Abstract
Proteases have garnered interest as candidate biomarkers and therapeutic targets for many human diseases. A key challenge is the identification and characterization of disease-relevant proteases in the complex milieu of biological fluids such as serum, plasma and bronchoalveolar lavage, in which a multitude of hydrolases act in concert. We have successfully utilized a concise combinatorial library of internally quenched fluorogenic peptide probes (IQFPs) to map the global proteolytic substrate specificities of complex biological samples. The substrate profiling approach provides a global and a quantitative comparison of protease specificities between different biological samples. Such a comparative analysis can lead to the identification of disease-specific ‘fingerprints’ of proteolytic activities with potential utility in diagnosis and therapy.
Keywords: FRET, Global proteolytic specificities, Proteases, Protease substrate identification
Introduction
Substrate profiling of proteases in clinically relevant biological fluids such as serum, plasma and bronchoalveolar lavage (BAL) may enable the development of novel disease-targeted therapies and diagnostics. One successful substrate profiling technique involves screening the proteases of interest against a combinatorial library of internally quenched fluorogenic peptide probes (IQFPs). These probes remain optically silent in the uncleaved state, but when recognized and cleaved by an appropriate protease they emit a fluorescent signal with the signal intensity proportional to the extent of cleavage. This technique was initially used to determine substrate specificities of purified recombinant proteases (Backes et al., 2000; Thomas et al., 2006) and we have extended it to map the proteolytic signatures of serum, BAL and other complex biological fluids (Watson et al., 2011a; Watson et al., 2011b).
Complex biological samples such as body fluids and tissue lysates represent a mixture of diverse proteolytic activities arising from proteases of many different functional classes. Accordingly, the protocol begins with determining the total proteolytic activity of each of the biological samples under consideration. Next it is important to assess the contribution of each of the four major classes of proteolytic enzymes (serine, cysteine, metallo- and aspartic) towards the total proteolytic activity. Consequently, the initial steps in assay development involve optimization of parameters such as buffer composition and pH to determine the most active protease class within the sample of interest. If a rationale exists to focus on a particular protease class in a given experiment, the buffer optimization space can be restricted accordingly. To assess total as well as class-specific proteolytic activity, and to identify the optimal dilution for the library screen, a range of dilutions of biological sample is initially screened against one or more generic peptide probes (examples listed in Table 1) or fluorescein isothiocyanate labeled casein (FITC-casein) in assay buffers representing the four major protease classes (serine, cysteine, metallo- and aspartic).
Table 1.
IQFPs for optimization of assay parameters from Anaspec
| Peptide | Amino acid sequence |
|---|---|
| Beta Secretase substrate 2 | Mca-EVKMDAEFK(DNP) |
| Cath D and E FRET Substrate | Mca-GKPILFFRLK(DNP)-r-NH2 |
| Amyloid Precursor protein (APP) | Mca-SEVKMDAEFR-Dap(DNP)-NH2 |
| Alpha Secretase substrate 1 | Mca-HQKLVFFAK(DNP) |
| TNF-FRET | Mca-PLAQAVRSSSDap(DNP)R-NH2 |
| Caspase-1 Substrate V | Mca-YVADAP(DNP) |
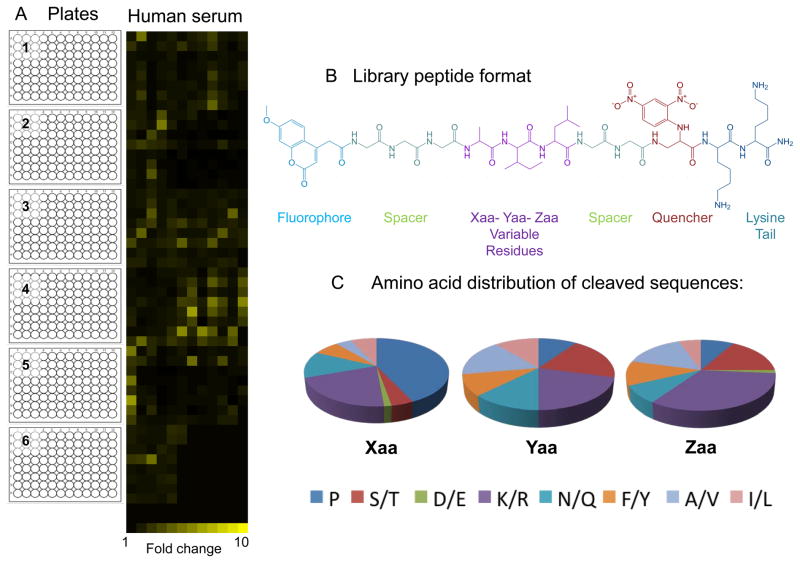
Following the initial assessment, the biological sample is then screened against the combinatorial library of IQFPs using the optimized assay conditions. The library utilized in our screen contains 3375 IQFP’s distributed into 512 wells (5 entirely filled 96 well plates and a partially filled sixth plate). The synthetic format of each IQFP is shown in Figure 3B, with variable positions Xaa, Yaa and Zaa corresponding to equimolar mixtures of similar amino acids: A/V, D/E, F/Y, I/L, K/R, N/Q, S/T or P. Thus, each well of the library contains eight peptides, except when one of the variable positions is proline. Proteolytic cleavage of the IQFP library is monitored as an increase in fluorescence as a function of time, typically for up to 6 hours.
