Abstract
Besides their genomic effects, oestrogens, 17β-oestradiol in particular, also activate cellular effects that may be too rapid (seconds to minutes) to result from de novo protein synthesis. Although the existence of such non-genomic actions has been extensively demonstrated in vitro, the understanding of their behavioural significance is only emerging. Recent findings provide evidence that acute oestrogen treatments significantly affect a variety of behavioural processes including sexual behaviour, social communication and cognition. One question arising from these results concerns the source of the oestrogens mediating non-genomic effects in vivo. In this review, data collected in vitro and in vivo are presented supporting the notion that fast modulations of local testosterone aromatisation can rapidly control the local oestrogen concentration in a time frame compatible with their rapid actions. Together, these data provide compelling evidence of how rapid changes in the local production and action of oestrogens can shape complex behaviours.
Keywords: oestrogens, oestrogen receptors, neuroactive steroids, oestrogen synthase, non-genomic, post-translational modification
INTRODUCTION
Although they have long been regarded as slow-acting messengers involved in the regulation of gene transcription through the activation of oestrogen receptors (ER; Fig.1), it is now clear that oestrogens, 17β-oestradiol (E2) in particular, also activate cellular effects that seems too rapid (seconds to minutes) to result from de novo protein synthesis (2, 3). Since the discovery of these rapid effects in the 70s (4, 5), considerable progress has been made and the assumption that non-genomic effects of oestrogens are associated with a large variety of cellular signalling pathways is now widely accepted (Fig.1, for review see (1)). Yet, the study of the consequences of such actions on physiology and behaviour is only emerging and, rather surprisingly, the origin of the oestrogens able to trigger these non-genomic actions is rarely addressed. In this review, we would like to (1) briefly summarize the current knowledge on the functional implications of these rapid effects of oestrogens and (2) propose that local testosterone aromatisation can rapidly control the local oestrogen concentration in a time frame compatible with their rapid actions.
Figure 1. Schematic representation of the control mechanisms of local oestrogen synthesis and some cellular mechanisms of genomic and non-genomic action of oestrogens.
Upper part: changes in intracellular Ca2+ levels, resulting notably from glutamatergic or dopaminergic inputs, regulate the phosphorylation status of aromatase through the modulation of kinase and phosphatase (PPase) activities, resulting in changes in its enzymatic activity. According to this model, the phosphorylated form of the enzyme (in red) is inhibited, while the dephosphorylated protein (in green) is active. Lower part: I. The classical mode of action of oestrogens involves the activation of specific nuclear receptors (oestrogen receptor [ER] α and β) that dimerize upon binding to their ligand (E2, represented by a yellow star). The ligand-receptor complex then binds to specific sequences of the DNA (oestrogen responsive element or ERE) resulting in the modulation of the transcription of a variety of target genes (2). II-VII. The non-genomic mode of action of oestrogens involves their interaction with membrane-associated receptors. Oestrogens can allosterically modulate the activity of G-protein coupled-receptors (GPCR; schematically represented as 7-transmembrane receptors in [II]) or ion-gated channels/receptors [III] (93). [IV] Novel G-protein-coupled membrane oestrogen receptors (mER) such as ER-X, STX-binding receptor or GPR30 have also been described (94–96). Finally [V], nuclear oestrogen receptors (ERα and ERβ) can also translocate and associate to the membrane where they activate intracellular cascades through the interaction with a G protein-coupled receptor (GPCR) (97, 98). The activated intracellular cascades [VI] include changes in intracellular concentrations of calcium and protein phosphorylations and ultimately lead to changes in enzymatic activity, G protein coupling, neuronal firing rate and neuronal activation. Importantly, these intracellular events can also result in the transcription of genes [VII] that do not depend on ER binding to the oestrogen response element (3, 99, 100).
Functional implications of non-genomic actions of oestrogens
Non-genomic effects of oestrogens on social behaviours
To our knowledge, the first account of a fast behavioural action of oestrogens was published in 1991 by Hayden-Hixson and Ferris who reported that an acute stereotaxic injection of E2 in the anterior-hypothalamus increased within 20 min the display of agonistic behaviours in male hamsters (6). Cross and Roselli then found that the acute administration of E2, but not testosterone, facilitates the expression of anogenital investigations and mounting behaviour within 35 min in castrated male rats (7). Similar effects were reported to occur within 10–15 min and vanish after 30 min in castrated quail and mice primed with a suboptimal concentration of testosterone (8, 9). The nature of the receptor involved in this response remains unknown. Male sexual behaviour is severely impaired in oestrogen receptor α (ERα) knock-out (ERαKO) mice (10, 11) as well as in the non-classical ER knock-in (or NERKI) mice possessing a mutated ERα knock-in which cannot bind to ERE and consequently signals only through membrane-initiated or ERE-independent genomic pathways (10). These results combined with the observation that fast oestrogenic actions on male sexual behaviour require some steroid priming to occur (8, 9) suggest that the acute control of male sexual behaviour by oestrogens probably involves an integration of non-genomic and genomic processes depending on ERα. In two mouse species in which males are more aggressive during winter-like days than in long days, aggressive behaviour appears to depend on non-genomic actions of oestrogens in short days only (12, 13). In midshipman fish, an intramuscular injection of E2 results within 5 min in a lengthening of the duration of fictive vocalizations in both sexes. These effects are also transient: they last for about 30 to 45 min and are no longer detected 1 hour after the injection (14, 15). In male goldfish, a systemic injection of E2 stimulates approach responses towards visual cues of females within 10–25 min (16). Finally, recent findings support the involvement of non-genomic actions of E2 in auditory processing in songbirds. In awake restrained zebra finches of both sexes, extracellular recordings of neurones from the caudomedial nidopallium (NCM, cortical-like auditory region) involved in song recognition revealed that acute treatment with E2 increases, while the ER antagonist tamoxifen reduces, the firing rate evoked by song playback within 5 min. Whole-cell patch-clamp recordings in slices revealed that E2 action in NCM is mediated through changes in GABA transmission probably involving a modulation of GABA release (17). A multi-unit recording study of auditory evoked activity in anaesthetized male zebra finches further indicated that these effects are transient and that the influence of E2 on song discrimination probably results from its ability to increase burst firing (see also below; (18)). In parallel to the modulation of firing rate, the application of E2 in NCM increases the expression of activity-dependent genes to the same extent as song, while tamoxifen suppresses this song-driven gene expression. Therefore, in addition to its short-term effects on auditory processing, E2 also induces hearing-driven gene expression (17).
