A pooled analysis of individual data from >5000 human immunodeficiency virus type 1 (HIV-1)–infected mothers and their infants from Africa and India who participated in 5 randomized trials shows that extended prophylaxis with nevirapine or with nevirapine and zidovudine significantly reduces postnatal HIV-1 infection.
Keywords: breast milk, HIV, nevirapine
Abstract
Background. In resource-limited settings, mothers infected with human immunodeficiency virus type 1 (HIV-1) face a difficult choice: breastfeed their infants but risk transmitting HIV-1 or not breastfeed their infants and risk the infants dying of other infectious diseases or malnutrition. Recent results from observational studies and randomized clinical trials indicate daily administration of nevirapine to the infant can prevent breast-milk HIV-1 transmission.
Methods. Data from 5396 mother-infant pairs who participated in 5 randomized trials where the infant was HIV-1 negative at birth were pooled to estimate the efficacy of infant nevirapine prophylaxis to prevent breast-milk HIV-1 transmission. Four daily regimens were compared: nevirapine for 6 weeks, 14 weeks, or 28 weeks, or nevirapine plus zidovudine for 14 weeks.
Results. The estimated 28-week risk of HIV-1 transmission was 5.8% (95% confidence interval [CI], 4.3%–7.9%) for the 6-week nevirapine regimen, 3.7% (95% CI, 2.5%–5.4%) for the 14-week nevirapine regimen, 4.8% (95% CI, 3.5%–6.7%) for the 14-week nevirapine plus zidovudine regimen, and 1.8% (95% CI, 1.0%–3.1%) for the 28-week nevirapine regimen (log-rank test for trend, P < .001). Cox regression models with nevirapine as a time-varying covariate, stratified by trial site and adjusted for maternal CD4 cell count and infant birth weight, indicated that nevirapine reduces the rate of HIV-1 infection by 71% (95% CI, 58%–80%; P < .001) and reduces the rate of HIV infection or death by 58% (95% CI, 45%–69%; P < .001).
Conclusions. Extended prophylaxis with nevirapine or with nevirapine and zidovudine significantly reduces postnatal HIV-1 infection. Longer duration of prophylaxis results in a greater reduction in the risk of infection.
(See the Editorial Commentary by Becquet and Dabis, on pages 140–42.)
UNAIDS estimates that 370 000 children became newly infected with human immunodeficiency virus type 1 (HIV-1) during 2009 [1]. How to prevent HIV-1 transmission associated with breastfeeding remains a concern in resource-constrained settings where the prevalence of HIV-1 is high among women of reproductive age, safe replacement feeding options are not available, and breastfeeding is critical for infant survival. In these settings, formula feeding is not affordable and is associated with increased child morbidity and mortality from diarrheal disease, pneumonia, and malnutrition, as shown in several studies from sub-Saharan Africa [2–6]. Thus, the World Health Organization (WHO) recommends exclusive breastfeeding during the first 6 months of life and continued breastfeeding with appropriate complementary food through at least 12 months for infants born to HIV-1–infected women in resource-limited settings [7].
Five recent randomized controlled clinical trials found that infant prophylaxis with daily nevirapine for 6, 14, or 28 weeks during breastfeeding reduced early postnatal HIV-1 transmission [8–10]. Herein we describe a pooled analysis of individual data from >5000 mother-infant pairs that participated in these 5 studies to evaluate the combined efficacy of the infant prophylaxis intervention and the effect of duration of infant prophylaxis.
Pooled analysis of individual-level data is considered the gold standard when combining data from multiple studies [11]. Pooled analysis of individual participant data has many advantages, both clinically and statistically, over meta-analytic methods that rely solely on reported aggregate data [12, 13]. For example, in the nevirapine prophylaxis setting it is difficult to compare the risk of postnatal HIV-1 transmission between mother-to-child transmission studies using only published results because of different study inclusion criteria for participating in the trials (eg, based on maternal CD4 cell count or infant birth weight) and different exclusion criteria for the primary analyses (eg, whether to include infants infected within the first week after birth). Additionally, certain analyses, such as those described below where the effect of infant prophylaxis is modeled as a time-varying covariate, are not possible to conduct without pooling individual-level data. Therefore, we initiated this pooled analysis of data from the 5 aforementioned trials to assess the efficacy of infant nevirapine prophylaxis to prevent breast-milk transmission of HIV-1. These 5 trials were included owing to similarity in study design; no other trials or studies were considered when this analysis was initiated. The HIV Prevention Trials Network (HPTN) study 046 [14] was not included in this analysis, as all infants in that study received at least 6 weeks of nevirapine; that is, 046 did not have a study arm comparable to the control arms of the other trials included in the pooled analysis.
