Abstract
Purpose
c-Src is an important adapter protein with estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2), which validates it as an attractive target for the treatment of breast cancer. A specific c-Src inhibitor, PP2, was utilized to block c-Src activity to identify targeted vulnerabilities affected by ER and HER2 in a panel of breast cancer cell lines.
Methods
ER, growth factor receptors, and signaling pathways were detected by Western-blot. The DNA content of the cells was determined by using a DNA fluorescence quantitation kit. Cell cycles were analyzed by flow cytometery.
Results
The antiproliferative effect of PP2 closely correlated with the inhibition of c-Src mediated ERK/MAPK and/or PI3K/Akt growth pathways. Inhibition of c-Src tyrosine kinase predominantly blocked ER negative breast cancer cell growth, particularly the triple (i.e. ER, PR, and HER2) negative cells. In contrast, ER negative Sk-Br-3 cells with highest HER2 phosphorylation were resistant to PP2, in which hyper-activated HER2 directly regulated growth pathways. However, blocking c-Src recovered ER expression and down-regulated HER2 which made Sk-Br-3 cells regain responsiveness to 4-hydroxytamoxifen. The majority of ER positive cells were not sensitive to PP2 regardless of wild-type or endocrine resistant cell lines.
Conclusions
c-Src mediates the essential role of growth pathways in ER negative breast cancer cells. The ER positive and HER2over-activationare two important predictive biomarkers for the resistance to a c-Src inhibitor. These data provided an important therapeutic rationale for patient selection in clinical trials with c-Src inhibitors in breast cancer.
Keywords: c-Src, estrogen receptor, HER2, breast cancer cell lines
1. Introduction
Targeting estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) are two successful therapies in the treatment of breast cancer patients expressing relevant target molecules (1,2). c-Src is a ubiquitously expressed intracellular tyrosine kinase that regulates protein-protein interactions and participates as a convergence point in different signaling pathways (3). c-Src functions as an important adapter protein between ER and receptor tyrosine kinases such as the epidermal growth factor receptor (EGFR) and HER2 in breast cancer (4–6). In this regard, c-Src acts as a critical component of the signaling cascades initiated by ER and HER2 to activate the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/AKT pathways (6,7), both of which cause ER phosphorylation and ER-dependent gene transcription (7).
Observations in vitro also support that multiple levels of association exist among ER, HER2, and c-Src in breast cancer. Targeting ER with tamoxifen increases c-Src activity which enhances cellular invasion and motility in breast cancer cells (8,9). Furthermore, c-Src is shown to be critical in mediating tamoxifen resistance since blocking its activity reverses tamoxifen resistance (10). A recent report indicates that c-Src is a common node downstream of multiple trastuzumab (targeting HER2) resistance pathways (11). These observations highlight c-Src as an important therapeutic target for the treatment of human breast cancer.
Dasatinib, a potent oral inhibitor of c-Src family tyrosine kinase, is approved for clinical use in imatinib-resistant and -intolerant chronic myeloid leukemia and solid tumor (12,13). Preclinical studies in breast cancer cell lines have shown that basal like triple negative (i.e. ER, PR, and HER2) breast cancer may have preferential sensitivity to the c-Src inhibitor (14,15). Two parallel phase II monotherapy studies of dasatinib in breast cancer were initiated in different breast cancer subtypes. In patients with triple-negative breast cancer (TNBC), dasatinib has good tolerability and modest activity (16), whereas dasatinib has limited single-agent activity in patients with HER2 positive and/or hormone receptors (HR) positive advanced breast cancer (17). These findings imply that HR and HER2 may prevent the therapeutic effects of the c-Src inhibitor in breast cancer. Thus, there is a need to identify patients who are unlikely to respond to the c-Src inhibitor treatment. More importantly, factors that cause c-Src inhibitor resistance will serve as molecular targets to improve the action of c-Src inhibitors. Unfortunately, there is little understanding of resistance to the c-Src inhibitors in breast cancer cells.
The goal of this study is to identify biological markers of resistance to a c-Src inhibitor in a panel of wild-type and endocrine resistant breast cancer cell lines. We demonstrate that c-Src has an essential role in mediating the growth pathways of ER negative breast cancer cells. ER positive and HER2 over-activation reduce the responsiveness to the c-Src inhibitor. Indeed, c-Src controls estrogen action in ER positive antihormone resistant cells. Our data provide an important therapeutic rationale for patient selection in future clinical trials of c-Src inhibitors in breast cancer.
2. Materials and methods
2.1 Materials
c-Src inhibitor PP2 was purchased from CalBiochem (San Diego, CA). Sources of antibodies for Western blot are as follows: ERα (sc-544) and PR (sc-810) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Total MAPK antibody (#9102), phosphorylation MAPK (#9101), total Akt (#9272), phosphorylated AktSer473 (#9271), phosphorylated c-SrcTyr416 (#2101L) antibodies and secondary antibodies conjugated with horseradish peroxidase (rabbit #7074, mouse #7076) were from Cell Signaling Technology (Beverly, MA). Phosphorylated HER2Tyr1248 and total c-Src mouse (GD11) antibodies were from Millipore (Temecula, CA). Antibodies to HER2 (Ab18) and EGFR (Ab15) were from NeoMarkers (Fremont, CA).
