Abstract
Methamphetamine (METH) exposure is primarily associated with deleterious effects to dopaminergic neurons. While several studies have implicated the endocannabinoid system in METH’s locomotor, rewarding and neurochemical effects, a role for this signaling system in METH’s effects on dopamine terminal dynamics has not been elucidated. Given that CB1 receptor blockade reduces the acute potentiation of phasic extracellular dopamine release from other psychomotor stimulant drugs and that the degree of acute METH-induced increases in extracellular dopamine levels is related to the severity of dopamine depletion, we predicted that pretreatment with the CB1 receptor antagonist rimonabant would reduce METH-induced alterations at dopamine terminals. Furthermore, we hypothesized that administration of METH in environments where reward associated-cues were present would potentiate METH’s acute effects on dopamine release in the nucleus accumbens and exacerbate changes in dopamine terminal activity. Fast-scan cyclic voltammetry was used to measure electrically-evoked dopamine release in the nucleus accumbens and revealed markers of compromised dopamine terminal integrity nine days after a single dose of METH. These were exacerbated in animals that received METH in the presence of reward-associated cues, and attenuated in rimonabant-pretreated animals. While these deficits in dopamine dynamics were associated with reduced operant responding on days following METH administration in animals treated with only METH, rimonabant-pretreated animals exhibited levels of operant responding comparable to control. Moreover, dopamine release correlated significantly with changes in lever pressing behavior that occurred on days following METH administration. Together these data suggest that the endocannabinoid system is involved in the subsecond dopaminergic response to METH.
1. Introduction
In 2008, methamphetamine use was associated with over an estimated 66,000 emergency room visits (SAMHSA, 2010). Repeated use and high doses of METH are associated with cognitive (Gonzalez et al., 2004; Scott et al., 2007), structural and neurochemical abnormalities (Chang et al. 2007; Wilson et al., 1996). The negative behavioral and cognitive effects of chronic use are attributed to enduring changes in brain structure, function and chemistry (Chang et al., 2007; Scott et al., 2007), and thus elucidation of the neural correlates of METH-induced alterations have profound implications for developing therapeutics to treat METH-abuse. METH exerts significant dopaminergic effects through blockade and reversal of the dopamine transporter (DAT) (Sulzer et al., 1995), leading to increased levels of extracellular dopamine, and subsequent free radical production (Yamamoto and Zhu, 1998). The resulting oxidative stress likely underlies dopamine alterations from METH, with the degree of acute METH-induced aberrant dopamine overflow predicting the severity of enduring dopamine depletions (Yamamoto and Zhu, 1998).
Neurochemical depletions associated with chronic METH exposure are particularly pronounced in the striatum (Chang et al., 2007). Amphetamines exert significant action on DAT in the nucleus accumbens (NAc, Wise and Bozarth, 1985), an area in which chronic METH users exhibit deficits in glucose metabolism (Wang et al., 2004), DAT density, dopamine content and activity of the rate-limiting enzyme, tyrosine hydroxylase (Wilson et al., 1996). We therefore hypothesized that any manipulation of dopaminergic activity in this brain region during METH’s acute effects would alter METH-induced dopaminergic effects. As drug users take substances in a wide range of environments, research into the impact of environmental stimuli (present during drug administration) on the effects of METH is warranted. As cues that predict access to food maintained responding contribute to increased phasic dopamine release in the NAc (Roitman et al., 2004), we hypothesized that administration of the drug in environments with such cues would augment acute METH-induced raises in dopamine in this brain region and thus exacerbate METH-induced neurochemical depletion.
The endocannabinoid system, comprised of its neurotransmitters, receptors and enzymes (Alger and Kim, 2011), has been implicated in the stereotypy and rewarding effects of METH, yet no work has investigated its role in METH-induced dopaminergic alterations. Studies have demonstrated that CB1 receptor blockade decreases METH self-administration systemically (Schindler et al., 2010) and into the NAc core (Rodriguez et al., 2011). In addition, local administration of the CB1R antagonists rimonabant or AM251 into the NAc reduces stereotypy from METH (Morra et al., 2010). Importantly, CB1 receptors are densely expressed in the NAc (Julian et al., 2003), and their activity modulates the mesolimbic dopamine system, the rostral dopaminergic projection from the ventral tegmental area (VTA) to the NAc (Cheer et al., 2003; Hungund et al., 2003; Wenger and Furst, 2004). Indeed, rats treated with rimonabant exhibit markedly attenuated cocaine-induced increases in frequency and amplitude of NAc dopamine transients (Cheer et al., 2007b). We therefore hypothesized that rimonabant pretreatment would be associated with a reduction in the rise in extracellular dopamine caused by METH and thus a decrease in the effects of METH in the NAc.
2. Matierals and Methods
2.1 Subjects
Male Sprague-Dawley rats (n = 3 1 ; 275 –350 g and 65 days old upon arrival) were ordered from Charles River (Charles River, Wilmington, MA), housed individually in a temperature-controlled environment on a 12-hour light/dark schedule (on at 7:00am, off at 7pm). Upon arrival, animals were allowed two days of rest and were provided food and water ad libitum. They were then weighed and brought down to 85% body-weight over 8 days.
2.2 Drugs
Methamphetamine HCL was obtained from Sigma-Aldrich and dissolved in saline at a concentration of 15 mg/mL. Rimonabant (SR141716A) (Research Triangle Institute–National Institute on Drug Abuse, Raleigh, NC) was freshly mixed in a 1:1:18 ratio of ethanol, emulphor (Alkamuls EL-620; Rhodia, Cran-bury, NJ), and saline (0.9%) at a concentration of 3 mg/mL. All drugs were injected intraperitoneally (IP) unless indicated otherwise.
2.3 Training boxes
Plexiglas chambers of 43 × 43 × 53 cm dimensions (Med. Associates, Inc., St Albans, VT, USA) were used for training. Chambers were individually housed in sound-attenuating cubicles. The beginning of training sessions was indicated by illumination of a cue light positioned above the right lever, a house light positioned at the top of the box opposite to the lever, lever extension of both the right and left levers and white noise. Pressing the right, but not the left lever induced 1) release of one chocolate pellet (F05984, 45mg Chocolate flavored, Bio-Serv, Frenchtown, NJ) into a food cup positioned between the two levers, 2) withdrawal of both levers, 3) initiation of a tone and 4) termination of both the house and cue lights. After an inter- trial interval of 10 seconds, the levers extended, the lights re-illuminated and the tone was terminated. Rats were permitted to press the lever during daily one hour sessions. White noise remained on throughout the session.
