Abstract
Objective
The aim of this study was to identify factors associated with the initiation of biologic agents for the treatment of rheumatoid arthritis (RA) in a large US observational cohort.
Methods
Semiannual patient-reported data in the ARAMIS (Arthritis, Rheumatism and Aging Medical Information System) data bank from January 1998 to January 2006 were analyzed retrospectively using pooled logistic regression (with adjustment for center-level and temporal effects) to identify patient-, disease-, and treatment-related characteristics associated with the initiation of biologics for the treatment of RA.
Results
The analysis included 1545 patients from 7 US centers. By 2006, 41.4% of 679 patients remaining in the sample had received biologics. Initiation of biologics was significantly associated with greater disability in the previous 6-month period (per 1-unit increase in Health Assessment Questionnaire score: odds ratio [OR] = 1.45; 95% CI, 1.22–1.72; P < 0.01) and treatment in the previous period with steroids (OR = 2.24; 95% CI, 1.76–2.85; P < 0.01) or nonbiologic disease-modifying antirheumatic drugs (OR = 2.43; 95% CI, 1.71–3.46; P < 0.01). Two sociodemographic factors were significant predictors of decreased use of biologics: older age (per 10 years: OR = 0.74; 95% CI, 0.66–0.82; P < 0.01) and lower annual income (per $10,000 reduction: OR = 0.95; 95% CI, 0.91–1.00; P = 0.04). There were no significant differences with respect to sex, race, employment status, comorbidity, previous NSAID use, or treatment center.
Conclusions
Disease- and treatment-related factors were significant predictors of the initiation of biologics for RA. Independent of these factors, however, biologics were less often used in patients who were older and those with lower incomes. Use of biologics increased steadily over the period studied.
Keywords: tumor necrosis factor–α antagonist, biologics, rheumatoid arthritis, prescription, age factors, inequalities
INTRODUCTION
Over the past decade, the US Food and Drug Administration (FDA) has approved numerous biologic agents for the treatment of patients with moderate to severe rheumatoid arthritis (RA) that has not responded to ≥1 nonbiologic disease-modifying antirheumatic drug (DMARD). Biologics are not routinely prescribed for all patients with RA, in part because they are expensive ($16,000–$20,000 per year)1 and because there was initial concern about their potential for long-term adverse effects.2,3
The first 4 biologics for the treatment of RA were approved over a 4-year period beginning in 1998—the 3 tumor necrosis factor–α (TNF-α) antagonists etanercept, infliximab, and adalimumab, and the interleukin (IL)-1 receptor antagonist anakinra (Table I).4,5 Abatacept, a modulator of T-cell activation, was approved in December 2005. Rituximab, a monoclonal antibody against the CD20 antigen that was initially marketed for the treatment of non-Hodgkin’s lymphoma, was approved for the treatment of RA in March 2006. A number of new biologics have since been approved for RA or are in clinical development, including an IL-6 inhibitor (tocilizumab), modified TNF-α antagonists (golimumab and certolizumab pegol), and monoclonal antibodies against various cytokines or targeting β-cells (ocrelizumab and ofatumumab).4,5
Table I.
Biologics used for the treatment of rheumatoid arthritis.5
| Mechanism/Agent | Description | Date of FDA Approval |
|---|---|---|
| TNF-α antagonism | ||
| Etanercept | Soluble TNF-receptor fusion protein | November 1998 |
| Infliximab | Monoclonal antibody to TNF-α (chimeric) | November 1999 |
| Adalimumab | Monoclonal antibody to TNF-α (human) | December 2002 |
| Golimumab | Monoclonal antibody to TNF-α (human) | April 2009 |
| Certolizumab pegol | Monoclonal antibody to TNF-α, pegylated (human) | May 2009 |
| IL-1 inhibition | ||
| Anakinra | IL-1 receptor antagonist | November 2001 |
| β-Cell depletion | ||
| Rituximab | Monoclonal antibody to CD20 | March 2006 |
| T-Cell costimulation inhibition | ||
| Abatacept | CTLA-4 immunoglobulin fusion protein | December 2005 |
FDA = US Food and Drug Administration; TNF-α = tumor necrosis factor–α; IL = interleukin; CTLA-4 = cytotoxic T-lymphocyte antigen 4.