2.2 Cells and culture conditions
Briefly, MCF-7:WS8 and T47D:A18 human mammary carcinoma cells, clonally selected from their parental counterparts for sensitivity to growth stimulation by E2 (18), were used in all experiments indicating MCF-7 and T47D cells. ZR-75-1, BT474, and Sk-Br-3 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). MDA-MB-231(10A) cells (19), clonally selected from parental MDA-MB-231 cells (obtained from ATCC), were used in this study indicating MDA-MB-231 cells. MCF-7:5C and MCF-7:2A cells were cloned from E2 deprived MCF-7 cells and maintained in E2-free RPMI medium which is phenol red-free RPMI 1640 supplemented with 10% dextran-coated charcoal-stripped fetal bovine serum (SFS) (20,21). T47D:C42 cells were cloned from E2 deprived T47D cells and maintained in E2-free RPMI 1640 medium (22). Pure antiestrogen fulvestrant resistant cell line MCF-7/F was derived from MCF-7 which was maintained in phenol red RPMI 1640 medium supplemented with 10% FBS (23).
2.3 Cell Proliferation Assays
Cell DNA content was determined as a measure of cell proliferation using the Fluorescent DNA Quantitation Kit (Bio-Rad, Hercules, CA) (24).
2.4 Immunoblotting
Proteins were extracted in cell lysis buffer (Cell Signaling Technology, Beverly, MA) supplemented with Protease Inhibitor Cocktail (Roche, Indianapolis, IN) and Phosphatase Inhibitor Cocktail Set I and Set II (Calbiochem, San Diego, CA). Total protein content of the lysate was determined by a standard BCA assay using the reagent from Bio-Rad Laboratories (Hercules, CA). Fifty micrograms of total protein were separated on 10% SDS polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was probed with primary antibodies followed by incubation with secondary antibody conjugated with HRP and reaction with Western Lighting™ plus-ECL enhanced chemiluminescent substrate (PerkinElmer Inc., Waltham MA). Protein bands were visualized by exposing the membrane to X-ray film.
2.5 Cell cycles analysis
Briefly, Sk-Br-3, BT474, T47D:C42, and MDA-MB-231 cells were cultured in dishes. They were treated with vehicle (0.1% DMSO), lapatinib (1μM), and PP2 (5μM) for 24h respectively. Cells were harvested and gradually fixed with 75% EtOH on ice. After staining with propidium iodide (PI), cells were analyzed using a fluorescence-activated cell sorter (FACS) flow cytometer (Becton Dickinson, San Jose, CA), and the data were analyzed with CellQuest software.
2.6 Quantitative Real-time RT-PCR
Cells were harvested in TRIzol. Total RNA, isolated with an RNeasy Micro kit (Qiagen, Valencia, CA), was converted to first-strand cDNA using a kit from Applied Biosystem (Foster City, CA). Quantitative real-time PCR assays were done with the SYBR Green PCR Master Mixes (Applied Biosystems, Foster City, CA) and a 7900HT Fast Real-time PCR System (Applied Biosystems, Foster City, CA). The PUM1 forward primer was 5'-AATGCAGGCGCGAGAAAT-3', PUM1 reverse primer was 5'-TTGTGCAGCTGAGGAACTAATGA-3'. The ERα forward primer was 5'-GGAGGGCAGGGGTGAA-3', ERα reverse primer was 5'-GGCCAGGCTGTTCTTCTTAGA-3'. All the data were normalized by PUM1.
2.7 Statistical Analysis
All reported values are the means ± SE. Statistical comparisons were determined with two-tailed Student's t tests. Results were considered statistically significant if the P value was <0.05.
3. Results
3.1 Baseline levels of ER, HER2, and c-Src activation in a panel of breast cancer cell lines
We addressed the question whether expression of ER and growth factor receptors would affect the therapeutic effects of the c-Src inhibitors in breast cancer cells. To answer this question, a panel of wild-type (MCF-7, T47D, ZR-75-1, BT474, MDA-MB-231, and Sk-Br-3) and endocrine resistant (MCF-7:5C, MCF-7:2A, MCF-7/F, and T47D:C42) breast cancer cell lines were investigated. Baseline levels of ER, HER2, EGFR, and c-Src were measured by immunoblot analysis. They all keep their biological characteristics with differential levels of ER, PR, HER2, and EGFR (Supplementary Fig. S1A and S1B). All cell lines expressed detectable levels of total c-Src, whereas they manifested different levels of phosphorylated c-Src (Supplementary Fig. S1C). The DNA fingerprinting pattern of all cell lines is consistent with the report by the ATCC (Supplementary Fig. S2).
3.2 Inhibitory effects of the c-Src inhibitor on ER positive wild-type breast cancer cells
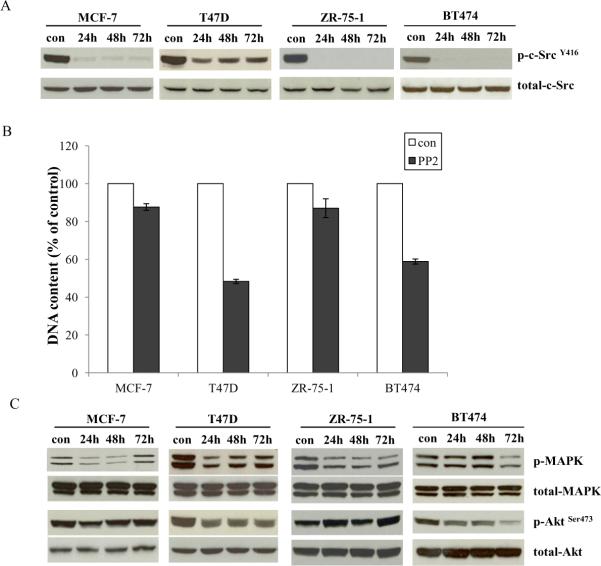
All ER positive wild-type breast cancer cells were cultured in estrogenized medium. The specific c-Src inhibitor, PP2, effectively blocked phosphorylation of c-Src in all cell lines (Fig. 1A). However, PP2 could not inhibit all cell growth (Fig. 1B). T47D and BT474 cells were responsive to PP2 with 50% and 40% inhibition after 7 days treatment, respectively (Fig.1B), whereas MCF-7 and ZR-75-1 cells were resistant to PP2 treatment (Fig.1B). Further investigation showed that antiproliferative effects of PP2 were correlated with inhibition of ERK/MAPK and/or PI3K/Akt pathways. PP2 could not continuously block growth pathways in resistant cells such as MCF-7 and ZR-75-1 (Fig. 1C). In contrast, PP2 effectively inhibited both signaling pathways in T47D and BT474 cells (Fig. 1C).