2.4 Experimental Design
2.4.1 Behavioral experiment
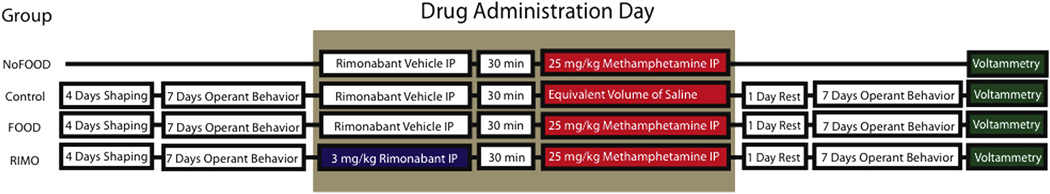
A between subjects design (n = 31) was used to assess how METH administration in an environment with reward-associated cues and rimonabant pretreatment modulates dopaminergic deficits that follow METH administration (Figure 1). Four groups, FOOD+Saline, FOOD+METH, NoFOOD+METH and FOOD+RIMO+METH were randomly created upon arrival. Chocolate pellets (F05984, 45mg Chocolate flavored, Bio-serv, Frenchtown, NJ) were placed in animal home cages 2 days prior to shaping to reduce neophobia on shaping days. Experimental groups (FOOD+Saline, FOOD+METH and FOOD+RIMO+METH) were shaped on a discrete 30 minute FR1 food-maintained responding task for 4 days (Inter-Trial Interval (ITI) = 1 second), at which point all subjects displayed robust operant responding. An additional group (NoFOOD+METH) was not shaped and did not receive any food in the training box, but was placed in the same environment to control for environmental variables. In order to closely replicate the training environment of experimental animals, cues associated with food delivery were delivered non-contingently to NoFOOD+METH animals at a frequency similar to the rate of responding of trained rats. Following removal from training boxes, this group was given food in their home cages to maintain 85% weight. Post-shaping, subjects of the experimental groups (FOOD+Saline, FOOD+METH, FOOD+RIMO+METH) continued f o r seven days on daily discrete FR1 food-maintained responding (ITI=10s) sessions for one hour. The NoFOOD+METH group was placed in the same environment, including non-contingent administration of cues associated with reward, but pellets were never dispensed. The NoFOOD+METH group did not engage in the operant behavior. All rats received IP injections of saline immediately preceding placement in training boxes, during shaping and training. Animals therefore did not experience an unusual amount of injection-related discomfort on days when they were administered drugs. On the eighth day of food-maintained responding, subjects were administered drugs before placement in their relative training environments. The FOOD+Saline (n = 12) group received behavioral training, vehicle pretreatment followed by saline injection on the day of drug administration. The FOOD+METH (n = 6) group received behavioral training, vehicle pretreatment followed by METH injection. The FOOD+RIMO+METH (n = 10) group received behavioral training, rimonabant pretreatment followed by METH injection on METH administration day. The NoFOOD+METH group (n = 7) was not trained to lever press for food, and received vehicle pretreatment followed by METH injection (Figure 1). Following drug administration, animals rested for one day in their home cages and then returned to hourly sessions for an additional seven days. Animals received seven days of training post METH administration so that the experiment was symmetrical in reference to drug administration. The day following the final session of food-maintained responding, animals were terminally anesthetized and voltammetry experiments were conducted (Figure 1). Thus neurochemical analysis was performed nine days after acute METH administration. Animal schedules were staggered so only a single voltammetry experiment was conducted on any given day; this allowed for experiments to begin at similar times.
Figure. 1.
Visual depiction of experimental design. A between subjects design (n = 31) was used to assess how the presence of reward-associated cues and rimonabant pretreatment impacts the dopaminergic deficits that follow METH administration. Animals in the different groups, FOOD+METH, FOOD+RIMO+METH, Food+Saline were shaped for 4 days, then maintained on hourly food-maintained responding sessions for seven days. On the following day animals were administered drugs, given one day of rest and returned to hourly sessions for seven days. Voltammetric experiments were then performed. T h e NoFOOD+METH group was not conditioned to lever press for food, was given food only in home cages and received vehicle pretreatment and METH administration; FOOD+Saline group was conditioned to lever press for food and received vehicle pretreatment and saline administration. FOOD+METH group was conditioned to lever press for food and received vehicle pretreatment and METH administration (25 mg/kg IP); FOOD+RIMO+METH group was conditioned to lever press for food and received rimonabant (3 mg/kg) pretreatment followed by METH. NoFOOD+METH group received equal exposure to the same environment with cues associated with reward, but lever press did not dispense food.
2.4.2 Drug Administration
Although previous rat studies using single METH boli to induce dopaminergic alterations have administered doses as high as 40 mg/kg IP (Imam & Ali, 2001; Jayanthi et al., 2005), our pilot studies revealed that such high doses induced high mortality rates. We employed a single dose regimen of 25 mg/kg because mortality occurred infrequently and the dose was sufficient to induce alterations in dopamine dynamics. The FOOD+RIMO+METH group received an IP injection of rimonabant (3 mg/kg) followed 30 minutes later by an IP injection of METH (25 mg/kg); the FOOD+METH group received an equivalent solution of vehicle followed 30 minutes later by METH (25 mg/kg IP); the NoFOOD+METH group received an equivalent solution of vehicle followed 30 minutes later by METH (25 mg/kg IP); the FOOD+Saline group received an equivalent solution of vehicle followed 30 minutes later by an equivalent solution of saline. A visual representation of the experimental design can be found in Figure 1. Pilot studies revealed that four minutes follwoing METH administration, rats ceased lever pressing. Therefore, on drug administration days, FOOD+METH and FOOD+RIMO+METH groups received a special training schedule: 4 minutes after session initiation, the cues associated with lever press were automatically elicited so as to replicate the normal training environment. Pellets were dispensed with the cues until the food cup was filled, after which the cues, but not pellet delivery continued automatically until the session finished. Sessions ran for the normal 60 minutes. Animals that received METH did not consume the rewards, consistent with our pilot studies. The NoFOOD+METH and FOOD+Saline groups were exposed to the training environments that they had been maintained on. Following training, rats were placed back in their home cages and kept in the lab until METH-induced effects had dissipated (i.e. hyperlocomotion) and were returned to animal housing rooms. In order to test the hypothesis that administration of a drug in an environment with reward- associated cues results in additive changes to dopamine release dynamics, we used a single high dose capable of provoking dopaminergic changes. If multiple doses had been used, the drug may have influenced associations between the environment and conditioning. As the drug was not self-administered and the dose was high, the regimen was not intended to mimic intake patterns of normal METH users.
2.5 Procedure
2.5.1 Electrode Preparation and Calibration
Carbon fiber microelectrodes for anesthetized experiments were prepared as previously described (Addy et al., 2010) using carbon fiber (T650, Amoco, Greenville, SC) filled into 1.2mm glass capillary tubes (A-M systems). Microelectrodes were cut at 75–120µm past the glass tip under a light microscope (Olympus, Center Valley, PA) with a scalpel blade (Cheer et al., 2004). Briefly, animals were anesthetized with urethane (1g/kg, i.p.) and placed in a stereotaxic apparatus. Burr holes were drilled for carbon fiber microelectrodes at the level of the NAc (+1.3 AP, +1.3 ML from bregma) and for a bipolar stimulating electrode above the VTA (−5.2 AP; + 0.5ML from bregma). One inside the brain, the carbon fiber electrode was held at −0.4 V in reference to an Ag/AgCl electrode (placed in the contralateral hemisphere). Ag/AgCl reference electrodes were made from 0.5mm Ag wire (Aldrich) through electrolysis in HCl (Sigma-Aldrich). Cyclic voltammograms were collected at 10 Hz by ramping up to +1.3 V and back in a triangular fashion at 400 V/s. Data collection, stimulation timing, and voltage application were controlled by interface boards and custom-written LabView routines (National Instruments) (Cheer et al. 2004). After experimentation, current changes were converted to dopamine concentration changes via pre- or post-calibration as previously described (Addy et al., 2010). A two-tailed independent samples t-test was conducted to ensure calibration factors did not differ significantly (p>.05) between pre- and post-calibrated electrodes.
2.6 Data Analysis
2.6.1 Behavior
Behavioral data from 14 days of training (7 before and 7 after drug administration) were collected. Latencies to press, total presses and initiation of response were analyzed. Latencies to press, the time between lever extension and operant response, were averaged within session to produce a single value per training day. Total presses per day were collected. The time from trial start to the first lever press constituted the initiation of response. To characterize behavioral effects of METH administration, values of before and after administration were compared. In order to control for changes in behavioral responding that occurred during learning, only the 2 sessions before drug administration (days 6 and 7) and 2 days after (days 8 and 9) were averaged together and compared. A ratio of after/before was calculated for each animal. A one-way ANOVA was conducted for rate of responding, initiation of response and latencies to press. A two-tailed product-moment Pearson’s correlation was run between the after/before ratio of total bar presses and mean peak amplitude of electrically-evoked dopamine release in the NAc.
2.6.2 Fast-Scan Cyclic Voltammetry
Voltammetric data was analyzed as previously described by Addy et al. (2010). Peak amplitude and rate of decay were calculated at every depth in which electrically-evoked dopamine was observed. Peak current was divided by calibration values, to calculate a dopamine concentration. Values from each depth measured were averaged across subjects, within group. A one-way ANOVA was conducted to assess differences between groups. Dopamine concentration vs. time plots were exported to Prism version 5 (Graph Pad, La Jolla, CA, USA). One-phase decay regression lines were fit to the data and software generated Tau values were collected for statistical analysis. Rate of decay was calculated from the Tau value of single exponential decay fits of all observed electrically- evoked dopamine. Peak amplitude and rate of decay were calculated at each stimulation point in the input-output experiment. A mixed model ANOVA and two-way repeated measures ANOVA were conducted, accounting for both within and between subject differences as a function of stimulation level and treatment.