The available biologics have different formulations and mechanisms of action, and no head-to-head randomized clinical trials have as yet been performed. Two recent meta-analyses found that the effectiveness of TNF-α antagonists in RA did not differ significantly, but all were more effective than anakinra.6,7 However, before the publication of these analyses, there were no data to guide the selection of one biologic over another based on efficacy.
Randomized controlled trials have suggested that TNF-α antagonists may, on rare occasions, be associated with an increased risk of serious infection or malignancy.8 TNF-α antagonists are to be used with caution in patients with a history of chronic or recurrent infections.5 TNF-α antagonists may exacerbate or precipitate congestive heart failure (CHF), and their use is contraindicated in patients with CHF.9 Because of the known risk for reactivation of latent tuberculosis with the use of TNF-α antagonists, candidates for therapy with a biologic should first be screened for the disease, and those with a positive result should receive antituberculosis treatment.10 Overall, patients with comorbidities may be less likely to be prescribed a biologic agent. However, there is no clinical rationale for the differential prescribing of biologics based on demographic characteristics such as age, race, and sex.11 Nonetheless, some studies have found that older age may be related to less aggressive treatment of RA,12,13 although this has not been a consistent finding.14
Previous research has suggested that among patients treated with biologics, insurance coverage may influence which type of biologic is prescribed (injectable vs infused).15 Although not specific to patterns of RA treatment, there is extensive literature documenting gender and racial disparities in many areas of health care.16,17 Given the high cost of biologics and the fact that they are prescribed by specialists (rheumatologists), it is plausible that similar disparities may occur in the use of biologics, with treatment disparities contributing to potentially worse outcomes in socioeconomically disadvantaged populations. This may be particularly important in RA, as it is now accepted that early, aggressive treatment is essential to reducing long-term damage associated with the disease.18
The objective of this study was to estimate the effects of sociodemographic characteristics, disease-related factors, and previous medication use on the initiation of biologics in a large observational cohort of RA patients under the care of rheumatologists in the United States, while controlling for center-level and temporal effects.
PATIENTS AND METHODS
This retrospective cohort study used data from the ARAMIS (Arthritis, Rheumatism and Aging Medical Information System) data bank, a large observational database managed by Stanford University that has been collecting longitudinal patient-reported data on medication use, disease status, and demographics in North America on a semiannual basis for nearly 3 decades.19,20 It includes data on >5000 patients with RA. The present study employed data collected from January 1998 to January 2006. Conduct of this study of ARAMIS data was approved by the Duke University Health System Institutional Review Board.
The study cohort consisted of consecutive RA patients recruited by participating study physicians during routine clinic visits to community-based clinics, private practices, and academic rheumatology referral centers located in various geographic regions of the United States (Northeast, South, Midwest, and West). Every 6 months, patients completed detailed multidimensional questionnaires on health status, medication use, adverse effects, disability, pain and other symptoms, socioeconomic status, and health care utilization. 20 Data collection was conducted primarily by mail. Each center included in the analysis represented multiple locations within a geographic region. Centers that contributed patients to the data bank for at least 2 sequential 6-month periods during the study period were included in the analysis.
Patient-related variables included age, any reported comorbidity, disease duration (years), education (years), employment status (employed vs not employed [disabled, homemaker, retired, student, other]), mean annual household income during the study (indexed to 1998 US dollars), insurance type (managed care, fee for-service, Medicare, Medicaid, government, other, none), race (black, Hispanic, white, other [Asian, Native American]), sex, and treatment center.
Disease-related variables included disability, as measured on the Health Assessment Questionnaire (HAQ) (score range, 0–3), and pain (scored from 0–3, 0–20, or 0–100 across different phases of data collection). Reported medications for the treatment of RA were grouped by drug class (ie, NSAIDs, steroids, nonbiologic DMARDs, and biologics).
Statistical Analysis
Baseline was defined as 1998 or, if the patient was enrolled later, the year of study entry. Baseline characteristics of patients who had ever used biologics during the study period (“ever users”) and patients who had never used biologics during the study period (“never users”) were reported as frequencies or means. Baseline comparisons of ever users and never users were conducted using t tests and χ2 tests, as appropriate.
To examine the relationship between potential predictive factors and the initiation of biologics, a pooled logistic regression was performed. In this method, repeated assessments for the same patient are treated as multiple observations in the data, and a logistic regression is performed that makes allowance for time-varying covariates and correlation among repeated observations.21 The outcome variable in the present model was dichotomous, indicating whether the patient had or had not initiated therapy with a biologic agent in the previous 6 months. A record was included in the analysis for each 6-month assessment until the patient received a biologic, at which point further assessments were excluded. Independent variables included the demographic-, disease-, and treatment-related characteristics listed in Table II. Variance inflation factors were used to assess multicollinearity before modeling. Two variables that exceeded the standard cutoff of 10 were excluded—education level (41.0) and insurance (24.1). The pain variable was excluded due to a change in response options (from ordinal to continuous) between survey phases.