METHODS
Pooled Analysis Study Population
The analysis presented in this paper includes data from 5 randomized studies: the Breastfeeding, Antiretrovirals and Nutrition (BAN; ClinicalTrials.gov number NCT00164736) trial in Lilongwe, Malawi; the Post-Exposure Prophylaxis of the Infant (PEPI; NCT00115648) trial in Blantyre, Malawi; and the 3 Six-Week Extended Nevirapine (SWEN; NCT00061321) trials in Ethiopia, India, and Uganda. The details of these studies, including randomization methods and study procedures, have been reported individually [8–10]. Because of similarities in the design and conduct of the trials, data from the SWEN trials were combined in the primary analysis publication [8]; the same convention is employed in this pooled analysis. All studies were approved by the appropriate institutional review boards. A summary of the major study design elements is provided in Table 1; the BAN trial provided infant prophylaxis for 28 weeks, PEPI for 14 weeks, and SWEN for 6 weeks. In these studies, infant HIV-1 infection status was determined by periodic HIV-1 DNA or RNA polymerase chain reaction (PCR) testing. All randomized infants were included in this pooled analysis with the following exceptions: if the infant was HIV-1 infected at birth (defined as HIV-1 first detected at birth to age 1 week); had no specimen available for HIV-1 testing at birth; had indeterminate HIV-1 status at birth or age 6 months; or died in the first 7 days after birth. In the case of multiple births (eg, twins), only firstborns were included. To ensure comparability between the studies, infants from SWEN and PEPI were excluded if their mothers had baseline CD4 cell counts <200 cells/µL at baseline (enrollment). The results presented below differ from the primary analyses of these trials [8–10] because of these exclusions (as well as minor updates to the study data sets).
Table 1.
Study Design Characteristics
| Characteristic | BANa | PEPI | SWEN |
|---|---|---|---|
| Trial site | Malawi (Lilongwe) | Malawi (Blantyre) | Ethiopia, India, Ugandab |
| Previous ARV exposure | Women with previous ART exposure excluded | No exclusions | Women with previous ART excluded (except India) |
| Postpartum regimen for mother | sd NVP (200 mg) + twice-daily zidovudine (300 mg) and lamivudine (150 mg) for 1 wk | sd NVP (200 mg) except late presenters | sd NVP (200 mg) + optional ART per local standard of care |
| Postpartum control regimen for infant | sd NVP (2 mg/kg) + twice-daily zidovudine (12 mg) and lamivudine (6 mg) for 1 wk | sd NVP (2 mg/kg) + twice-daily oral zidovudine (4 mg/kg) for 1 wk | sd NVP (2 mg/kg) |
| Postpartum intervention regimen for infant | Control + daily NVP for 28 wk (10 mg/d weeks 1–2 increasing to 30 mg/d weeks 19–28) | Control + daily NVP for 14 wk (2 mg/kg/d during wk 2; 4 mg/kg/d weeks 3–14) Control + daily NVP as above + oral zidovudine for 14 wk (4 mg/kg twice daily for weeks 2–5, 3 times daily for weeks 6–8, and 6 mg/kg weeks 9–14) |
Control + daily NVP for 6 wk (5 mg/d) |
| HIV testing schedule | Birth + weeks 1, 2, 4, 6, 8, 12, 18, 21, 24, 28, 32, 36, 42, 48 | Birth + weeks 6, 9, 14 + months 6, 12, 18, 24 | Birth + weeks 1c, 2, 6, 10c, 14 + month 6 |
| Infants randomized | 1520 | 3397d | 2037 |
| Breastfeeding counseling | Exclusive BF to 24 wk, then rapid weaning by 28 wk | Exclusive BF to 6 mo, told to consider weaning | Encouraged to wean between 4 and 6 moe |
| Maternal CD4 count at enrollment | CD4 <200/250 cells/μL excludedf | No exclusions | No exclusions |
| Infant birth weight | > 2 kg | No exclusions | No exclusions |
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; BAN, Breastfeeding, Antiretrovirals and Nutrition study; BF, breastfeeding; HIV, human immunodeficiency virus; PEPI, Post-Exposure Prophylaxis of the Infant study; sd NVP, single-dose nevirapine; SWEN, Six-Week Extended Nevirapine study.
a Mother antiretroviral arm of BAN excluded.
b Immunoglobulin arm from Uganda site excluded.
c India only.
d Only 3276 randomized in the analysis reported by Kumwenda et al [10]; here n = 3397 since enrollment in PEPI continued beyond the cutoff date (7 August 2007) used in the data analysis by Kumwenda et al.
e Weaning advised at 4 months in India, 6 months or earlier in Uganda, 4–6 months in Ethiopia.
f Exclusion threshold changed from 200 to 250 cells/μL midway through the study (24 July 2006).
For the BAN trial, 1520 infants were randomized into the infant nevirapine or control arms (excluding the maternal antiretroviral arm); 72 infants were excluded because they were HIV-1 infected at birth. Of the remaining 1448 infants, 632 were randomized to receive the control regimen of single-dose nevirapine and 7 days of zidovudine/lamivudine and 816 were randomized to receive the control regimen plus 28 weeks of infant nevirapine.