2.6.3 Statistics
All statistics were performed on SPSS 17 (SPSS, Chicago IL) and Prism version 5 (Graph Pad, La Jolla, CA, USA). All significance levels were set to 0.05. Bonferroni post hoc tests were conducted when omnibus tests exhibited significant results (p < 0.05).
3. Results
3.1 Methamphetamine administration is associated with reduced motivated behavior
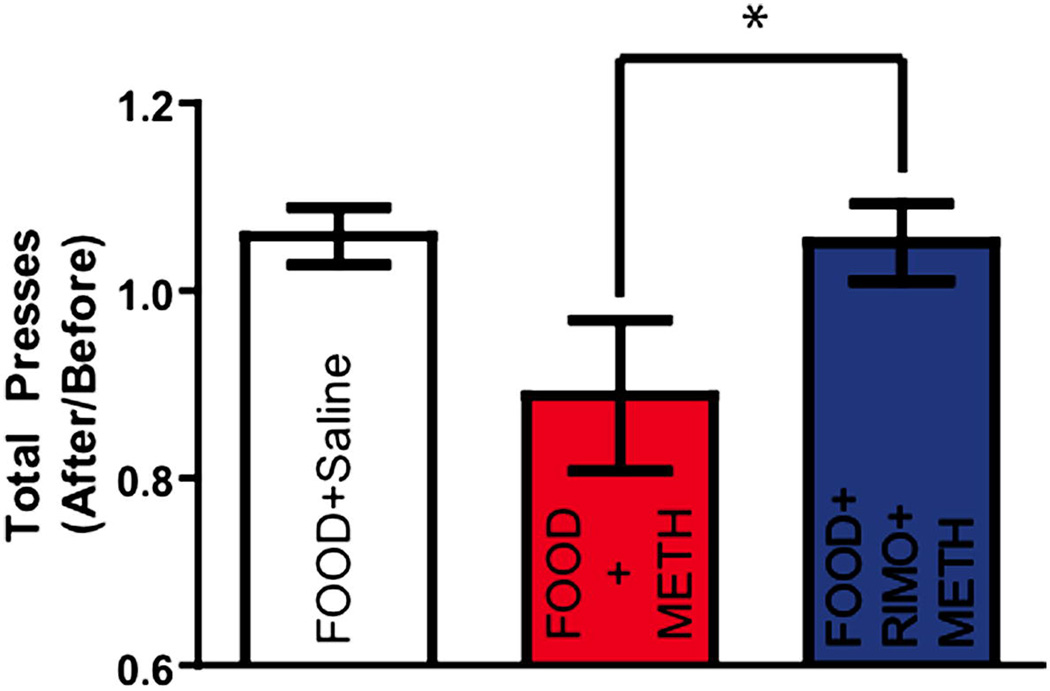
To assess whether METH was associated with reductions in operant responding, and whether RIMO offered protection against this, total bar presses were collected within-subject by training day. The values for each behavioral parameter on each of the two training days before drug administration (training days 6 and 7) were averaged together. This value was divided by the average of the two training days after METH administration (training days 8 and 9) to produce a single value representing the change from baseline as a result of the drug. Given that our pilot studies revealed that rats did not lever press after METH injection, operant behavior on drug administration days was not included in the analysis. This ratio was analyzed with a one-way ANOVA to assess how METH altered behavior. Statistical analysis revealed a significant treatment effect for total bar presses (F(2,32) = 5.242, p = 0.0112) (Figure 2). Post-hoc analyses revealed that while the FOOD+METH group displayed significantly less lever presses than FOOD+RIMO+METH, neither group displayed significant differences from control (FOOD+Saline) (Bonferroni post hoc: FOOD+Saline vs. FOOD+METH p > 0.05; FOOD+METH vs. FOOD+RIMO+METH p < 0.05; FOOD+Saline vs. FOOD+RIMO+METH p > 0.05). FOOD+METH was, however, associated with a trend towards reduced lever presses. Therefore, while animals given METH showed nonsignificant reductions in lever pressing, RIMO prevented diminished responding.
Figure. 2. METH is associated with nonsignificant reductions in lever pressing that is reversed by rimonabant pretreatment.
Ratio of total bar presses two days before and after drug administration (Mean ±SEM, *p<0.05, One-Way ANOVA, Bonferroni post hoc test.).
3.2 METH reduces electrically-evoked NAc dopamine release and uptake nine days after administration
3.2.1 METH administration in environments paired with reward-associated cues is linked with markers of reduced dopamine reuptake and this effect is attenuated by rimonabant
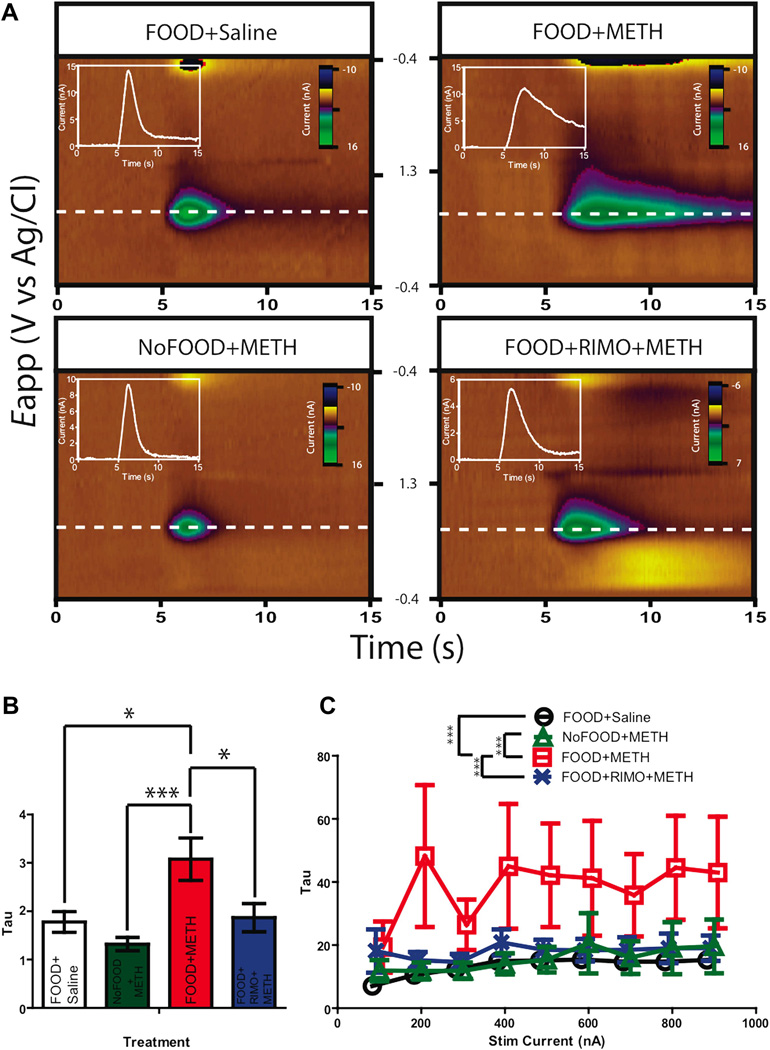
To investigate neurochemical correlates of the deficits in operant responding associated with METH treatment as well as the effects of rimonabant pretreatment, we measured dopamine release in the NAc on the four treatment groups. As deficits in DAT density are associated with METH toxicity (O’Neil et al., 2006; Wilson et al., 1996), we analyzed the rate of decay (Tau) of electrically-evoked dopamine release as a measure of DAT activity. Indeed, a previous anesthetized voltammetry experiment has assessed METH-induced DAT function alterations via somewhat similar kinetic analysis (Howard et al., 2011). We conducted a depth analysis to measure dopamine terminal integrity in the NAc. Given the heterogeneity of dopamine release in the NAc (Wightman et al., 2007), electrically-evoked release was not observed at every depth and thus statistical analysis could not be conducted as a function of both depth and subject. Thus, in order to fully represent the measured release as a product of depth in statistical analysis, all the values at a given depth were averaged across subjects within a group. A one-way ANOVA revealed a significant effect of treatment on rates of decay (F(3, 44) = 6.582, p = 0.0009). Post hoc analyses revealed that the Tau values of electrically-evoked dopamine release (a quantification of decay rate) were significantly larger in animals receiving the drug in the presence of reward-associated cues (FOOD+METH) (Figure 3A–B), (Bonferroni’s post hoc test: FOOD+Saline vs. FOOD+METH p < 0.05; FOOD+Saline vs. NoFOOD+METH p > 0.05; FOOD+Saline vs. FOOD+RIMO+METH p > 0.05; FOOD+METH vs. NoFOOD+METH p < 0.001; FOOD+METH vs. FOOD+RIMO+METH p < 0.05; FOOD+RIMO+METH vs. NoFOOD+METH p < 0.05). This suggests that under these experimental conditions, METH administration in the presence of reward-associated cues (FOOD+METH) was associated with signs of reduced DAT activity. Rimonabant pretreated animals (FOOD+RIMO+METH) received METH in the same environment (with food- predicting cues), but exhibited reuptake rates comparable to control animals only injected with saline (FOOD+Saline), suggesting t h at CB1 receptor blockade was linked to an attenuation of the changes in DAT function that accompanied METH administration in environments with reward-paired cues. Because animals that received METH in the presence of cues that were not associated with reward (NoFOOD+METH) exhibited rates of decay comparable to those of the FOOD+Saline group, this suggest that METH administration in the presence of these cues (independent of operant training) was not inherently sufficient to elicit indices of compromised DAT functioning; i.e., these cues must have been paired with reward to produce the observed changes in markers of DAT function. Thus, when controlling for environmental variables, METH treatment was associated with markers of compromised DAT functioning only when administered in environments where animals were exposed to conditioned reward-associated cues.
