factors could not be fully eliminated. The proportion of the total energy intake derived from sugar-sweetened beverages was not evaluated. Our genetic-predisposition score captured the combined information from all the established BMI-associated loci that have been identified to date, but it accounts for only a small amount of variation in BMI.5 Our study cohorts were restricted to persons of European ancestry, and it is unknown whether our results can be generalized to other ethnic groups.
In conclusion, our data provide consistent evidence from three separate cohorts that greater consumption of sugar-sweetened beverages was associated with a more pronounced genetic predisposition to elevated BMI and obesity risk among women and men.
Supplementary Material
Acknowledgments
Supported by grants (DK091718, HL071981, HL073168, CA87969, CA49449, CA055075, HL34594, HL088521, U01HG004399, DK080140, 5P30DK46200, U54CA155626, DK58845, U01HG004728-02, EY015473, DK70756, and DK46200) from the National Institutes of Health, with additional support for genotyping from Merck Research Laboratories; an American Heart Association Scientist Development Award (0730094N, to Dr. L. Qi); and a Research to Prevent Blindness award and a Harvard Ophthalmology Scholar Award (both to Dr. Pasquale) from the Harvard Glaucoma Center of Excellence. The WGHS is supported by grants (HL043851, HL69757, and CA047988) from the National Institutes of Health, with collaborative scientific support and funding for genotyping provided by Amgen.
Dr. Ridker reports receiving consulting fees from Genzyme, Isis Pharmaceuticals, Vascular Biogenics, Merck, and Abbott and grant support to his institution from Novartis and Astra-Zeneca and being listed as a co-inventor on patents held by his institution that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes (patent and royalty payments are received by Brigham and Women’s Hospital); Dr. Hu, receiving consulting fees from Novo Nordisk and grant support to his institution from Merck and the California Walnut Commission; and Dr. L. Qi, receiving lecture fees from Kellogg. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Ms. Lynda M. Rose for assistance with statistical programming; and all the participants of the NHS, the HPFS, and the WGHS for their continued cooperation.
(Funded by the National Institutes of Health and others.)
References
- 1.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9. 1 million participants. Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi L, Cho YA. Gene-environment interaction and obesity. Nutr Rev. 2008;66:684–94. doi: 10.1111/j.1753-4887.2008.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363:2339–50. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- 4.Willer CJ, Speliotes EK, Loos RJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik VS, Popkin BM, Bray GA, Després JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–64. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–34. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 8.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–88. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2007;97:667–75. doi: 10.2105/AJPH.2005.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malik VS, Hu FB. Sugar-sweetened beverages and health: where does the evidence stand? Am J Clin Nutr. 2011;94:1161–2. doi: 10.3945/ajcn.111.025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popkin BM. Patterns of beverage use across the lifecycle. Physiol Behav. 2010;100:4–9. doi: 10.1016/j.physbeh.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 14.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–8. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 15.Hunter DJ, Kraft P, Jacobs KB, et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39:870–4. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi L, Cornelis MC, Kraft P, et al. Genetic variants at 2q24 are associated with susceptibility to type 2 diabetes. Hum Mol Genet. 2010;19:2706–15. doi: 10.1093/hmg/ddq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelis MC, Monda KL, Yu K, et al. Genome-wide meta-analysis identifies regions on 7p21 (AHR) and 15q24 (CYP1A2) as determinants of habitual caffeine consumption. PLoS Genet. 2011;7(4):e1002033. doi: 10.1371/journal.pgen.1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiggs JL, Kang JH, Yaspan BL, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum Mol Genet. 2011;20:4707–13. doi: 10.1093/hmg/ddr382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen MK, Pers TH, Dworzynski P, Girman CJ, Brunak S, Rimm EB. Protein interaction-based genome-wide analysis of incident coronary heart disease. Circ Cardiovasc Genet. 2011;4:549–56. doi: 10.1161/CIRCGENETICS.111.960393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM, Chasman DI, Zee RYL, et al. Rationale, design, and methodology of the Women’s Genome Health Study: a genome-wide association study of more than 25,000 initially healthy American women. Clin Chem. 2008;54:249–55. doi: 10.1373/clinchem.2007.099366. [DOI] [PubMed] [Google Scholar]
- 21.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;35:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 22.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 23.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semi-quantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 24.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–73. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Pirie P, Jacobs D, Jeffery R, Hannan P. Distortion in self-reported height and weight data. J Am Diet Assoc. 1981;78:601–6. [PubMed] [Google Scholar]
- 26.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–9. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 27.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–71. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 28.Ahmad T, Lee IM, Paré G, et al. Lifestyle interaction with fat mass and obesity-associated (FTO) genotype and risk of obesity in apparently healthy U.S. women. Diabetes Care. 2011;34:675–80. doi: 10.2337/dc10-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstein AR, Sesso HD, Lee IM, et al. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA. 2004;292:1188–94. doi: 10.1001/jama.292.10.1188. [DOI] [PubMed] [Google Scholar]
- 30.Andreasen CH, Stender-Petersen KL, Mogensen MS, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57:95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- 31.Rampersaud E, Mitchell BD, Pollin TI, et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Arch Intern Med. 2008;168:1791–7. doi: 10.1001/archinte.168.16.1791. [Erratum, Arch Intern Med 2009;169:453.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan JT, Dorajoo R, Seielstad M, et al. FTO variants are associated with obesity in the Chinese and Malay populations in Singapore. Diabetes. 2008;57:2851–7. doi: 10.2337/db08-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hakanen M, Raitakari OT, Lehtimäki T, et al. FTO genotype is associated with body mass index after the age of seven years but not with energy intake or leisure-time physical activity. J Clin Endocrinol Metab. 2009;94:1281–7. doi: 10.1210/jc.2008-1199. [DOI] [PubMed] [Google Scholar]
- 34.Jonsson A, Renström F, Lyssenko V, et al. Assessing the effect of interaction between an FTO variant (rs9939609) and physical activity on obesity in 15,925 Swedish and 2,511 Finnish adults. Diabetologia. 2009;52:1334–8. doi: 10.1007/s00125-009-1355-2. [Erratum, Diabetologia 2010;53:1244.] [DOI] [PubMed] [Google Scholar]
- 35.Kilpeläinen TO, Qi L, Brage S, et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 2011;8(11):e1001116. doi: 10.1371/journal.pmed.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S, Zhao JH, Luan J, et al. Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk Prospective Population Study. PLoS Med. 2010;7(8):e1000332. doi: 10.1371/journal.pmed.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manolio TA, Bailey-Wilson JE, Collins FS. Genes, environment and the value of prospective cohort studies. Nat Rev Genet. 2006;7:812–20. doi: 10.1038/nrg1919. [DOI] [PubMed] [Google Scholar]
- 38.Ebbeling CB, Feldman HA, Chomitz VR, et al. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367:1407–16. doi: 10.1056/NEJMoa1203388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367:1397–406. doi: 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- 40.Mattes RD. Dietary compensation by humans for supplemental energy provided as ethanol or carbohydrate in fluids. Physiol Behav. 1996;59:179–87. doi: 10.1016/0031-9384(95)02007-1. [DOI] [PubMed] [Google Scholar]
- 41.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord. 2000;24:794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- 42.Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80:348–56. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 43.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–34. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.