For the PEPI trial, 3397 infants were randomized; 949 were excluded because they were HIV-1 infected at birth (n = 232), infant HIV-1 status at birth was unknown (n = 40), the infant died in the first week (n = 9), maternal baseline CD4 cell count was <200 cells/µL (n = 627), or the infant was not firstborn (n = 41). Among the remaining 2448 infants, 792 were randomized to receive the control regimen of single-dose nevirapine and 7 days of zidovudine, 823 were randomized to receive the control regimen plus 14 weeks of infant nevirapine, and 833 were randomized to receive 14 weeks of daily nevirapine plus zidovudine.
For the SWEN trials, 2037 infants were randomized in the combined 3 trials; 13 were excluded because they had no specimens before 6 months, and an additional 143 were excluded because they were HIV-1-infected at birth (n = 94), there were no birth specimens (n = 36), HIV-1 infection status was indeterminate at birth or 6 months (n = 6), or the infant died in the first week of life (n = 7). Of the remaining 1881 infants, 361 infants had mothers with baseline CD4 cell counts <200 cells/µL or missing, and an additional 20 infants were not firstborn. Among the remaining 1500 infants, 769 were randomized to receive the control regimen of single-dose nevirapine and 731 were randomized to receive the control regimen plus 6 weeks of infant nevirapine.
Pooled Analysis Endpoints
The primary study endpoints for the pooled analysis were infant HIV-1 infection and the composite endpoint infant HIV-1 infection or death (as in [8–10]).
Statistical Methods
The main focus of this pooled analysis was to assess the efficacy of nevirapine in preventing HIV-1 infection or death by age 28 weeks. In the BAN protocol, which had the longest infant prophylaxis period of all the studies, the week 28 visit could occur up to 7 days after the infant was 28 weeks old. Therefore HIV-1 infection and death up to 203 days after birth were included in the analysis. Similarly, the primary endpoint for the SWEN trials was HIV-1 infection and death up to day 202. The primary endpoint of the PEPI trial was HIV-1 infection and death by 9 months of age.
The Kaplan-Meier method was used to estimate the probability of infant HIV-1 infection or death by 203 days. For the HIV-1 infection endpoint, time until the first positive HIV-1 test was used in the analysis. Infants who did not reach a study endpoint by 203 days (HIV-1 infection or death) were right-censored at the last negative HIV-1 test or at 203 days, whichever occurred first. Estimates allowing for interval censored endpoints with intervals formed by the last negative and first positive HIV-1 tests were similar (results not shown) to those presented below using the Kaplan-Meier estimator. HIV-1 infection risk estimates treating HIV-1–free death as a competing risk also yielded similar results (not shown). For SWEN, similar risk estimates were obtained (not shown) on the basis of combining country-specific Kaplan-Meier estimates using inverse variance-weights [8]. Differences between trial arms were assessed using the log-rank test. Cox proportional-hazards models, stratified by trial site, were used to estimate hazard ratios (HRs) for the study outcomes comparing the different intervention and control groups while adjusting for the potentially prognostic baseline factors listed in Table 2. Stratified Cox regression models were also fit with nevirapine as a time-varying covariate equal to 1 for the first 6 weeks for infants in the intervention arm of SWEN, for the first 14 weeks for infants in the intervention arms of PEPI, and for the first 28 weeks for infants in the intervention arm in BAN, and equal to 0 otherwise. Because infants were assigned randomly to different durations of prophylaxis, modeling nevirapine duration as a time-varying covariate provides a valid method for assessing the effect of nevirapine; this is in contrast to observational studies where time-varying confounding may be present. Proportional hazard models with trial site as a fixed effect yielded similar results (not shown).
Table 2.