Figure. 3.
Animals administered METH in environments of food-maintained responding (FOOD+METH) exhibit increased rate of decay of electrically-evoked NAc dopamine release. Rimonabant pretreated animals show rates of decay comparable to control. A, Representative false color plots of electrically evoked dopamine in the NAc, where green is hot; plots are normalized to maximal release to emphasize change in uptake. Insets are Current vs. Time traces of color along +0.6V. FOOD+METH exhibits a longer tail of electrically-evoked dopamine release, demonstrating compromised reuptake. B, Mean values of rate of decay in the NAc (Mean ±SEM, One-Way ANOVA, Bonferroni post hoc test). C, Rate of decay in an input-output experiment (Mean ±SEM, One-Way ANOVA, Bonferroni post hoc test). Points are shifted in reference to NoFOOD+METH to display error bars: FOOD+Saline 6 points shifted right, FOOD+RIMO+METH 3 points shifted right, FOOD+METH is 3 points shifted left, NoFOOD+METH no shift. [*p<0.05; **p<0.01; ***p<0.001]
To further characterize NAc DAT activity, an input-output curve was generated in which VTA stimulation level was varied and NAc dopamine release was recorded. The rate of decay (Tau) was calculated for dopamine signals at each level of stimulation. To account for within and between subject differences, a mixed model ANOVA was employed to compare DAT activity as a function of treatment. Statistical analysis revealed a significant group effect (F(3,35) = 54.94, p < 0.0001). There was, however, no significant interaction between stimulation level and treatment (F(24,143) = .3297, p > 0.05) and thus values were collapsed across stimulation to determine the main effects of treatment. Post hoc analyses indicated that animals that received METH in environments with reward-associated cues (FOOD+METH) exhibited significantly longer decay rates than other groups, (Figure 3C) (Bonferroni multiple comparison test: FOOD+METH vs. FOOD+Saline p < .0001; FOOD+METH vs. NoFOOD+METH p < .0001; FOOD+METH vs. FOOD+RIMO+METH p < 0.001; FOOD+Saline vs. NoFOOD+METH p > 0.05; FOOD+Saline vs. FOOD+RIMO+METH p > 0.05; NoFOOD+METH vs. FOOD+RIMO+METH p > 0.05). Taken together these data suggest that METH administration in environments with reward predicting cues (FOOD+METH) was linked with reduced dopamine reuptake, and pretreatment with rimonabant was associated with attenuated METH-induced effects.
3.2.2 Rimonabant pretreatment attenuates deficits in electrically- evoked dopamine release that accompany METH administration
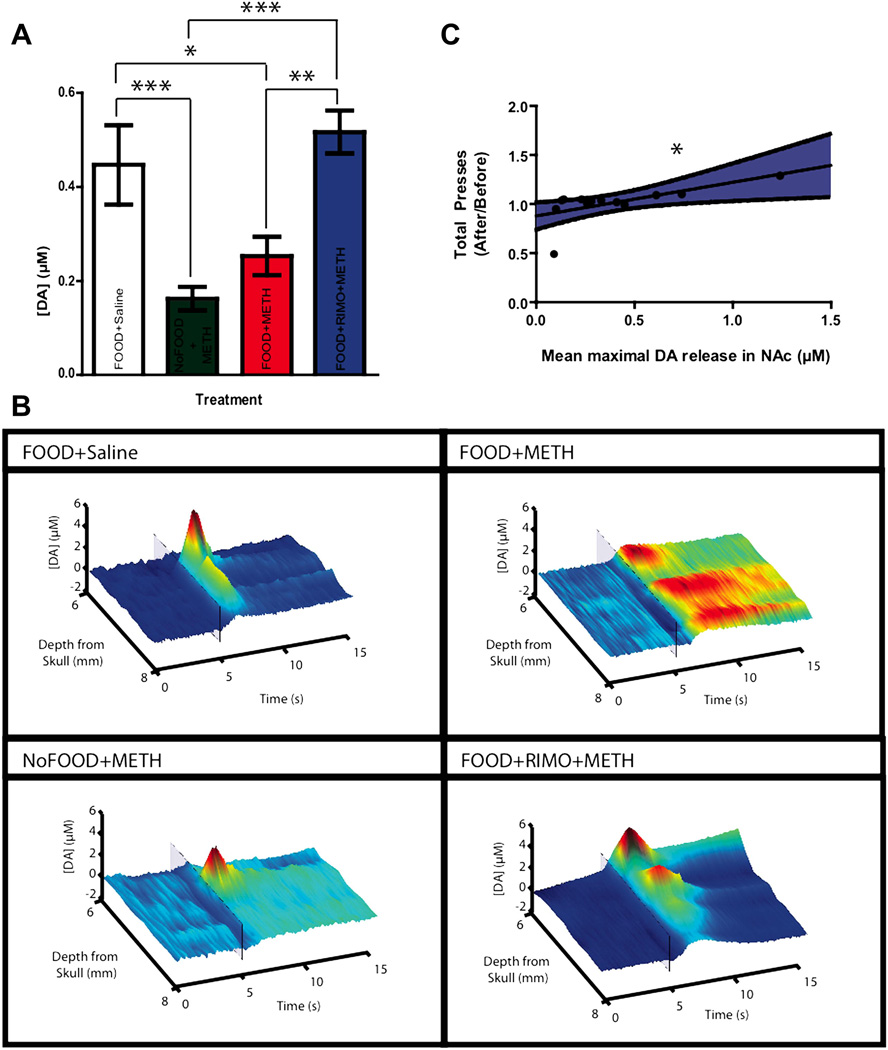
To further analyze terminal activity, we examined the amplitude of electrically-evoked dopamine release throughout the NAc to measure the capacity of mesolimbic neurons to release dopamine. As mentioned above, dopamine release was not observed at every depth due to the heterogeneous nature of release in this brain region (Wightman et al., 2007), thus dopamine release was not analyzed as a function of both depth and treatment. In order to fully represent release throughout the NAc, all the values at a given depth were averaged across subjects within a group. A one-way ANOVA revealed a significant effect of treatment (F(3,44) = 7.106, p = 0.001). Post hoc analyses indicated that animals which received METH without rimonabant pretreatment (FOOD+METH and NoFOOD+METH) exhibited amplitudes of electrically-evoked dopamine that were significantly lower than FOOD+Saline and FOOD+RIMO+METH groups (Figure 4AB), (Bonferroni post hoc: FOOD+Saline vs. NoFOOD+METH, p < 0.05; FOOD+Saline vs. FOOD+METH p < 0.05; FOOD+Saline vs. FOOD+RIMO+METH p = 0.240;FOOD+METH vs. NoFOOD+METH p = 0.851; NoFOOD+METH vs. FOOD+RIMO+METH p < 0.001; FOOD+METH vs. FOOD+RIMO+METH p < 0.01). As NAc dopamine activity is associated with reward-directed behaviors (Cheer et al., 2007a; Roitman et al., 2004), we sought to examine whether average levels of electrically-evoked dopamine release throughout the NAc correlated with the measures of behavior that we had obtained previously. As both operant responding (albeit nonsignificantly) and electrically evoked dopamine release were reduced in animals treated with METH (FOOD+METH) compared to FOOD+Saline and rimonabant- pretreated animals (FOOD+RIMO+METH), we investigated a correlation between behavioral and neurochemical indices of METH activity. Peak amplitude of electrically-evoked dopamine release from the depth analysis was averaged within subject and correlated with the ratio of total bar press changes that was associated with drug treatment. A two-tailed product- moment Pearson’s correlation revealed a significant within subject relationship between mean peak amplitude of NAc electrically-evoked dopamine release and total bar presses (r = 0.63, n = 13, p = 0.02), suggesting that greater NAc dopamine release was associated with higher operant responding (Figure 4C). As the NoFOOD+METH group did not engage in operant behavior, they were not included in statistical analysis.
