Abstract
Besides their well-known genomic actions, oestrogens also exert effects through the activation of receptors associated to the plasma membrane that are too fast to be mediated by transcriptional activation (non-genomic effects). While the existence of such rapid effects of oestrogens and their involvement in various biological processes are not in doubt anymore, questions remain about the mechanisms responsible for the rapid modulations of oestrogen production that are required to sustain their non-genomic effects. Recent data indicate that the conversion of androgens into oestrogens in the brain by the enzyme aromatase can be rapidly modulated by conformational changes of the enzyme thus providing a possible mechanism for rapid controls of the effects of oestrogens on male sexual behaviour. In this review, I describe the data supporting this hypothesis. I then discuss a few unanswered questions such as the mechanism of oestrogen inactivation or the potential cellular sites of action of brain-derived oestrogens on male sexual behaviour.
Keywords: Male sexual behaviour, aromatase, non-genomic effect
Introduction
Besides their traditional long-lasting genomic effects, oestrogens also produce rapid and short-lived actions through an interaction with the cell membrane (non-genomic effects). Some of these actions can, however, ultimately activate genomic effects (indirect genomic effects) [1]. In parallel, in the brain, two mechanisms regulate oestrogen production by local aromatisation of androgens that correspond to the two time-frames of actions of oestrogens (Genomic Vs. Non-genomic): 1/a transcriptional control of the enzyme concentration and 2/conformational changes leading to rapid modulations of enzyme kinetics without changes in aromatase concentration. Evidence supports the involvement of these fast changes in oestrogen bioavailability in the control of male sexual behaviour [2]. However, questions remain regarding the cellular mechanisms underlying these effects. In this paper, I first summarise the experiments that led to the demonstration of genomic and non-genomic controls of oestrogen production and action with an emphasis on the control of male sexual behaviour. Then I discuss some mechanistic hypotheses that should guide future investigations.
Genomic effects of oestrogens and transcriptional control of aromatase concentration
The best example of a genomic action of oestrogens on behaviour is the activating effect of oestrogens originating from the ovary at pro-oestrus on female sexual behaviour [3]. In males, many effects of testosterone in the brain are mediated via its local conversion into 17β-oestradiol by the enzyme aromatase (a conversion resulting from a series of complex reactions [4, 5]). For instance, the activation of male sexual behaviour by testosterone requires its aromatisation into 17β-estradiol in many vertebrate species. This conclusion is supported by experiments demonstrating that behavioural effects of testosterone are mimicked in castrates by oestrogens or aromatisable androgens, but not by non-aromatisable androgens, and that they are blocked by aromatase inhibitors and anti-oestrogens but not, or less so by anti-androgens [4].
A key site of steroid action in the control of male sexual behaviour is the medial preoptic area (POA), which contains one of the richest populations of aromatase-immunoreactive cells [6–8] as well as oestrogen receptors [9, 10]. The classic mode of steroid action involves binding to their cognate nuclear receptors acting as transcription factors for various target genes. Such genomic effects are relatively slow and develop with latencies ranging from a few hours to several days. One example of such genomic actions of oestrogens on behaviour is the regulation of brain aromatase activity (AA) that is largely mediated by changes in enzyme concentration resulting from an increased transcription after exposure to testosterone. These effects on aromatase transcription appear to result from a synergistic action of the oestrogenic and androgenic metabolites of testosterone with a prevalence for oestrogens in birds and androgens in mammals [4, 7]. Changes in sexual behaviour are tied to these steroid-dependent changes in aromatase concentration. In quail, for example, AA peaks 48 hours after the onset of testosterone treatment while the activation of copulatory behaviour by testosterone is delayed by a few days. The behavioural effects of testosterone thus seem to be explained, to a large extent, by the transcriptional modulation of aromatase concentration and the subsequent genomic effects of oestrogens produced by testosterone aromatisation.
Non-genomic effects of oestrogens on behaviour
Besides their genomic actions, oestrogens also exert effects that are too rapid (seconds to minutes) to be mediated through the activation of protein synthesis. Although purely cytoplasmic effects have also been described, non-genomic effects are generally initiated at the plasma membrane resulting in the activation of a wide variety of intracellular signalling pathways [1, 11]. In this article, I focus on membrane receptors, but the existence of non-genomic effects occurring completely intracellularly should not be ignored and the hypotheses presented in this paper remain valid for such cytoplasmic effects. Multiple receptor systems have been implicated in mediating the effects of oestrogens at the membrane level. First, the nuclear receptors can associate with the plasma membrane (some studies suggest an interaction with caveolae) and generate intracellular signals through the activation of a G protein-coupled receptor (GPCR)[2, 12]. Novel membrane receptors such as GPR30 [13], ER-X [14] or another nameless GPCR [15] have also been proposed as candidates for mediating oestrogen actions at the membrane. Finally, oestrogens can also act as co-agonists or allosteric modulators of GPCR or ion-gated channels/receptors [16]. The signalling pathways activated by these receptors result in phosphorylations of enzymes or receptors leading, for example, to changes in enzymatic activities or receptor uncoupling from their effectors.