Baseline Characteristics and Endpoints (HIV Infections and Deaths) of Infants in Pooled Analysisa
| BAN |
PEPI |
SWEN |
|||||
|---|---|---|---|---|---|---|---|
| 28 wk NVP | Control | 14 wk NVP | 14 wk NVP/AZT | Control | 6 wk NVP | Control | |
| No. of mother-infant pairs | 816 | 632 | 823 | 833 | 792 | 731 | 769 |
| Baseline characteristics | |||||||
| Maternal age, yb | 25 (22–29) | 26 (22–29) | 25 (23–29) | 25 (23–29) | 25 (22–29) | 24 (22–27) | 24 (21–27) |
| Maternal CD4 count at baseline/enrollmentb | 440 (330.5–594) | 443.5 (335–590) | 431 (311–609) | 447 (334–610) | 442 (330.5–618) | 446 (312–601) | 434 (317–620) |
| Mode of delivery (%) | |||||||
| Vaginal | 93.7 | 91.9 | 96.1 | 96.5 | 96.3 | 83.6 | 81.8 |
| Cesarean | 1.0 | 1.9 | 2.2 | 2.8 | 2.5 | 14.4 | 15.7 |
| Instrumental | 5.3 | 6.2 | 1.6 | 0.6 | 1.0 | 2.0 | 2.5 |
| Infant sex (%) | |||||||
| Male | 50.0 | 51.4 | 49.7 | 49.9 | 54.4 | 48.4 | 55.0 |
| Female | 50.0 | 48.6 | 50.3 | 50.1 | 45.6 | 51.6 | 45.0 |
| Infant birth weight (kg)b | 3.0 (2.7–3.3) | 3.0 (2.7–3.3) | 3.1 (2.8–3.3) | 3.0 (2.8–3.3) | 3.0 (2.8–3.3) | 2.9 (2.6–3.2) | 3.0 (2.6–3.2) |
| Endpoints | |||||||
| HIV-1 infection | 13 | 32 | 26 | 34 | 61 | 39 | 54 |
| Death (in children without confirmed HIV-1 infection) | 9 | 9 | 16 | 18 | 17 | 6 | 13 |
Abbreviations: AZT, zidovudine; BAN, Breastfeeding, Antiretrovirals and Nutrition study; HIV-1, human immunodeficiency virus type 1; NVP, nevirapine; PEPI, Post-Exposure Prophylaxis of the Infant study; SWEN, Six-Week Extended Nevirapine study.
a Mode of delivery missing for 2 infants in BAN and 3 infants in PEPI; birth weight missing for 2 infants in PEPI and 8 infants in SWEN.
b Median (interquartile range).
In the BAN study, the independent data and safety monitoring board (DSMB) halted enrollment in the control group after 668 of the planned 806 mother-infant pairs had been enrolled in the control arm [9]. Women in the control arm at the time of the DSMB recommendation were offered a choice of remaining on the control arm or switching to either the maternal or infant antiretroviral interventions [15]. Data regarding mother-infant pairs who switched to an intervention group after the DSMB recommendation were censored at that time.
RESULTS