Table II.
Characteristics of patients at study entry.
| Characteristic | Total Cohort (n = 1545) |
Ever Users of Biologics (n = 515) |
Never Users of Biologics (n = 1030) |
P, Ever Users Versus Never Users* |
|---|---|---|---|---|
| Age in 1998, mean, y | 57.0 | 52.1 | 59.5 | <0.01 |
| Sex, (%) | <0.01 | |||
| Female | 78.4 | 82.9 | 76.2 | |
| Male | 21.6 | 17.1 | 23.8 | |
| Race, (%) | 0.07 | |||
| White | 86.8 | 88.1 | 86.0 | |
| Black/African-American | 6.5 | 5.7 | 6.8 | |
| Hispanic | 4.3 | 4.9 | 3.9 | |
| Other | 2.5 | 1.2 | 3.2 | |
| Education, mean, y | 13.8 | 14.1 | 13.7 | 0.02 |
| Employed, (%) | 36.7 | 45.9 | 32.1 | <0.01 |
| Total income, mean, $000s† | 46.6 | 52.4 | 42.8 | <0.01 |
| Comorbidity (ever reported another medical problem), % | 68.3 | 79.4 | 62.7 | <0.01 |
| Disease duration, mean, y | 16.2 | 14.1 | 17.3 | <0.01 |
| HAQ score,‡ mean | ||||
| Baseline | 1.08 | 1.08 | 1.07 | 0.82 |
| Maximum during study | 1.44 | 1.53 | 1.39 | <0.01 |
HAQ = Health Assessment Questionnaire.
t Tests were used to compare continuous variables (age, disease duration, HAQ, income, years of education), and χ2 tests were used to compare categorical variables (comorbidity, employment, race/ethnicity, sex).
Indexed to 1998 dollars.
HAQ scores range from 0 to 3.
In the model, the explanatory variables any comorbidity, HAQ scores, and type of RA treatment (ie, NSAIDs, nonbiologic DMARDs, and steroids) could vary across 6-month intervals. Lagged values (ie, recorded 6 months earlier) for the measure of functional disability (HAQ) were used because the value at the visit at which use of a biologic was first recorded might have been affected by use of the biologic in the preceding interval. Similarly, variables for previous drug treatment reflected drug use recorded at the previous assessment. To control for potentially disproportionate use of biologics across participating centers, indicator variables were included in the regression model to represent individual centers, except for the grouping of 3 centers that represented only 3% of the study sample. Finally, variables representing calendar years were included to account for any secular trend representing increased use of biologics over time.
Comorbidity data were not collected in 2 assessment periods, and income was collected in only a few assessment periods. Missing comorbidity data were imputed using the last-observation-carried-forward method. There was a moderate amount of missing data for mean income (15.1%) and disease duration (10.7%), both of which were addressed using the missing-indicator method.22
All statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, North Carolina). The results are presented as odds ratios (OR) and 95% CIs. P values <0.05 were considered statistically significant.
RESULTS
The analysis included a cumulative total of 1545 patients from 7 US centers. One Canadian center and 1 US center without sequential data were excluded. The mean duration of follow-up was 4.2 years. Of the 863 participants who contributed data in 1998, ~40% remained in the cohort in 2006.