Interaction between non-genomic and genomic actions on social behaviours
The regulation of a behavioural response involves a chain of events precisely organized in time and space (from the intracellular space to neuronal networks controlling motivation or motor patterns). The non-genomic effects of oestrogens identified in vivo may thus be expected to develop after longer latencies than the intracellular actions that triggered them. As a consequence, behavioural changes induced by oestrogens within a few minutes to an hour could probably be considered as extremely rapid (See (1) for further discussion of the issue). Given the very short time course of the oestrogen effects reported so far, it is thus very likely that they depend on a non-genomic action rather than a regulation of gene transcription.
As illustrated by the parallel effects of E2 on firing and auditory-driven gene expression described in zebra finches, non-genomic and genomic actions are generally undissociable (see also Fig.1). Indeed, although the initial cellular event triggered by E2 does not involve protein synthesis, the intracellular cascades activated by this event very often lead to transcriptional activation and both events are usually required for the expression of a given behaviour. One good example of such effects is provided by studies of female sexual receptivity. In 2004, Kow and Pfaff demonstrated that a pulse of E2 as short as 15 min delivered in the ventromedial nucleus of the hypothalamus potentiates the effect of a second pulse of 1 hour (given 5 hours after the first pulse) on receptivity in ovariectomized rats (19). Similar effects, but of lower magnitude, are observed if E2-BSA is substituted for E2 in the first, but not the second pulse, indicating that this non-genomic action is initiated at the membrane. The activated intracellular cascade involves PKA and PKC as activators of these protein kinases mimic the effect of the first pulse of E2 as previously demonstrated in vitro (20). Lordosis is also facilitated by the activation of a membrane-bound oestrogen receptor α (mERα) associated to a metabotropic glutamate receptor in the arcuate nucleus of the hypothalamus (21). The behavioural consequences of this non-genomic activation were only investigated 30 hours after the administration of the hormone (21). Previous data showed that the subcutaneous administration of estradiol benzoate (EB) results in an increased internalisation of mu opioid receptors (µ-OR) in the medial preoptic nucleus of ovariectomized rats after 30 min of treatment, an effect that lasts 24 hours at least (22). Interestingly, although the rapid and prolonged preoptic internalisation of µ-OR induced by EB attenuates sexual receptivity, this phenomenon seems to be critical for the induction of lordosis by progesterone (23). Together, this series of studies indicates that, although they are initiated early in the cascade of events involved in lordosis facilitation, the intracellular events activated by E2 appear to trigger long lasting effects that probably require protein synthesis (non-classical genomic mechanisms involving CRE binding by pCREB; (17, 24, 25)) and also interact with classical genomic actions involving the binding to ERE (e.g. activation of progesterone receptor required for progesterone action). Preliminary studies using NERKI mice indeed suggest that non-classical ERα signalling might be sufficient to induce the expression of specific genes and trigger some aspects of female sexual behaviour through ERE-independent mechanisms (26).
Membrane-initiated actions of oestrogens on non-social behaviours
The non-genomic influence of oestrogens is not limited to social behaviour. Tonic immobility, dorsal immobility and amphetamine-elicited rotational behaviours vary across the oestrus cycle in females and are affected within 4 hours of the administration of E2 in the striatum of both sexes (27–32). Given that (1) apomorphine-induced postural deviation and rotational behaviour is modulated by E2 within an hour and that (2) the striatum is apparently devoid of nuclear ER, these effects were initially attributed to non-genomic mechanisms. Recent findings suggest that the enhanced rotational behaviour is mediated by mERα-dependent modulation of striatal GABA affecting dopamine release (33). Thus, in the striatum, oestrogen action appears to potentiate, acutely and non-genomically, dopaminergic activity that in turn enhances sensorimotor processing. This mechanism has been proposed to play a role in the control of paced mating behaviour in female rats. However, since the potentiation of pacing behaviour was investigated following several hours of treatment, it is not known whether it could occur faster or whether it requires some time to develop as is the case for the non-genomic actions of E2 described on lordosis behaviour (34).
Another example of rapid action of oestrogens concerns their effects on cognition. Acute in vitro or in vivo treatment with E2 non-genomically enhances the electrical activity, synaptic plasticity (spine morphology and long-term potentiation [LTP]) and synaptic transmission in hippocampal neurons (35–37) suggesting that oestrogens might rapidly enhance hippocampus-dependent learning and memory. This hypothesis is supported by experiments showing that systemic or intra-hippocampus E2 administration 30 min prior to or immediately after, but not 2 hours after, training enhances spatial memory assessed several hours later by hippocampus-dependent tasks such as the Morris water maze test (38), visual object recognition (39), object placement recognition (39) and contextual fear conditioning (40). Further analyses of the intracellular events involved in these effects of E2 on synaptic plasticity and hippocampus-dependent tasks suggest that they are mediated by ERβ activation, initiated at the membrane and depend on the activation of the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) pathway (41, 42).