Overall, 5396 mother-infant pairs were included in this pooled analysis. Table 2 shows baseline characteristics of the study populations. Maternal age and CD4 cell count at baseline were similar across all 5 trials. The proportion of infants delivered vaginally was significantly lower in the SWEN trials (Pearson χ2 test, P < .0001) as cesarean delivery was more common in the SWEN than BAN or PEPI studies (15.1% vs 2.1%). Infant sex and birth weight were similar across trials, with slightly more males in the PEPI and SWEN control arms (P = .07).
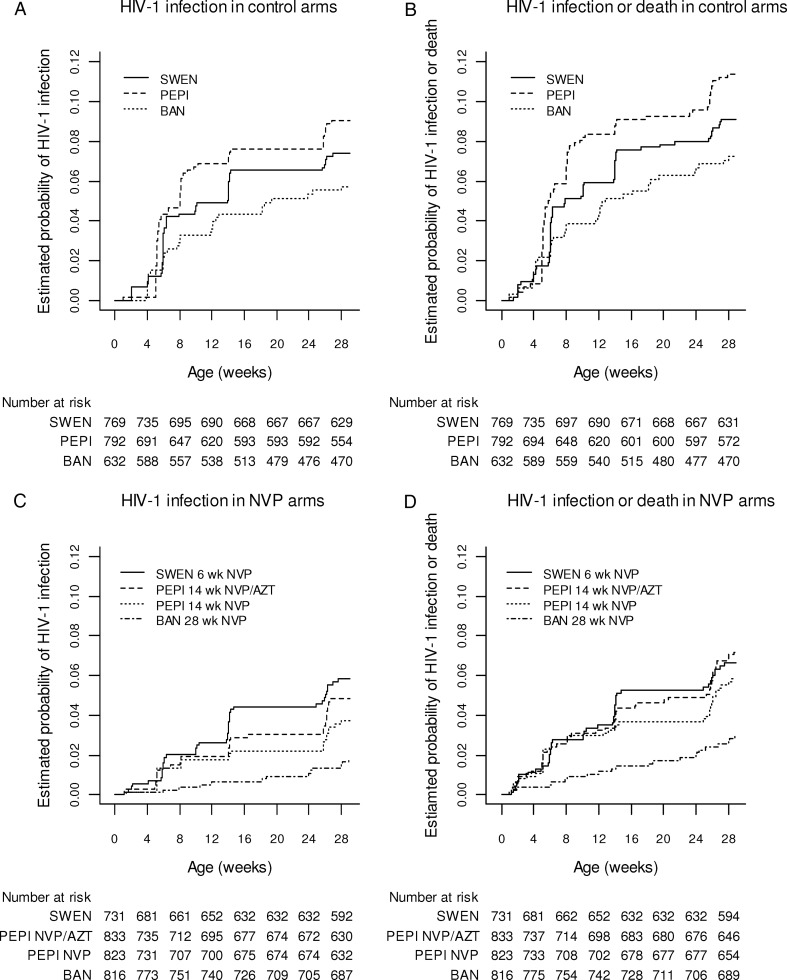
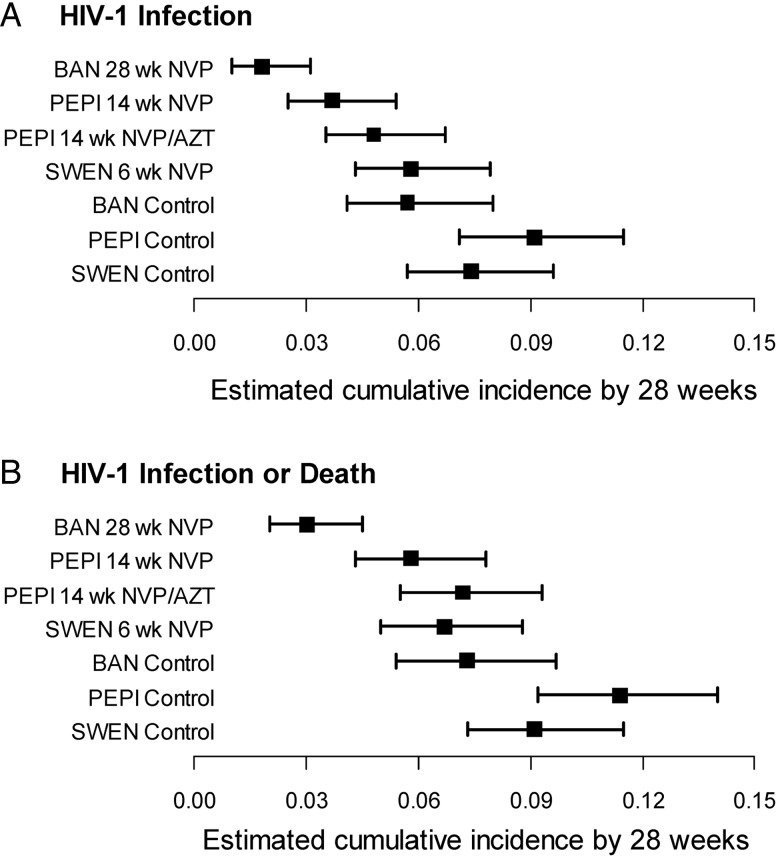
The numbers of infants with HIV-1 infection or death by age 28 weeks are shown in Table 2. Kaplan-Meier estimates of the cumulative risk of HIV-1 infection are shown in Figure 1A and 1C and Figure 2A. The estimated risk of HIV-1 infection by 28 weeks in the control arms of the trials was 9.1% (95% CI, 7.1%–11.5%) in PEPI, 7.4% (95% CI, 5.7%–9.6%) in SWEN, and 5.7% (95% CI, 4.1%–8.0%) in BAN (log-rank test P = .08). Risk of HIV-1 infection by 28 weeks in the SWEN nevirapine arms was 5.8% (95% CI, 4.3%–7.9%), in the PEPI nevirapine arm was 3.7% (95% CI, 2.5%–5.4%), in the PEPI nevirapine plus zidovudine arm was 4.8% (95% CI, 3.5%–6.7%), and in the BAN nevirapine arm was 1.8% (95% CI, 1.0%–3.1%) (log-rank test for trend, P < .001). Similar results were obtained for the risk of HIV-1 infection or death (Figure 1B and 1D and Figure 2B). Risk of HIV-1 infection or death by 28 weeks in the SWEN nevirapine arms was 6.7% (95% CI, 5.0%–8.8%), in the PEPI nevirapine arm was 5.8% (95% CI, 4.3%–7.8%), in the PEPI nevirapine plus zidovudine arm was 7.2% (95% CI, 5.5%–9.3%), and in the BAN nevirapine arm was 3.0% (95% CI, 2.0%–4.5%).
Figure 1.
Kaplan-Meier estimates of cumulative risk of human immunodeficiency virus type 1 (HIV-1) infection (A and C) and HIV-1 infection or death (B and D) among infants HIV-1 uninfected at birth, by study arm. Abbreviations: AZT, zidovudine; BAN, Breastfeeding, Antiretrovirals and Nutrition study; HIV-1, human immunodeficiency virus type 1; NVP, nevirapine; PEPI, Post-Exposure Prophylaxis of the Infant study; SWEN, Six-Week Extended Nevirapine study.
Figure 2.
Estimated cumulative risk (with 95% confidence intervals) of (A) human immunodeficiency virus type 1 (HIV-1) infection or (B) HIV-1 infection or death by 28 weeks among infants HIV-1 uninfected at birth by study arm. Abbreviations: AZT, zidovudine; BAN, Breastfeeding, Antiretrovirals and Nutrition study; HIV-1, human immunodeficiency virus type 1; NVP, nevirapine; PEPI, Post-Exposure Prophylaxis of the Infant study; SWEN, Six-Week Extended Nevirapine study.
