Abstract
There is a need for more cost-effective options to more accurately discriminate among members of the Anopheles gambiae complex, particularly An. gambiae and Anopheles arabiensis. These species are morphologically indistinguishable in the adult stage, have overlapping distributions, but are behaviorally and ecologically different, yet both are efficient vectors of malaria in equatorial Africa. The method described here, High-Resolution Melt (HRM) analysis, takes advantage of minute differences in DNA melting characteristics, depending on the number of incongruent single nucleotide polymorphisms in an intragenic spacer region of the X-chromosome-based ribosomal DNA. The two species in question differ by an average of 13 single-nucleotide polymorphisms giving widely divergent melting curves. A real-time PCR system, Bio-Rad CFX96, was used in combination with a dsDNA-specific dye, EvaGreen, to detect and measure the melting properties of the amplicon generated from leg-extracted DNA of selected mosquitoes. Results with seven individuals from pure colonies of known species, as well as 10 field-captured individuals unambiguously identified by DNA sequencing, demonstrated that the method provided a high level of accuracy. The method was used to identify 86 field mosquitoes through the assignment of each to the two common clusters with a high degree of certainty. Each cluster was defined by individuals from pure colonies. HRM analysis is simpler to use than most other methods and provides comparable or more accurate discrimination between the two sibling species but requires a specialized melt-analysis instrument and software.
Keywords: rDNA, single nucleotide polymorphism, AS-PCR, mosquito
INTRODUCTION
The mosquito An. gambiae Giles is the major vector of the malaria parasite Plasmodium falciparum in equatorial Africa, where its global impact is by far the most severe.1,2 An. gambiae sensu lato (s.l.) is a complex that includes seven biological sibling species, which are genetically and behaviorally distinct and vary widely in their importance as malaria vectors. Particularly important are two members of the complex, An. gambiae sensu stricto (s.s.) and An. arabiensis Patton, which are morphologically indistinguishable as adults, are widely distributed, and have broadly overlapping geographical ranges.2–5 Despite their sympatry, their roles in transmitting malaria often are different, partly reflecting differences in use of hosts, willingness to enter and rest in houses, and tolerance of dry climates. To complicate matters, the behavior of An. arabiensis is variable across its range,2,6–8, and the distributions, abundances, and relative proportions of An. gambiae and An. arabiensis can shift seasonally9 or long-term, the latter perhaps a result of recent extensive use of insecticide-treated bed nets10–13 or environmental change. To implement and evaluate vector-based malaria-control measures where these two species coexist, it is essential to have an identification method that is fast and simple.14
Identification of sibling species of An. gambiae s.l. was achieved originally by polytene chromosome analysis3,4 and followed by other techniques, such as isoenzyme electrophoresis15 and HPLC of cuticular hydrocarbons.16 Current identification methods make use of PCR to detect specific DNA nucleotide differences in the intergenic spacer of the ribosomal DNA (rDNA).17,18 In this paper, we have integrated the allele-specific (AS)-PCR and HRM analysis to discriminate An. gambiae s.s. from An. arabiensis rapidly and with high precision. HRM is not only rapid but a cost-effective technique compared with other genotyping methods, such as sequencing17–19 and TaqMan single nucleotide polymorphism (SNP) typing.20,21 Our method is simple, fast, and thus able to identify many samples to species level rapidly and accurately, allowing high-throughput processing of large field collections.
MATERIALS AND METHODS
Mosquito Collections
Specimens of An. gambiae s.l. were collected in the field by mouth aspirator and backpack aspirator during 2006–2008 from houses and resting pots at Mbita (00° 26–27′ S, 34° 12–13′ E) and Luanda (00° 28′ S, 34° 16–17′ E; Suba District, Nyanza, western Kenya), where An. gambiae s.s. and An. arabiensis have overlapping distributions and seasonally varying proportions. The specimens were held in a cold chest and carried to the laboratory within a few hours of collection and stored at −20°C in a freezer, except when it temporarily failed, possibly causing temporary thawing. They were later transferred to 95% ethanol until processed. Maintenance of mosquitoes has been approved under Institutional Review Board Permit 2004H0193 and Institutional Biosafety Committee Permit 2005R0020 from The Ohio State University (Columbus, OH, USA).