Figure 3.
IQFP library screen of pooled complement preserved normal human serum at 1:10 dilution (A) Heat map generated from fluorescence fold change values calculated at the assay end point (t6h). The heat map corresponds to stacked 96 well library plates as indicated. Each yellow square corresponds to a library well and the brightness of each square corresponds to calculated fold change value for that well (B) Example of IQFP library peptide containing the variable amino acid sequence of ‘AIL’. (C) Variable position amino acid distribution profile for library sequences cleaved by human serum.
Fluorescence fold change values for each library well are then calculated as the ratio of fluorescence measured at the experimental endpoint (t6h) to fluorescence measured at the initial time point (t0). Wells that exhibit a fluorescence fold change of 2 or greater are considered to be cleaved by the sample. For a single biological sample, if multiple library plates are assayed simultaneously, the library screen can be completed within a day, followed by detailed data analysis. To represent the data visually for ease of comparison between the samples, the proteolytic fingerprint of each sample can be represented in a heatmap format. For example, we have used the free software developed by Dr. Euan Ashley’s laboratory at Stanford University (see Figure 3A for an example) (King et al., 2011). IQFP library wells of interest are then selected for further analysis and sequence deconvolution to define the precise substrate specificities of the proteases in each sample. This is accomplished by synthesizing and individually testing the constituent peptides in each selected library well.
Materials
Fluorescein isothiocyanate-labeled casein (FITC casein) (Anaspec)
IQFP Probe MCA-RPPGFSAFK (DNP) (Anaspec)
Milli-Q water
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)
Tris (hydroxymethyl) amino methane (Tris base)
Sodium citrate
Sodium chloride (NaCl)
Calcium chloride (CaCl2)
Zinc chloride (ZnCl2)
Dithiothreitol (DTT)
Ethylene diamine tetra acetic acid (EDTA)
Trichloroacetic acid, 10% (w/v) in water
Serine protease buffer, HEPES pH 8.0. (refer to section Reagents and Solutions)
Metallo protease buffer, Tris pH 7.5. (refer to section Reagents and Solutions)
Cysteine protease buffer, Citrate pH 5.5. (refer to section Reagents and Solutions)
Aspartic protease buffer, Citrate pH 4. (refer to section Reagents and Solutions)
50 nmol REPLi library (Mimotopes, Australia)
1:1 acetonitrile: water solution
Dimethyl sulfoxide (DMSO), molecular biology grade
Complement preserved normal human serum (Innovative Research)
96 well low-volume black microtiter plates (Molecular Devices)
1 L filter flasks
1 L glass bottles
50 mL conical tubes
1.7 mL microcentrifuge tubes (Eppendorf)
Microcentrifuge tube racks
Solvent reservoir
Seal & sample aluminum foil lids
5 – 50 μL 8-channel pipette
1–10 μL 8-channel pipette
5–50 μL multichannel pipette tips
1–10 μL multichannel pipette tips
Analyst HT plate reader (Molecular Devices) or any other plate reader with time resolved fluorescence capability and appropriate instrument software
Excitation and emission fluorescence filters (485 nm/530 nm for FITC-Casein; 320 nm/420 nm for MCA/DNP)
Vortexer
Eppendorf table-top centrifuge or any other table-top centrifuge capable of spinning at 21000 × g.
Vacuum pump for buffer sterile filtration
pH meter
Microsoft excel
Heat map builder (Ashley Lab, Stanford University)
Basic Protocol 1: Determination of optimum assay condition for library screen
Assay optimization with FITC-casein
-
All buffers and reagents must be prepared before starting the experiment. Five dilutions (1:1, 1:5, 1:10, 1:50 and 1:100) of the biological samples to be screened are prepared in each of four assay buffers (one for each protease class) for a total of 20 assay conditions per biological sample.
Typically, the ideal sample dilution for the library screen falls within suggested dilution range (1:1 to 1:100). This can be further confirmed by screening the sample against one or more of the generic peptide probes listed in Table 1 as described in next section of the protocol. -
Combine 10 μL of a 100 μM solution of FITC -casein (in water) and 10 μL of an appropriate dilution of the biological sample of interest (culture supernatant, serum, plasma or BAL) in a 1.7 mL microcentrifuge tube. Prepare one such tube for every dilution to be screened. Incubate the tubes for 5 hours in the dark at room temperature. Two types of negative controls are used to calculate the assay background: tubes with only FITC-casein in assay buffer (i.e., lacking biological sample) and tubes with only assay buffer (i.e., lacking FITC-casein and biological sample). Tubes with FITC-casein and trypsin can be utilized as positive controls.
The tubes should be protected from light during the incubation time by covering them in aluminum foil and storing them in a dark-room or closed drawer.The lamp in the plate reader that will be utilized to monitor fluorescence should be allowed to warm up for 20 minutes prior to reading the plate. -
After incubation add 50 μL of a 10% (w/v) trichloroacetic acid solution and incubate the tubes for 30 minutes at room temperature. Centrifuge the tubes for 15 minutes at 21000 × g on a table-top centrifuge.
The addition of trichloroacetic acid will terminate the enzymatic reaction by causing proteins in the solution to precipitate. The solution will become visibly cloudy. A 30 minutes incubation period is required to ensure complete protein precipitation. Centrifugation will pellet out the precipitated protein leaving the cleaved FITC dye in the supernatant. -
After centrifugation, pipette 40 μL of the supernatant to a 1.7 mL microcentrifuge tube containing 1 mL HEPES buffer pH 8.0.