Results of the Cox proportional-hazards analyses of risk factors for postnatal HIV-1 infection are shown in Table 3. In both unadjusted and adjusted analyses, daily nevirapine for 14 weeks (with or without zidovudine) or 28 weeks was significantly associated with a lower rate of HIV-1 infection. Maternal age, mode of delivery, and infant sex were not significant risk factors for HIV-1 infection (P > .2 for each covariate). For the stratified proportional hazards model, the adjusted HR estimate for 28 weeks of daily nevirapine vs control was 0.28 (95% CI, .15–.54). Similarly, for a stratified Cox regression model with nevirapine as a time-varying covariate, the corresponding adjusted HR estimate was 0.29 (95% CI, .20–.42; P < .001), indicating that extended infant nevirapine prophylaxis results in a 71% decrease in the rate of HIV-1 infection during the period it is being given. There was no evidence that this decrease in infection rate was modified by maternal CD4 cell count (P = .42). For the model in Table 3, after adjusting for infant prophylaxis, maternal CD4 cell count < 350 cells/µL was associated with an increased rate of transmission (adjusted HR estimate, 2.19 [95% CI, 1.71–2.80]; P < .001), and higher birth weight was associated with a lower rate of transmission (adjusted HR estimate, 0.64 [95% CI, .48–.85] per 1-kg increase in birth weight; P = .002).
Table 3.
Risk Factors of Infant HIV-1 Infection and a Composite Outcome of HIV-1 Infection or Death by 28 Weeks Among Infants Who Were HIV-1 Uninfected at Birth
| Risk Factor | Unadjusteda |
Adjustedb |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| For HIV-1 infection by 28 wk | ||||
| Infant NVP 6 wk vs control | .80 (.53–1.20) | .27 | .80 (.53–1.21) | .29 |
| Infant NVP 14 wk vs control | .39 (.24–.61) | <.001 | .36 (.23–.57) | <.001 |
| Infant NVP + AZT 14 wk vs control | .50 (.33–.77) | .001c | .49 (.32–.75) | .001c |
| Infant NVP 28 wk vs control | .29 (.15–.56) | <.001 | .28 (.15–.54) | <.001 |
| Maternal baseline CD4 count (<350 vs ≥350 cells/μL) | 2.14 (1.68–2.74) | <.001 | 2.19 (1.71–2.80) | <.001 |
| Infant birth weight (per increase of 1 kg) | .65 (.49–.86) | .002 | .64 (.48–.85) | .002 |
| For HIV-1 infection or death by 28 wk | ||||
| Infant NVP 6 wk vs control | .73 (.50–1.06) | .10 | .73 (.50–1.07) | .10 |
| Infant NVP 14 wk vs control | .49 (.34–.71) | <.001 | .46 (.32–.67) | <.001 |
| Infant NVP + AZT 14 wk vs control | .61 (.43–.86) | .005c | .59 (.42–.84) | .003c |
| Infant NVP 28 wk vs control | .39 (.23–.65) | <.001 | .38 (.23–.64) | <.001 |
| Maternal baseline CD4 count (<350 vs ≥350 cells/μL) | 1.83 (1.48–2.27) | <.001 | 1.83 (1.50–2.30) | <.001 |
| Infant birth weight (per increase of 1 kg) | .61 (.49–.78) | <.001 | .60 (.47–.77) | <.001 |
Abbreviations: AZT, zidovudine; BAN, Breastfeeding, Antiretrovirals and Nutrition study; CI, confidence interval; HIV-1, human immunodeficiency virus type 1; NVP, nevirapine; PEPI, Post-Exposure Prophylaxis of the Infant study; SWEN, Six-Week Extended Nevirapine study.
a Proportional hazards models with each risk factor as the sole covariate, stratified by trial site. Infant NVP 6 weeks vs control using SWEN data only, stratified by trial site; infant NVP 14 weeks vs control and infant NPV + AZT 14 weeks vs control using PEPI data only; infant NVP 28 weeks vs control using BAN data only.
b Proportional hazards models adjusted for all variables listed, stratified by trial site.
c Test for proportional hazards assumption 0.025 <P < .05 for all models with infant NPV + AZT; for all other covariates and models P > .05.
Table 3 also shows the results of the Cox proportional hazards analyses of risk factors for the composite endpoint HIV-1 infection or death. Hazard ratio estimates are similar to those for the HIV-1 infection endpoint. Maternal age, mode of delivery, and infant sex were not significant risk factors for HIV-1 infection or death. The estimated HR for 28 weeks of daily nevirapine vs control was 0.38 (95% CI, .23–.64). Similarly, for a Cox regression model with nevirapine as a time-varying covariate, the adjusted HR estimate for nevirapine was 0.42 (95% CI, .31–.55; P < .001), indicating that extended infant nevirapine prophylaxis results in a 58% decrease in the rate of HIV-1 infection or death.