Control Samples
Specimens from previously identified colonies served as positive controls. The standards for An. gambiae s.s. originated from a local population in Mbita, Kenya. This Mbita strain was established in 2001 at the International Centre of Insect Physiology and Ecology (ICIPE; Kenya) and verified by molecular techniques.17 Specimens for analysis were taken from our Mbita-strain colony at The Ohio State University. Standard specimens of An. arabiensis (MRA-856 Dongola) were supplied by the Malaria Research and Reference Reagent Resource Center (MR4) from a colony maintained in Manassas, VA, USA.
DNA Purification and Quantification
Individual mosquito specimens from field and laboratory strains were prepared for identification by removing two legs from each specimen with sterile forceps and placing them into a sterile, 1.5-ml microtube. Each sample was ground and homogenized by a disposable pestle in a tube containing 180 μl PBS solution (pH 7.2, 50 mM potassium phosphate, 150 mM NaCl). DNA was purified with DNeasy Blood & Tissue Kit (Qiagen, Germantown, MD, USA; Insect Protocol DY14, Aug. 2006). The concentration of DNA for a subset of samples was measured with a Qubit fluorometer (Invitrogen, Life Technologies, Carlsbad, CA, USA; according to the manufacturer's procedure for the high-sensitivity dsDNA kit; probes.invitrogen.com/qubit). The DNA yield from two legs of a mosquito was ca. 10 μg; however, the quality and quantity of DNA obtained from these specimens varied according to consistency of the storage conditions, with many below the detection limit of the Qubit fluorometer.
AS-PCR and HRM Analysis
Five microliters of unknown samples (∼5 μg DNA, based on 10% that had been randomly selected to quantify their DNA contents), along with the same volume of positive and negative controls, was placed in a 96-well PCR plate. The following Invitrogen primers were used in the amplification step, which generated a 165-bp-long amplicon: (1) universal forward 5′-GTGAAGCTTGGTGCGTGCT-3′ and (2) universal reverse 5′-GCACGCCGACAAGCTCA-3′.20 They correspond, respectively, to the 623–641 and 772–788 positions of the 5′ end of the intergenic spacer region.20 Each reaction consisted of 5 μl genomic DNA (gDNA) extract and 5 μl HRM master mix, which contained 1× of SsoFast EvaGreen supermix (Bio-Rad Laboratories, Hercules, CA, USA; P/N 172-5200) and 8 pmoles each of the primers listed above. All reactions were performed using the Bio-Rad CFX96 real-time PCR system with the following thermal cycle protocol: (1) 95°C for 180 s; (2) 92° for 15 s, 60° for 60 s, 40×; (3) 95° for 30 s; and (4) 65° for 10 s with a 0.2° increase for each repetition, 150×. Fluorescence data were collected during the 60° stage of Step #2 above and during all repetitions of Step #4 above. The resulting melting curves were analyzed with Precision Melt Analysis v1.0 (Bio-Rad Laboratories) with the following settings: 0.2 melt-curve shape sensitivity and 0.5 for melting temperature (Tm) difference threshold.
Sequencing of Amplicons and Alignment
All DNA sequencing reactions were performed with 1 μl of the resulting amplicon product from the AS-PCR/HRM procedure above, 4 pmoles primer, 2.0 μl 5× dilution buffer (Applied Biosystems, Life Technologies, Foster City, CA, USA), and 0.5 μl BigDye Terminator v3.1 master mix (Applied Biosystems, Life Technologies) in a 10.5-μl reaction with the following thermal cycling conditions: (1) 1 min at 95°C and (2) 95° for 10 s, 50° for 5 s, and 60° for 120 s (35×). Each amplicon was sequenced twice with each of the two primers described above, forward and reverse. The sequencing reactions were subsequently purified with Performa v3 dye terminator removal plates (Edge BioSystems, Gaithersburg, MD, USA), according to the manufacturer's protocol. The purified extension products were subjected to electrophoresis on the 3730 DNA Analyzer (Applied Biosystems, Life Technologies) and analyzed with Sequencing Analysis software v2.5 (Applied Biosystems, Life Technologies). The sequences were aligned with the program ClustalW2 and the slow default parameters (http://www.ebi.ac.uk/Tools/msa/clustalw2/). A cladogram was generated from the same software with default conditions.