Summary
The past decade has thus witnessed the emergence of studies attesting the behavioural relevance of the membrane-initiated effects of oestrogens in various vertebrate species. Most membrane-initiated effects are detected about 15–30 min after oestrogen treatment although some actions require more time to be observed. Compared to the several days typically required for genomic activation of behavioural responses, such latencies are thus extremely rapid. Their transient nature also contrast with the prolonged actions of genomic effects. Moreover, fast actions of oestrogens appear to be somewhat region specific (1). Finally, it is interesting to notice that a large number of the fast behavioural effects of oestrogens identified so far have been described in males. Because males are not exposed to the dramatic changes in circulating concentrations of oestrogens that females experience across the oestrus cycle, the source of the oestrogens able to trigger these rapid effects is questionable (but see below).
Brain aromatase as a source of oestrogens
Intuitively, the pre-ovulatory surge of E2 experienced by females provides a model of a fast and transient rise in circulating oestrogens (but see also (1)). Likewise, males of several species show similar rises in plasma testosterone in response to social encounters such as territorial intrusion and sexual interactions (43–45); for review see (46, 47)). Such rapid increases in peripheral androgen concentration could translate into rapid and transient changes in local oestrogen concentrations following local conversion into an oestrogen by aromatase (figure 2; for a detailed discussion of this hypothesis see (1)). In the present section, we would like to propose that rapid changes in local aromatization of androgens is a region-specific source of oestrogens compatible with a large number of their non-genomic actions.
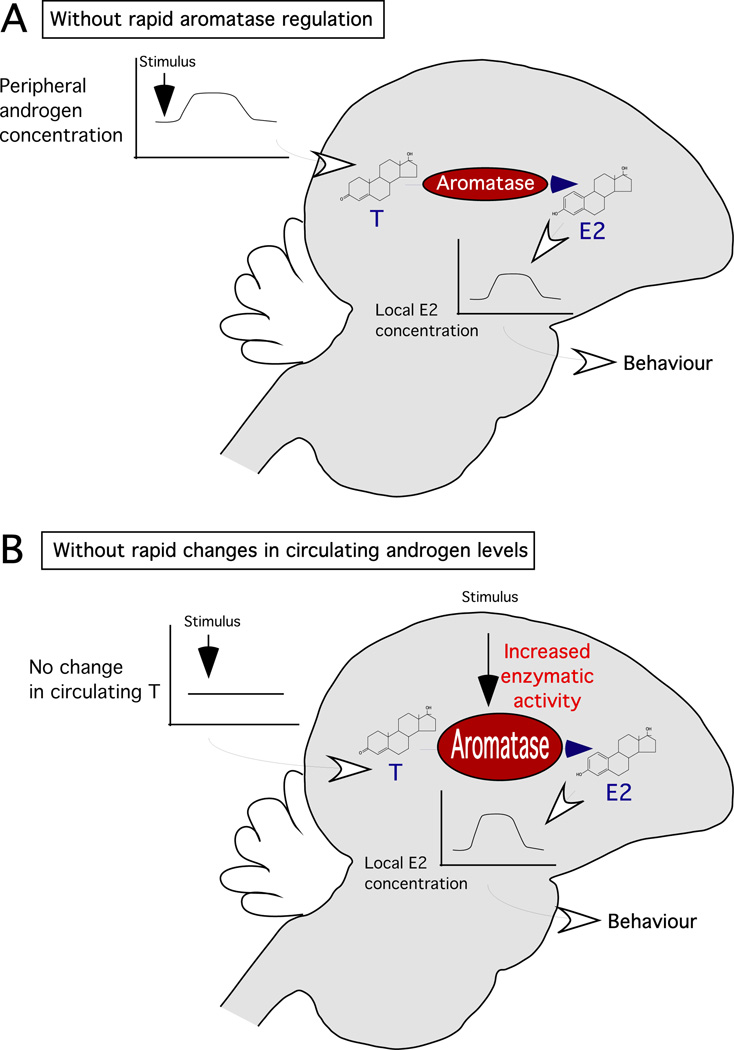
Figure 2. Potential sources of oestrogens associated with non-genomic effects.
(A). On the one hand, rapid changes in local oestrogen production can result from variations of plasma testosterone following social/environmental stimuli such as sexual interaction (43, 44, 101) or territorial intrusion (101–103). Upon brain aromatization through a steady-state enzymatic activity, elevated testosterone can translate into a local rise of oestradiol independently of rapid change in aromatase kinetics. (B). On the other hand, in the absence of rapid changes in circulating steroid levels, socially- and/or environmentally-elicited modulations of neurotransmitter activity, such as glutamate or dopamine, can trigger modifications of the phosphorylation status of aromatase leading to a rapid change of its enzymatic activity. In these two models, aromatase concentration remains constant while either the enzymatic substrate (i.e. testosterone) or the rate of aromatization rapidly increases resulting in a rise in the enzymatic product. The fast changes in local oestradiol concentration could in turn induce fast actions in specific cell populations that would ultimately result in fast behavioural effects. Although these two processes are presented independently, they could occur in parallel, resulting in an even higher increase in local oestrogen synthesis.
The enzyme aromatase is found in both sexes (although not to the same extent) in various tissues including the brain. Under normal physiological conditions, its expression in the avian and mammalian brain is restricted to specific neuronal populations mainly located in the hypothalamic/preoptic (medial preoptic nucleus, ventromedial nucleus) and limbic system (bed nucleus of stria terminalis, amygdala, hippocampus, septum, etc). More scattered groups of neurones expressing the enzyme are also present in the cerebral cortex (48–52). In songbirds, high concentrations of aromatase are detected in NCM, nucleus adjacent to the song control nucleus HVC (53–55). Aromatase is also abundantly expressed in the brain of teleost fishes where the enzyme is not expressed in the neurones but in radial glia (56). Interestingly, the protein and its enzymatic activity have also been described in presynaptic boutons in several brain nuclei of vertebrates (57–59). Because testosterone produced by the gonads circulates in relatively high levels in the bloodstream of males and even females of many species (1), the presence of aromatase in discrete brain regions likely allows the local conversion of androgen reaching the central nervous system into oestradiol. In addition, the enzymes involved in the synthesis of several steroids (including dehydroepiandrosterone [DHEA], androgens or oestrogens) from cholesterol have also been described in several brain areas, suggesting that local oestrogen synthesis could occur even in the absence of detectable concentrations of androgens in the circulation (60).