Pipetting should be carried out with care to prevent disrupting the precipitate at the bottom of the tube. FITC fluorescence is pH dependent and is brightest in pH 8.0 HEPES buffer. -
Transfer 50 μL of each sample to a single well of a low-volume black 96 well microtiter plate and measure fluorescence intensity using a fluorescence plate reader with excitation and emission filters of 485 nm and 530 nm. Optimized Analyst HT instrument parameters for FITC are as listed below,
Integration time is 100,000 μsec
Readings per well is 1
Delay after flash is 0 μsec
Plot the measured fluorescence values for each sample dilution and assay buffer condition as a bar chart and select ideal assay conditions for library screen.
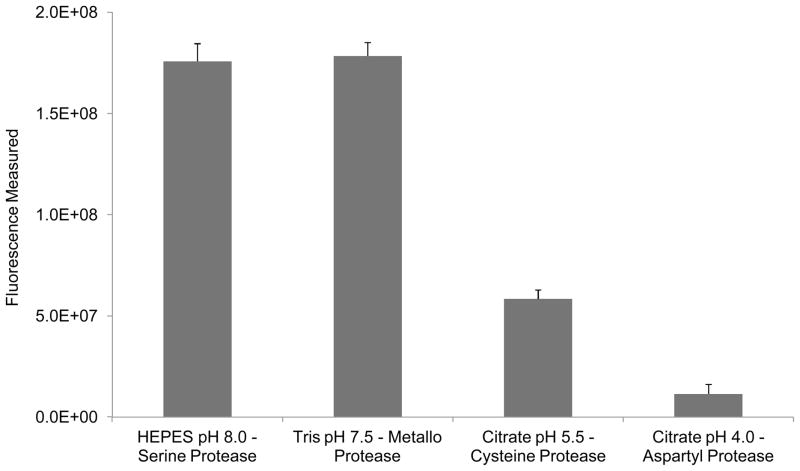
Figure 1 shows the cleavage of FITC-casein by a 1:50 dilution of Aspergillus fumigatus (AF) fungal culture supernatant in assay buffers representing each of the four major protease classes. This result in combination with the available literature regarding the proteases known to be abundant in AF, HEPES buffer pH 8.0 was selected for library screens of the AF culture supernatant (Watson et al., 2011b).
Figure 1.
Endpoint fluorescence measurement for cleavage of FITC-casein by Aspergillus fumigatus culture supernatant diluted 1:50 in assay buffers representing each of the four major protease classes. Maximum proteolytic activity was observed in both Serine protease buffer (HEPES, pH 8.0) and Metallo-protease buffer (Tris, pH 7.4). These experimental results along with literature indicating that serine proteases are the most abundant class of proteases secreted in AF culture supernatant led to the selection of HEPES buffer pH 8.0 for IQFP library screens.
If high background fluorescence is observed due to contaminants or interfering materials present in the sample, assay optimization can be carried out alternatively using generic peptide probes as described below.
Alternate Protocol 1: Assay optimization utilizing generic peptide probes
In addition to the FITC-casein optimization experiment described above, assay conditions can be further optimized using one or more of the generic peptide probes listed in Table 1. In comparison to FITC-casein, these probes provide a more direct approach to assay optimization because they contain the same fluorophore- quencher pair as the IQFPs in the library – methoxy coumarin (MCA) and dinitrophenyl (DNP) respectively. Although these peptide probes are expensive they do provide a higher level of confidence in selection of appropriate assay parameters for the library screen.
Materials
IQFP Probe MCA-RPPGFSAFK (DNP) (Anaspec) (Refer Table 1 for other peptides)
Milli-Q water
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)
Tris (hydroxymethyl) amino methane (Tris base)
Sodium citrate
Sodium chloride (NaCl)
Calcium chloride (CaCl2)
Zinc chloride (ZnCl2)
Dithiothreitol (DTT)
Ethylene diamine tetra acetic acid (EDTA)
Serine protease buffer, HEPES pH 8.0. (refer to section Reagents and Solutions)
Metallo protease buffer, Tris pH 7.5. (refer to section Reagents and Solutions)
Cysteine protease buffer, Citrate pH 5.5. (refer to section Reagents and Solutions)
Aspartic protease buffer, Citrate pH 4. (refer to section Reagents and Solutions)
Dimethyl sulfoxide (DMSO), molecular biology grade
96 well low-volume black microtiter plates (Molecular Devices)
1 L filter flasks
1 L glass bottles
50 mL conical tubes
1.7 mL microcentrifuge tubes (Eppendorf)
Microcentrifuge tube racks
Solvent reservoir
Seal & sample aluminum foil lids
5 – 50 μL 8-channel pipette
1–10 μL 8-channel pipette
5–50 μL multichannel pipette tips
1–10 μL multichannel pipette tips
Analyst HT plate reader (Molecular Devices) or any other plate reader with time resolved fluorescence capability and appropriate instrument software
Excitation and emission fluorescence filters (485 nm/530 nm for FITC-Casein; 320 nm/420 nm for MCA/DNP)
Vortexer
Vacuum pump for buffer sterile filtration
pH meter
Microsoft excel
Protocol
-
Prepare a 10 mM stock solution of the generic peptide probe (IQFP) in DMSO (or a solvent specified by the manufacturer). From this stock solution prepare a 1mM working stock solution in all four assay buffers.