RESULTS
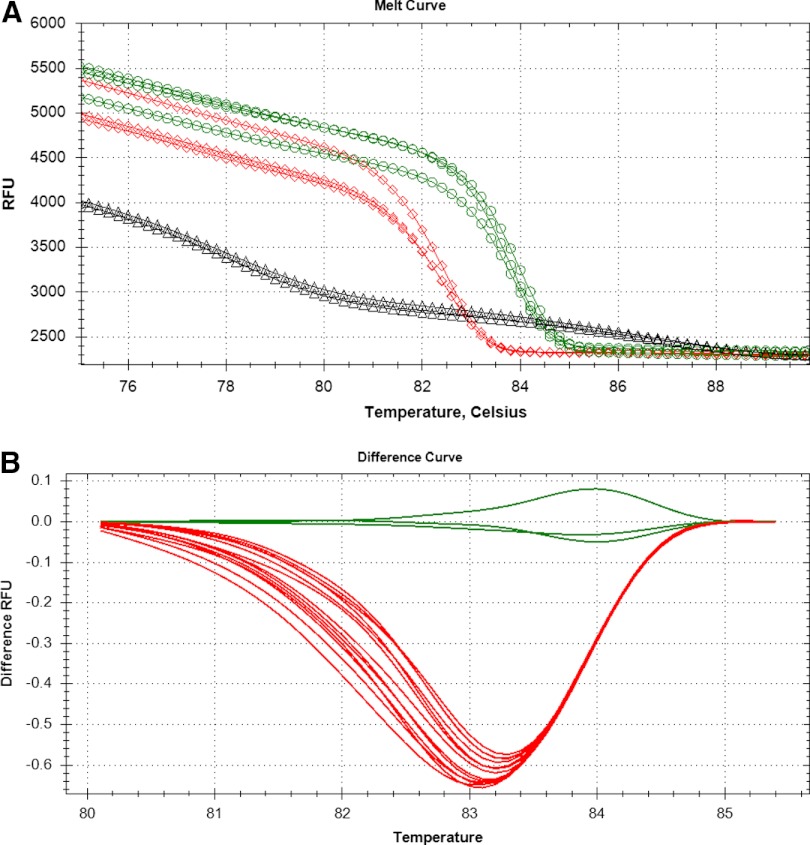
Figure 1, shows the melt curves from the 17 samples listed in Table 1. Figure 1A is based on the raw fluorescence data for the controls (positive, negative, and no template) only, whereas Fig. 1B is the difference curve for normalized data of all samples. In Fig. 1A, the differences between the two species are obvious as a result of the shape of the curve and the difference in the Tm, i.e., 84°C for An. gambiae and 83°C for the An. arabiensis curves, although they have an identical number of bases. The samples included seven positive controls from maintained cultures and 10 randomly selected individuals (unknowns) obtained from the field (Table 1). Two negative control wells (Fig. 1A; triangles) were included that also had a dsDNA product, which is clearly not the same as the 165-bp product from the mosquitoes. The Tm for the negative control was 72°C as compared with 83°C for An. arabiensis and 84°C for An. gambiae and the melt-curve shape is indicative of two primers annealing, i.e., a primer dimer (data not shown). Primer dimers are not uncommon in the absence of template during PCR, and the melt curves did not indicate a secondary or primer-dimer peak in the presence of gDNA. Figure 1B includes the same samples as displayed in Fig. 1A, although shown after analysis for melt-curve differences. An. arabiensis traces are below −0.5 RFU, and the An. gambiae traces are at or above −0.05 RFU. An. arabiensis and An. gambiae clearly cluster together as indicated by the color of the lines—red and green, respectively. In addition, the Table 1 data, with their Percent confidence values, indicate how reliable the assignment is to a particular cluster, with all values >97%.
FIGURE 1.
HRM analysis of Anopheles sp. based on X-chromosome-based rDNA amplicons. The data from the same samples are displayed in A and B. (A) Raw melt-curve data. Green lines with circles are from An. gambiae, red lines with diamonds are from An. arabiensis, and black lines with triangles are from water (negative control). (B) The difference-melt curves normalized to the cluster An. gambiae. The negative control is not present in B as a result of the absolute signal being too low for comparison. RFU, Relative fluorescence units.