Figure 1. Effects of the c-Src inhibitor on ER positive wild-type cell lines.
1A. Blocking c-Src phosphorylation in ER positive wild-type cell lines by PP2. ER positive wild-type cells were treated with PP2 (5μM) in estrogenized medium at time points as indicated and cell lysates were harvested. Phosphorylated c-Src was detected by immunoblotting with primary antibody. Immunoblotting for total c-Src was used for loading control. 1B. Inhibitory effects of PP2 on wild-type ER positive cells. Wild-type ER positive cells were seeded in 24-well plates in triplicate in estrogenized medium. After one day, the cells were treated with vehicle (0.1%DMSO) and PP2 (5μM) respectively. The cells were harvested after 7 days treatment and total DNA was determined using a DNA fluorescence quantitation kit. 1C. Signaling pathways changes in ER positive wild-type cells after PP2 treatment. Cell lysates were harvested as above. Phosphorylated MAPK and Akt were examined by immunoblotting with primary antibodies. Immunoblotting for total MAPK and Akt were used for loading controls.
3.3 Inhibitory effects of the c-Src inhibitor varied under conditions with or without basal E2 in ER positive wild-type breast cancer cells
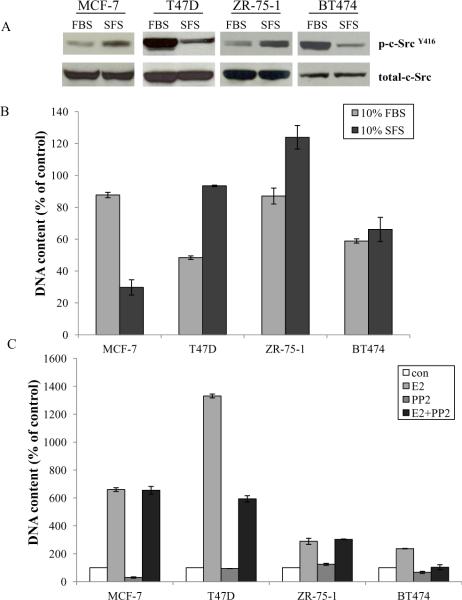
Since basal estrogen levels in the culture medium affect the biological function of the ER positive wild-type breast cancer cells (18) (Supplementary Fig. S3), we investigated inhibitory effects of the c-Src inhibitor on ER positive wild-type cells under conditions with (10% FBS) or without (10% SFS) basal estrogen. Two distinct modulations of c-Src phosphorylation existed in ER positive wild-type cells after short-term absence of E2. MCF-7 and ZR-75-1 cells had the same pattern with enhanced c-Src phosphorylation, conversely, c-Src phosphorylation was down-regulated in T47D and BT474 cells (Fig. 2A). Therefore, inhibition by PP2 varied in ER positive wild-type cells under these two conditions (Fig. 2B). MCF-7 cells were effectively responsive to PP2 under conditions without basal E2 (10% SFS), conversely, T47D cells were completely resistant to PP2 in phenol red free medium (Fig. 2B). Four ER positive wild-type breast cancer cells were stimulated by E2 to grow with different sensitivity (Fig.2C). Notably, PP2 could not block the proliferation induced by E2 in MCF-7 and ZR-75-1 cells but partially abolished E2 stimulation in T47D and BT474 cells (Fig. 2C). These results indicated that c-Src might play a distinct role in mediating E2 signaling in wild-type cells (4, 25).
Figure 2. Effects of the c-Src inhibitor on ER positive wild-type cell lines under conditions with or without basal E2.
2A. c-Src phosphorylation changed after short-term absence of E2 in ER positive wild-type cells. Wild-type ER positive cells were cultured under conditions with basal estrogen (10% FBS) or without basal estrogen (10% SFS) for 3 days, respectively. Cell lysates were harvested. Phosphorylated c-Src was examined by immunoblotting with primary antibody. Immunoblotting for total c-Src was determined as loading control. 2B. Growth inhibitory effects of PP2 on ER positive wild-type cells under conditions with or without basal E2. Wild-type ER positive cells were cultured under conditions with basal estrogen (10%FBS) or without basal estrogen (10% SFS) for 3 days, respectively. Then, they were seeded in 24-well plates in triplicate. After one day, the cells were treated with vehicle (0.1%DMSO) and PP2 (5μM) in estrogenized medium (10%FBS) or E2 free medium (10%SFS), respectively. The cells were harvested after 7 days treatment and total DNA was determined as above. 2C. The PP2 had different effects on E2 stimulation in ER positive wild-type cells. Wild-type ER positive cells were changed to E2 free medium for 3 days. Then, they were seeded in 24-well plates. After one day, the cells were treated with vehicle (0.1% EtOH), E2 (10−9 mol/L), PP2 (5μM), and E2 (10−9 mol/L) plus PP2 (5μM) respectively in E2 free culture medium. The cells were harvested after 7 days treatment and total DNA was determined as above.