Both 10 mM and 1 mM peptide stock solutions prepared are stable for up to six months when stored at −20°C. To avoid repeated freeze thaw cycles, it is preferable to aliquot them appropriately before storing in −20°C.To avoid confusion when handling multiple biological samples, generate a 96 well plate map with the information about sample description, dilution and assay buffer for each well of both the experimental plate and the sample –plate. Refer to Table 2, which is a sample 96 well plate map generated for screening three generic peptide probes against plasma samples representing different disease states. -
Dispense 16 μL of appropriate assay buffer to each well of a black 96 well low-volume microtiter plate. To each well, add 4 μL of a 1 mM IQFP stock solution prepared in the same buffer such that the final IQFP concentration in each well is 100 μM in a 40 μL total reaction volume. Label this plate as the ‘experimental plate’.
Sample dilution should be selected so that a gradual increase in fluorescence is observed over the duration of the experiment (typically 6 hours) so that significant differences in fold change values between biological samples can be observed. To a new black 96 well low volume microtiter plate add, 25 μL of each biological sample in appropriate assay buffers and dilutions as per the plate map. Label this plate the ‘sample plate’.
-
Using the appropriate 8-channel pipettor transfer 20 μL of the biological sample to the experimental plate as quickly as possible.
With proper technique, the entire contents of the ‘sample’ plate (12 columns) can be transferred in less than 2 minutes using the multichannel pipettor. To account for this slight shift in the true t0 time point value track and record the time required to complete the transfer to each plate. -
As soon the transfer is complete, record the initial t0 fluorescence in time resolved fluorescence mode on the plate reader with excitation and emission filters of 320 nm and 420 nm. Emitted fluorescence can be monitored as a function of time for up to 6 hours. Optimized Analyst HT instrument parameters for the MCA/DNP fluorophore/quencher pair is as listed below,
Integration time is 1000 μsec
Flashes per well is 60
Interval between flashes is 10 msec
Delay after flash is 35 μsec
The interval between different time points of the plate read can be selected depending upon the number of experimental plates being read consecutively. We typically monitor fluorescence every 10 minutes for 6 hours. From the measured fluorescence values, calculate the fold change as a ratio of final fluorescence (t6h) to initial fluorescence (t0). Plot the fold change values for each biological sample as a function of assay buffer and sample dilution to determine the ideal conditions for screening the IQFP library. Enzyme kinetics can be further probed by analyzing fold change rates at earlier time points.
Table 2.
96 well plate map for screening of generic peptide probes against disease state 1 and disease state 2 plasma samples from two different patients
| Patient 1 | Patient 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease State 1 Plasma | Disease State 2 Plasma | Disease State 1 Plasma | Disease State 2 Plasma | ||||||||||
| Pep 1 | Pep 2 | Pep 3 | Pep 1 | Pep 2 | Pep 3 | Pep 1 | Pep 2 | Pep 3 | Pep 1 | Pep 2 | Pep 3 | ||
| Row/Column | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Buffer 1-HEPES pH 8 - Serine Protease |
A | ||||||||||||
| B | |||||||||||||
| Buffer 2-Tris pH 7.5 - Metallo Protease |
C | ||||||||||||
| D | |||||||||||||
| Buffer 3-Citrate pH 5.5 - Cysteine Protease |
E | ||||||||||||
| F | |||||||||||||
| Buffer 4 -Citrate pH 4.0 - Aspartyl Protease |
G | ||||||||||||
| H | |||||||||||||
A sample 96 well plate for screening of three generic peptide probes against plasma samples collected from two different patients representing different disease states. Peptide 1, 2 and 3 corresponds to the generic peptide probes Mca-RPPGFSAFK (Dnp), CathD and E substrate, and Amyloid precursor protein (peptide sequences listed in Table 1) respectively. The final concentration of the peptides in each reaction well is 62.5 μM and final dilution of all the plasma samples was 1:4. The ‘Experimental plate’ is generated by pipetting respective volumes of buffer and peptides in a 96 well plate as per the plate map mentioned above. The ‘Sample plate’ is prepared in a fresh 96 well plate by pipetting 25 μL of 1:1 dilution of plasma sample as per the plate map defined above. Using an 8-channel pipettor 20 μL of plasma from each of the 8 wells in the column of the ‘Sample plate’ is transferred to the corresponding column in the ‘Experimental plate’.
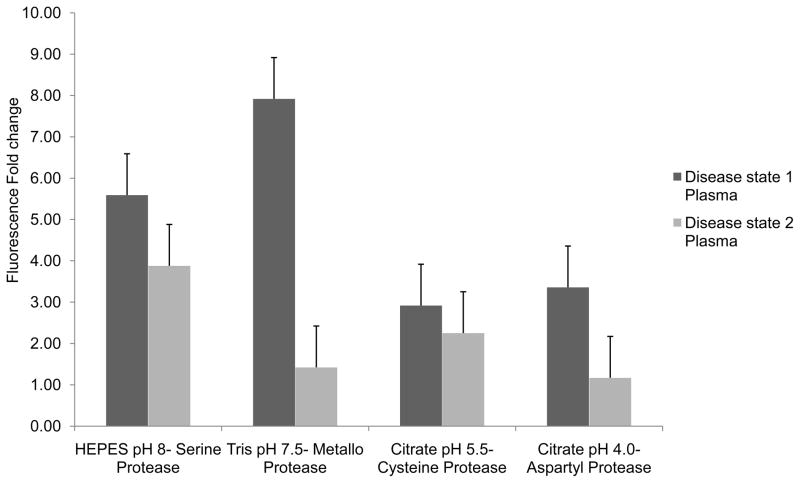
Results for assay optimization of human plasma samples using generic peptide probe MCA-RPPGFSAFK (DNP) are presented in Figure 2. Plasma samples representing two different disease states were screened against this peptide probe at 1:1 dilution. Based on these results HEPES buffer, pH 8.0 was selected for the library screens.