TABLE 1.
Results of High-Resolution Melt Analysis
| Sample | Content | Cluster | Color | Percent confidence |
|---|---|---|---|---|
| 1992 | Unkn | Arabiensis #2 | Red | 98.1 |
| 2119 | Unkn | Arabiensis #2 | Red | 98.7 |
| 2142 | Unkn | Arabiensis #2 | Red | 98.5 |
| 2159 | Unkn | Arabiensis #2 | Red | 99.1 |
| Arabiensis #5 | Pos Ctrl | Arabiensis #2 | Red | 98.7 |
| 4184 | Unkn | Arabiensis #2 | Red | 99.6 |
| 2271 | Unkn | Arabiensis #2 | Red | 99.7 |
| 5194 | Unkn | Arabiensis #2 | Red | 98.9 |
| Arabiensis #1 | Pos Ctrl | Arabiensis #2 | Red | 99.6 |
| Arabiensis #2 | Pos Ctrl | Arabiensis #2 | Red | 99.1 |
| Arabiensis #8 | Pos Ctrl | Arabiensis #2 | Red | 98.8 |
| 2226 | Unkn | Arabiensis #2 | Red | 97.5 |
| 5711 | Unkn | Arabiensis #2 | Red | 97.8 |
| 2063 | Unkn | Arabiensis #2 | Red | 98.8 |
| Gambiae #2 | Pos Ctrl | Gambiae #2 | Green | 98.4 |
| Gambiae #1 | Pos Ctrl | Gambiae #2 | Green | 97.9 |
| Gambiae #3 | Pos Ctrl | Gambiae #2 | Green | 97.8 |
Results of 10 unknown (Unkn) Anopheles individuals collected from the field, as well as six individuals (Pos Ctrl) from laboratory-maintained colonies. The Percent confidence is reported by the Precision Melt Analysis software as to how similar/dissimilar the cluster is to adjacent clusters.
To confirm that the clustering pattern presented in the difference-melt curves represented two different species, both strands of each amplicon were sequenced, with the resulting consensus sequences listed in Fig. 2. Two control sequences were included from the GenBank database, which had been submitted in conjunction with additional published research when the sequences were aligned.22 An. arabiensis is represented by Submission Number EU091306.1 and An. gambiae by AF470116.1. The sequences from the positive control and unknown samples clearly align with GenBank controls as well as each other, confirming that the HRM analysis worked correctly for all of the samples. The relationship between the individuals is demonstrated in the cladogram (Fig. 3). The An. gambiae individuals clearly group together with AF470116.1, and the An. arabiensis samples, as well as the 10 unknowns, clearly group together with EU091306.1. The clustering of sequences also was confirmed with SeqScape software (Applied Biosystems, Life Technologies), which uses a different algorithm from ClustalW2 (data not shown). In total, there are 13 SNPs between An. gambiae and An. arabiensis whereas there is one SNP, A/T, at Position 92 within the An. arabiensis group, and some individuals are heterozygous with a W (an A or T).
FIGURE 2.
Alignment of X-chromosome-based rDNA sequences. Each individual can be placed in one of four groups listed above with the corresponding sequence. Among the An. arabiensis groups, only one base differs, at position 89, whereas the An. gambiae group has 13 differences from the An. arabiensis groups, as indicated by the letters in row G. Reference sequences from GenBank submissions are AF470116.1 for An. gambiae and EU091306.1 for An. arabiensis. Name–number combinations are specimens from identified colonies. Un-named numbers are record codes of field-collected samples previously unidentified.
FIGURE 3.
Cladogram of X-chromosome-based rDNA sequences. The same relationship is confirmed with the HRM analysis and sequence alignment. Control sequences from GenBank submissions are AF470116.1 for An. gambiae and EU091306.1 for An. arabiensis. Name–number combinations are specimens from identified colonies. Un-named numbers are record numbers of field-collected unknowns.