3.4 Effects of the c-Src inhibitor on ER positive endocrine resistant breast cancer cells
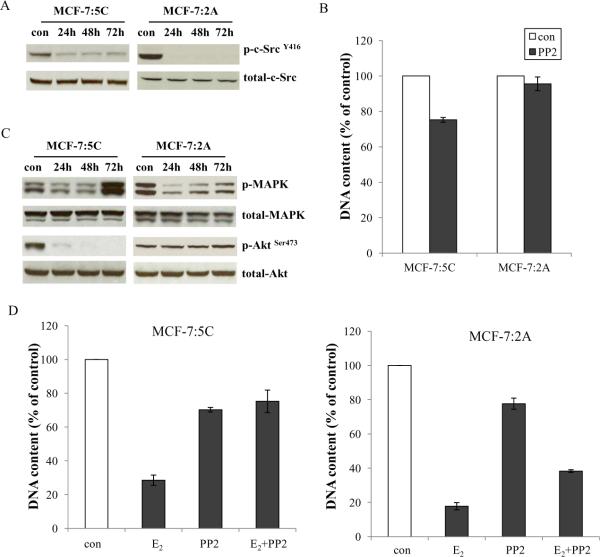
In two endocrine resistant cells (MCF-7:5C and MCF-7:2A), that overexpress ER, PP2 could block c-Src activation (Fig. 3A) and abolished about 25% of proliferation in MCF-7:5C cells but without any inhibition in MCF-7:2A cells (Fig.3B). The inhibitory effects of PP2 were consistent with blocking growth pathways in different cells. Phosphorylated Akt was abolished in MCF-7:5C cells but without continuous inhibition of MAPK. PP2 could not continuously block both growth pathways in MCF-7:2A cells (Fig. 3C). Our previous data showed that E2 has therapeutic function to induce apoptosis in long-term E2 deprived breast cancer cells (24). We reasoned that a combination of PP2 with E2 would enhance E2-induced apoptosis. Surprisingly, PP2 did not enhance the growth inhibitory effects of E2 on these two cell lines but blocked the growth inhibition induced by E2 (Fig. 3D). These data implied that E2-triggered apoptosis might be utilizing c-Src tyrosine kinase as an important signaling pathway. We are currently investigating the mechanisms of how the c-Src inhibitor blocks E2-triggered apoptosis.
Figure 3. Effects of the c-Src inhibitor on ER positive endocrine resistant cell lines.
3A. Blocking c-Src phosphorylation in endocrine resistant ER positive cells. MCF-7:5C and MCF-7:2A cells were treated with PP2 (5μM) at time points as indicated and cell lysates were harvested. Phosphorylated c-Src was detected by immunoblotting with primary antibody. Immunoblotting for total c-Src was used for loading control. 3B. Growth inhibitory effects of PP2 on endocrine resistant ER positive cells. MCF-7:5C and MCF-7:2A cells were seeded in 24-well plates in triplicate. After one day, the cells were treated with vehicle (0.1% DMSO) and PP2 (5μM) respectively in culture medium. The cells were harvested after 7 days treatment and total DNA was determined as above. 3C. Signaling pathways changes in endocrine resistant ER positive cells after PP2 treatment. Cell lysates were harvested as above. Phosphorylated MAPK and Akt were examined by immunoblotting with primary antibodies. Immunoblotting for total MAPK and Akt were used for loading controls. 3D. The PP2 blocked E2-induced inhibition in MCF-7:5C and MCF-7:2A cells. MCF-7:5C cells were seeded in 24-well plates as above. After one day, the cells were treated with vehicle (0.1% EtOH), E2 (10−9mol/L), PP2 (5μM), and E2 (10−9mol/L) plus PP2 (5μM) respectively. The cells were harvested after 7 days treatment and total DNA was determined as above. MCF-7:2A cells were seeded in 6-well plates. After one day, the cells were similarly treated as in MCF-7:5C cells. The cells were harvested after 14 days treatment and total DNA was determined as above.
3.5 The c-Src inhibitor effectively blocked ER negative breast cancer cell growth
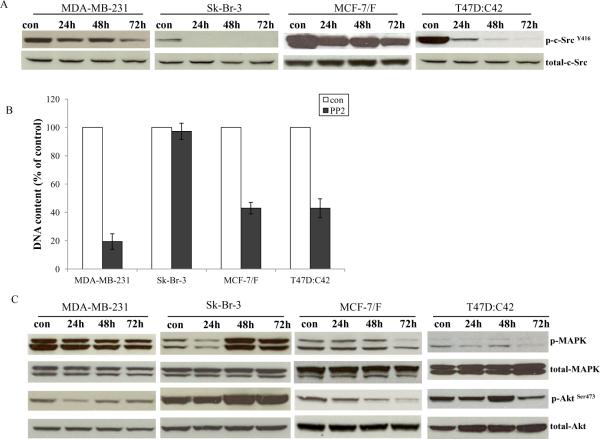
The inhibitory effects of the c-Src inhibitor, PP2, on ER negative breast cancer cell lines were examined in two wild-type MDA-MB-231 and Sk-Br-3 and two endocrine resistant cell lines MCF-7/F and T47D:C42. PP2 blocked the phosphorylation of c-Src in all ER negative cells (Fig. 4A). However, the growth inhibitory effects of the c-Src inhibitor were different. PP2 could inhibit 80% of cell growth in MDA-MB-231 cells. In contrast, PP2 exerted no inhibitory effects on Sk-Br-3 cells with HER2 overexpression (Fig. 4B). Inhibition of c-Src could efficiently suppress around 60% of cell growth in both endocrine resistant cells, MCF-7/F and T47D:C42 (Fig. 4B). The triple negative MDA-MB-231 cell line was the most sensitive to PP2. These results demonstrated that HER2 amplification might be an indicator for resistance to the c-Src inhibitors in clinical trials. Further investigation indicated that PP2 effectively blocked the MAPK and Akt pathways in the c-Src inhibitor sensitive cells, whereas MAPK and Akt phosphorylation were increased in Sk-Br-3 cells (Fig.4C). The data implied that HER2 might drive the growth pathways in Sk-Br-3 cells.