DISCUSSION
This analysis indicates extended infant prophylaxis with nevirapine or with nevirapine and zidovudine significantly reduces postnatal HIV-1 infection by 28 weeks. Compared with the control arms of BAN, SWEN, and PEPI trials, daily nevirapine for 28 weeks reduces the rate of HIV infection by 71% (95% CI, 58%–80%; P < .001) and reduces the rate of HIV infection or death by 58% (95% CI, 45%–69%; P < .001). This pooled analysis indicates that longer duration of prophylaxis results in a greater reduction in infection risk in the first 28 weeks of an infant's life, in agreement with the HPTN 046 trial [14], which found that extending infant nevirapine from 6 weeks to 6 months significantly decreased postnatal HIV-1 infection by 54%.
Although this pooled analysis does not include a formal comparison of safety data from the SWEN, PEPI, and BAN trials, the individual primary analyses for each of the trials all led to the conclusion that extended infant daily antiretroviral prophylaxis is safe. Although data on maternal viral load were not available for this analysis, confounding of the effect of nevirapine by maternal viral load seems unlikely given that mother-infant pairs were randomized to nevirapine or control at each trial site and our adjusted pooled analysis included data on maternal CD4 cell counts at baseline. Another potential limitation of this analysis is that no formal adjustment is made for reported breastfeeding. No significant differences in breastfeeding rates through 28 weeks between trial arms were reported in any of the studies. In BAN, mothers were specifically counseled to wean their babies from 24 to 28 weeks with approximately only 33% still breastfeeding at 28 weeks. In SWEN, mothers were encouraged to wean between 4 and 6 months, with only 31% reporting breastfeeding by 6 months. In PEPI, women were told to exclusively breastfeed for 6 months and consider weaning thereafter; at 6 months approximately 90% of infants were breastfeeding, with most women weaning their infants between ages 6 and 9 months.
CONCLUSIONS
This pooled analysis of 5 randomized clinical trials that used daily infant nevirapine prophylaxis during breastfeeding in resource-limited settings confirms the significant efficacy of this mode of prophylaxis in preventing infant HIV-1 infection and HIV-1 infection or death by 28 weeks. However, after prophylaxis was discontinued, the HIV-1 transmission risk returns if breastfeeding is continued. Longer duration of prophylaxis was associated with greater protection. Following the results of the trials included in this analysis, WHO changed its guidelines to recommend daily infant nevirapine prophylaxis during breastfeeding through age 12 months as one of the options for prevention of transmission in breastfeeding infants of HIV-1–infected women who do not require treatment for their own health [16]. This analysis supports the recommendation that if breastfeeding continues through age 12 months, prophylaxis should also continue, although data on the safety of extending prophylaxis for longer than 6 months are needed.
Many countries are moving quickly to implement highly active antiretroviral therapy (HAART) for all HIV-positive pregnant women prepartum, regardless of CD4 cell count, and continuing for the duration of breastfeeding [17–19]. This early treatment strategy also decreases tuberculosis incidence and transmission of HIV to uninfected sexual partners [20]. However, a large percentage of HIV-positive pregnant women in developing countries do not access voluntary counseling and testing until late in pregnancy, thus lessening the impact of HAART [9, 21]. Furthermore, many countries are reaching <50% of those patients who need HAART for their own health [22].
Although maternal treatment is being rapidly adopted, in settings where maternal treatment is not affordable, available, or acceptable, infant nevirapine prophylaxis may be a feasible alternative. Infant nevirapine prophylaxis is an inexpensive, safe, and effective intervention that allows HIV-1–infected mothers who do not require treatment for their own health to breastfeed their infants with reduced risk of HIV transmission. Infant nevirapine does not require any lab monitoring and allows overburdened and under-resourced treatment programs to focus on those women who need treatment for their own health. While nevirapine is known to select for resistant virus in infants who become infected despite prophylaxis (resulting in limited treatment options, especially in resource-limited settings), resistance may also occur in infants who become infected while their mothers are receiving HAART. Therefore, alternative prevention strategies should be pursued. Until such effective alternatives are available, infant nevirapine prophylaxis offers a simple intervention that will contribute to meeting the goal of eliminating mother-to-child HIV-1 transmission worldwide.
Notes
Disclaimer. Centers for Disease Control and Prevention (CDC) and National Institutes of Health (NIH) representatives were part of the study teams and were involved in study design, coordination, data collection, data analysis, data interpretation, and the writing of this report. The CDC did review and approve this manuscript for publication; however, the findings and conclusions of this study are those of the authors and do not necessarily represent the official position of the CDC. The study drug manufacturers had no role in the design of the study, the collection or analysis of the data, or the decision to submit this manuscript for publication.