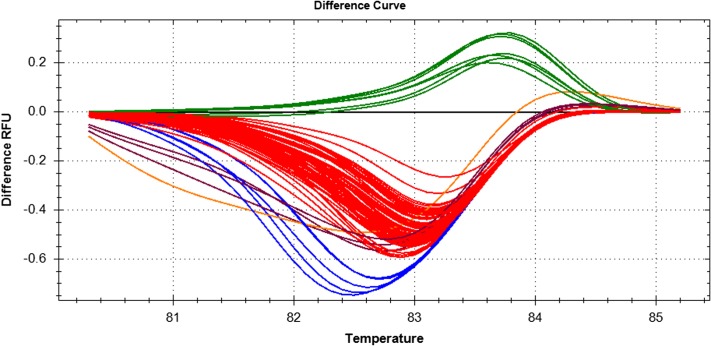
To further test the usefulness of this technique, a larger set of individuals, previously collected from the field, was subjected to HRM analysis. In total, 88 individuals (Fig. 4), were analyzed, and two of them produced an insufficient fluorescence signal to be assigned by the Precision Melt software, although from the amplification curves from the PCR step, we know that some DNA amplification did occur (data not shown). The same occurred for the negative control reactions, i.e., water instead of gDNA, and in this case, the products were again primer dimers. The six positive-control reactions (three for each species) performed correctly and as expected. Of the 86 mosquitoes that generated measurable data, four were found to be An. gambiae, 81 were An. arabiensis, and one was a putative hybrid, as the curve lies midway between the An. gambiae and An. arabiensis clusters. Based on a score of 85 successful, confirmed identifications out of 86 samples, HRM analysis had an accuracy of ∼99%.
FIGURE 4.
Difference curves for multiple individuals from the field, including positive control samples for An. arabiensis and An. gambiae. Of the 86 unknowns that were tested, four are An. gambiae, i.e., at or above 0.0; 81 are An. arabiensis, i.e., below 0.0; and one is a putative hybrid (black line), in between the two clusters. Each color, six in total, represents a different cluster, with each member having a confidence value of 0.95 or higher on a scale of 0.0 to 1.0. Temperature is Celsius.
DISCUSSION
HRM analysis is an established technique for determining genotypic variation, as indicated by a recent search of the National Center for Biotechnology Information database that yielded over 2000 published research articles. For example, HRM can readily distinguish as subtle a genetic difference as one SNP.23,24 This technique was used to determine whether it would be effective in distinguishing field-captured An. gambiae from An. arabiensis, as these species are morphologically inseparable and overlap geographically but have distinctive behaviors. To monitor the melting process in real-time, we used a fluorescent dye, EvaGreen, which binds specifically to dsDNA and has several advantages over the older and commonly used dye, SYBR Green.25,26 For example, EvaGreen intercalates into the DNA molecule and therefore, provides greater resolution during melting, as compared with SYBR Green, which binds in the minor groove of the helix in a less-consistent manner.
The An gambiae genome has 278 million base pairs.27 So far, among its many regions used in studies of An. gambiae s.l. population genetics, only the X-linked rDNA portion contains molecular variations that reliably differentiate among chromosomal forms.28 This region has been used by many researchers, starting in 1993,17 through the use of AS-PCR. The primers reported in that article were considered to be the “gold standard” for many years and were tried initially in our work but unfortunately, with unsatisfactory success, as the fluorescently labeled fragment pattern following electrophoresis from many individuals often indicated it was both species simultaneously (data not shown). Yet, cytogenetic studies and more recent molecular studies indicate that hybrids are rare.29–31
After further review of the literature, we used the primers and PCR conditions from the Walker laboratory,20 with some modifications, to take advantage of local instrumentation and with the goal of lowering costs. Previously, the detection method relied on a TaqMan assay32 to interrogate the SNPs in this 165-bp region. So, the dual-labeled oligonucleotide (TaqMan) probe was replaced with EvaGreen as the detection method. Therefore, increased fluorescence, caused by the release of a quenched dye by exonuclease activity, was replaced with the measurement of the melting characteristics by a decrease in fluorescence as the dye is released by the separating DNA strands. The two techniques have similar accuracy (∼98%) and use the same instruments and bench techniques, but HRM has a significant advantage over TaqMan: a lower cost by ∼25%/reaction. HRM does not require the use of a relatively expensive, dual-labeled, sequence-specific probe but instead, uses a saturating fluorescent dye that costs less.