Figure 4. Effects of the c-Src inhibitor on ER negative cell lines.
4A. Blocking c-Src phosphorylation in ER negative cell lines by PP2. ER negative cells were treated with PP2 (5μM) for different times as indicated and cell lysates were harvested. Phosphorylated c-Src was detected by immunoblotting with primary antibody. Immunoblotting for total c-Src was used for loading control. 4B. Inhibitory effects of PP2 on ER negative cells. ER negative cells were seeded in 24-well plates in triplicate. After one day, the cells were treated with vehicle (0.1%DMSO) and PP2 (5μM) in 10% SFS medium. The cells were harvested after 7 days treatment and total DNA was determined as above. 4C. Signaling pathways were changed in ER negative cells after PP2 treatment. ER negative cells were treated with PP2 (5μM) for different times as indicated and cell lysates were harvested. Phosphorylated MAPK and Akt were examined by immunoblotting with primary antibodies. Immunoblotting for total MAPK and Akt were determined for loading controls.
3.6 Activation status of HER2 determined the inhibitory effects of the c-Src inhibitor
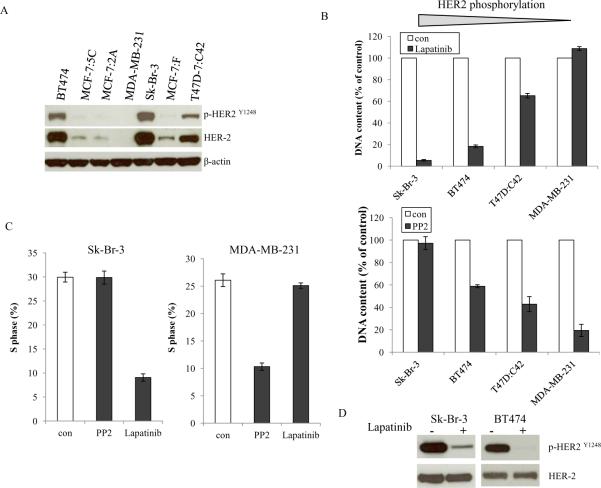
HER2 overexpression leads to a very aggressive cancer phenotype and poor patient survival (26). c-Src is known to bind to HER2 and is thus activated in HER2-overexpressing cancer cells (27,28). BT474 and Sk-Br-3 cells overexpress endogenous HER2 (Supplementary Fig. S1B), however, they had different responses to PP2 (Fig.1B and 4B). To examine whether HER2 activation affects the inhibitory rate of PP2, phosphorylation of HER2 was evaluated. Among tested cell lines, Sk-Br-3, BT474, and T47D:C42 cells had elevated though different levels of HER2 activation. As a control, HER2 was undetectable in MDA-MB-231 cells (Fig.5A). HER2 was highly activated in Sk-Br-3 cells compared with BT474 cells which made it hypersensitive to lapatinib, a dual tyrosine kinase inhibitor of HER2 and EGFR (Fig.5B). The growth inhibitory effects by lapatinib corresponded to the levels of phosphorylated HER2 (Fig. 5B). We observed that HER2 hyper-activation rendered breast cancer cell completely resistant to PP2, the higher HER phosphorylation, the lower responsive rate to PP2 (Fig. 5B). This was further confirmed by S phase changes through flow cytometric analysis (Fig. 5C and Supplementary Fig. S4). Lapatinib reduced S phase in cells with higher HER2 phosphorylation, conversely, PP2 was effective in cells with lower HER2 phosphorylation (Fig. 5C and Supplementary Fig. S4). Lapatinib's antitumor activity was associated with blocking phosphorylation of HER2 and the subsequent inhibition of its downstream signaling pathways (Fig. 5D and Supplementary Fig. S5). Lapatinib blocked MAPK and Akt pathways in Sk-Br-3 and BT474 cells, but it exerted no inhihition in MDA-MB-231 cells (Supplementary Fig. S5), which demonstrated that antiproliferative effects of lapatinib also correlated with inhibitory ability of growth pathways.
Figure 5. Activation status of HER2 determined the inhibitory effects of the c-Src inhibitor.
5A. Baseline HER2 phosphorylation in different cell lines. Cell lysates were harvested from different cells. Phosphorylated HER2 and total HER2 were examined by immunoblotting with primary antibodies. Immunoblotting for β-actin was determined for loading control. 5B. Inhibitory effects of the HER2 inhibitor and the c-Src inhibitor on cells with elevated HER2 phosphorylation. Sk-Br-3, BT474, T47D:C42, and MDA-MB-231 cells were seeded in 24-well plates in triplicate. After one day, the cells were treated with vehicle (0.1%DMSO), lapatinib (1μM), and PP2 (5μM) in 10% SFS medium. The cells were harvested after 7 days treatment and total DNA was determined as above. 5C. S phase changes after lapatinib and PP2 treatment. Sk-Br-3 and MDA-MB-231 cells were treated with vehicle (0.1% DMSO), lapatinib (1μM), and PP2 (5μM) for 24h. Cells were harvested and fixed with 75% EtOH. Cell cycles were analyzed through flow cytometery. 5D. Blocking HER2 phosphorylation after lapatinib treatment. Sk-Br-3 and BT474 cells were treated with vehicle (0.1%DMSO) and lapatinib (1μM) for 24h. HER2 phosphorylation was examined by immunoblotting with primary antibody. Immunoblotting for total HER2 was determined for loading control.