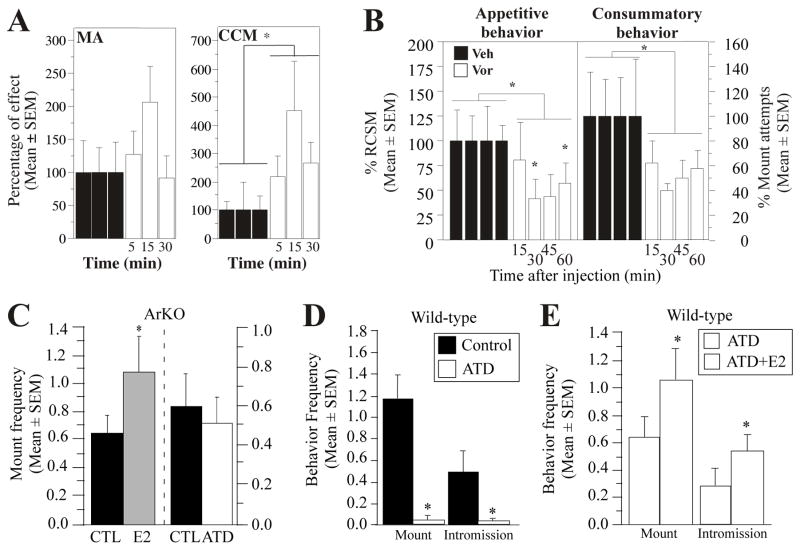
The functional significance of non-genomic actions of oestrogens remains largely unknown. Evidence is accumulating that oestrogens acutely influence processes such as pain, aggressive and sexual behaviours [17–19]. In females, acute administration of 17β-oestradiol facilitates lordosis behaviour partly through non-genomic actions [20] potentially involving an interaction with metabotropic glutamate receptors and ERα [18]. In males, Cross and Roselli first showed that a subcutaneous injection of a high dose of oestradiol (100μg/kg) stimulates within 35 min mounts and anogenital investigations in castrated rats [21]. Subsequent studies demonstrated that a single injection of a bolus 17β-oestradiol facilitates the expression of most aspects of male sexual behaviour within 10–15 min in quail (500μg/kg) and mice (500μg/subject) (Fig. 1) [22, 23]. These effects are best observed in animals pre-treated with a sub-optimal dose of testosterone or oestradiol-benzoate suggesting that these non-genomic actions require some steroid priming to occur.
Figure 1. Acute effects of oestrogens and of aromatase inhibition on male sexual behaviour in quail and mice.
A. Time-response curve of the acute effects of 17β-oestradiol (E2, 500μg/kg) on consummatory behaviour assessed by mount attempt (MA) and cloacal contact movements (CCM) frequency. B. Time-response curve of the acute effects of the aromatase inhibitor, vorozole™ (30mg/kg), on appetitive (as assessed by the frequency of the rhythmic cloacal sphincter movements [RCSM]) and consummatory sexual behaviour (as assessed by the frequency of mount attempts) expressed as percentage of controls. C. Acute effect of E2 (500μg/subject) and absence of acute effect of the aromatase inhibitor, ATD (1,4,6-androstatriene-3,17-dione, 4mg/subject), on male sexual behaviour in aromatase knock-out mice (ArKO) injected 10 minutes before the test. D. Acute effect of ATD (4mg/subjects) on male sexual behaviour in wild-type mouse injected 10 minutes before the test. E. Acute effect of E2 (500μg/subject) on male sexual behaviour in wild-type mouse chronically treated with ATD (0.5mg/subject) to inhibit the genomic effects of estrogens. Redrawn from [22, 23, 41].
Many acute cellular and behavioural effects of oestrogens appear to require concentrations higher than normal circulating levels to be triggered. This conclusion is based on the observation that 1/numerous non-genomic effects of oestrogens observed in vitro are triggered by concentrations exceeding physiological levels, 2/oestrogen concentrations greater than circulating levels have been reported in certain brain regions and 3/the doses of oestradiol used in acute injection studies are estimated to reach circulating concentrations ranging between 100 and 500 ng/ml which is about 2 to 3 orders of magnitude above the endogenous concentrations found in the plasma (in quail 500 μg/kg would give 500 ng/ml of plasma assuming that the distribution is immediate and the volume of distribution equals the entire body [1liter/kg body weight]). Such observations led some to question the physiological relevance of these acute effects of oestrogens (for more details and a discussion on whether these concentrations should be considered as physiological or pharmacological, see [2]). Another issue concerns the mechanism(s) able to produce fluctuations of endogenous oestrogens on a timescale compatible with their acute effects [2, 24]. The next section suggests potential solutions to this question.
Rapid modulations of oestrogen production influence male sexual behaviour
Brain AA can be rapidly (within minutes) modulated by calcium-dependent phosphorylations that do not change the enzyme concentration but only affect the activity of pre-existing enzymatic molecules. The enzymatic activity measured in quail preoptic/hypothalamic homogenates submitted to phosphorylating conditions (increased but physiological concentrations of ATP, Mg2+ and Ca2+) is profoundly inhibited within 10–15 minutes. This inhibition is blocked by compounds chelating divalent ions or by kinase inhibitors indicating that it is indeed caused by phosphorylation processes [25, 26]. This interpretation is consistent with the presence in the aromatase sequence of several consensus sites of phosphorylation [26, 27]. Similar effects were observed in preoptic/hypothalamic explants in which the cellular integrity of the neurones and a large part of their connectivity was maintained. In these explants, AA is rapidly (within 5 minutes) and reversibly inhibited by conditions increasing the intracellular calcium concentration or by application of glutamatergic agonists [25, 28]. A recent microdialysis study performed on zebra finches also demonstrated that retrodialysis of glutamatergic agonists results in reduced concentrations of oestrogen within the caudo-medial nidopallium thus confirming the inhibitory role of glutamate on oestrogen synthesis [29]. Given that stimulation of glutamate receptors can rapidly lead to their desensitization, one could, however, hypothesize that the enzymatic inhibition results from the stimulation or the desensitization of these receptors.