Although brain aromatase was traditionally associated with the regulation of reproductive behaviours, it has recently become clear that androgen aromatization in the brain is also involved in the control of non-reproductive processes such as mood and cognition (61). Yet, most studies investigated the role of aromatase in the control of relatively slow, presumably genomic, effects of oestrogens. New evidence now supports the notion that aromatase also plays a pivotal role in the control of non-genomic effects of oestrogens. Here, we briefly summarize pharmacological and enzymatic evidence supporting a role of androgen aromatization in the acute control of four social behaviours. The acute blockade of oestrogen synthesis by systemic administration of aromatase inhibitors (Vorozole or ATD [Androstatrienedione]) significantly reduced appetitive and consummatory aspects of male sexual behaviour in sexually active quail and mice within 30 and 10 min, respectively (8, 62). Similar pharmacological treatments were also shown to impair approach behaviour towards females in male goldfish (16), vocalization-like processes in midshipman fish (15) and aggressive behaviour in Beach and California mice, two species displaying higher level of aggressiveness in winter-like photoperiod (12, 13). In parallel, a transient decrease (20%) in aromatase activity was also detected in preoptic-hypothalamic homogenates of quail collected immediately after a 1–5 min sexual encounter with a receptive female, supporting the idea that rapid changes in local oestrogen concentration are behaviourally relevant (63). Subsequent experiments suggest that this socially induced reduction of enzymatic activity arises from more pronounced decreases restricted to the medial preoptic nucleus and the mediobasal hypothalamus (Unpublished data, Cornil and Balthazart). This socially-induced inhibition of aromatase activity seems to contradict the hypothesis that locally synthesized oestrogens play a stimulatory role on male sexual behaviour as evidenced by acute injections of oestradiol or aromatase inhibitors. This apparent discrepancy between the excitatory effects of oestrogens on male sexual behaviour and the rapid inhibition of oestrogen synthesis following copulation probably reflects the different time frames at which these two effects have been examined. Indeed, the enzymatic activity could not be assessed during the expression of the behaviour, not even immediately thereafter, since a minimum of 1 min is necessary to isolate brain samples. It is thus currently hypothesized that pharmacological experiments mimic processes involved in the initiation of the behaviour, whereas changes in oestrogen synthesis observed quickly after a sexual encounter might depend on mechanisms associated to the termination of the behaviour (but see (64)).
Several findings also support the involvement of local aromatisation in the control of fast actions of oestrogens on sensory processing. As described previously, acute actions of E2 have been implicated in the processing of auditory stimuli by the NCM of songbirds. This cortical-like region contains an extremely dense population of neurones expressing aromatase (53, 59, 65). Retrodialysis of the aromatase inhibitor fadrozole in NCM suppressed within 30 min the preference for a bird’s own song compared with a conspecific male song in male zebra finches (18). Fadrozole also reduced song-elicited firing rate in awake zebra finches and prevented the expression of hearing-driven gene expression in NCM (17). These acute effects of local aromatase blockade on sensory processing suggest that endogenous changes in E2 concentration influence neuronal responses to auditory stimuli in NCM. This conclusion is supported by the recent demonstration that female presentation as well as male song playback results within 30 min in an increased E2 concentration recovered from microdialysis probes implanted within, but not outside of, NCM (66). A subsequent study showed that a brief bout of singing behaviour (30 min) resulted in an enhanced aromatase activity (AA) in homogenates containing NCM. Interestingly, this effect would mainly arise from an increased enzymatic activity measured in synaptic terminals rather than cell bodies (67). Together, these data indicate that the auditory processing of socially relevant cues is accompanied by rapid local changes in oestrogen synthesis that might be critical to song recognition and preference. Another example of acute regulation of sensory processing by locally produced oestrogens is the rapid modulation of nociception. Non-genomic effects of E2 have been reported in the dorsal root ganglion, a region involved in nociception (68, 69). In parallel, aromatase is found in pain-sensitive neurons in the quail spinal cord (70) suggesting that local synthesis of oestrogens might regulate the perception of pain. In agreement with this hypothesis, intrathecal injections of aromatase inhibitors markedly increased the latency of foot withdrawal from a hot water bath in less than 5 min. This effect was no longer observed 30 min after the injection and was prevented by the concurrent infusion of E2 (71). Finally, recent data collected in vitro indicate that oestrogens derived from local aromatization might also contribute to sensory integration in the vestibular system (72).
Finally, rapid E2 effects have also been described on hippocampal synaptic plasticity and hippocampus-dependent learning and memory (see above). Although, we have not found any report of learning or memory impairment following aromatase inhibition, rapid glutamatergic-dependent changes of enzymatic activity (73) as well as a reduction of synaptogenesis after inhibition of oestrogen synthesis (74) have been demonstrated in hippocampus slices. Local oestrogen production might thus be involved in rapid synaptic modelling and memory task relying on hippocampus.
Therefore, many non-genomic effects of E2 appear to critically depend on the local aromatisation of testosterone. Moreover, enzymatic and microdialysis studies have uncovered rapid changes in endogenous oestrogen concentrations in response to socially relevant cues. Together, these data suggest that, in order to acutely regulate the local concentration of oestrogens and their subsequent effects, there must exist some mechanism(s) able to rapidly regulate (1) the local synthesis of oestrogens and (2) their elimination. The following sections examine mechanisms involved the acute control of aromatase activity and in the subsequent catabolism of oestrogens.