3.7 Blocking c-Src tyrosine kinase recovered ERα expression and reduced HER2 levels in ER negative Sk-Br-3 cells
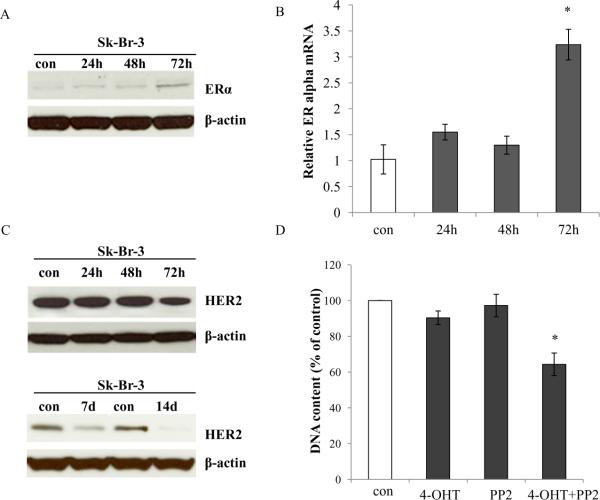
c-Src may drive estrogen-dependent ERα proteolysis in a subset of ER negative breast cancer (29). c-Src did not play a critical role in mediating growth pathways in Sk-Br-3 cells (Fig. 4B). To study whether the c-Src inhibitor can regulate ER turn-over in breast cancer cells with HER2 amplification, we found that PP2 could recover ERα expression in Sk-Br-3 cells (Fig. 6A). Real-time PCR analysis showed that mRNA levels of ERα was increased after PP2 treatment in Sk-Br-3 cells (Fig. 6B) which implied that c-Src was involved in the regulation of ERα not only in the protein level but also at the transcription level. We further demonstrated that PP2 decreased HER2 levels in Sk-Br-3 cells after extending treatment time (Fig. 6C). This result also implied a complicated feedback loop existed between c-Src and HER2 in Sk-Br-3 cells. Importantly, Sk-Br-3 cells acquired responses to 4-hydroxytamoxifen and ICI 182,780 after short-term treatment with PP2 (Fig. 6D and Supplementary Fig. S6). Therefore, it is plausible that the simultaneous interruption of c-Src tyrosine kinase and targeting ER might be an effective treatment for breast cancer cells with HER2 amplification (30).
Figure 6. Blocking c-Src sensitized cell to antiestrogen in Sk-Br-3 cells.
6A. ERα expression was elevated in Sk-Br-3 cells after PP2 treatment. Sk-Br-3 cells were treated with PP2 as indicated. ERα expression was examined by immunoblotting with primary antibody. Immunoblotting for β-actin was determined for loading control. 6B. ERα mRNA was increased in Sk-Br-3 cells after PP2 treatment. Sk-Br-3 cells were treated with PP2 (5μM) for the times as indicated. The RNA was harvested in TRIzol for real-time PCR analysis. P<0.05, * compared with control. 6C. HER2 expression was downregualted in Sk-Br-3 cells after PP2 treatment. Sk-Br-3 cells were treated with PP2 for the times as indicated. HER2 was examined by immunoblotting with primary antibody. Immunoblotting for β-actin was determined for loading control. 6D. The PP2 sensitized Sk-Br-3 cells to 4-hydroxytamoxifen. Sk-Br-3 cells were treated with vehicle, 4-OHT (1μΜ), PP2 (5μM), and 4-OHT (1μΜ) plus PP2 (5μM) in 10% FBS medium. The cells were harvested after 7 days treatment and total DNA was determined as above. P<0.05, * compared with control.
4. Discussion
We employed a panel of well characterized breast cancer cell lines (MCF-7, T47D, ZR-75-1, BT474, MDA-MB-231, and Sk-Br-3) and endocrine resistant cell lines (MCF-7:5C, MCF-7:2A, MCF-7/F, and T47D:C42) to identify biomarkers associated with the inhibitory actions of a specific c-Src inhibitor, PP2. PP2 effectively blocked c-Src tyrosine kinase activity in all cell lines tested. However, the antiproliferative effects of PP2 were associated with the inhibition of ERK/MAPK and/or PI3K/Akt growth pathways. ER positive and HER2 hyperactivation were two important clinically related markers that were associated with the inability of PP2 to inhibit both wild-type and endocrine resistant breast cancer cells. Triple-negative breast cancer cells, defined by a lack of expression of estrogen, progesterone and HER2 receptors, were the most sensitive to the c-Src inhibitor.
The therapeutic mechanisms of the c-Src inhibitor are to block its phosphorylation and subsequent growth pathways (31). It has been reported that cancer cells which do not manifest detectable c-Src phosphorylation are resistant to the c-Src inhibitor (32). Generally, cells with higher c-Src activity were more sensitive to PP2 (Fig. 4B), but not all cells with elevated c-Src tyrosine kinase activity were able to be effectively inhibited by the c-Src inhibitor such as ZR-75-1, MCF-7:2A, and Sk-Br-3 cells (Fig. 1B and 4B). Thus, the level of c-Src phosphorylation is not sufficient to distinguish responsive cells from cells resistant to the c-Src inhibitor. Growth inhibition also depends on whether c-Src directly mediates growth pathways in a special type of cell. We consistently found that the levels of MAPK phosphorylation and/or Akt phosphorylation were reduced by PP2 in responsive cell lines but not in resistant cell lines (Fig. 1C,3C, and 4C).