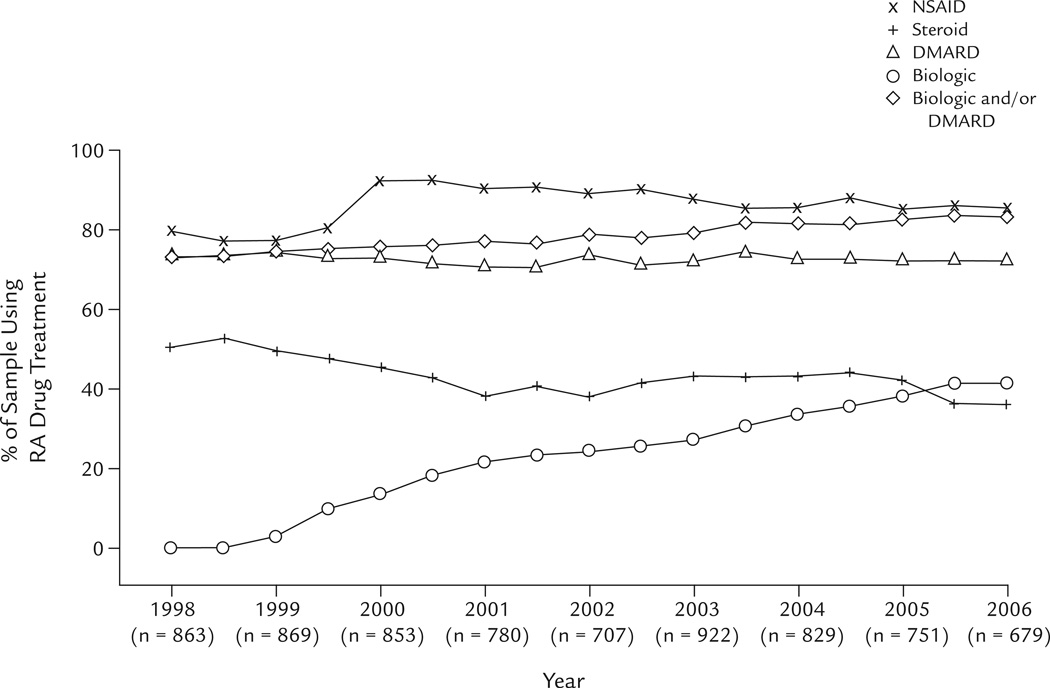
The percentages of the sample using NSAIDs, steroids, nonbiologic DMARDs, and biologics in each year of the study are displayed in the figure. Use of biologics increased monotonically from 13.5% (115/853) in 2000 to 41.4% (281/679) in 2006, although the rate of increase appeared to decline over the last 6 months of the study. NSAID use increased from 79.6% in 1998, peaked at 92.0% in 2000 (reflecting the introduction of the cyclooxygenase-2–selective inhibitors celecoxib [December 1998] and rofecoxib [May 1999]), and decreased to 85.3% in 2006. The percentage of nonbiologic DMARD users remained roughly constant at 73.0% in 1998 and 72.2% in 2006. In contrast, use of steroids declined from 50.5% in 1998 to 35.9% in 2006. Among the cumulative sample, 33.3% initiated a biologic at some point during their participation in the longitudinal study.
Figure 1.
Percentages of the sample receiving drug treatment for rheumatoid arthritis (RA) from 1998 to 2006. DMARD = nonbiologic disease-modifying antirheumatic drug.
Characteristics of users and nonusers of biologics are summarized in Table II. Compared with nonusers, users of biologics were significantly younger (P < 0.01), were more likely to be female (P < 0.01), had more years of education (P = 0.02), were more likely to be employed (P < 0.01), had a higher mean income (P < 0.01), and had a shorter duration of disease (P < 0.01). A higher percentage of users of biologics reported having a comorbid condition (P < 0.01). Baseline HAQ scores were statistically similar across groups, but maximal reported HAQ scores during the study period were higher among biologic users (P < 0.01). The highest reported HAQ score occurred after the initiation of biologics in 41.8% of biologic users. Use of biologics was proportionately balanced across centers during the study period (Table III).
Table III.
Use of biologics, by treatment center (N = 1545; n = 515 biologic users).*
| Biologic Users |
||||
|---|---|---|---|---|
| Region/ Treatment Center |
No. of Patients (% of Total Cohort) |
No. | % of Cohort |
% of Center |
| West 1 | 330 (21.4) | 114 | 22.1 | 34.5 |
| West 2 | 413 (26.7) | 133 | 25.8 | 32.2 |
| West 3 | 8 (0.5) | 1 | 0.2 | 12.5 |
| Midwest 1 | 176 (11.4) | 58 | 11.3 | 33.0 |
| Midwest 2 | 583 (37.7) | 195 | 37.9 | 33.4 |
| Northeast | 22 (1.4) | 8 | 1.6 | 36.4 |
| South | 13 (0.8) | 6 | 1.2 | 46.2 |
Difference between centers: P = 0.80, χ2 test.