New insight on mechanisms of rapid control of aromatase activity
It is well established that aromatase is regulated at the transcriptional level through the use of alternate tissue-specific promoters. Notably, sex steroids significantly induce the expression of the enzyme in the hypothalamus of all vertebrates investigated so far. Accordingly, low levels of aromatase expression are detected after gonadectomy, while testosterone replacement therapy markedly increases aromatase transcript, protein and activity in several brain regions in both sexes (48, 75, 76). This effect appears to be mediated by androgen receptors in rat (77), while ERs seem more important in quail (75). These changes in aromatase activity (AA) generally occurred after several hours or days of treatment and were paralleled by the progressive occurrence of male sexual behaviour (78). However, the rapid effects of E2 described above strongly suggest that oestrogen concentrations need to be regulated more rapidly, thus implying the existence of rapid modulations of the enzymatic activity.
Rapid changes of enzymatic activity can be obtained through post-translational modifications of the protein rather than through changes in protein concentration that are too slow to explain the kinetic of oestrogen synthesis. Observations from cell lines expressing aromatase confirmed that its enzymatic activity can be modulated independently of it transcriptional control. The selective inhibitor of the protein kinase MEK-1/2 PD98059 reduces AA without a corresponding effect upon the mRNA level of aromatase (79), thus providing insights into the regulation of aromatase through post-translational modulation. Other studies implicated protein kinases as potential regulators of AA. Indeed, the blockade of MAPK or phosphoinositide 3-kinase reduces AA (Yue et al 2003). Likewise, growth factors increase AA through the stimulation of cAMP-dependent protein kinases and PKC in vitro (80, 81). However, all these actions are rather slow and cannot explain the very rapid changes of E2 availability described in vivo.
Our lab demonstrated that AA can be down-regulated by calcium-dependent processes in preoptic-hypothalamic brain homogenates and explants from male quail exposed to treatments that increase intracellular calcium levels such as thapsigargin and potassium-induced depolarization (Fig.1; (82)). Interestingly, these effects were observed after only 15 min of treatment, a time scale compatible with rapid behavioural oestrogen actions. Moreover, the calcium-induced inhibition is more pronounced in the presence of physiological concentrations of ATP and magnesium, providing additional evidence to support the hypothesis that rapid phosphorylation processes are involved (82). The physiological relevance of these observations was investigated in preoptic-hypothalamic explants of male quail in which the cellular integrity and neuronal connectivity are preserved. In this preparation, the activation of AMPA, kainate and, to a lower extent, NMDA glutamatergic receptors rapidly (within 5 min) and reversibly down-regulates AA (83), while the inhibitory amino acid GABA does not affect the enzymatic activity. Very interestingly, in vivo retrodialysis of glutamate in the zebra finch telencephalon significantly reduces the synthesis of local oestradiol (66), consistent with the glutamatergic inhibition of AA observed in quail preoptic-hypothalamic explants. It is speculated that the activation of glutamate receptors triggers the release of calcium from intracellular stores since the removal of calcium from the incubation medium at the time of kainate application has no effect on AA inhibition (83). Pharmacological experiments further demonstrated that these calcium-dependent phosphorylations are directly controlled by the activity of multiple protein kinases, including PKC and PKA (84) and treatment with acid phosphatase completely blocks the inhibitory effect of ATP/magnesium/calcium (85). Western blot experiments on immunoprecipitated aromatase revealed the presence of phosphorylated serine, threonine and tyrosine residues when aromatase was subjected to phosphorylating conditions, confirming that aromatase itself is phosphorylated (84).
The recent bioinformatic analysis of the quail aromatase coding sequence identified several potential phosphorylation sites, highly conserved amongst several avian and mammalian species. Of these potential sites, only two (T455 and T486, corresponding to T462 and T493 in human and mouse) seem to be in an environment that corresponds to the consensus sites of PKA and PKC, thought to be involved in the control of AA based on pharmacological experiments. However, the importance of these 2 residues remains to be experimentally tested. A recent in vitro study of the mouse aromatase suggests that 2 other amino acid residues, namely serine S118 (86) or tyrosine Y361 (87), play a crucial role in the phosphorylation of aromatase, albeit leading to different outcomes. The phosphorylation of S188 by AGC-like kinases (such as PKC and PKA) seems important for the stabilization of the protein (86). Independently, the phosphorylation of Y361 triggered by the tyrosine-kinase c-Src seems involved in the E2-dependent up-regulation of AA in breast cancer cell lines (87). However, the effect of a rapid change of the phosphorylation status of S188 and Y361 residues is currently unknown.
In addition to phosphorylation, we have shown that calmodulin significantly inhibits quail preoptic-hypothalamic AA both in the presence and in absence of phosphorylating conditions, suggesting that calmodulin itself interact directly with aromatase rather than through a modulation of calcium/calmodulin-dependent protein kinases (88). This interpretation is reinforced by the presence of calcium-independent and -dependent calmodulin binding motifs on the quail aromatase sequence (88).
Altogether, numerous biochemical and pharmacological evidence confirms that AA can be rapidly modulated via post-translational modifications. Although the mechanisms leading to these modifications remain poorly understood, this suggests that the enzymatic change must result in a local rapid modulation of oestrogens availability and consequently in a modification of cellular events that do not require protein synthesis.