Figure 2.
Fluorescence fold change values observed for cleavage of the generic IQFP MCA-RPPGFSAFK (DNP) by human plasma samples collected from the same patient at two different disease stage time points. The plasma samples were diluted 1:1 in four assay buffers. Maximum proteolytic activity for both plasma samples was observed in HEPES buffer, pH 8.0 which was chosen for library screens of the plasma samples.
Basic Protocol 2: IQFP library screen of biological samples
Our library of choice contains 3375 IQFP’s distributed in 512 assay wells arrayed across 6 microtiter plates. In this format the library occupies all 96 wells of library plates 1–5, except wells G12 and H12, and the first 5 columns of plate 6 plus wells A6 and B6. Each well of the library plate contains 1–8 IQFPs. In the absence of proteases that recognize and cleave the IQFPs in a particular well, no fluorescence is observed in that well. However, in the presence of proteases that recognize and cleave one or more IQFPs present in the well, fluorescence is observed. The change in fluorescence as a function of time is monitored by measuring the fluorescence every hour for up to 6 hours. Fluorescence fold change values are calculated as described in the data analysis section and a fold change values of 2 or greater is indicative of IQFP cleavage.
Materials
Milli-Q water
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)
Sodium chloride (NaCl)
Calcium chloride (CaCl2)
Serine protease buffer, HEPES pH 8.0. (refer to section Reagents and Solutions)
50 nmol REPLi library (Mimotopes, Australia)
1:1 acetonitrile: water solution
Complement preserved normal human serum (Innovative Research)
96 well low-volume black microtiter plates (Molecular Devices)
1 L filter flasks
1 L glass bottles
50 mL conical tubes
1.7 mL microcentrifuge tubes (Eppendorf)
Microcentrifuge tube racks
Solvent reservoir
Seal & sample aluminum foil lids
5 – 50 μL 8-channel pipette
1–10 μL 8-channel pipette
5–50 μL multichannel pipette tips
1–10 μL multichannel pipette tips
Analyst HT plate reader (Molecular Devices) or any other plate reader with time resolved fluorescence capability and appropriate instrument software
Excitation and emission fluorescence filters (485 nm/530 nm for FITC-Casein; 320 nm/420 nm for MCA/DNP)
Vortexer
Vacuum pump for buffer sterile filtration
pH meter
Microsoft excel
Heat map builder (Ashley Lab, Stanford University)
Protocol
-
1
Peptides are obtained in lyophilized form and stored at −20°C before use. Hence, the plates need to be warmed to room temperature prior to adding 1:1 acetonitrile-water solution.
-
2
Dissolve library contents (50 nmol) by adding 100 μL of a 1:1 acetonitrile- water solution to each well of six library plates, resulting in a final IQFP concentration of 0.5 mM in each well.
-
3
Add 20 μL of the chosen assay buffer to each well of clean black 96 well low-volume microtiter plates. Label these plates as ‘experimental plates 1 to 6’. Using an 8-channel pipettor (1–10 μL capacity) transfer 5 μL of each column of the library plate in to the same column of the corresponding experiment plate. For example, wells A1-H1 (8 wells) of a 96 well plate constitute a column. Transfer 5 μl from each well of this column in the library plate to the same column in the experimental plate
Up to six experiment plates can be screened simultaneously. When screening multiple biological samples, screen the same library plates for each sample to avoid subjecting the IQFP library to multiple freeze thaw cycles. Final IQFP concentration in each well of the experiment plate is 55.5 μM. -
4
Pipette 25 μL of appropriately diluted biological sample in a new black 96 well low-volume microtiter plate and label it the ‘sample plate’. Transfer 20 μL of this sample solution to the experiment plate containing the lQFP library using the 8-channel pipettor (5–50 μL capacity).The transfer should be completed as quickly as possible to minimize the elapsed time between the t0 value and the initial fluorescence time-point read
Typically transferring the biological samples to the experimental plate using an 8-channel pipettor is accomplished in approximately 2–4 minutes. Note the elapsed time of transfer if it is greater than 4 minutes.For screens spanning multiple days prepare the required biological sample dilution fresh each day. Prepare all the experiment plates and the sample plates before transferring the biological sample to the experimental plates. -
5
Immediately after transferring the biological sample to the experimental plate record the fluorescence on the plate reader in time-resolved fluorescence mode using excitation and emission filters of 320 nm and 420 nm respectively. Instrument set up is as described in step 5 of the assay optimization utilizing generic peptide probes section. Record fluorescence of the experiment plates every hour for up to 6 hours.