To assess whether such enzymatic modulations occur in vivo and participate in the control of male sexual behaviour, we employed two different but complementary approaches. First, given that acute oestradiol injections facilitate the expression of both appetitive and consummatory aspects of male sexual behaviour, we investigated whether acute injections of an aromatase inhibitor would disrupt the expression of these behaviours. Accordingly, systemic injections of aromatase inhibitors significantly reduced both aspects of male sexual behaviour in sexually active male quail and mice within 10 and 30 minutes, respectively (see figure 1 for details on inhibitors and their specific dosage). Similar inhibitions could not be obtained in aromatase knock-out mice, while the acute treatment with oestradiol stimulated their copulatory behaviour further supporting the specific involvement of aromatase in these behavioural effects (Fig. 1B) [22, 23]. Second, we investigated whether preoptic AA is rapidly modulated in vivo during sexual activity and showed that visual access to, as well as copulation with, a receptive female results in a decrease in AA within 1 minute. This enzymatic inhibition reached a maximum (−20%) after 5 minutes of interaction and was back to normal after 15 minutes (Fig. 1E)[30]. Hence, AA varies very rapidly in vivo in physiologically relevant situations.
Together, these data indicate that beside the transcriptional control of aromatase concentration, mechanisms involving conformation changes are able to rapidly regulate brain oestrogen synthesis. Such mechanisms should play a key role in the control of acute effects of oestrogens in that it makes it possible to reach locally the high concentrations required to trigger non-genomic actions and allow changing levels of endogenous oestrogens on a timescale compatible with their acute effects.
A new model and mechanistic hypotheses
The work described so far indicates that brain oestrogen synthesis is rapidly modulated in vitro and in vivo by changes in AA presumably influenced by glutamatergic inputs. Oestrogen receptors associated with the plasma membrane and signalling pathways sustaining non-genomic oestrogenic actions are currently being unravelled. Some physiological and behavioural responses to oestrogens occur at latencies incompatible with changes in protein synthesis and are blocked by aromatase inhibitors or oestrogen receptors antagonists. Also, aromatase immunoreactivity and activity have been identified in presynaptic boutons thus indicating that rapid changes in enzymatic activity can modulate the bioavailability of oestrogens at the synapse [31–33]. Taken together, these observations suggested that brain-derived oestrogens produced by local aromatisation of testosterone might fulfil at least some of the criteria defining neurotransmitters, or neuromodulators (for more discussion on this topic see Figure 2 and [34]). Many questions, however, remain unanswered. For example, neurotransmitters should possess appropriate mechanisms for inactivation. This question is important independently of whether or not oestrogens are considered as neurotransmitters: if steroids hormones do exert rapid effects on behaviour, mechanisms must indeed exist to rapidly inactivate their effects.
Figure 2. Should oestrogens be considered as neurotransmitters/neuromodulators? – A potential model.
Several criteria have been established for a molecule to be considered as a neurotransmitter/neuromodulator. These criteria involve 1) synthesis and storage in presynaptic vesicles, 2) release upon stimulation in the synaptic cleft in concentrations sufficient to activate post-synaptic receptors, 3) specificity for a defined receptor so that action can be blocked by specific antagonists, 4) presence of inactivation mechanisms (e.g., catabolism or reuptake). Recent evidence suggests that oestrogens might fulfil some, if not all, of these criteria. (1) Oestrogens are formed via aromatisation of androgens catalysed by the enzyme aromatase. Aromatase-immunoreactive material and enzymatic activity have been detected in presynaptic terminals, indicating that oestrogens are synthesised and present in synapses [32, 33]. Although estrogens are probably not stored in vesicles, this does not necessarily disqualify them as transmitters. The gaseous transmitter nitric oxide (NO) is similarly not stored in vesicles; its synthesis and release directly depend on neuronal activation [61]. This may also be the case for brain-derived oestrogens. (2) Oestrogen synthesis is rapidly modulated by conformational changes of aromatase [25, 26, 28] themselves triggered by various behavioural as well as chemical stimuli [29, 30]. Given that oestrogens are liposoluble and easily cross the cell membrane, rapid changes in oestrogen production should regulate their bioavailability in the synaptic cleft. (3) Various membrane/cytoplasmic effects of oestrogens have been identified that are supported by a diversity of intracellular cascades through the activation of nuclear receptors associated or not to the membrane of novel G protein coupled membrane oestrogen receptors (receptors characterized by 7 transmembrane domains) [13–15]. The intracellular pathways triggered by such non-genomic actions of oestrogens may affect neuronal firing through the modulation of ionic conductance via phosphorylation of ionotropic receptors or the uncoupling of G protein-coupled receptors from their ionic channels or intracellular effectors [1, 16, 47]. Some behavioural effects of oestrogens are rapidly blocked by aromatase inhibition or by antagonists of nuclear receptors [18, 41] indicating that events triggered by oestrogens are rapidly blocked by known antagonists. Together, these data support the specific nature of the actions triggered by brain-derived oestrogens. (4) Finally, mechanisms of rapid inactivation of oestrogens exist [35, 36] but their role in the control of non-genomic actions of oestrogens in the brain has not formerly been tested yet. It is also possible that locally synthesised oestrogens might simply diffuse away from their target sites. For further discussion of these issues, the interested readers should refer to previously published papers critically discussing the definition of neurotransmitters [61] and the potential extension of this definition to neuro-oestrogens [34]. The nuclear receptor is schematically represented only in association with the membrane for simplicity but non-genomic effects can also occur in the cytoplasm.
Additional questions still pending concern the specific functional impact of non-genomic actions of oestrogens as well as the neural site(s) where they take place. It is also not clearly established whether non-genomic effects of oestrogens can be activated independently of their genomic actions. Possible answers to these questions are considered in the following paragraphs.