Mechanisms of oestrogen elimination
The behavioural inhibition resulting from the pharmacological blockade of aromatase suggests that there must exist some mechanism(s) able to rapidly clear locally-produced oestrogens to terminate their effects. The elimination of plasma oestrogen is ensured through excretion following their conversion into inactive (or less active) water-soluble metabolites by oxidative metabolism and conjugation (89). This metabolism mainly occurs in the liver but detectable levels of catabolic activity are also observed in the brain (90–92). Interestingly, in addition to its oestrogen synthase activity, purified aromatase from human placental microsomes also catalyses oestrogen 2-hydroxylation. It is thus possible that the same enzymatic protein is involved in both the production and the conversion of oestrogens (for further information, see (64)). Finally, passive dilution could also contribute to the rapid equilibration of the high locally synthesized oestrogen concentrations with the much lower brain concentrations. At such concentrations, brain-derived oestrogens would then no longer be able to sustain membrane effects resulting in their termination. The half-life of oestradiol in the brain is not known, but calculations derived from pharmacokinetic data estimated that its half-life in the blood ranges from 5 to 15 min (63). Therefore, it is conceivable that oestrogen dilution combined with its enzymatic degradation could interrupt oestrogen-dependent signalling in the brain within minutes.
Conclusions
Besides traditionally studied long-lasting effects, the data summarized in the present review strongly indicates that oestrogens also exert rapid, presumably non-genomic, effects on various physiological and behavioural processes including sexual behaviour, sensory processing and cognition. These non-genomic E2 effects are generally characterized by a behavioural onset shorter than an hour, a transient nature and a certain regional specificity. The critical question of the source of the oestrogens able to trigger these fast behavioural and physiological actions had until recently received little attention. The demonstration that AA can be rapidly modulated in vitro through post-translational processes such as phosphorylation provides a first line of evidence indicating that local oestrogen production can vary in a time scale compatible with their rapid behavioural actions. This notion is further supported by the observation of rapid changes in endogenous oestrogen concentrations in response to socially relevant cues. Although, the past few years have witnessed an increasing interest for the study of the behavioural effects of brain-derived estrogens, the cellular mechanisms through which oestrogens acutely modulate these physiological and behavioural effects remain largely unknown. Understanding these cellular mechanisms, how the rapid regulation of local oestrogen synthesis interacts with circulating steroid concentrations and how non-genomic and genomic events activated in different brain regions converge on the activation of a complex behaviour will constitute a challenge for future studies. Based on the flourishing studies published during the past decade, the coming years are offering exciting perspectives.
Acknowledgements
CAC is a Research Associate from the Fonds de la Recherche Scientifique (F.R.S.-FNRS). TDC is Postdoctoral Researcher from the Fonds de la Recherche Scientifique (F.R.S.-FNRS). We would like to thank Dr Jacques Balthazart for his input and continuous support. Some of the experimental work described in this review was supported by grants from the NIH (R01 MH 50388), the Belgian Fonds de la Recherche Fondamentale Collective (FRFC 2.4537.09) and the University of Liège (Fonds Spéciaux 2009) to JB.
References
- 1.Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: Where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine reviews. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 3.Vasudevan N, Pfaff DW. Membrane Initiated Actions of Estrogens in Neuroendocrinology: Emerging Principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- 4.Yagi K. Changes in firing rates of single preoptic and hypothalamic units following an intravenous administration of estrogen in the castrated female rat. Brain Research. 1973;53:343–352. doi: 10.1016/0006-8993(73)90219-9. [DOI] [PubMed] [Google Scholar]
- 5.Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Research. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- 6.Hayden-Hixson DM, Ferris CF. Steroid-specific regulation of agonistic responding in the anterior hypothalamus of male hamsters. Physiology & Behavior. 1991;50:793–799. doi: 10.1016/0031-9384(91)90020-o. [DOI] [PubMed] [Google Scholar]
- 7.Cross E, Roselli CE. 17b-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. American Journal of Physiology. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- 8.Taziaux M, Keller M, Bakker J, Balthazart J. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J Neurosci. 2007;27:6563–6572. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behavioural Brain Research. 2006;66:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 10.McDevitt MA, Glidewell-Kenney C, Weiss J, Chambon P, Jameson JL, Levine JE. Estrogen response element-independent estrogen receptor (ER)-alpha signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERalpha knockout mice. Endocrinology. 2007;148:5288–5294. doi: 10.1210/en.2007-0673. [DOI] [PubMed] [Google Scholar]
- 11.Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm Behav. 1997;32:176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- 12.Trainor BC, Finy MS, Nelson RJ. Rapid effects of estradiol on male aggression depend on photoperiod in reproductively non-responsive mice. Horm Behav. 2008;53:192–199. doi: 10.1016/j.yhbeh.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trainor BC, Lin S, Finy MS, Rowland MR, Nelson RJ. Photoperiod reverses the effects of estrogens on male aggression via genomic and nongenomic pathways. Proc Natl Acad Sci U S A. 2007;104:9840–9845. doi: 10.1073/pnas.0701819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. Journal of Neuroscience. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lord LD, Bond J, Thompson RR. Rapid steroid influences on visually guided sexual behavior in male goldfish. Horm Behav. 2009;56:519–526. doi: 10.1016/j.yhbeh.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kow L-M, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. PNAS. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasudevan N, Kow L-M, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. PNAS. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signalling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 26.McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol. 2008;290:24–30. doi: 10.1016/j.mce.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Hartesveldt C, Cottrell GA, Meyer ME. The effects of intrastriatal hormones on the dorsal immobility response in gonadectomized male and female rats. Pharmacol Biochem Behav. 1989;34:459–463. doi: 10.1016/0091-3057(89)90541-8. [DOI] [PubMed] [Google Scholar]