At the end of the experiment seal the plates with aluminum foil lids and store them at −20°C until all data analysis is completed.
Data Analysis of library screen
-
6Copy the raw fluorescence data collected at each time point for each library plate to an Excel spread sheet. Calculate the fluorescence fold change value for each plate using the following formulaA fold change value of greater than 2 in a particular well of the library indicates that the IQFPs in that well are being cleaved by a protease or proteases in the biological sample. When comparing multiple biological samples, the total number of wells containing cleaved IQFP for each biological sample can be calculated. The number of such wells that are common to both samples and to unique to each can also be estimated.
-
7
For each biological sample, identify all the IQFP motif’s cleaved in the library screen and plot the amino acid distribution at each variable position.
As an example Figure 3C corresponds to the amino acid distribution of the variable positions of IQFPs cleaved by complement preserved human serum. Typically, IQFP motif’s that display the highest fold change values are selected for further analysis and characterization.A comparison of the IQFP library screen result of two different biological samples can reveal proteolytic specificity differences between them, which may identify/indicate interesting targets for further analysis even if they do not exhibit the highest overall fold change values. -
8
Generate heat-map depictions of each IQFP library screen to represent the data visually.
The heat-map in Figure 3A was generated using Heat-map builder developed by Dr. Euan Ashley’s laboratory (King et al., 2005). Detailed instructions for use of the software are provided with the software.
Basic Protocol 3: Deconvolution of IQFP peptide motifs identified in the library screen
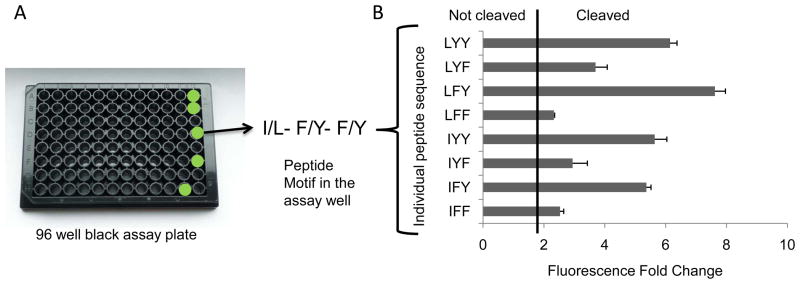
Selected library hits, typically either those exhibiting the greatest extent of cleavage by a particular biological sample or those demonstrating selectivity between multiple samples, may be further characterized by individually synthesizing and testing each constituent IQFP in the chosen library well. In one example, an IQFP motif extensively cleaved by guinea pig BAL, I/L-F/Y-F/Y was deconvoluted to define the substrate specificities of BAL proteases more precisely by individually synthesizing and testing each constituent IQFP from the chosen library well.
Materials
Individual IQFP peptides identified from library screen
Milli-Q water
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)
Sodium chloride (NaCl)
Calcium chloride (CaCl2)
Serine protease buffer, HEPES pH 8.0. (refer to section Reagents and Solutions)
Dimethyl sulfoxide (DMSO), molecular biology grade
96 well low-volume black microtiter plates (Molecular Devices)
1 L filter flasks
1 L glass bottles
50 mL conical tubes
1.7 mL microcentrifuge tubes (Eppendorf)
Microcentrifuge tube racks
Solvent reservoir
Seal & sample aluminum foil lids
5–50 μL 8-channel pipette
1–10 μL 8-channel pipette
5–50 μL multichannel pipette tips
1–10 μL multichannel pipette tips
Analyst HT plate reader (Molecular Devices) or any other plate reader with time resolved fluorescence capability and appropriate instrument software
Excitation and emission fluorescence filters (485 nm/530 nm for FITC-Casein; 320 nm/420 nm for MCA/DNP)
Vortexer
Vacuum pump for buffer sterile filtration
pH meter
Microsoft excel
Protocol
Prepare 10 mM IQFP stock solution in DMSO. From this the main stock solution, prepare a 1 mM working stock solution in the assay buffer used in the library screen. Negative control should include assay wells containing only IQFPs in buffer (i.e., lacking biological sample) and assay wells containing only assay buffer (i.e., lacking biological sample and IQFP).
Combine 4 μL of 1 mM IQFP stock solution and 16 μL of assay buffer in a clean black 96 well low-volume microtiter plate. The assay buffer is identical to that in which the library screen was carried out. Label this plate the ‘experimental plate’
-
Add 25 μL of appropriately diluted biological sample (single dilution) to a new black 96 well low-volume microtiter plate and label it as the ‘sample plate’. Using an 8-channel pipettor quickly transfer 20 μL of the sample to the corresponding column in the experiment plate.
Before conducting the experiment, generate a plate map as previously described to avoid confusion while preparing the experimental and the sample plate. Refer to Table 2, which provides a sample 96 well plate map generated for screening of three generic peptide probes screened against plasma samples representing different disease states. -
As soon as the transfer is complete, record the fluorescence of the experimental plate on the plate reader in time-resolved fluorescence mode with excitation and emission filter of wavelength of 320 nm and 420 nm respectively. Instrument set up is as described in step 5 of the assay optimization utilizing generic peptide probes section. Monitor the fluorescence every 10 minutes for up to 6 hours.
IQFPs exhibiting a fold change value greater than 2 are considered to be cleaved by the biological sample. By comparing the fold change values of closely related IQFP sequences, the substrate specificity preferences of the sample can be defined.