Although a real-time PCR system is expensive, the cost and portability of these instruments are decreasing and increasing, respectively, each year, as the technology improves with the use of light-emitting diode technology, single-dye platforms, and more robust cooling/heating systems. With the placement of instrumentation in local laboratories in areas indigenous to An. gambiae s.s., identification can be achieved for hundreds of individuals in 1 day by one technician at a cost of approximately $0.50/sample for reagents, i.e., plates, films, EvaGreen kit, primers, and others. This cost includes neither the technician's labor nor the DNA extraction. For example, with sonication (which adds only the cost of labor), it takes minutes to extract DNA from each set of samples and ∼90 min to analyze 96 samples in the real-time PCR instrument. It is possible to process samples stored at −20°C or in ethanol, but fresh samples are better by having a higher yield of gDNA (unpublished data).
In conclusion, HRM analysis, in combination with AS-PCR, is an effective and efficient method to distinguish between An. gambiae and An. arabiensis.
ACKNOWLEDGMENTS
This research was supported, in part, by U.S. National Institutes of Health (NIH) grants R21-AI062957 and R01-AI077722 from the National Institute of Allergy & Infectious Diseases (NIAID) to W.A.F. Hortance Manda and Tom Guda and their ICIPE-Mbita team in Kenya were essential to the collection and storage of specimens tested here. We thank Edward Walker of Michigan State University for his comments on the method developed. The assistance of Jonathan Corbett in preparation of specimens for analysis is gratefully acknowledged. The supply of specimens of An. arabiensis, MRA-856 Dongola, deposited by M.Q. Benedict and obtained through the MR4 as part of the Biodefense and Emerging Infections (BEI) Resources Repository, NIAID, NIH, is gratefully acknowledged. Bryan Albright and Steve Wowk of Bio-Rad Laboratories generously provided resources for this research.
Footnotes
The content of this paper is solely the responsibility of the authors and does not represent the official views of NIAID or NIH. There is no conflict of interest.
REFERENCES
- 1. WHO: World Malaria Report 2011 Geneva: World Health Organization, 2011. [http://www.who.int/malaria/world_malaria_report_2011/en/index.html] [Google Scholar]
- 2. Sinka ME, Bangs MJ, Manguin S, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors 2010;3:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. White GB. Anopheles gambiae complex and disease transmission in Africa. Trans R Soc Trop Med Hyg 1974;68:278–298 [DOI] [PubMed] [Google Scholar]
- 4. Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environment in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg 1979;73:483–497 [DOI] [PubMed] [Google Scholar]
- 5. Service MW. Anopheles gambiae: Africa's principal malaria vector, 1902–1984. Bull Entomol Soc Am 1985;31:8–12 [Google Scholar]
- 6. Gibson G. Genetics, ecology, and behavior of anophelines. In Brock GR, Cardew G. (eds): Olfaction in Mosquito-Host Interactions. Chichester, UK: John Wiley & Sons, 1996;22–45 [Google Scholar]
- 7. Ng'habi KR, Knols BGJ, Lee Y, Ferguson HM, Lanzaro GC. Population genetic structure of Anopheles arabiensis and Anopheles gambiae in a malaria endemic region of southern Tanzania. Malaria J 2011;10:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fornadel CM, Norris LC, Glass GE, Norris DE. Analysis of Anopheles arabiensis blood feeding behavior in southern Zambia during the two years after introduction of insecticide-treated bed nets. Am J Trop Med Hyg 2010;83:848–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koenraadt CJM, Githeko AK, Takken W.The effects of rainfall and evapotranspiration on the temporal dynamics of Anopheles gambiae s. s. and Anopheles arabiensis in a Kenyan village. Acta Tropica 2004;90:141–153 [DOI] [PubMed] [Google Scholar]