- 28.Van Hartesveldt C, Cottrell GA, Meyer ME. Effects of intrastriatal hormones on the dorsal immobility response in male rats. Pharmacol Biochem Behav. 1990;35:307–310. doi: 10.1016/0091-3057(90)90160-j. [DOI] [PubMed] [Google Scholar]
- 29.Meyer ME, Van Hartesveldt C. Differential effects of intrastriatal estradiol on the dorsal immobility response in male rats. Pharmacol Biochem Behav. 1992;43:303–306. doi: 10.1016/0091-3057(92)90672-3. [DOI] [PubMed] [Google Scholar]
- 30.Becker JB, Snyder PJ, Miller MM, Westgate SA, Jenuwine MJ. The influence of estrous cycle and intrastriatal estradiol on sensorimotor performance in the female rat. Pharmacol Biochem Behav. 1987;27:53–59. doi: 10.1016/0091-3057(87)90476-x. [DOI] [PubMed] [Google Scholar]
- 31.Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- 32.Smith RL, Webster DG, Van Hartesveldt C, Meyer ME. Effects of estrus, estrogen-progesterone priming, and vaginal stimulation on tonic immobility, dorsal immobility, and lordosis in the female rat. Physiol Behav. 1985;35:577–581. doi: 10.1016/0031-9384(85)90143-x. [DOI] [PubMed] [Google Scholar]
- 33.Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29:1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao L, Becker JB. Hormonal activation of the striatum and the nucleus accumbens modulates paced mating behavior in the female rat. Horm Behav. 1997;32:114–124. doi: 10.1006/hbeh.1997.1412. [DOI] [PubMed] [Google Scholar]
- 35.Moss RL, Gu Q, Wong M. Estrogen : nontranscriptional signaling pathway. Recent progress in hormone research. 1997;52:33–69. [PubMed] [Google Scholar]
- 36.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 37.MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 38.Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- 39.Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 40.Barha CK, Dalton GL, Galea LA. Low doses of 17alpha-estradiol and 17beta-estradiol facilitate, whereas higher doses of estrone and 17alpha- and 17beta-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology. 2010;35:547–559. doi: 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 43.Batty J. Acute changes in plasma testosterone levels and their relation to measures of sexual behaviour in the male house mouse (Mus musculus) Anim Behav. 1978;26:349–357. doi: 10.1016/0003-3472(78)90053-2. [DOI] [PubMed] [Google Scholar]
- 44.Purvis K, Haynes NB. Short-term effects of copulation, human chorionic gonadotrophin injection and non-tactile association with a female on testosterone levels in the male rat. Journal of Endocrinology. 1974;60:429–439. doi: 10.1677/joe.0.0600429. [DOI] [PubMed] [Google Scholar]
- 45.Saginor M, Horton R. Reflex Release of Gonadotropin and Increased Plasma Testosterone Concentration in Male Rabbits during Copulation. Endocrinology. 1968;82:627. doi: 10.1210/endo-82-3-627. [DOI] [PubMed] [Google Scholar]
- 46.Harding CF. Social modulation of circulating hormone levels in the male. AmerZool. 1981;21:223–231. [Google Scholar]
- 47.Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AV. Social modulation of androgen levels in male teleost fish. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:203–215. doi: 10.1016/s1096-4959(01)00523-1. [DOI] [PubMed] [Google Scholar]
- 48.Roselli CE, Resko JA. Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain. JSteroid BiochemMolBiol. 1997;61:365–374. [PubMed] [Google Scholar]
- 49.Roselli CE, Resko JA. Cytochrome P450 aromatase (CYP19) in the non-human primate brain: distribution, regulation, and functional significance. Journal of Steroid Biochemistry & Molecular Biology. 2001;79:247–253. doi: 10.1016/s0960-0760(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 50.Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. JChemNeuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- 51.Wagner CK, Morrell JI. Neuroanatomical distribution of aromatase mRNA in the rat brain : indications of regional regulation. JSteroid BiochemMolBiol. 1997;61:307–314. [PubMed] [Google Scholar]
- 52.Stoffel-Wagner B. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann N Y Acad Sci. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- 53.Balthazart J, Absil P, Foidart A, Houbart M, Harada N, Ball GF. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): implications for the neural action of steroids and nuclear definition in the avian hypothalamus. Journal of Neurobiology. 1996;31:129–148. doi: 10.1002/(SICI)1097-4695(199610)31:2<129::AID-NEU1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 54.Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol. 1999;407:115–129. [PubMed] [Google Scholar]
- 55.Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An atlas of aromatase mRNA expression in the zebra finch brain. Journal of Comparative Neurology. 1995;360:172–184. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- 56.Forlano PM, Schlinger BA, Bass AH. Brain aromatase: new lessons from non-mammalian model systems. Front Neuroendocrinol. 2006;27:247–274. doi: 10.1016/j.yfrne.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. Neuroendocrinol. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- 58.Schlinger BA, Callard GV. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinol. 1989:434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- 59.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol. 2009;30:259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Segura LM. Aromatase in the brain: not just for reproduction anymore. J Neuroendocrinol. 2008;20:705–712. doi: 10.1111/j.1365-2826.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 62.Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav. 2006;49:45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Balthazart J. Sexual behavior affects preoptic aromatase activity and brain monoamines’ levels. Endocrinology. 2005;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cornil CA. Rapid regulation of brain oestrogen synthesis: the behavioural roles of oestrogens and their fates. J Neuroendocrinol. 2009;21:217–226. doi: 10.1111/j.1365-2826.2009.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 66.