Table IV reports the results of the adjusted regression model. Significant independent predictors of the initiation of biologics were age (per 10 years: OR = 0.74; 95% CI, 0.66–0.82; P < 0.01), annual household income (per $10,000 reduction: OR = 0.95; 95% CI, 0.91–1.00; P = 0.04), HAQ in the previous 6-month period (per unit increase: OR = 1.45; 95% CI, 1.22 – 1.72; P < 0.01), previous steroid use (OR = 2.24; 95% CI, 1.76–2.85; P < 0.01), and previous DMARD use (OR = 2.43; 95% CI, 1.71–3.46; P < 0.01). Variables that did not have a significant independent effect in the model were sex, race, employment status, comorbidity, disease duration, previous NSAID use, and treatment center.
Table IV.
Pooled logistic regression model of the initiation of treatment with biologics for rheumatoid arthritis.
| Variable | Adjusted OR (95% CI) | P |
|---|---|---|
| Age (per 10 y) | 0.74 (0.66–0.82) | <0.01 |
| Female sex | 1.15 (0.86–1.54) | 0.34 |
| Race | 0.43 | |
| White (reference) | 1.00 | |
| Black/African American | 1.03 (0.58–1.84) | |
| Hispanic | 0.82 (0.49–1.36) | |
| Other | 0.50 (0.19–1.36) | |
| Employment status | 0.52 | |
| Employed (reference) | 1.00 | |
| Not employed (disabled, homemaker, | ||
| retired, student, other) | 0.90 (0.66–1.23) | |
| Income (per $10,000 reduction) | 0.95 (0.91–1.00) | 0.04 |
| Comorbidity | 0.83 (0.66–1.04) | 0.11 |
| Disease duration (per 10 y) | 0.91 (0.81–1.03) | 0.12 |
| Calendar year | <0.01 | |
| 1999 (reference) | 1.00 | |
| 2000 | 2.68 (1.41–5.10) | |
| 2001 | 2.77 (1.44–5.30) | |
| 2002 | 1.95 (0.98–3.88) | |
| 2003 | 1.51 (0.74–3.06) | |
| 2004 | 2.47 (1.26–4.84) | |
| 2005 | 2.13 (1.06–4.25) | |
| 2006 | 2.31 (1.14–4.68) | |
| Lagged HAQ scores (per 1 unit) | 1.45 (1.22–1.72) | <0.01 |
| Previous NSAID use | 0.98 (0.73–1.33) | 0.91 |
| Previous steroid use | 2.24 (1.76–2.85) | <0.01 |
| Previous DMARD use | 2.43 (1.71–3.46) | <0.01 |
| Treatment center | 0.18 | |
| West 1 (reference) | 1.00 | |
| West 2 | 0.77 (0.57–1.05) | |
| Midwest 1 | 0.87 (0.54–1.38) | |
| Midwest 2 | 0.67 (0.48–0.92) | |
| Northeast, South, and West 3 | 0.68 (0.33–1.41) |
OR = odds ratio; HAQ = Health Assessment Questionnaire; DMARD = nonbiologic disease-modifying anti-rheumatic drug.
DISCUSSION
Based on FDA indications for the use of biologics in RA, it was anticipated that users of biologics would have moderate to severe disease that did not respond to ≥1 DMARD. In this retrospective cohort study, previous steroid use, previous nonbiologic DMARD use, and higher levels of disability (based on HAQ scores) were independent predictors of the initiation of biologics. However, disease factors alone did not govern who received biologics in this observational patient cohort; age and income also had an influence.
In 2006, at the end of the 8-year study period, 41.4% of patients remaining in the study cohort had reported using biologics, reflecting rapid adoption in the United States. This is consistent with the findings of a large survey of US rheumatologists (N = 1023) published in 2005, in which 42.3% of respondents reported prescribing biologics for 20% to 33% of their RA patients, and 44.2% reported prescribing biologics for ≥50% of patients.23 These estimates from the United States far exceed estimates from other countries. For example, estimates from Germany24 and France25 suggest use of biologics in ~10% of the RA population, and an Australian study suggests an estimate of ~1%.26 These practice variations may stem from relatively restricted access to these agents in countries where utilization is guided by advisory boards that consider the cost-effectiveness of therapies.26,27
In the present study, younger age was a significant predictor of the use of biologics, despite the fact that clinical trials have reported no difference in the efficacy or tolerability of biologics in patients aged <65 and ≥65 years.5 Although it is unclear why younger patients were preferentially treated with biologics, this finding is consistent with other claims-based13 and observational12 studies that have found relative under-use of nonbiologic DMARDs and biologics with increasing patient age. A potential explanation is the increased prevalence of comorbid conditions among older patients, although this factor should have been accounted for in the regression model. Other possibilities include patient or physician preferences, a perception that older patients may not be amenable to treatment with biologics, a fear of increased risk of infection among the elderly, or a potential age effect on the part of treating physicians. In a study by Fraenkel et al28 in which 204 US rheumatologists were presented with a standard case scenario that differed only in terms of patient age, respondents were less likely to prefer more aggressive treatment for the older RA patient than for the younger RA patient (71% vs 87%, respectively; P < 0.01). The present study’s finding of a decreased use of biologics in older patients (after adjustment for factors such as comorbidity, disability, disease duration, and income) supports the existence of an age bias in RA treatment decisions.