The non-receptor tyrosine kinase c-Src acts as a critical molecule in relaying ER signaling, including nongenomic and genomic actions (4,25). Its activity is modulated by E2 through multiple mechanisms, leading to breast cancer cell proliferation, invasion, and metastasis (3,7). Consistently, the growth inhibitory effects by the c-Src inhibitor on ER positive cells appear to be more complex than on ER negative cells in present work. Most ER negative breast cancer cells were sensitive to the inhibition by PP2 (Fig. 4B). However, the majority of ER positive cells were not sensitive to PP2 regardless of whether they were wild-type or endocrine resistant (Fig. 1B and 3B). Although PP2 had moderate ability to inhibit some ER positive wild-type cell growth (Fig. 1B), inhibitory effects by it varied under conditions with or without basal E2 (Fig. 2B). Our results also demonstrated that c-Src mainly mediated E2 responses which included E2-stimualted growth and E2-induced apoptosis in ER positive cells (Fig. 2C and 3D). These functions might disturb the therapeutic effects of the c-Src inhibitor on ER positive cells.
The function of c-Src has been linked to its association with the HER2/Neu epidermal growth factor receptor family members (33). In this study, increased expression of EGFR (MDA-MB-231 and MCF-7/F) did not affect the inhibitory effects of PP2, but HER2 overexpression was an indicator for the resistance to PP2 (Fig. 4B). Finn et al (15) also reported HER2 amplification was a predictive marker for resistance to a c-Src inhibitor, dasatinib, in breast cancer cells. However, both BT474 and Sk-Br-3 cells overexpress endogenous HER2, they had differential responses to PP2 (Fig.1B and 4B). Further investigation demonstrated that status of HER2 activation determined the inhibitory rate of PP2, the higher HER2 phosphorylation, the lower inhibitory rate of PP2 (Fig. 5B and 5C). HER2 was highly activated in Sk-Br-3 cells compared with BT474 cells which made it hypersensitive to the HER2 inhibitor but not the c-Src inhibitor (Fig.5A and 5B). Therefore, status of HER2 activation may be a better predictive biomarker for resistance to the c-Src inhibitor than currently available total HER2 determined by immunohistochemistry (IHC) or fluorescent in situ hybridization (FISH) (16).
The triple negative MDA-MB-231 cells are characterized by a point mutation at codon 13 in the K-RAS gene (34). This mutation is responsible for the constitutive phosphorylation of ERK1/2 which leads to a very aggressive cancer phenotype (35). The c-Src inhibitor, PP2, effectively suppressed growth pathways in MDA-MB-231cells, which demonstrated that triple negative breast cancer cells depend on c-Src to proliferate (Fig. 4B). Two independent studies support our observation by showing that the majority of dasatinib sensitive breast cancer cell lines were “basal” type or “triple-negative” (14,15). The hyper-sensitivity to the c-Src inhibitors provides a good therapeutic option for the clinical triple negative breast cancer (TNBC) patient. However, the TNBC is actually a highly diverse group of cancer (36), so that the determining of ER, PR and HER2 is not a precise classification to subtype this aggressive disease. Recent Phase II clinical trial shows that single-agent dasatinib has limited activity in unselected patients with TNBC (17), which suggests that a strategy of better patient selection with gene signatures is required to further evaluate the potential of the c-Src inhibitors in TNBC patient (36).
In summary, this study demonstrated a complex association exists among ER, HER2, and c-Src in different breast cancer cell lines. Moreover, our results underscored that ER expression and HER2 overexpression (especially over-activation) might be causes of resistance to a c-Src inhibitor in breast cancer. Our findings may be of value for future clinical investigation to determine the therapeutic efficacy of c-Src inhibitors in ER negative breast cancer with or without HER2 over-activation.
Supplementary Material
Acknowledgements
This work (VCJ) is supported by the Department of Defense Breast Program under Award number W81XWH-06-1-0590 Center of Excellence; subcontract under the SU2C (AACR) Grant number SU2C-AACR-DT0409; the Susan G Komen For The Cure Foundation under Award number SAC100009; GHUCCTS CTSA (Grant # UL1RR031975) and the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008. The views and opinions of the author(s) do not reflect those of the US Army or the Department of Defense.
Role of the funding source Ping Fan, Russell E. McDaniel, and Helen R. Kim's salaries, as well as laboratory supplies supported by the following: the Department of Defense Breast Program under award number W81XWH-06-1-0590 Center of Excellence (VCJ); subcontract under the SU2C (AACR) Grant number SU2C-AACR-DT0409 (VCJ); the Susan G Komen For The Cure Foundation under Award number SAC 100009 (VCJ) and the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008 (VCJ).
Footnotes
Conflict of interest statement None declared.