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Remage-Healey L, Oyama RK, Schlinger BA. Elevated aromatase activity in forebrain synaptic terminals during song. J Neuroendocrinol. 2009;21:191–199. doi: 10.1111/j.1365-2826.2009.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- 69.Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005;81:31–37. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- 70.Evrard H, Baillien M, Foidart A, Absil P, Harada N, Balthazart J. Localization and controls of aromatase in the quail spinal cord. Journal of Comparative Neurology. 2000:552–564. doi: 10.1002/1096-9861(20000807)423:4<552::aid-cne2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 71.Evrard HC, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. Journal of Neuroscience. 2004;24:9225–9229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grassi S, Frondaroli A, Dieni C, Scarduzio M, Pettorossi VE. Long-term potentiation in the rat medial vestibular nuclei depends on locally synthesized 17beta-estradiol. J Neurosci. 2009;29:10779–10783. doi: 10.1523/JNEUROSCI.1697-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. Journal of Neuroscience. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. JSteroid BiochemMolecBiol. 1993;44:521–540. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- 76.Roselli CE, Resko JA. Testosterone regulates aromatase activity in discrete brain areas of male rhesus macaques. Biol Reprod. 1989;40:929–934. doi: 10.1095/biolreprod40.5.929. [DOI] [PubMed] [Google Scholar]
- 77.Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- 78.Charlier TD, Ball GF, Balthazart J. Rapid action on neuroplasticity precedes behavioral activation by testosterone. Horm Behav. 2008;54:488–495. doi: 10.1016/j.yhbeh.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shozu M, Sumitani H, Murakami K, Segawa T, Yang HJ, Inoue M. Regulation of aromatase activity in bone-derived cells: possible role of mitogen-activated protein kinase. J Steroid Biochem Mol Biol. 2001;79:61–65. doi: 10.1016/s0960-0760(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 80.Ryde CM, Nicholls JE, Dowsett M. Steroid and growth factor modulation of aromatase activity in MCF7 and T47D breast carcinoma cell lines. Cancer Res. 1992;52:1411–1415. [PubMed] [Google Scholar]
- 81.Mendelson CR, Corbin CJ, Smith ME, Smith J, Simpson ER. Growth factors suppress and phorbol esters potentiate the action of dibutyryl adenosine 3',5'-monophosphate to stimulate aromatase activity of human adipose stromal cells. Endocrinology. 1986;118:968–973. doi: 10.1210/endo-118-3-968. [DOI] [PubMed] [Google Scholar]
- 82.Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. JNeuroendocrinol. 2001;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- 83.Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- 84.Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. European Journal of Neuroscience. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- 85.Balthazart J, Baillien M, Ball GF. Interactions between kinases and phosphatases in the rapid control of brain aromatase. J Neuroendocrinol. 2005;17:553–559. doi: 10.1111/j.1365-2826.2005.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miller TW, Shin I, Kagawa N, Evans DB, Waterman MR, Arteaga CL. Aromatase is phosphorylated in situ at serine-118. J Steroid Biochem Mol Biol. 2008;112:95–101. doi: 10.1016/j.jsbmb.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Catalano S, Barone I, Giordano C, Rizza P, Qi H, Gu G, Malivindi R, Bonofiglio D, Ando S. Rapid estradiol/ERalpha signaling enhances aromatase enzymatic activity in breast cancer cells. Mol Endocrinol. 2009;23:1634–1645. doi: 10.1210/me.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Balthazart J, Baillien M, Charlier TD, Ball GF. Effects of calmoduline on aromatase activity in the preoptic area. Journal of Neuroendocrinology. 2005;17:664–671. doi: 10.1111/j.1365-2826.2005.01355.x. [DOI] [PubMed] [Google Scholar]
- 89.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells : review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 90.Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacity of the new selective COMT inhibitors. Pharmacological Reviews. 1999;51:593–628. [PubMed] [Google Scholar]
- 91.Timmers RJ, Granneman JC, Lambert JG, van Oordt PG. Estrogen-2-hydroxylase in the brain of the male African catfish, Clarias gariepinus. Gen Comp Endocrinol. 1988;72:190–203. doi: 10.1016/0016-6480(88)90202-x. [DOI] [PubMed] [Google Scholar]
- 92.Balthazart J, Stoop R, Foidart A, Granneman JCM, Lambert JGD. Distribution and regulation of estrogen-2-hydroxylase in the quail brain. Brain ResBull. 1994;35:339–345. doi: 10.1016/0361-9230(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 93.Nadal A, Diaz M, Valverde MA. The estrogen trinity: membrane, cytosolic, and nuclear effects. News in Physiological Sciences. 2001;16:251–255. doi: 10.1152/physiologyonline.2001.16.6.251. [DOI] [PubMed] [Google Scholar]
- 94.Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73 doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Sander Connolly EJ, Nethrapalli IS, Tinnikov AA. ER-X : a novel membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. Journal of Neuroscience. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prossnitz ER, Sklar LA, Oprea TI, Arterburn JB. GPR30: a novel therapeutic target in estrogen-related disease. Trends Pharmacol Sci. 2008;29:116–123. doi: 10.1016/j.tips.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 97.Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. Estrogen induces rapid translocation of estrogen receptor beta, but not estrogen receptor alpha, to the neuronal plasma membrane. Neuroscience. 2008;153:751–761. doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Molecular Endocrinology. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 100.Levin ER. Membrane oestrogen receptor alpha signalling to cell functions. J Physiol. 2009;587:5019–5023. doi: 10.1113/jphysiol.2009.177097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wingfield JC. Short-term changes in plasma levels of hormones during establishment and defense of a breeding territory in male song sparrows, Melospiza melodia. Horm Behav. 1985;19:174–187. doi: 10.1016/0018-506x(85)90017-0. [DOI] [PubMed] [Google Scholar]
- 102.Harding CF, Follett BK. Hormone changes triggered by aggression in a natural population of blackbirds. Science. 1979;203:918–920. doi: 10.1126/science.570304. [DOI] [PubMed] [Google Scholar]
- 103.Remage-Healey L, Bass AH. Rapid elevations in both steroid hormones and vocal signaling during playback challenge: a field experiment in Gulf toadfish. Hormones and Behavior. 2005;47:297–305. doi: 10.1016/j.yhbeh.2004.11.017. [DOI] [PubMed] [Google Scholar]