Figure 4 is a bar chart representation of data obtained by screening the eight individual peptides from the motif I/L-F/Y-F/Y against guinea pig BAL.
Figure 4.
Deconvolution profile for the peptide motif I/L-F/Y-F/Y selected from the IQFP library screen of guinea pig BAL (A) A representation of a 96 well assay plate with cleaved wells highlighted in green. The selected peptide motif written in single amino acid code. (B) Guinea pig BAL cleavage profile of the 8 individual constituent peptides from the motif. A fluorescence fold change value of 2 or higher is considered to be indicative of peptide cleavage (a ‘positive hit’).
Reagents and Solutions
Aspartic protease buffer – 50 mM Citrate, 100 mM NaCl, pH 4.0
Cysteine protease buffer – 50 mM Citrate, 100 mM NaCl, 1 mM DTT, 1 mM EDTA, pH 5.5
Metallo-protease buffer – 50 mM Tris-base, 100 mM NaCl, 10 mM CaCl2, 10 μM ZnCl2, pH 7.5
Serine protease buffer – 50 mM HEPES, 100 mM NaCl, 10 mM CaCl2, pH 8.0
All buffers must be sterile filtered and can be stored at room temperature for up to 6 months.
IQFP library stock solutions are prepared by dissolving the contents of lyophilized wells in 100 μL of 1:1 acetonitrile: water solution to achieve a final concentration of 0.5 mM. Individual IQFP’s synthesized for the deconvolution of library hits are dissolved in DMSO to generate a 10 mM stock solution. These stock solutions must be stored at −80°C when not in use and are stable in this form for up to a year. All protease-containing biological samples must be subdivided in to appropriate aliquots and stored at −80°C. Unopened aliquots can be stored indefinitely, but once opened an aliquot must be used within a day.
Commentary
Background Information
Proteases are involved in the regulation of a wide variety of physiological processes and their disregulation has been implicated in a plethora of human diseases such as rheumatoid arthritis, Alzheimer’s disease and cancer. Serum, plasma and BAL are three of the most clinically relevant complex biological samples utilized routinely in disease diagnosis. Hence, the key challenge is identification and characterization of disease-relevant proteases in these samples.
Mass spectrometry-based proteomics and activity-based protein profiling have been used to detect proteases and their cleavage products in complex biological mixtures (Cravatt et al., 2007; Cravatt et al., 2008). However each technique has notable limitations. The major disadvantage of mass spectrometry-based techniques is that they often focus only on abundant proteins and thus, may not be sufficient for identification of diagnostic or therapeutic targets which are less abundant. Also, mass spectrometry may be unable to differentiate catalytically active proteases from those that are inactive or degraded. In contrast activity-based protein profiling is not a global method because it targets a select few proteases and detects them specifically. The principal shortcoming of this approach is that it may overlook important disease-relevant proteases as a result of its narrow, targeted focus.
The substrate specificity profiling technique described in this protocol can complement these existing techniques in the assessment of differences in protease specificity between complex samples. It can provide information based on a broad quantitative comparison across a large proteolytic space and may reveal differences that could be missed when focusing on specific proteases. One of the potential limitations of this technique is that the existing IQFP library is restricted to the identification of endoproteases. Another limitation of this technique is that the proteolytic activity in the biological samples are dependent on sample preparation and assay screening conditions and may not always accurately represent the in vivo situation. In addition, the synthetic octapeptide sequences of the library may not accurately capture the folded structures of endogenous substrates. However, the robust technique described in the protocol can be utilized to identify of proteolytic specificities of interest which can then be further characterized using the activity-based protein profiling or mass spectrometry-based techniques.
Critical Parameters and Troubleshooting
Both the IQFP library and the individual IQFPs are obtained as lyophilized powders and can be stored indefinitely in this state at −80°C. Once the peptides are dissolved in DMSO they must be used within six months and when not in use must be stored at −80°C. The 1 mM working stock solutions of individual IQFPs prepared in assay buffers must be used within a month of their preparation and when not in use must be stored at −20°C. Some peptides may have solubility issues in some assay buffers and that can be mitigated by preparing working stock solutions of lower concentrations (e.g., 500 μM).
Samples to be screened must be well characterized and should not contain protease inhibitors or other additives known to inhibit or otherwise affect proteolytic activity. Screening the IQFP library with a single biological sample requires about 15 ml of appropriately diluted sample. It is essential to ensure that sufficient sample volume is available before starting the experiment. Avoid subjecting the samples to multiple freeze-thaw cycles as this is known to affect proteolytic activity. To avoid this issue altogether, aliquot samples into small fractions (500 μL – 1 mL) on initial collection and store them at −80°C. If a sample contains tissues and other insoluble material, centrifuge for 10 minutes at 5000 × g and collect the supernatant for the library screen. Estimation of the total protein content of the biological samples using Bicinchoninic acid (BCA) protein assay kit (Thermo Scientific Pierce, Product # 23225) will provide a good correlation to the observed proteolytic activity of the samples.