In this analysis, use of biologics was significantly decreased in those with a lower income (P < 0.01). Given the increased cost-shifting to consumers by health care plans,29 the large price differential between biologics and nonbiologic DMARDs, and the out-of-pocket costs to patients, it is not unexpected that income would be a significant predictor of the initiation of biologics.
The possibility of differential access to biologics based on income and age raises questions of equity. As reported by Goldman et al,30 increased patient cost-sharing is associated with decreased drug adherence and persistence with many prescription drugs. It is unfortunate that the data set did not provide information on prescription drug coverage, or, more specifically, on the generosity of this coverage. As insurance companies continue to shift a larger proportion of costs to their beneficiaries through higher deductibles, copayments, and coinsurance, patient income may become an increasing barrier to the initiation of biologics or a factor in their early discontinuation, potentially affecting ultimate patient outcomes.
This study was limited by a lack of generalizability, given its inclusion of 7 centers, underrepresentation of the nonwhite population (13.4% of the study population vs an estimated 24.9% of the US population in 200031), and no separate categories for race and ethnicity. In a cohort study of RA patients treated in rheumatology clinics in Texas between 1994 and 2000, Suarez-Almazor et al32 found that nonwhite patients were more likely to have delayed initiation of nonbiologic DMARD and biologic therapy. Although the multivariable regression analysis in the present study found no significant racial disparity after controlling for other patient-level factors, the potential effect on the use of biologics may have been underestimated relative to the general population if nonspecialist providers are less likely to initiate nonbiologic DMARD and biologic therapy than were the rheumatologists in this sample.13,33 Other studies have found that black patients are less likely than white patients to be referred to a specialist.34,35 If the same referral patterns exist for RA, black RA patients, as a whole, may be less likely to initiate biologics than are white patients.
The study data were limited in that all data except the RA diagnosis and referral center were provided by patient self-report. Beyond specialty, physician characteristics were not available. Laboratory values or joint counts (tender and/or swollen joint counts, loss of motion) were not available as measures of disease activity. The income variable may not have adequately reflected assets and, by extension, the ability to pay. As mentioned previously, the health insurance variable was omitted due to multicollinearity. In any event, this variable did not specify useful information such as drug coverage or levels of coinsurance. Collection of these types of data in future database projects could be useful from a policy perspective.
The results of this study highlight the importance of following standard guidelines for RA treatment and implementing quality measures to reinforce adherence and promote the best outcomes for all patients with RA. In a climate of diminishing insurance coverage and more cost-shifting to patients, it will be increasingly important to be aware of potential financial barriers to the use of this class of therapy.
CONCLUSIONS
In this observational cohort of RA patients in the care of US rheumatologists, use of biologics was significantly and independently associated with previous use of steroids and DMARDs and greater disability (as measured on the HAQ). Some factors unrelated to the disease and its treatment were also significantly associated with receipt of biologics: younger age and higher income were predictors of an increased likelihood of initiating treatment with biologics.
ACKNOWLEDGMENTS
This analysis was supported by an Engalitcheff Outcomes Initiative grant from the Maryland chapter of the Arthritis Foundation. Dr. Morgan DeWitt is also supported by an award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (5U01-AR-52186).
The authors thank Bharathi Lingala of Stanford University for assistance with database management and James F. Fries and Bonnie Bruce for access to the ARAMIS data bank. They also thank the following current or former research assistants and fellows at Duke Clinical Research Institute: Chia-Hung Chou, Michaela Dinan, Maria Fawzy, and Alice Fortune-Greeley.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have indicated that they have no conflicts of interest with regard to the content of this article.