Mechanisms of oestrogen inactivation
In males, once aromatase concentrations reach a maximum under the influence of the seasonal increase in testosterone circulating levels, its activity could be turned on and off on a shorter-time frame by conformational changes of the enzymatic protein. These rapid changes in oestrogen synthesis should result in rapid changes in oestrogen release. But what terminates the non-genomic effects when oestrogen production ceases? Two theoretical possibilities can be considered: (1) oestrogens might be degraded into less potent/inactive molecules and/or (2) oestrogens might simply diffuse away from their targets, the dilution of the steroid resulting in the disappearance of the effect.
Passive diffusion could indeed rapidly equilibrate the high concentrations of oestrogens produced locally with the much lower average brain concentration reflecting peripheral concentrations. As already mentioned above, this lower concentration is presumably unable to trigger the fast effects of oestrogens that appear to depend on concentrations much higher than those found in the periphery. At a lower concentration, brain-synthesised oestrogens might no longer be able to activate their membrane receptors thus resulting in the disappearance of the effects.
Alternatively (or additionally) the termination of oestrogen action could also result from its catabolism. In the periphery, oestrogenic hormones are catabolised to hormonally inactive (or less active) water-soluble metabolites excreted in urine and/or feces. The metabolic elimination of oestrogens relies on oxidative metabolism (largely hydroxylations by cytochrome P450 enzymes) and conjugations by glucuronidation, sulfonation and/or O-methylation [35]. While hydroxylated products retain some oestrogenic activity, conjugations appear to markedly decrease hormonal action. This metabolism of oestrogens takes place primarily in the liver, but detectable levels of metabolic activity are also expressed in the brain. In order to rapidly clear out the synapse and switch off oestrogenic signalling, it is expected that these enzymes would co-exist with aromatase. Whether this is the case or not is unknown for most enzymes.
An interesting hypothesis emerges from the observation that aromatase purified from human placental microsomes displays both androgen aromatisation and oestrogen 2-hydroxylase activities depending on pH [36]. Importantly, transfection of cell lines devoid of aromatase and 2-hydroxylase activities with the aromatase gene also results in the appearance of both types of enzymatic activities, thus indicating that the same enzymatic protein can catalyse the two reactions [36]. Although other enzymatic conversions or conjugations are necessary to fully eliminate 2-hydroxy-metabolites from the tissue, this metabolic transformation alone has profound consequences on the activity of oestrogens. 2-hydroxyoestrogens still bind to classical oestrogen receptors, but with a markedly reduced affinity and consequently they display a much weaker hormonal potency as compared to the parent hormone [35]. Further investigations are clearly necessary to confirm that brain aromatase possesses the same properties as placental aromatase, but available data already indicate that this enzyme has the potential to catalyse both the synthesis and part of the catabolism of oestrogens providing a mechanism for a rapid inactivation of oestrogens compatible with their action as neurotransmitters. The fact that the distribution of aromatase and 2-hydroxylase activities are nearly identical in the quail brain when measured in micro-dissected brain samples is consistent with this conclusion [37, 38].
There is indirect experimental evidence available suggesting that brain oestrogens are rapidly metabolised. This evidence can be derived from (1) the comparison of two methods used to quantify aromatase activity and (2) the comparison of the time-course of aromatase inhibitors effects in quail and mice, two species displaying different amounts of aromatase in the POA.
(1) Comparison of results from different aromatase assays
Although it is technically challenging to measure local concentrations of oestrogens in the brain and even more so to assess the turn-over of the hormone, indirect assessments of this turnover can be obtained by comparing results of two independent types of aromatase assays. The tritiated water assay measures the tritiated water produced during the aromatisation of [1β-3H]-androstenedione (the tritium on carbon 1 in β-position is incorporated into water). Alternatively, the product formation assay quantifies the tritiated oestrogens produced by aromatisation of androgens carrying tritium in a different position. If the newly formed oestrogens are rapidly metabolised (and water is not), a difference should exist in the enzymatic activity measured by these two assays. This seems to be the case since AA measured in the POA of male quail by the 3H-water assay [39] appears 10–20 fold higher than the activity assessed by oestrogen production [37, 40]. These data suggest that oestrogens formed by aromatisation are metabolised rapidly after their formation but these comparisons are so far indirect and should be reproduced on the same samples.
(2) Time-course of behavioural effects of aromatase inhibitors in quail and mice
The non-steroidal aromatase inhibitor, vorozole™, inhibits AA within 5 minutes in preoptic-hypothalamic explants but the first signs of behavioural inhibition are only seen after 30–45 min in vivo [41]. This latency of action is attributed to the time necessary for the inhibitor to first reach the enzyme, inhibit its activity and then for the resulting oestrogenic stimulation to disappear due to catabolism and/or dilution of the locally produced oestrogens. In mice, a systemic injection of vorozole™ almost completely inhibits copulatory behaviour within 10 minutes [23], while, in quail, the maximal behavioural inhibition (a 60% decrease as compared to basal behavioural level) occurs 30–45 minutes only after the injection [41]. The behavioural inhibition following the acute injection of vorozole thus appears slower and reaches a smaller magnitude in quail than in mice. This species difference could be explained by the difference in preoptic aromatase activity (and presumably concentration). The quail POA indeed expresses a much higher degree of AA than is the case in mice. This higher enzymatic activity is likely to generate higher oestrogen concentrations. Their clearance by dilution or catabolism could very well take more time thus explaining the longer latency of the behavioural inhibition. This would then contradict the idea that catabolism results from the action of the same enzymatic protein expressing both aromatase and 2-hydroxylase activities, assuming that these two activities are proportional irrespective of the enzyme concentration. Other processes may however be involved in the termination of oestrogen action or, alternatively, aromatase and 2-hydroxylase activities may be subjected to specific control mechanisms (e.g. by local pH) even if based on the activity of a same molecule. More work is definitely warranted on this topic.