Figure. 4.
Rimonabant pretreatment is associated with attenuated METH-induced reductions in the peak amplitude of electrically-evoked dopamine release throughout the NAc. A, Electrically evoked NAc dopamine release (Mean ±SEM, One-Way ANOVA, Bonferroni post hoc). B, Representative concentration traces of dopamine concentration vs. time vs. depth. Color plots demonstrate the effect of treatment throughout the NAc. Transparent boxes represent the electrical stimulation at 5 seconds. C, Correlation of mean dopamine release in the NAc and total bar presses ratio. Shaded region indicates 95% confidence interval (*p<0.05, 2-tailed Pearson’s correlation). [*p<.05, **p<.01, ***p<.001]
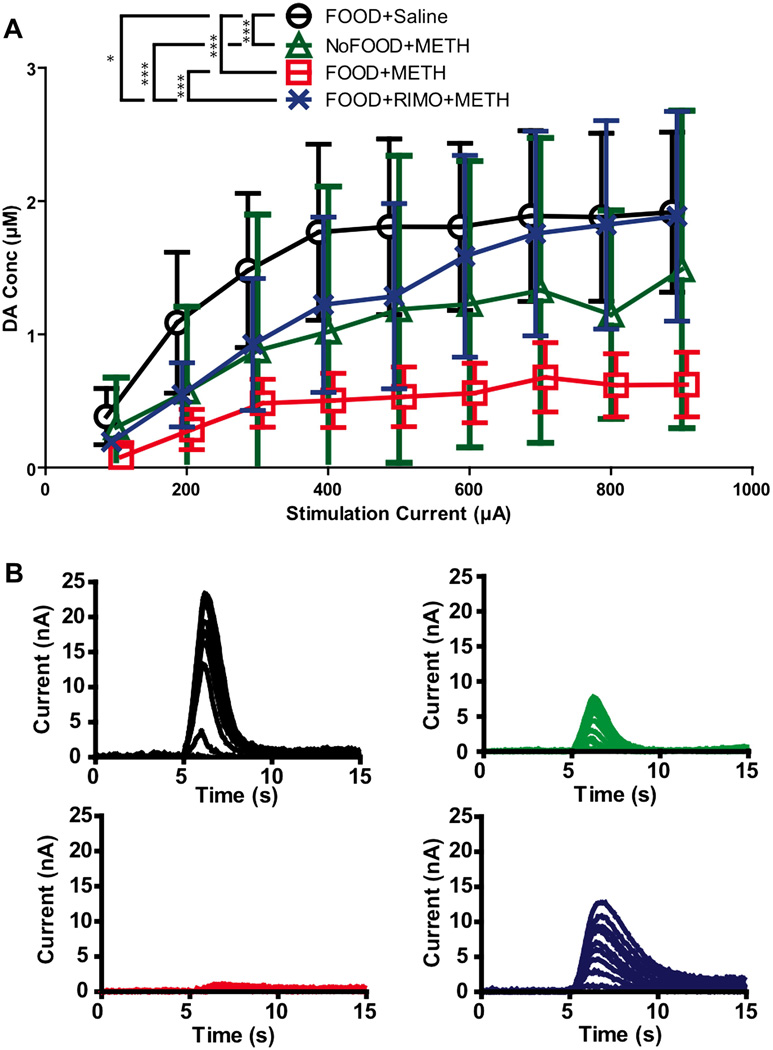
To assess whether these neurochemical changes persisted throughout a range of stimulations, the peak amplitude of the input-output experiment was also collected and analyzed. High stimulation parameters allowed for the assessment of the capacity of neurons to engage in phasic dopamine release, and the mobilization of the readily releasable pool (Zweifel et al., 2009). As phasic release plays a crucial role in the initiation of motivated behavior (Cheer et al., 2007a), this experiment bears strong behavioral relevance. Because there is a range of electrical activity that neurons exhibit and respond to, testing release at multiple stimulation amplitudes allowed for a robust assessment of terminal function. A two-way repeated measures ANOVA revealed a significant effect of treatment for peak amplitude in the input-output experiment (F(3,27) = 37.5, p < 0.0001). Since there was no significant interaction between stimulation and treatment (F(3,27) = 0.66, p = 0.88), values were collapsed across stimulations to examine the main effect of treatment. Post hoc analyses (Bonferroni: FOOD+Saline vs. NoFOOD+METH p < 0.001; FOOD+Saline vs. FOOD+METH p < 0.001; FOOD+Saline vs. FOOD+RIMO+METH p < 0.05; FOOD+METH vs. NoFOOD+METH p > 0.05; NoFOOD+METH vs. FOOD+RIMO+METH p < 0.001; FOOD+METH vs. FOOD+RIMO+METH p < 0.001) indicated that animals who received vehicle pretreatment and METH (FOOD+METH and NoFOOD+METH groups) exhibited reduced electrically-evoked dopamine release throughout the input output curve compared to animals who received either rimonabant pretreatment and METH (FOOD+RIMO+METH) or who received vehicle pretreatment and saline (FOOD+Saline). Rimonabant attenuated these METH-induced deficits (Figure 5), but animals pretreated with rimonabant still exhibited peak amplitudes that were lower than controls (FOOD+Saline). Thus, METH treatment was associated with a reduction in the peak amplitude of NAc dopamine release, and rimonabant pretreatment partially attenuated METH’s effect. This suggests that METH treatment is associated with a compromised releasable pool of dopamine nine days after administration and that this process involves CB1 receptors.
Figure. 5.