References
- 1.Bansback NJ, Regier DA, Ara R, et al. An overview of economic evaluations for drugs used in rheumatoid arthritis: Focus on tumour necrosis factor-alpha antagonists. Drugs. 2005;65:473–496. doi: 10.2165/00003495-200565040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Pressman Lovinger S. Use of biologics for rheumatoid arthritis tempered by concerns over safety, cost. JAMA. 2003;289:3229–3230. doi: 10.1001/jama.289.24.3229. [DOI] [PubMed] [Google Scholar]
- 3.American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the man- agement of rheumatoid arthritis: 2002 Update. Arthritis Rheum. 2002;46:328–346. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 4.Senolt L, Vencovský J, Pavelka K, et al. Prospective new biological therapies for rheumatoid arthritis. Autoimmun Rev. doi: 10.1016/j.autrev.2009.03.010. [published online ahead of print March 26] [DOI] [PubMed] [Google Scholar]
- 5.Drugs@FDA. [Accessed July 31, 2009];FDA approved drug products. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- 6.Gartlehner G, Hansen RA, Jonas BL, et al. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: A systematic review and metaanalysis. J Rheumatol. 2006;33:2398–2408. [PubMed] [Google Scholar]
- 7.Nixon R, Bansback N, Brennan A. The efficacy of inhibiting tumour necrosis factor alpha and interleukin 1 in patients with rheumatoid arthritis: A meta-analysis and adjusted indirect comparisons. Rheumatology (Oxford) 2007;46:1140–1147. doi: 10.1093/rheumatology/kem072. [DOI] [PubMed] [Google Scholar]
- 8.Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and metaanalysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [published correction appears in JAMA 2006;295:2482] [DOI] [PubMed] [Google Scholar]
- 9.Kwon HJ, Coté TR, Cuffe MS, et al. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med. 2003;138:807–811. doi: 10.7326/0003-4819-138-10-200305200-00008. [DOI] [PubMed] [Google Scholar]
- 10.Gardam MA, Keystone EC, Menzies R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: Mechanisms of action and clinical management. Lancet Infect Dis. 2003;3:148–155. doi: 10.1016/s1473-3099(03)00545-0. [DOI] [PubMed] [Google Scholar]
- 11.Genevay S, Finckh A, Ciurea A, et al. for the Physicians of the Swiss Clinical Quality Management Program for Rheumatoid Arthritis. Tolerance and effectiveness of anti-tumor necrosis factor alpha therapies in elderly patients with rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007;57:679–685. doi: 10.1002/art.22688. [DOI] [PubMed] [Google Scholar]
- 12.Tutuncu Z, Reed G, Kremer J, Kavanaugh A. Do patients with olderonset rheumatoid arthritis receive less aggressive treatment? Ann Rheum Dis. 2006;65:1226–1229. doi: 10.1136/ard.2005.051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmajuk G, Schneeweiss S, Katz JN, et al. Treatment of older adult patients diagnosed with rheumatoid arthritis: Improved but not optimal. Arthritis Rheum. 2007;57:928–934. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- 14.Harrison MJ, Kim CA, Silverberg M, Paget SA. Does age bias the aggressive treatment of elderly patients with rheumatoid arthritis? J Rheumatol. 2005;32:1243–1248. [PubMed] [Google Scholar]
- 15.DeWitt EM, Glick HA, Albert DA, et al. Medicare coverage of tumor necrosis factor alpha inhibitors as an influence on physicians’ prescribing behavior. Arch Intern Med. 2006;166:57–63. doi: 10.1001/archinte.166.1.57. [published correction appears in Arch Intern Med. 2006;166:954] [DOI] [PubMed] [Google Scholar]
- 16.Schulman KA, Berlin JA, Harless W, et al. The effect of race and sex on physicians’ recommendations for cardiac catheterization. N Engl J Med. 1999;340:618–626. doi: 10.1056/NEJM199902253400806. [published correction appears in N Engl J Med. 1999;340:1130] [DOI] [PubMed] [Google Scholar]
- 17.Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Trends in the quality of care and racial disparities in Medicare managed care. N Engl J Med. 2005;353:692–700. doi: 10.1056/NEJMsa051207. [DOI] [PubMed] [Google Scholar]
- 18.Sokka T, Mäkinen H. Drug management of early rheumatoid arthritis—2008. Best Pract Res Clin Rheumatol. 2009;23:93–102. doi: 10.1016/j.berh.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 19.ARAMIS. [Accessed August 11, 2009]; http://aramis.stanford.edu.