Discrepancies between effects of injection experiments and measures of enzymatic activity
In our attempt to link rapid modulations of brain AA and acute control of male sexual behaviour by oestrogens, we encountered contradictory results that seem difficult to reconcile within one simple functional model. Studies based on acute injections of oestradiol or aromatase inhibitors suggest that locally synthesised oestrogens play a stimulatory role on male sexual behaviour. It was thus anticipated that AA should rapidly rise during sexual behaviour to sustain its expression. However, a rapid enzymatic decline was observed, 1 to 5 min following copulation. To this date, it is not technically possible to assess the enzymatic activity in freely behaving animals. AA thus cannot be quantified during the expression of the behaviour nor immediately thereafter (a minimum of one min is required for brain collection and preservation on dry ice). The apparent discrepancy between the stimulatory effects of oestrogens on sexual behaviour and the inhibition of oestrogen synthesis observed after copulation could thus simply reflect the different time points at which these two effects have been studied. Injection experiments could mimic processes involved in the initiation of the behaviour (potentially a very short-lived increase in enzyme activity), while the changes in AA observed rapidly after copulation would reflect processes associated with the termination of copulation.
It must be noted that AA is already inhibited in males that have been visually exposed to a female but were not allowed to copulate with her [30]. This suggests therefore that the enzymatic inhibition cannot reflect satiation processes that would take place in the brain. It remains, however, possible that a form of negative feedback blocks or decreases the activity of the enzymatic molecules immediately after they have been active (due for example to liberation of the enzyme and of the synthesised oestrogens in the synaptic cleft, which would modify the ionic and pH environment of the enzyme). More experiments carefully manipulating the behavioural test situations and the conditions of enzyme assays could provide answers to these questions.
What are the cellular mechanisms of rapid oestrogen action on sexual behaviour in males?
The cellular mechanisms responsible for the acute behavioural effects of oestrogens are not known. However, based on the fast growing literature on non-genomic cellular actions of oestrogens, several potential mechanisms can be proposed.
Extranuclear ER have been identified in presynaptic boutons [42] as well as at post-synaptic locations [43]. Oestradiol rapidly potentiates excitatory post-synaptic potentials [44], i.e., electrophysiological responses relying on synaptically released transmitters, thus suggesting that oestrogens can modulate synaptic transmission through the control of transmitter release. Examples of changes in neuronal excitability produced by post-synaptic actions have also been documented [45–47]. These data thus suggest that signalling pathways mediating non-genomic effects of oestrogens (mediated by membrane or cytoplasmic receptors) identified so far are compatible with both a pre- and a post-synaptic modulation of synaptic transmission.
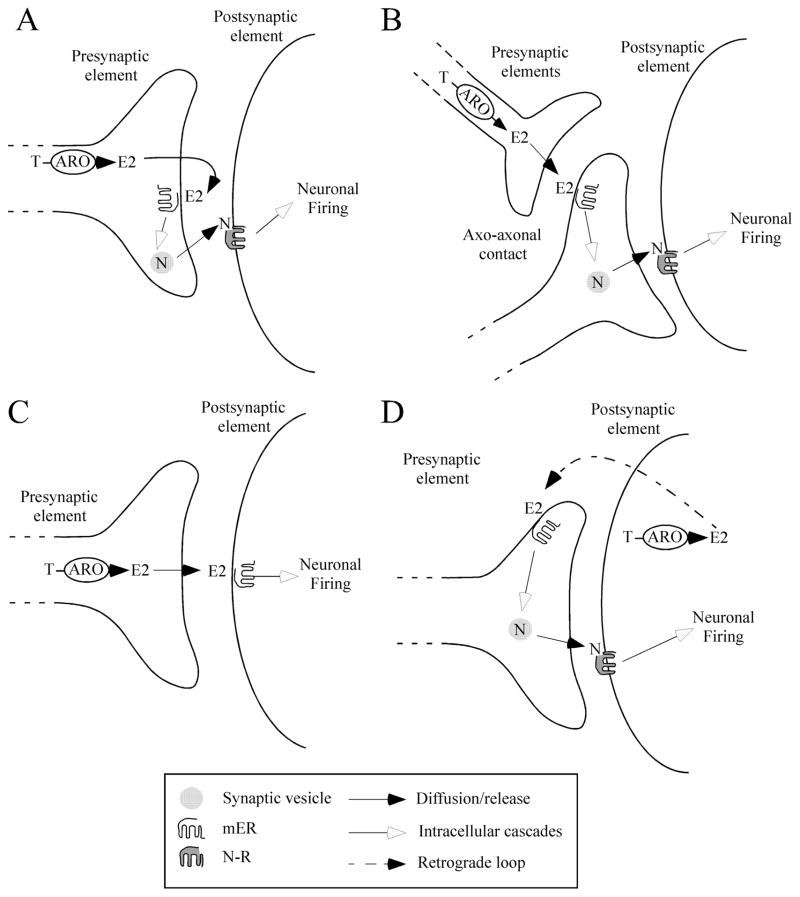
How do then these mechanisms fit with what we know about aromatase and non-genomic oestrogen effects on male sexual behaviour? In a model based on pre-synaptic actions of oestrogens, it can be hypothesised that the transmitter whose release is altered could be located either in the aromatase-containing cells (Fig. 3A) or in synapses contacted by the aromatase synapses (axo-axonal contacts; Fig. 3B). This latter option seems, however, unlikely since aromatase has been so far detected in axo-dendritic and axo-somatic synapses only [32]. We do not unfortunately know what is (are) the transmitter(s) released by aromatase-positive synapses. The distribution of several neurotransmitters/neuropeptides implicated in the control of male sexual behaviour (e.g. vasotocine, vasoactive intestinal polypeptide, substance P, serotonine, dopamine, noradrenaline, GnRH and neurotensine) has been investigated in the quail brain with special attention given to areas containing high densities of aromatase-immunoreactive cells. However, a high degree of co-localisation has not been detected to date either because the neurotransmitter/peptide was not present at all in aromatase-expressing areas or it was present but double-label studies revealed no co-localisation [48–51]. GABA is broadly expressed in the brain and is a potential candidate for co-localisation with aromatase. Although this has not been formally demonstrated yet, evidence suggests that it might be the case. Indeed, in the different species and brain regions investigated, the majority of pre-synaptic sites that are immunoreactive for aromatase based on electron microscope studies were found to be symmetrical synapses. These are thought to represent inhibitory synapses, suggesting that aromatase-positive cells may well be primarily GABAergic in nature. Non-genomic modulations of inhibitory or GABA currents by oestrogens have been reported in the hypothalamus [47, 52]. It is thus tempting to postulate that direct non-genomic action of oestrogens on GABA release could be implicated in the control of male sexual behaviour through a modulation of the tonic descending inhibition of motor outputs associated with erection, ejaculation or postural changes involved in male sexual behaviour [53, 54].