Input-output experiment: effects of METH and rimonabant. A, VTA stimulation current (100–900µA) vs. peak dopamine concentration in the NAc. (Mean ±SEM, Repeated Measures, One-Way ANOVA, Bonferroni’s post hoc test). B, Representative within- subject input output experiment plotted in current vs. time traces. Colors from A indicate group. Points are shifted in reference to NoFOOD+METH to display error bars: FOOD+Saline 6 points shifted right, FOOD+RIMO+METH 3 points shifted right, FOOD+METH is 3 points shifted left, NoFOOD+METH no shift. Differences in averaging are responsible for discrepancy between figure 5 and 3: FOOD+METH displays non significant but greater average peak amplitudes in the depth analysis, where values were averaged between subject within a group at each depth and analyzed by one-way ANOVA; NoFOOD+METH displays higher peak amplitudes in Figure 3 as it was analyzed by a two-way ANOVA as data was obtained from only one depth for each subject. [*p<.05, **p<.01,***p<.001]
4. Discussion
The novel findings described herein suggest that a single dose of METH is associated with neurochemical deficits at NAc dopamine terminals as long as nine days after administration. METH administration in an environment with reward-associated cues exacerbates markers of reduced dopamine terminal health. Importantly, CB1R blockade is associated with an attenuation of neurochemical markers of METH administration, implicating the endocannabinoid system in METH’s subsecond dopaminergic effects. While METH administration is associated with only a nonsignificant reduction in behavioral responding, animals pretreated with a CB1 receptor antagonist exhibit significantly greater behavioral responding than animals only treated with METH. The exact nature of the behavioral deficits exhibited by animals that received METH in the presence of reward-paired cues (FOOD+METH) cannot be determined given the experimental design. The high dose of METH used may have exerted motor impairments, lingering effects on appetite, or conditioned aversion which may underlie the marginal reductions in lever pressing on days following METH administration. Moreover, the large dose of METH may have been aversive; since animals were non-contigentally delivered pellets and consumed pellets on the day of drug administration, the association between the aversive qualities of METH and the food may have contributed to the reduced lever pressing for food on subsequent days through conditioned aversion. If RIMO did attenuate neurochemical effects of METH it may have also reduced associated aversive effects and therefore decreased the METH-induced reduction in lever pressing through inhibition of conditioned aversion. Animals did, however, work for food on subsequent days and thus any potential conditioned aversion must have been subtle if this interpretation is accurate. The experimental design cannot address the precise mechanism through which METH modulated behavior. However, the within-subject correlation between electrically-evoked dopamine levels and operant performance suggests that the behavioral effect may be dopamine-related, but this cannot be determined with the present results.
The finding that METH administration in the presence of reward-associated cues is accompanied by slower dopamine uptake supports our hypothesis that such cues are associated with greater dopaminergic effects when compared with animals that received the drug in the same environment in the absence of cue-driven responding. Furthermore, rimonabant pretreated animals do not exhibit indices of compromised DAT activity that accompany METH treatment in environments with reward-paired cues. We wish to caution the reader in the interpretation of both results as dopamine release was not measured when animals were administered METH or rimonabant and thus the cause of the observed effects cannot be determined. We propose, however, that the presentation of food-associated cues on METH administration days augmented METH-induced elevations in dopamine release and possibly free radical production. Dopamine is released in the NAc in response to reward-associated cues (Phillips et al., 2003; Roitman et al.,2004) and phasic midbrain dopamine neuron activity accompanies motivated behavior (Cheer et al., 2007a; Zweifel et al., 2009). Interestingly, animals that received METH without exposure to reward-associated cues (NoFOOD+METH) did not exhibit altered rates of reuptake. Although the mechanism cannot be ascertained with the experimental design, the proposed cue-induced elevation in dopamine may have been necessary to push the rises in dopamine from METH to a level that resulted in measurable deficits. In other words, the METH-induced increases in dopamine may have not been sufficient to cause alterations in release dynamics without the cue-induced augmentation of dopamine.
Of note, METH treatment was associated with equivalent reductions in the peak amplitude of dopamine release compared to FOOD+Saline and rimonabant-pretreated animals (FOOD+RIMO+METH), irrespective of whether cues were associated with reward. This suggests that METH treatment was accompanied by depletions to the releasable dopamine pool, which were uniformly attenuated by rimonabant. Reward-associated cues, however, were not linked with greater deficits of the peak amplitude of electrically-evoked release as FOOD+METH and NoFOOD+METH exhibited equal deficits of electrically-evoked release. Since only one report has assessed METH-induced dopaminergic alterations with anesthetized voltammetry (Howard et al., 2011), it is difficult to address this discrepancy between release and cues in the present report. Reductions in DAT are more sensitive than dopamine content alterations are to METH’s effects (O’Neil et al., 2006; Bjorklund et al., 2008) and may be more sensitive to lower doses of METH (Schwendt et al., 2009). Striatal DAT function is rapidly compromised by reactive oxygen species (Fleckenstein et al., 1997; Kiss et al., 2004). Thus, moderate augmentation of dopaminergic activity from reward-associated cues, and greater subsequent oxidative stress may be sufficient to exacerbate DAT loss of function, without significantly altering the ability of terminals to release. The peak amplitude of electrically-evoked release may therefore be less sensitive to minor changes in dopamine content than markers of DAT activity (Tau). Given the experimental design and assay used, however, we cannot determine the cause of this discrepancy. Operant training prior to METH administration may have influenced the dopamine alterations associated with METH given in environments paired with reward paired cues. We feel it is improbable that training influenced DAT function, given that enriched environments do not influence METH toxicity (Thiriet, 2011). Motor effects cannot be ruled out but appear to be an unlikely cause because lever pressing ceased four minutes following METH injection.
Rimonabant was associated with attenuated reductions in dopamine release and uptake that accompanied METH treatment. Rimonabant pretreatment was, however, linked with only partial protection against the diminished peak amplitude of dopamine release in the input output curve. While this group did show greater dopamine release than animals given METH without rimonabant (FOOD+METH and NoFOOD+METH), the RIMO group still showed lower peak amplitudes than control animals (FOOD+Saline). While rimonabant appears fully protective in the depth analysis, the high levels of stimulation used in the input output curve may reveal subtle alterations in terminal dynamics not visible at the lower stimulation levels used in the depth analysis. This indicates that while rimonabant pretreatment is associated with attenuated METH-induced alterations in dopamine release dynamics, it is not fully protective.
Although CB1R antagonism acutely reduces reward-directed behavior (Trujillo-Pisanty et al., 2011), it is unlikely that rimonabant influenced lever pressing three days after administration. If rimonabant did exert enduring effects it would likely result in reduced lever pressing, due to its anorectic effects (see De Vry, et al., 2004) instead of mitigating the decrease in lever pressing associated with METH. Moreover, rimonabant likely did not independently influence dopamine terminal activity, but rather modulated METH’s acute subsecond dopamine releasing effects. This is supported by our past work showing that rimonabant lacks acute effects at DAT (Cheer et al., 2007a) and data demonstrating the absence of CB1Rs on dopamine terminals (Lupica and Riegel, 2005). Since rimonabant is effective at reducing stimulant-induced elevations in NAc dopamine release (Cheer et al, 2007b), and phasic dopamine transients from cues associated with reward (Oleson et al, 2012), we argue that rimonabant may exert the effects seen here through attenuation of cue-evoked phasic release and reduced drug-induced dopamine augmentation, which are difficult to dissociate with our design. As diminished DAT activity is a result of oxidative stress (Fleckenstein et al., 1997; Kiss et al., 2004), putative rimonabant attenuation of acute METH-induced dopamine release may underlie rimonabant’s protection against markers of DAT depletion. We stress that this is speculation based upon sound inductive reasoning, given the experimental design and that rimonabant’s effects on free radical production under our specific experimental conditions are unknown.
Rimonabant’s effects are consistent with prior findings that endocannabinoids modulate psychostimulants’ effects; rimonabant inhibits relapse to both cocaine (De Vries et al., 2003) and methamphetamine (Anggadiredja et al., 2004). It reduces the acquisition and reinstatement of METH self-administration (Schindler et al., 2010), and METH-related reward memory in conditioned place preference experiments (Yu et al., 2011). CB1R blockade of stimulant reward may occur, at least in part, through attenuation of drug-induced increases in dopamine release. CB1R blockade in the NAc core also reduces self-administration of METH into that region (Rodriguez et al., 2011), suggesting that not only is METH capable of exerting rewarding effects when injected locally in NAc core, but that CB1Rs in this region modulate METH’s rewarding qualities. Likewise, we have shown CB1R antagonism into the NAc to decrease METH-induced stereotypy (Morra et al., 2010). These findings show that there is likely an interaction between METH and the endocannabinoid system in the NAc that bears behavioral significance. Although CB1Rs may modulate dopamine release locally in the NAc, rimonabant’s effects may also be mediated by the VTA (Lupica and Riegel., 2005; Oleson et al., 2012), where CB1Rs influence excitability of dopaminergic neurons (Lupica and Riegel, 2005; Szabo et al., 2002).