Anticipated Results
A typical IQFP library screen of human serum or guinea pig BALF will identify approximately 100–150 motifs that are cleaved by the proteases in each of these fluids. Two independent IQFP library screens of the complement preserved human serum revealed a modest variability; 63% of hits were identified in both the experiments with 21% of hits identified only in experiment 1 and 14% of hits identified only experiment 2 (Watson et al., 2011a). This could be accounted for by inherent biological variability arising from different donors in the two independent lots pooled serum (Diamandis, 2004).The library itself is subjected to rigorous quality control. Thus, variability can be minimized by utilizing samples that are similar and well defined, as well as by running the assay replicates on the same day. Further confirmation of peptide hits can be obtained by secondary screening of individually synthesized IQFPs from the selected library hits against each biological sample. Depending on sample availability and budget it is recommended to conduct a single assay screen of the biological sample followed by rigorous deconvolution and confirmation of individual hits. It is also recommended that the deconvolution of library hits be carried out at least in duplicates and repeated in a minimum of two independent experiments. Further confirmation of IQFP cleavage and the cleavage site can be determined by identification of cleaved IQFP fragments by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS).
Time Considerations
Sample preparation for the assay optimization phase requires 1–2 hours and the experimental component requires 7–8 hours. Data analysis requires an additional 1–3 hours, depending on number of biological samples screened.
The IQFP library screen, which includes preparation of IQFP stock solutions (including thawing of IQFP stocks stored at −80°C), sample dilution in appropriate buffer (including thawing sample on ice before diluting inappropriate assay buffer) and preparation of experimental and sample plates, requires approximately 3–4 hours. Time-resolved fluorescence measurements of the experimental plate(s) require a time-point measured every hour for 6 hours. Up to six plates can be screened in a day, thus a single biological sample library screen will require approximately 8–10 hours. It recommended to avoid screening more than six plates at once to minimize the risks of sample contamination and operator error. For screens in which multiple replicates of the same biological sample are being screened or different biological samples are being compared it is recommended to screen each library plate against all the samples (not to exceed six plates) on the same day to prevent exposing library stock solutions to multiple freeze-thaw cycle. For example, when comparing two biological samples, it is advised to screen both samples against library plates 1–3 (6 total plates) on Day 1 and then screen both samples against library plates 4–6 (6 total plates) on Day 2.
Screening of a single experiment plate of deconvoluted peptides against the samples requires 8–10 hours including sample preparation. Data analysis requires an additional 1–3 hours.
Acknowledgments
This work was supported by grant R21AI08502 from the National Institute of Allergy and Infectious Diseases (NIAID). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAID or National Institutes of Health (NIH). Guinea pig BAL was kindly provided by Dr. Tom Patterson, University of Texas Health Science Center at San Antonio, supported by contract NO 1-AI-30041 from the NIAID, NIH. Thanks are also extended to members of his laboratory; Laura Najvar and Rick Kirkpatrick. Aspergillus fumigatus culture supernatants were kindly provided by Dr. David Askew, University of Cincinnati. Human plasma samples were provided by Dr. Moin Saleem, University of Bristol, UK. The Center for Advanced Drug Research was established through funding support from commonwealth of Virginia to SRI International. Encouragement and guidance from Dr. Walter Moos, Vice President of Biosciences at SRI is also gratefully acknowledged.
References
- Backes BJ, Harris JL, Leonetti F, Craik CS, Ellman JA. Synthesis of positional-scanning libraries of fluorogenic peptide substrates to define the extended substrate specificity of plasmin and thrombin. Nat Biotechnol. 2000;18:187–193. doi: 10.1038/72642. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Simon BM, Yates JR., III The biological impact of mass spectrometry-based proteomics. Nature. 2007;450:991–1000. doi: 10.1038/nature06525. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Wright AT, Kozarich JW. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu Rev Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- Diamandis EP. Mass spectrometry as a diagnostic and a cancer biomarker discovery tool: opportunities and potential limitations. Mol Cell proteomics. 2004;3:367–378. doi: 10.1074/mcp.R400007-MCP200. [DOI] [PubMed] [Google Scholar]
- King JY, Ferrara R, Tabibiazar R, Spin JM, Chen MM, Kuchinsky A, Vailaya A, Kincaid R, Tsalenko A, Deng DX, Connolly A, Zhang P, Yang E, Watt C, Yakhini Z, Ben-Dor A, Adler A, Bruhn L, Tsao P, Quertermous T, Ashley EA. Pathway analysis of coronary atherosclerosis. Physiol Genomics. 2005;23:103–118. doi: 10.1152/physiolgenomics.00101.2005. [DOI] [PubMed] [Google Scholar]
- Thomas DA, Francis P, Smith C, Ratcliffe S, Ede NJ, Kay C, Wayne G, Marin SL, Moore K, Amour A, Hooper NM. A broad spectrum fluorescence based peptide library for the rapid identification of protease substrates. Proteomics. 2006;6:2112–2120. doi: 10.1002/pmic.200500153. [DOI] [PubMed] [Google Scholar]
- Watson DS, Jambunathan K, Askew DS, Kodukula K, Galande AK. Robust Substrate profiling method reveals striking differences in specificities of serum and lung fluid proteases. 2011. Bio Techniques. 2011;51:95–104. doi: 10.2144/000113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DS, Feng X, Askew DS, Jambunathan K, Kodukula K, Galande AK. Substrate specificity profiling of the Aspergillus fumigates proteolytic secretome reveals consensus motifs with predominance of Ile/Leu and Phe/Tyr. PLoS ONE. 2011;6:1–16. doi: 10.1371/journal.pone.0021001. [DOI] [PMC free article] [PubMed] [Google Scholar]