Figure 3. Potential models of the modulation of synaptic transmission by brain-synthesised oestrogens involved in the acute control of male sexual behaviour.
A. Presynaptic control of the release of a neurotransmitter (N) co-expressed in the aromatase-imunoreactive neurone. Oestrogen synthesized in the terminal could act pre-synaptically on its own neurone (the mER is then called an autoreceptor) to control the release of neurotransmitter N which could then act post-synaptically to modulate neuronal excitability. B. Presynaptic control of the release of a neurotransmitter (N) from a neurone on which the aromatase-positive synapse makes axo-axonal contacts. In this case, oestrogens would act on the pre-synaptic terminal of the neurotransmitter N-containing neuron (the mER then being called an heteroreceptor) and modulate the release of neurotransmitter N which could then act post-synaptically to modulate neuronal excitability. C. Post-synaptic control of neuronal excitability. The oestrogens synthesized in the pre-synaptic neurone would be released in the synaptic cleft synthesis and could then activate membrane-associated receptors at the post-synaptic level resulting in the modulation of the neuronal firing rate. D. Retrograde modulation of synaptic transmission through release of post-synaptic oestrogens that could act on the pre-synaptic element to influence the neurotransmitter’s release. In these models, the membrane oestrogen receptor is schematically represented as a G-protein coupled receptor (GPCR) for simplicity. These receptors are diverse and can include a nuclear receptor associated with another GPCR, a novel membrane ER or a known GPCR or ion-gated receptor whose activity would be modulated by oestrogens. In addition, purely cytoplasmic non-genomic effects of oestrogens have also been identified and should be considered as well.
Post-synaptic non-genomic actions of brain-derived oestrogens could also modulate glutamatergic transmission (Fig. 3C). Indeed, electrophysiological evidence indicates that oestradiol potentiates excitatory post-synaptic responses to glutamate recorded from hippocampal or hypothalamic preparations [45–47]. In addition, it is known that preoptic glutamate release facilitates male sexual behaviour through the elicitation of dopamine release [55]. One can thus hypothesise that oestrogens synthesised in the POA modulate post-synaptic responses to glutamate. Alternatively, such modulations could occur downstream of POA via one of its many projections.
Finally, it should be noted that recent evidence indicates that oestrogens also exert non-genomic effects on glial cells [56, 57] that might be implicated in the activation of behaviour. In female rats, for example, oestrogens have been shown to induce via non-genomic mechanisms a de novo synthesis of progesterone by astrocytes which is thought to play an important role in the control of female physiology and behaviour [58].
What is the site of rapid oestrogen action?
In females, Kow and colleagues identified the ventromedial nucleus of the hypothalamus as a potential for non-genomic regulation of lordosis behaviour by oestrogens [20, 46]. In males, although the actual site of acute action of oestrogens is unknown, the mesencephalic periaqueductal grey constitutes a likely candidate. It receives projections from preoptic aromatase cells [59] and expresses classical oestrogen receptors that could associate with the membrane to signal non-genomic effects [53]. Alternatively, this regulation could occur in the POA where aromatase is expressed both in the soma and in pre-synaptic terminals. Additionally, it cannot be excluded that other brain regions receiving aromatase inputs might be involved. As a matter of fact, it is likely that oestrogens exert their effects at more than one site, so this question remains largely open.
The subcellular site of action of oestrogens also remains unidentified. Aromatase is present both in synaptic terminals and in cell bodies. Previous studies showed that somatic and synaptic aromatase are both active and display similar kinetics [31, 33, 60] but, at present, it is not known whether they are both rapidly modulated by conformational changes. If this was the case, another mode of action of locally synthesised oestrogens could be proposed. As shown for nitric oxide and endocannabinoids, oestrogens produced in the perikarya could act as retrograde modulators of synaptic transmission by acting on pre-synaptic terminals [61] (Fig. 3D).
Potentiation of genomic effects
Apart from their effects on neuronal excitability, oestrogens exert genomic effects. Oestrogen-receptor complexes classically modulate the expression of target genes with an oestrogen-responsive element (ERE) in their promoter region. The intracellular cascades triggered at the membrane by oestrogens may also activate gene transcription through phosphorylation processes resulting in transcription regulation [1, 11]. Non-genomic effects of oestrogens may thus influence genomic actions either through the potentiation of specific transcriptional events dependent on nuclear ER or through the activation of genes that would not be transcribed otherwise by nuclear ER dependent mechanisms.