Factors other than those that acutely modulate monoaminergic activity can significantly influence METH-induced neurochemical alterations. For example, rises in body temperature exacerbate neurochemical depletions from METH (Albers and Sonsalla, 1995; Yuan et al., 2006), and reductions in METH-induced hyperthermia protect against dopamine deficits (Bowyer et al., 1993). However, rimonabant likely does not attenuate METH-induced alterations in dopamine dynamics through the prevention of hyperthermia. Hyperthermia is not required for METH-induced neurochemical deficits and modulation of neurotransmitter depletion does not always accompany temperature changes (see Krasanova and Cadet, 2009). Co-administration of rimonabant does not prevent hyperthermia from MDMA (Morley et al., 2004), a substituted amphetamine (Cadet et al., 2007) with similar hyperthermic, behavioral, neurotoxic and neurochemical effects as METH (Fantegrossi et al., 2008; Gough et al., 2002). Moreover, CB1R agonism is associated with hypothermia (Morley et al., 2004; Touriño et al., 2010) and it is thus unlikely that CB1 receptor blockade also induces hypothermia, which might otherwise explain the attenuation of METH-induced dopamine depletion associated with rimonabant pretreatment.
Although antioxidants mitigate METH-induced monoaminergic depletions (De Vito and Wagner, 1989; Hashimoto et al., 2004;), rimonabant lacks antioxidant activity (Tsvetanova et al. 2006). Thus, rimonabant’s amelioration of METH-induced potential oxidative stress and reduction of dopamine function is more likely a product of decreased dopamine release than inherent antioxidant characteristics.
As glutamate plays a crucial role in dopamine depletions from METH (see Riddle et al., 2006; Stephans and Yamamoto, 1994), rimonabant could exert its effects through prevention of METH-induced glutamate elevations. It is difficult to determine the role of glutamate in rimonabant’s associated effects given that the effects of METH on NAc glutamate are unclear. For example, systemic METH administration is not associated with a rise in glutamate in the NAc (Ago et al., 2011), and METH conditioned placed paradigms are not associated with changes in metabotropic glutamate receptor expression in the NAc (Herrold et al., 2011). While glutamate may play a role in striatal dopaminergic deficits that follow METH (see Riddle et al., 2006; Stephens and Yamamoto, 1994), its role in accumbal depletions is unclear. Future studies are required to ascertain glutamate’s precise role in the effects of rimonabant seen here. Likewise, VMAT2 has been implicated in dopaminergic alterations from METH (Fumagalli et al., 1999; Fleckenstein and Hanson, 2003), but we are unaware of any evidence implicating VMAT2 in accumbal dopaminergic deficits.
Our findings that METH is associated with compromised NAc dopamine terminal function conflicts with reports suggesting this region is relatively insensitive to METH’s dopamine-depleting effects (Eisch et al., 1992; Volz et al., 2007). Even within electrochemical measurements, our results are at odds with past amperometric work (Cass, 1997; Cass and Manning, 1999), reporting only modest METH effects on dopamine release in the NAc. Beyond potential differences between voltammetry and amperometry, our results may conflict on the bases of rat strain (Sprague-Dawley vs. Fisher), stimulation (electrical vs. potassium) and dosing regimen (high single-dose vs. low binge dose). Differences in assays employed may explain why the NAc is traditionally not thought to be susceptible to METHs effects: electrically- evoked dopamine release may detect more nuanced indices of compromised terminal function than binding assays (Anderson and Itzhak, 2006), immunohistochemical (Thomas et al., 2010) techniques, and HPLC (Thomas et al., 2010) that are frequently used in neurotoxicity research. Neurons may exhibit reduced capacity to release dopamine in response to electrical stimulation, while tissue content of dopamine markers may remain consistent. Finally, the high spatial and temporal resolution of voltammetry allows more specific detection of alterations in terminal function throughout the neuropil of the NAc.
The present study contributes significantly to present knowledge on the dopamine sequelae of METH exposure. We demonstrate that we can record neurochemical effects of METH nine days following administration, suggesting that a single METH bolus can initiate a cascade of accumbal dopamine terminal adaptations. We show that METH administration in environments with reward-associated cues exacerbates METH-induced neurochemical depletions, suggesting that the environment METH is taken in can have a negative impact that lasts beyond its acute effects. This is the first voltammetric demonstration of CB1 receptor-dependent METH-induced dopamine depletions in the NAc, an area previously thought to be resistant to such effects. Furthermore, this work adds to the growing literature suggesting the endocannabinoid system is a viable target for the modulation of the dopaminergic effects of abused drugs such as METH.
Methamphetamine produces enduring dopaminergic deficits in the accumbens
These effects are potentiated by reward predicting cues
And attenuated by CB1 receptor blockade
The endocannabinoid system is required for the subsecond dopamine response to methamphetamine
Acknowledgments
The authors would like to thank Mr. David Bernstein for expert technical assistance and Drs Bryan Yamamoto and Kristen Keefe for valuable input and comments. This research was funded by NIH grant DA022340 to JFC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Addy NA, Daberkow DP, Ford JN, Garris PA, Wightman RM. Sensitization of rapid dopamine signaling in the nucleus accumbens core and shell after repeated cocaine in rats. J Neurophysiol. 2010;104:922–931. doi: 10.1152/jn.00413.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ago Y, Araki R, Yano K, Hiramatsu N, Kawasaki T, Chaki S, Nakazato A, Onoe H, Hashimoto H, Baba A, Takuma K, Matsuda T. Activation of metabotropic glutamate 2/3 receptors attenuates methamphetamine-induced hyperlocomotion and increase in prefrontal serotonergic neurotransmission. Psychopharmacology (Berl) 2011;217:443–452. doi: 10.1007/s00213-011-2295-3. [DOI] [PubMed] [Google Scholar]
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. [PubMed] [Google Scholar]
- Alger BE, Kim J. Supply and demand for endocannabinoids. Trends Neurosci. 2011;34:304–315. doi: 10.1016/j.tins.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KL, Itzhak Y. Methamphetamine-induced selective dopaminergic neurotoxicity is accompanied by an increase in striatal nitrate in the mouse. Ann N Y Acad Sci. 2006;1074:225–233. doi: 10.1196/annals.1369.021. [DOI] [PubMed] [Google Scholar]
- Anggadiredja K, Nakamichi M, Hiranita T, Tanaka H, Shoyama Y, Watanabe S, Yamamoto T. Endocannabinoid system modulates relapse to methamphetamine seeking: possible mediation by the arachidonic acid cascade. Neuropsychopharmacology. 2004;29:1470–1478. doi: 10.1038/sj.npp.1300454. [DOI] [PubMed] [Google Scholar]
- Bjorklund NL, Sorg BA, Schenk JO. Neuronal dopamine transporter activity, density and methamphetamine inhibition are differentially altered in the nucleus accumbens and striatum with no changes in glycosylation in rats behaviorally sensitized to methamphetamine. Synapse. 2008;62:736–745. doi: 10.1002/syn.20528. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Gough B, Slikker W, Lipe GW, Newport GD, Holson RR. Effects of a cold environment or age on methamphetamine-induced dopamine release in the caudate putamen of female rats. Pharmacol Biochem Behav. 1993;44:87–98. doi: 10.1016/0091-3057(93)90284-z. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Cass WA. Decreases in evoked overflow of dopamine in rat striatum after neurotoxic doses of methamphetamine. J Pharmacol Exp Ther. 1997;280:105–113. [PubMed] [Google Scholar]
- Cass WA, Manning MW. Recovery of presynaptic dopaminergic functioning in rats treated with neurotoxic doses of methamphetamine. J Neurosci. 1999;19:7653–7660. doi: 10.1523/JNEUROSCI.19-17-07653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007a;54:237–244. doi: 10.1016/j.neuron.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Kendall DA, Mason R, Marsden CA. Differential cannabinoid-induced electrophysiological effects in rat ventral tegmentum. Neuropharmacology. 2003;44:633–641. doi: 10.1016/s0028-3908(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007b;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vito MJ, Wagner GC. Methamphetamine-induced neuronal damage: a possible role for free radicals. Neuropharmacology. 1989;28:1145–1150. doi: 10.1016/0028-3908(89)90130-5. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Homberg JR, Binnekade R, Raasø H, Schoffelmeer AN. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology (Berl) 2003;168:164–169. doi: 10.1007/s00213-003-1422-1. [DOI] [PubMed] [Google Scholar]