- 10. Bayoh MN, Mathias DK, Odiere MR, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malaria J 2010;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Derua YA, Alifrangis M, Hosea KM, et al. Change in composition of the Anopheles gambiae complex and its possible implications for the transmission of malaria and lymphatic filariasis in north-eastern Tanzania. Malaria J 2012;11:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kitau J, Oxborough RM, Tungu PK, et al. Species shifts in the Anopheles gambiae complex: do LLINs successfully control Anopheles arabiensis? PLoS ONE 2012;7:e31481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mutuku FM, King CH, Mungai P, et al. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malaria J 2011;10:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonizzoni M, Afrane Y, Yan G. Loop-mediated isothermal amplification (LAMP) for rapid identification of Anopheles gambiae and Anopheles arabiensis mosquitoes. Am J Trop Med Hyg 2009;8:1030–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coosemans M, Smits A, Roelants P. Intraspecific isozyme polymorphism of Anopheles gambiae in relation to environment, behavior, and malaria transmission in southwestern Burkina Faso. Am J Trop Med Hyg 1998;58:70–74 [DOI] [PubMed] [Google Scholar]
- 16. Carlson DA, Service M. Differentiation between species of the Anopheles gambiae Giles complex (Diptera: Culicidae) by analysis of cuticular hydrocarbons. Ann Trop Med Parasitol 1979;73:589–592 [DOI] [PubMed] [Google Scholar]
- 17. Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg 1993;49:520–529 [DOI] [PubMed] [Google Scholar]
- 18. Townson H, Onapa AW. Identification by rDNA-PCR of Anopheles bwambae, a geothermal spring species of the An. gambiae complex. Insect Mol Biol 1994;3:279–282 [DOI] [PubMed] [Google Scholar]
- 19. Djadid ND, Gholizadeh S, Tafsiri E, Romi R, Gordeev M, Zakeri S. Molecular identification of Palearctic members of Anopheles maculipennis in northern Iran. Malaria J 2007;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walker ED, Thibault AR, Thelen AP, et al. Identification of field caught Anopheles gambiae s. s. and Anopheles arabiensis by TaqMan single nucleotide polymorphism genotyping. Malaria J 2007;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bass C, Williamson MS, Wilding CS, Donnelly MJ, Field LM. Identification of the main malaria vectors in the Anopheles gambiae species complex using a TaqMan real-time PCR assay. Malaria J 2007;6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gentile G, Della Torre A, Maegga B, Powell J, Caccone A.Genetic differentiation in the African malaria vector, Anopheles gambiae s. s., and the problem of taxonomic status. Genetics 2002;161:1561–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pasay C, Arlian L, Morgan M, et al. High-resolution melt analysis for the detection of a mutation associated with permethrin resistance in a population of scabies mites. Med Vet Entomol 2008;22:82–88 [DOI] [PubMed] [Google Scholar]
- 24. Lochlainn S, Amoah S, Graham N, et al. High resolution (HRM) analysis is an efficient tool to genotype EMS mutants in complex crop genomes. Plant Methods 2011;7:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mao F, Leung W, Xin X. Characterization of EvaGreen and the implication of its physicochemical properties for qPCR applications. BMC Biotechnol 2007;7:76–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Escheid A. SYTO dyes and EvaGreen out perform SYBR Green in real-time PCR. BMC Res Notes 2011;4:263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holt RA, Subramanian GM, Halpern A, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science 2002;298:129–149 [DOI] [PubMed] [Google Scholar]
- 28. Wang R, Zheng L, Touré YT, Dandekar T, Kafatos FC. When genetic distance matters: measuring genetic differentiation at microsatellite loci in whole genome scans of recent and incipient mosquito species. Proc Natl Acad Sci USA 2001;98:10769–10774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Temu EA, Hunt RH, Coetzee M, Minjas JM, Shiff CJ. Detection of hybrids in natural populations of the Anopheles gambiae complex by the rDNA-based, PCR method. Ann Trop Med Parasitol 1997;91:963–965 [DOI] [PubMed] [Google Scholar]
- 30. Bezansky NJ, Krzywinski J, Lehmann T, et al. Semipermeable species boundaries between Anopheles gambiae and Anopheles arabiensis: evidence from multilocus DNA sequence variation. Proc Natl Acad Sci USA 2003;100:10818–10823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stump AD, Shoener JA, Costantini C, Sagnon N, Besansky NJ. Sex-linked differentiation between incipient species of Anopheles gambiae. Genetics 2005;169:1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res 1996;6:986–994 [DOI] [PubMed] [Google Scholar]