As mentioned above, rapid behavioural effects of oestrogens that we identified in quail and mice seem to depend on the simultaneous activation of oestrogen-dependent genomic mechanisms [22, 23, 41]. Using a two-pulse oestrogen administration paradigm, Vasudevan and colleagues demonstrated that the membrane-initiated actions of oestradiol potentiate their transcriptional actions both in an artificial cell model where the oestrogenic response is assessed by the transcription of the ERE-driven luciferase gene [62] and in the hypothalamus of female rats where the dependent response was the activation of lordosis behaviour [20]. Therefore, in addition to their short-lived actions on neuronal firing and behaviour, locally produced oestrogens could potentiate their own genomic actions and/or induce long lasting effects susceptible to further enhance sexual behaviour.
Conclusions
Besides their well-known long lasting actions, mounting evidence indicates that oestrogens also exert rapid effects on physiological and behavioural processes. These effects are activated within minutes and have been described in both sexes. The fundamental issue of the source of rapid changes in oestrogen concentrations required to sustain these fast effects has so far received little attention. The demonstration of rapid changes in AA resulting in the fast regulation of oestrogen availability in the brain provides a source of rapid and transient production of high local concentrations of oestrogen that seems to perfectly match the requirements for non-genomic effects. These findings open new avenues in the understanding of the action of oestrogens on the brain. While our knowledge of the cellular mechanisms of non-genomic actions of oestrogens is increasing everyday, numerous questions still have to be addressed about how these effects affect physiological and behavioural processes. So far, rapid changes in aromatase activity have been described in the preoptic/hypothalamic area and future studies should continue to investigate the mechanisms underlying these rapid activity changes and determine whether they take place in all brain regions where aromatase is present. It would be important to identify the cellular mechanisms through which oestrogens acutely modulate male sexual behaviour. The subcellular location of non-genomic actions as well as the brain regions involved in the acute control of male sexual behaviour by oestrogens should also be determined. Finally, little is known about the mechanism(s) that control oestrogens catabolism in the brain and their impacts on their acute actions. Characterisation of the rapid action of oestrogens on physiology and behaviour and of the rapid modulation of their synthesis and catabolism will certainly have a profound impact on the design of future research programs and of clinical interventions that involve oestrogen actions in the brain.
Acknowledgments
CAC is a FNRS postdoctoral researcher. Research in our laboratories is supported by grants NIH/NIMH R01 MH50388 to Dr Gregory F. Ball and FRFC 2.4562.05 to Jacques Balthazart. I thank Drs Jacques Balthazart and Gregory F. Ball for discussion and comments on the manuscript.
References
- 1.Vasudevan N, Pfaff DW. Membrane Initiated Actions of Estrogens in Neuroendocrinology: Emerging Principles. Endocr Rev. 2007;28(1):1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- 2.Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: Where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfaff DW. Drive: Neurobiological and Molecular Mechanisms of Sexual Motivation. 1. The MIT Press; 1999. p. 328. [Google Scholar]
- 4.Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiology and Behavior. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR, Bulun SE. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 6.Balthazart J, Foidart A, Surlemont C, Vockel A, Harada N. Distribution of aromatase in the brain of the japanese quail, ring dove, and zebra finch: an immunocytochemical study. J Comp Neurol. 1990;301:276–288. doi: 10.1002/cne.903010210. [DOI] [PubMed] [Google Scholar]
- 7.Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Research Bulletin. 1997;44(4):351–357. doi: 10.1016/s0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 8.Roselli CE, Klosterman SA, Resko JA. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J Comp Neurol. 2001;439:208–223. doi: 10.1002/cne.1343. [DOI] [PubMed] [Google Scholar]
- 9.Halldin K, Axelsson J, Holmgren C, Brunstrom B. Localization of estrogen receptor-alpha and -betamRNA in brain areas controlling sexual behavior in Japanese quail. J Neurobiol. 2006;66(2):148–54. doi: 10.1002/neu.20199. [DOI] [PubMed] [Google Scholar]
- 10.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Molecular Endocrinology. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 12.Evinger AJ, 3rd, Levin ER. Requirements for estrogen receptor alpha membrane localization and function. Steroids. 2005;70(5–7):361–3. doi: 10.1016/j.steroids.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Prossnitz ER, Sklar LA, Oprea TI, Arterburn JB. GPR30: a novel therapeutic target in estrogen-related disease. Trends Pharmacol Sci. 2008;29(3):116–23. doi: 10.1016/j.tips.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Sander Connolly EJ, Nethrapalli IS, Tinnikov AA. ER-X: a novel membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. Journal of Neuroscience. 2002;22(19):8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadal A, Diaz M, Valverde MA. The estrogen trinity: membrane, cytosolic, and nuclear effects. News in Physiological Sciences. 2001;16:251–255. doi: 10.1152/physiologyonline.2001.16.6.251. [DOI] [PubMed] [Google Scholar]
- 17.Evrard HC, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. Journal of Neuroscience. 2004;24(33):9225–9229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27(35):9294–300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trainor BC, Finy MS, Nelson RJ. Rapid effects of estradiol on male aggression depend on photoperiod in reproductively non-responsive mice. Horm Behav. 2008;53(1):192–9. doi: 10.1016/j.yhbeh.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. PNAS. 2004;101(33):12354–1235. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cross E, Roselli CE. 17β-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. American Journal of Physiology. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- 22.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behavioural Brain Research. 2006;66(1):110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Taziaux M, Keller M, Bakker J, Balthazart J. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J Neurosci. 2007;27(24):6563–72. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–80. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 25.Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. JNeuroendocrinol. 2001;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- 26.Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. European Journal of Neuroscience. 2003;17(8):1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- 27.Harada N. Cloning of a complete cDNA encoding human aromatase: Immunochemical identification and sequence analysis. Biochem Biophys Res Commun. 1988;156:725–732. doi: 10.1016/s0006-291x(88)80903-3. [DOI] [PubMed] [Google Scholar]