Financial support. The Breastfeeding, Antiretrovirals and Nutrition (BAN) study was supported by the Prevention Research Centers Special Interest Project of the CDC (SIP 13-01 U48-CCU409660-09, SIP 26-04 U48-DP000059-01, and SIP 22-09 U48-DP001944), the National Institute of Allergy and Infectious Diseases (NIAID), the University of North Carolina Center for AIDS Research (P30-AI50410), the NIH Fogarty AIDS International Training and Research Program (DHHS/NIH/FIC 5-D43 TW001039 and 5-R24 TW007988), Abbott Laboratories, GlaxoSmithKline, Boehringer Ingelheim, Roche Pharmaceuticals, Bristol-Myers Squibb, the Elizabeth Glaser Pediatric AIDS Foundation, the United Nations Children's Fund, the World Food Program, the Malawi Ministry of Health and Population, Johnson & Johnson, and the US Agency for International Development. The SWEN study was supported by grants from the NIH, NIAID (R01AI45462, R01AI3857601A, R01AI34235) and the NIH Fogarty International Center NIH Program of International Training Grants in Epidemiology Related to AIDS (D43-TW0000). The PEPI study was supported by a Cooperative Agreement (5 U50 PS022061-05; award U50/CC0222061) from the CDC and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH. The CDC and NIH were sponsors of the studies included in this pooled analysis.
Potential conflicts of interest. During the follow-up phase of the BAN study, the University of North Carolina received cash grants from GlaxoSmithKline and Abbott Laboratories to conduct pharmacokinetic and resistance analysis substudies. In addition, GlaxoSmithKline, Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, and Roche Pharmaceuticals provided the study drugs. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. Available at http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf . Accessed 19 June 2012. [Google Scholar]
- 2.Thior I, Lockman S, Smeaton LM, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana. A randomized trial: the Mashi study. JAMA. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355:451–5. [PubMed] [Google Scholar]
- 4.Fawzy A, Arpadi S, Kankasa C, et al. Early weaning increases diarrheal morbidity and mortality among uninfected children born to HIV-infected mothers in Zambia. J Infect Dis. 2011;203:1222–30. doi: 10.1093/infdis/jir019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creek TL, Kim A, Lu L, et al. Hospitalization and mortality among primarily non-breastfed children during a large outbreak of diarrhea and malnutrition in Botswana, 2006. J Acquir Immune Defic Syndr. 2010;53:14–9. doi: 10.1097/QAI.0b013e3181bdf676. [DOI] [PubMed] [Google Scholar]
- 6.Kafulafula G, Hoover DR, Taha TE, et al. Frequency of gastroenteritis and gastroenteritis-associated mortality with early weaning in HIV-1-uninfected children born to HIV-infected women in Malawi. J Acquir Immune Defic Syndr. 2010;53:6–13. doi: 10.1097/QAI.0b013e3181bd5a47. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Geneva, Switzerland: WHO; 2009. HIV and infant feeding: revised principles and recommendations. Rapid advice. Available at http://whqlibdoc.who.int/publications/2009/9789241598873_eng.pdf. Accessed 14 February 2012. [Google Scholar]
- 8.Bedri A, Gudetta B, Isehak A, et al. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–13. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 9.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–29. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 11.Clarke MJ, Stewart LA. Systematic reviews: obtaining data from randomised controlled trials: how much do we need for reliable and informative meta-analyses? BMJ. 1994;309:1007–10. doi: 10.1136/bmj.309.6960.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitehead A. Meta-analysis of controlled clinical trials. Oxford, UK: John Wiley and Sons; 2002. [Google Scholar]
- 13.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 14.Coovadia HM, Brown ER, Fowler MG, et al. for the HPTN 046 Protocol Team. Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:221–28. doi: 10.1016/S0140-6736(11)61653-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavula C, Long D, Kayira D, et al. Stopping the control arm in response to the DSMB: mother's choice of HIV prophylaxis during breastfeeding in the BAN study. Contemp Clin Trials. 2012;33:55–59. doi: 10.1016/j.cct.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Geneva, Switzerland: WHO; 2010. Antiretroviral drugs for treating pregnant women and preventing HIV infections in infants: recommendations for a public health approach—2010 version. Available at http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf . Accessed 14 February 2012. [PubMed] [Google Scholar]
- 17.Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378:282–4. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- 18.Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–80. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–94. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chibwesha CJ, Giganti MJ, Putta N, et al. Optimal time on HAART for prevention of mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011;58:224–8. doi: 10.1097/QAI.0b013e318229147e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. The treatment 2.0 framework for action: catalysing the next phase of treatment, care and support. Geneva, Switzerland: WHO, 2011. Available at: http://whqlibdoc.who.int/publications/2011/9789241501934_eng.pdf . Accessed 27 September 2012. [Google Scholar]