References
- 1.Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Thomas SN, Brugge JS. Cellular functions regulated by Src family kinases. Ann Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 4.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci USA. 2002;99:14783–8. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Belsches-Jablonski AP, Biscardi JS, Peavy DR, Tice DA, Romney DA, Parsons SJ. Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene. 2001;20:1465–75. doi: 10.1038/sj.onc.1204205. [DOI] [PubMed] [Google Scholar]
- 6.Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–68. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- 7.Shupnik MA. Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene. 2004;23:7979–89. doi: 10.1038/sj.onc.1208076. [DOI] [PubMed] [Google Scholar]
- 8.Hiscox S, Morgan L, Green TP, Barrow D, Gee J, Nicholson RI. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat. 2006;97:263–74. doi: 10.1007/s10549-005-9120-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Planas-Silva MD. Mislocalizaion of cell-cell adhesion complexes in tamoxifen-resistant breast cancer cells with elevated c-Src tyrosine kinase activity. Cancer Lett. 2009;275:204–12. doi: 10.1016/j.canlet.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Fan P, Wang J, Santen RJ, Yue W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res. 2007;67:1352–60. doi: 10.1158/0008-5472.CAN-06-1020. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Huang WC, Li P, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17:461–9. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 13.Demetri GD, Lo Russo P, Macpherson IR, et al. Phase I dose-escalation and pharmacokinetic study of dasatinib (BMS-354825), a Src and multi-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2009;15:6232–40. doi: 10.1158/1078-0432.CCR-09-0224. [DOI] [PubMed] [Google Scholar]
- 14.Huang F, Reeves K, Han X, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–38. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 15.Finn RS, Dering J, Ginther C, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/”triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–26. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 16.Finn RS, Bengala C, Ibrahim N, Roché H, Sparano J, Strauss LC, Fairchild J, Sy O, Goldstein LJ. Dasatinib as a Single Agent in Triple-Negative Breast Cancer: Results of an Open-Label Phase 2 Study. Clin Cancer Res. 2011;17:6905–13. doi: 10.1158/1078-0432.CCR-11-0288. [DOI] [PubMed] [Google Scholar]
- 17.Mayer E, Baurain JF, Sparano JA, Strauss LC, Campone M, Fumoleau P, Rugo H, Awada A, Sy O, Llombart-Cussac A. A phase 2 trial of dasatinib in patients with advanced HER2-positive and/or hormone receptor-positive breast cancer. Clin Cancer Res. 2011;17:6897–904. doi: 10.1158/1078-0432.CCR-11-0070. [DOI] [PubMed] [Google Scholar]
- 18.Pink JJ, Jordan VC. Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Res. 1996;56:2321–30. [PubMed] [Google Scholar]
- 19.Jiang SY, Jordan VC. Growth regulation of estrogen receptor negative breast cancer cells transfected with complementary DNAs for estrogen receptor. J Natl Cancer Inst. 1992;84:580–91. doi: 10.1093/jnci/84.8.580. [DOI] [PubMed] [Google Scholar]
- 20.Jiang SY, Wolf DM, Yingling JM, Chang C, Jordan VC. An estrogen receptor positive MCF-7 clone that is resistant to antiestrogens and estradiol. Mol Cell Endocrinol. 1992;90:77–86. doi: 10.1016/0303-7207(92)90104-e. [DOI] [PubMed] [Google Scholar]
- 21.Pink JJ, Jiang SY, Fritsch M, Jordan VC. An estrogen-independent MCF-7 breast cancer cell line which contains a novel 80-kilodalton estrogen receptor-related protein. Cancer Res. 1995;55:2583–90. [PubMed] [Google Scholar]
- 22.Murphy CS, Pink JJ, Jordan VC. Characterization of a receptor-negative, hormone-nonresponsive clone derived from a T47D human breast cancer cell line kept under estrogen-free conditions. Cancer Res. 1990;50:7285–92. [PubMed] [Google Scholar]
- 23.Liu H, Cheng D, Weichel AK, et al. Cooperative effect of gefitinib and fumitremorgin c on cell growth and chemosensitivity in estrogen receptor alpha negative fulvestrant-resistant MCF-7 cells. Int J Oncol. 2006;29:1237–46. [PubMed] [Google Scholar]
- 24.Lewis JS, Meeke K, Osipo C, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746–59. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- 25.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27:466–80. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu D, Hung MC. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene. 2000;19:6115–21. doi: 10.1038/sj.onc.1203972. [DOI] [PubMed] [Google Scholar]
- 27.Muthuswamy SK, Muller WJ. Activation of Src family kinases in Neu-induced mammary tumors correlates with their association with distinct sets of tyrosine phosphorylated proteins in vivo. Oncogene. 1995;11:1801–10. [PubMed] [Google Scholar]
- 28.Belsches-Jablonski AP, Biscardi JS, Peavy DR, Tice DA, Romney DA, Parsons SJ. Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene. 2001;20:1465–75. doi: 10.1038/sj.onc.1204205. [DOI] [PubMed] [Google Scholar]
- 29.Chu I, Arnaout A, Loiseau S, et al. Src promotes estrogen-dependent estrogen receptor alpha proteolysis in human breast cancer. J Clin Invest. 2007;117:2205–15. doi: 10.1172/JCI21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page K, Hava N, Ward B, Brown J, et al. Detection of HER2 amplification in circulating free DNA in patients with breast cancer. Br J Cancer. 2011;104:1342–8. doi: 10.1038/bjc.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luttrell DK, Lee A, Lansing TJ, et al. Involvement of pp60c-src with two major signaling pathways in human breast cancer. Proc Natl Acad Sci USA. 1994;91:83–7. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto W, Okamoto I, Yoshida T, et al. Identification of c-Src as a potential therapeutic target for gastric cancer and of MET activation as a cause of resistance to c-Src inhibition. Mol Cancer Ther. 2010;9:1188–97. doi: 10.1158/1535-7163.MCT-10-0002. [DOI] [PubMed] [Google Scholar]
- 33.Tan M, Li P, Klos KS, Lu J, Lan KH, Nagata Y, Fang D, Jing T, Yu D. ErbB2 promotes Src synthesis and stability: novel mechanisms of Src activation that confer breast cancer metastasis. Cancer Res. 2005;65:1858–67. doi: 10.1158/0008-5472.CAN-04-2353. [DOI] [PubMed] [Google Scholar]
- 34.Eckert LB, Repasky GA, Ülkü AS, McFall A, Zhou H, Sartor CI, Der CJ. Involvement of Ras activation in human breast cancer cell signaling, invasion, and anoikis. Cancer Res. 2004;64:4585–4592. doi: 10.1158/0008-5472.CAN-04-0396. [DOI] [PubMed] [Google Scholar]
- 35.Toulany M, Baumann M, Rodemann HP. Stimulated PI3K-AKT signaling mediated through ligand or radiation-induced EGFR depends indirectly, but not directly, on constitutive K-Ras activity. Mol Cancer Res. 2007;5:863–872. doi: 10.1158/1541-7786.MCR-06-0297. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.