- 28.Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147(1):359–66. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- 29.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels uctuate rapidly during social interactions. Nature Neuroscience. 2008 doi: 10.1038/nn.2200. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Balthazart J. Sexual behavior affects preoptic aromatase activity and brain monoamines’ levels. Endocrinology. 2005;146(9):3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roselli CE. Subcellular localization and kinetic properties of aromatase activity in rat brain. J Steroid Biochem Mol Biol. 1995;52:469–477. doi: 10.1016/0960-0760(94)00192-o. [DOI] [PubMed] [Google Scholar]
- 32.Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. Neuroendocrinol. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- 33.Schlinger BA, Callard GV. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinol. 1989;49:434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- 34.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006:241–9. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19(1):1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Osawa Y, Higashiyama T, Shimizu Y, Yarborough C. Multiple functions of aromatase and the active site structure; aromatase is the placental estrogen 2-hydroxylase. Biochem Molec Biol. 1993;44(4–6):469–480. doi: 10.1016/0960-0760(93)90252-r. [DOI] [PubMed] [Google Scholar]
- 37.Schumacher M, Balthazart J. Neuroanatomical distribution of testosterone metabolizing enzymes in the Japanese quail. Brain Research. 1987;422:137–148. doi: 10.1016/0006-8993(87)90548-8. [DOI] [PubMed] [Google Scholar]
- 38.Balthazart J, Stoop R, Foidart A, Granneman JCM, Lambert JGD. Distribution and regulation of estrogen-2-hydroxylase in the quail brain. Brain Res Bull. 1994;35:339–345. doi: 10.1016/0361-9230(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 39.Cornil CA, Balthazart J. Sexual experience and rapid effects of copulatory behavior on preoptic aromatase activity. Abstract of Society For Neuroscience. 2008;34:866.22. [Google Scholar]
- 40.Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain Res. 1986;370:285–293. doi: 10.1016/0006-8993(86)90483-x. [DOI] [PubMed] [Google Scholar]
- 41.Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav. 2006;49(1):45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27(8):2102–11. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. Journal of Comparative Neurology. 2001;429:355–371. [PubMed] [Google Scholar]
- 44.Fugger HN, Kumar A, Lubahn DB, Korach KS, Foster TC. Examination of estradiol effects on the rapid estradiol mediated increase in hippocampal synaptic transmission in estrogen receptor alpha knockout mice. Neurosci Lett. 2001;309(3):207–9. doi: 10.1016/s0304-3940(01)02083-3. [DOI] [PubMed] [Google Scholar]
- 45.Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. Journal of Neuroscience. 1992;12(8):3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kow L-M, Pfaff DW. Acute estrogen potentiates excitatory responses of neurons in rats hypothalamic ventromedial nucleus. Brain Research. 2005;1043:124–131. doi: 10.1016/j.brainres.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 47.Minami T, Oomura Y, Nabekura J, Fukuda A. 17β-estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res. 1990;519:301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- 48.Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on the male sexual behavior. Frontiers in Neuroendocrinology. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- 49.Pozzi L, Invernizzi R, Cervo L, Vallebuona F, Samanin R. Evidence that extracellular concentrations of dopamine are regulated by noradrenergic neurons in the frontal cortex of rats. J Neurochem. 1994;63(1):195–200. doi: 10.1046/j.1471-4159.1994.63010195.x. [DOI] [PubMed] [Google Scholar]
- 50.Moons L, D’Hondt E, Pijcke K, Vandesande F. Noradrenergic system in the chicken brain: immunocytochemical study with antibodies to noradrenaline and dopamine–β-hydroxylase. Journal of Comparative Neurology. 1995;360:331–348. doi: 10.1002/cne.903600210. [DOI] [PubMed] [Google Scholar]
- 51.Moons L, Van Gils J, Ghijsels E, Vandesande F. Immunocytochemical localization of L-DOPA and dopamine in the brain of the chicken (Gallus domesticus) Journal of Comparative Neurology. 1994;346:97–118. doi: 10.1002/cne.903460107. [DOI] [PubMed] [Google Scholar]
- 52.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signalling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. Journal of Neuroscience. 2003;23(29):9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy AZ, Shupnik MA, Hoffman GE. Androgen and estrogen (alpha) receptor distribution in the periaqueductal gray of the male Rat. Horm Behav. 1999;36(2):98–108. doi: 10.1006/hbeh.1999.1528. [DOI] [PubMed] [Google Scholar]
- 54.Normandin JJ, Murphy AZ. Nucleus paragigantocellularis afferents in male and female rats: organization, gonadal steroid receptor expression, and activation during sexual behavior. Journal of Comparative Neurology. 2008;508(5):771–794. doi: 10.1002/cne.21704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hull EM, Dominguez JM. Getting his act together: Roles of glutamate, nitric oxide, and dopamine in the medial preoptic area. Brain Res. 2006;1126:66–75. doi: 10.1016/j.brainres.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 56.Hirahara Y, Matsuda KI, Gao W, Arvanitis DN, Kawata M, Boggs JM. The Localization and Non-Genomic Function of the Membrane-Associated Estrogen Receptor in Oligodendrocytes. Glia. 2008 doi: 10.1002/glia.20742. Epub. [DOI] [PubMed] [Google Scholar]
- 57.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145(8):3788–95. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 58.Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148(2):782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- 59.Absil P, Riters L, Balthazart J. Preoptic aromatase cells project to the mesencephalic central gray in the male japanese quail (Coturnix japonica) Hormones and Behavior. 2001;40:369–383. doi: 10.1006/hbeh.2001.1702. [DOI] [PubMed] [Google Scholar]
- 60.Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol. 2007;67(1):1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- 61.Boehning D, Snyder MA. Novel neural modulators. Annu Rev Neurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- 62.Vasudevan N, Kow LM, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. PNAS. 2001;98(21):12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]