- 20.Singh G. Arthritis, Rheumatism and Aging Medical Information System Post-Marketing Surveillance Program. J Rheumatol. 2001;28:1174–1179. [PubMed] [Google Scholar]
- 21.D’Agostino RB, Lee ML, Belanger AJ, et al. Relation of pooled logistic regression to time dependent Cox regression analysis: The Framingham Heart Study. Stat Med. 1990;9:1501–1515. doi: 10.1002/sim.4780091214. [DOI] [PubMed] [Google Scholar]
- 22.Huberman M, Langholz B. Application of the missing-indicator method in matched case-control studies with incomplete data. Am J Epidemiol. 1999;150:1340–1345. doi: 10.1093/oxfordjournals.aje.a009966. [DOI] [PubMed] [Google Scholar]
- 23.Cush JJ. Biological drug use: US perspectives on indications and monitoring. Ann Rheum Dis. 2005;64(Suppl 4):iv18–iv23. doi: 10.1136/ard.2005.042549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zink A, Strangfeld A, Schneider M, et al. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: Comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheum. 2006;54:3399–3407. doi: 10.1002/art.22193. [DOI] [PubMed] [Google Scholar]
- 25.Fautrel B, Flipo RM, Saraux A. Eligibility of rheumatoid arthritis patients for anti-TNF-alpha therapy according to the 2005 recommendations of the French and British Societies for Rheumatology. Rheumatology (Oxford) 2008;47:1698–1703. doi: 10.1093/rheumatology/ken348. [DOI] [PubMed] [Google Scholar]
- 26.Lu CY, Williams KM, Day RO. The funding and use of high-cost medicines in Australia: The example of anti-rheumatic biological medicines. Aust New Zealand Health Policy. 2007;4:2. doi: 10.1186/1743-8462-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kay LJ, Griffiths ID. for the BSR Biologics Register Management Committee. UK consultant rheumatologists’ access to biological agents and views on the BSR Biologics Register. Rheumatology (Oxford) 2006;45:1376–1379. doi: 10.1093/rheumatology/kel333. [DOI] [PubMed] [Google Scholar]
- 28.Fraenkel L, Rabidou N, Dhar R. Are rheumatologists’ treatment decisions influenced by patients’ age? Rheumatology (Oxford) 2006;45:1555–1557. doi: 10.1093/rheumatology/kel144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klepser DG, Huether JR, Handke LJ, Williams CE. Effect on drug utilization and expenditures of a costshare change from copayment to coinsurance. J Manag Care Pharm. 2007;13:765–777. doi: 10.18553/jmcp.2007.13.9.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: Associations with medication and medical utilization and spending and health. JAMA. 2007;298:61–69. doi: 10.1001/jama.298.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Census Bureau. [Accessed July 31, 2009];Fact sheet: Census 2000 demographic profile highlights. http://factfinder.census.gov/servlet/SAFFFacts?_event=&geo_id=01000US&_geoContext=01000US&_street=&_county=&_cityTown=&_state=&_zip=&_lang=en&_sse=on&ActiveGeoDiv=&_useEV=&pctxt=fph&pgsl=010&_submenuld=factsheet_0&ds_name=ACS_2007_3YR_SAFF&_ci_nbr=null&qr_name=null®=&_keyword=&_industry=
- 32.Suarez-Almazor ME, Berrios-Rivera JP, Cox V, et al. Initiation of diseasemodifying antirheumatic drug thera- py in minority and disadvantaged patients with rheumatoid arthritis. J Rheumatol. 2007;34:2400–2407. [PubMed] [Google Scholar]
- 33.Shipton D, Glazier RH, Guan J, Badley EM. Effects of use of specialty services on disease-modifying antirheumatic drug use in the treatment of rheumatoid arthritis in an insured elderly population. Med Care. 2004;42:907–913. doi: 10.1097/01.mlr.0000135810.39691.f6. [DOI] [PubMed] [Google Scholar]
- 34.Earle CC, Neumann PJ, Gelber RD, et al. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20:1786–1792. doi: 10.1200/JCO.2002.07.142. [DOI] [PubMed] [Google Scholar]
- 35.Einbinder LC, Schulman KA. The effect of race on the referral process for invasive cardiac procedures. Med Care Res Rev. 2000;57(Suppl 1):162–180. doi: 10.1177/1077558700057001S08. [DOI] [PubMed] [Google Scholar]