- De Vry J, Schreiber R, Eckel G, Jentzsch KR. Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur J Pharmacol. 2004;483:55–63. doi: 10.1016/j.ejphar.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Gaffney M, Weihmuller FB, O'Dell SJ, Marshall JF. Striatal subregions are differentially vulnerable to the neurotoxic effects of methamphetamine. Brain Res. 1992;598:321–326. doi: 10.1016/0006-8993(92)90201-j. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza R, Traynor JR, Woods JH. A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience. 2008;151:533–543. doi: 10.1016/j.neuroscience.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein AE, Hanson GR. Impact of psychostimulants on vesicular monoamine transporter function. Eur J Pharmacol. 2003;479:283–289. doi: 10.1016/j.ejphar.2003.08.077. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Beyeler ML, Gibb JW, Hanson GR. Oxygen radicals diminish dopamine transporter function in rat striatum. Eur J Pharmacol. 1997;334:111–114. doi: 10.1016/s0014-2999(97)01175-8. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci. 1999;19:2424–2431. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC, Cherner M, Grant I. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug Alcohol Depend. 2004;76:181–190. doi: 10.1016/j.drugalcdep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Gough B, Imam SZ, Blough B, Slikker W, Ali SF. Comparative effects of substituted amphetamines (PMA, MDMA, and METH) on monoamines in rat caudate: a microdialysis study. Ann N Y Acad Sci. 2002;965:410–420. doi: 10.1111/j.1749-6632.2002.tb04182.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Shimizu E, Iyo M. Protective effects of N-acetyl-L-cysteine on the reduction of dopamine transporters in the striatum of monkeys treated with methamphetamine. Neuropsychopharmacology. 2004;29:2018–2023. doi: 10.1038/sj.npp.1300512. [DOI] [PubMed] [Google Scholar]
- Herrold AA, Voigt RM, Napier TC. Brain region-selective cellular redistribution of mGlu5 but not GABA(B) receptors following methamphetamine-induced associative learning. Synapse. 2011;65:1333–1343. doi: 10.1002/syn.20968. [DOI] [PubMed] [Google Scholar]
- Howard CD, Keefe KA, Garris PA, Daberkow DP. Methamphetamine neurotoxicity decreases phasic, but not tonic, dopaminergic signaling in the rat striatum. J Neurochem. 2011;118:668–676. doi: 10.1111/j.1471-4159.2011.07342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Ali SF. Aging increases the susceptiblity to methamphetamine-induced dopaminergic neurotoxicity in rats: correlation with peroxynitrite production and hyperthermia. J Neurochem. 2001;78:952–959. doi: 10.1046/j.1471-4159.2001.00477.x. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 2005;102:868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian MD, Martin AB, Cuellar B, Rodriguez De Fonseca F, Navarro M, Moratalla R, Garcia-Segura LM. Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience. 2003;119:309–318. doi: 10.1016/s0306-4522(03)00070-8. [DOI] [PubMed] [Google Scholar]
- Kiss JP, Zsilla G, Vizi ES. Inhibitory effect of nitric oxide on dopamine transporters: interneuronal communication without receptors. Neurochem Int. 2004;45:485–489. doi: 10.1016/j.neuint.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48:1105–1116. doi: 10.1016/j.neuropharm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Morley KC, Li KM, Hunt GE, Mallet PE, McGregor IS. Cannabinoids prevent the acute hyperthermia and partially protect against the 5-HT depleting effects of MDMA ("Ecstasy") in rats. Neuropharmacology. 2004;46:954–965. doi: 10.1016/j.neuropharm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Morra JT, Glick SD, Cheer JF. Neural encoding of psychomotor activation in the nucleus accumbens core, but not the shell, requires cannabinoid receptor signaling. J Neurosci. 2010;30:5102–5107. doi: 10.1523/JNEUROSCI.5335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Beckert MV, Morra JT, Lansink CS, Cachope R, Abdullah RA, Loriaux AL, Schetters D, Pattij T, Roitman MF, Lichtman AH, Cheer JF. Endocannabinoids shape accumbal encoding of cue-motivated behavior via CB1 receptor activation in the ventral tegmentum. Neuron. 2012;73:360–373. doi: 10.1016/j.neuron.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil ML, Kuczenski R, Segal DS, Cho AK, Lacan G, Melega WP. Escalating dose pretreatment induces pharmacodynamic and not pharmacokinetic tolerance to a subsequent high-dose methamphetamine binge. Synapse. 2006;60:465–473. doi: 10.1002/syn.20320. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. AAPS J. 2006;8:E413–E418. doi: 10.1007/BF02854914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JS, Boctor SY, Flores LC, Phelix CF, Martinez JL. Local pretreatment with the cannabinoid CB1 receptor antagonist AM251 attenuates methamphetamine intra-accumbens self-administration. Neurosci Lett. 2011;489:187–191. doi: 10.1016/j.neulet.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Gilman JP, Justinova Z, Vemuri VK, Makriyannis A, Goldberg SR. Effects of cannabinoid receptor antagonists on maintenance and reinstatement of methamphetamine self-administration in rhesus monkeys. Eur J Pharmacol. 2010;633:44–49. doi: 10.1016/j.ejphar.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: roles for glutamate and dopamine efflux. Synapse. 1994;17:203–209. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Rockville, MD: 2010. The DAWN Report: Emergency Department Visits Involving Methamphetamine: 2004 to 2008. [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Siemes S, Wallmichrath I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci. 2002;15:2057–2061. doi: 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- Thiriet N, Gennequin B, Lardeux V, Chauvet C, Decressac M, Janet T, Jaber M, Solinas M. Environmental enrichment does not reduce the rewarding and neurotoxic effects of methamphetamine. Neurotox Res. 2011;19:172–182. doi: 10.1007/s12640-010-9158-2. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Angoa Pérez M, Francescutti-Verbeem DM, Shah MM, Kuhn DM. The role of endogenous serotonin in methamphetamine-induced neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2010;115:595–605. doi: 10.1111/j.1471-4159.2010.06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touriño C, Zimmer A, Valverde O. THC Prevents MDMA Neurotoxicity in Mice. PLoS One. 2010;5:e9143. doi: 10.1371/journal.pone.0009143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo-Pisanty I, Hernandez G, Moreau-Debord I, Cossette MP, Conover K, Cheer JF, Shizgal P. Cannabinoid receptor blockade reduces the opportunity cost at which rats maintain operant performance for rewarding brain stimulation. J Neurosci. 2011;31:5426–5435. doi: 10.1523/JNEUROSCI.0079-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanova E, Kessiova M, Alexandrova A, Petrov L, Kirkova M, Todorov S. In vivo effects of CB1 receptor ligands on lipid peroxidation and antioxidant defense systems in the rat brain of healthy and ethanol-treated rats. Pharmacol Rep. 2006;58:876–883. [PubMed] [Google Scholar]
- Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007;102(Suppl 1):44–48. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Wenger T, Fürst S. Role of endogenous cannabinoids in cerebral reward mechanisms. Neuropsychopharmacol Hung. 2004;6:26–29. [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatr Med. 1985;3:445–460. [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther. 1998;287:107–114. [PubMed] [Google Scholar]
- Yu LL, Zhou SJ, Wang XY, Liu JF, Xue YX, Jiang W, Lu L. Effects of cannabinoid CB• receptor antagonist rimonabant on acquisition and reinstatement of psychostimulant reward memory in mice. Behav Brain Res. 2011;217:111–116. doi: 10.1016/j.bbr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Yuan J, Hatzidimitriou G, Suthar P, Mueller M, McCann U, Ricaurte G. Relationship between temperature, dopaminergic neurotoxicity, and plasma drug concentrations in methamphetamine-treated squirrel monkeys. J Pharmacol Exp Ther. 2006;316:1210–1218. doi: 10.1124/jpet.105.096503. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]