Host-induced flares are associated with presence of only wild-type hepatitis B virus (HBV) and result in decline and clearance of HBV DNA, hepatitis B e antigen, and hepatitis B surface antigen (HBsAg). Monitoring of HBsAg levels during and after flares may help predict a favorable treatment outcome.
Keywords: peginterferon, prediction of response, precore, basal core promoter, hepatitis B surface antigen
Abstract
Background. Alanine aminotransferase (ALT) flares occur frequently during peginterferon (PEG-IFN) therapy. We related occurrence of flares to presence of precore (PC) and/or basal core promoter (BCP) mutants and studied kinetics of hepatitis B e antigen (HBeAg) and hepatitis B surface antigen (HBsAg) levels during flares.
Methods. Fifty of 214 (23%) patients treated with PEG-IFN ± lamivudine for 52 weeks experienced flares. Flares were host-induced (ALT elevation followed by HBV DNA decline, n = 19), virus-induced (HBV DNA increase with subsequent ALT elevation, n = 17) or indeterminate (n = 14). Presence of wild-type (WT) or non-WT (detectable PC/BCP mutants) was studied by lineprobe assay.
Results. Fifty-eight percent of host-induced flares occurred in WT HBV patients, whereas 94% of virus-induced flares occurred in patients with PC and/or BCP mutants (P = .003). HBsAg loss was only achieved in patients with a host-induced flare, and WT patients with a host-induced flare cleared HBsAg in 64% of cases. Serum HBsAg levels declined after a host-induced flare, whereas virus-induced flares were accompanied by stable or increasing levels of HBsAg. Patients with a host-induced flare achieved a mean HBsAg reduction of 3.24 log IU/mL, compared with 0.25 log IU/mL in virus-induced flares (P < .001). Patients who achieved a decline in HBsAg of >0.5 log IU/mL within 4 weeks after the flare cleared HBsAg in 64% (7 of 11) of cases.
Conclusions. Host-induced flares are associated with WT virus and may result in decline and clearance of HBV DNA, HBeAg, and HBsAg. Monitoring of HBsAg levels during and after flares may help predict a favorable treatment outcome.
Pegylated interferon (PEG-IFN) is a first-line treatment option for hepatitis B e antigen (HBeAg)–positive chronic hepatitis B but results in a response in only a limited number of patients [1–3]. Spontaneous elevations of alanine aminotransferase (ALT), or flares, are a well-recognized phenomenon in patients treated with PEG-IFN, although the pathogenesis is not well understood [4]. Flares may occur in up to 25% of patients treated with a 1-year course of PEG-IFN and have been associated with an increased probability of serological response [5–6]. However, flares can also have considerable detrimental effects and may result in hepatic decompensation and death in patients with advanced cirrhosis [7]. We previously recognized different types of flares during PEG-IFN therapy of HBeAg-positive chronic hepatitis B [5, 8]. In that study, host-induced flares, characterized by an ALT flare followed by a decline in hepatitis B virus (HBV) DNA, were associated with response to treatment, whereas virus-induced flares, ALT elevations that were preceded by an increase in HBV DNA, were not [5]. Why some patients develop host-induced flares whereas others experience virus-induced flares is currently unclear. Acute flares in untreated chronic hepatitis B patients were recently shown to be associated with presence of precore (PC) and basal core promoter (BCP) mutant virus [9]. These mutants are associated with impaired or absent production of HBeAg and may be less susceptible to an immune response against HBeAg. Because ALT flares that are preceded by an increase in HBV DNA levels do not trigger an effective immune response [10], it is possible that these virus-induced flares during PEG-IFN therapy are associated with presence of PC and BCP mutant virus.
It has recently become clear that serum levels of hepatitis B surface antigen (HBsAg) reflect intrahepatic covalently closed circular DNA and may be a marker for response to PEG-IFN [11–14]. Monitoring of serum HBsAg levels may, therefore, provide additional insight into the differences of host- vs virus-induced flares and may help identify patients with a high likelihood of a favorable outcome after flares.
The aims of this study were therefore to (1) relate presence of PC and BCP mutants before PEG-IFN therapy to occurrence of host- or virus-induced flares, (2) study the kinetics of HBeAg and HBsAg levels during these flares, and (3) investigate whether ALT and HBsAg monitoring can help predict therapeutic outcome.
PATIENTS AND METHODS
Patients
Patients treated with PEG-IFN alpha-2b alone or in combination with lamivudine in an investigator-initiated, multicenter, randomized trial were enrolled into this study [1, 14, 15]. The inclusion and exclusion criteria for the original trial have previously been described elsewhere [1]. Patients were HBsAg positive for at least 6 months before randomization, were HBeAg positive, had elevated serum ALT levels of >2 but <10 times the upper limit of normal, and had a serum HBV DNA concentration of >1.0 × 105 copies/mL. Patients were treated with 100 μg of PEG-IFN alpha-2b weekly (PegIntron, Schering-Plough) in combination with placebo or 100 mg of lamivudine (Zeffix, GlaxoSmithKline) daily for 52 weeks. Of the 266 patients in the original study, 214 had available data on baseline presence of PC and/or BCP mutants. The study was conducted in accordance with the guidelines of the Declaration of Helsinki and the principles of good clinical practice. All patients gave written informed consent according to the standards of the local ethics committees.
Laboratory Measurements
Presence of PC and BCP mutants was assessed using the INNO-LiPA HBV PreCore assay (Innogenetics). This line probe assay allows for easy detection of PC (at nucleotide position G1896) and BCP (at nucleotide positions A1762 and G1764) mutants, even when only present as minority species [16]. Patients were subsequently classified as having wild-type (WT; only WT virus detectable) virus, or non-WT (PC, BCP, or both mutants detectable) virus. Serum HBV DNA, HBeAg, and HBsAg were quantified in samples taken at baseline, during treatment, and during 6 months of off-treatment follow-up. Patients were seen at the outpatient clinic at least every 4 weeks during the study. HBV DNA quantification was performed using an in-house–developed TaqMan polymerase chain reaction assay (lower limit of quantification, 400 copies/mL) based on the EuroHep standard [17]. HBsAg and HBeAg were measured using the Roche ELECSYS assay using a quantitative protocol (Roche Diagnostics).
Classification of Flares
A flare was defined as a 3-fold increase in serum ALT compared with baseline levels [5]. The time point of the flare was defined as the time of the peak level of serum ALT. If a patient experienced multiple flares, the first was used for classification. Two types of flares were recognized, as described previously by Flink et al [5]. A flare was designated as virus-induced when the ALT peak was preceded by at least a 1 log increase in HBV DNA levels within 4 months. Host-induced flares were characterized as an ALT peak without a preceding increase in HBV DNA and were typically followed by a subsequent decrease in HBV DNA of at least 1 log within the next 4 months. Flares that could not be classified were designated indeterminate [5].
Statistical Analysis
Associations between variables were tested using Student t test, χ2 test, Pearson correlation test, or their nonparametric equivalents when appropriate. SPSS version 15.0 (SPSS Inc) and the SAS 9.2 program (SAS Institute Inc) were used to perform statistical analyses. All statistical tests were 2-sided and were evaluated at the .05 level of significance.
RESULTS
Patient Characteristics
Flares occurred in 50 of 214 patients with available PC and/or BCP data (23%). The characteristics of patients with a host-induced, virus-induced, or indeterminate flare are shown in Table 1. The frequency of flares was similar for patients with WT virus when compared with those with PC and/or BCP mutants (25% vs 23%; P = .68).
Table 1.
Characteristics of the Study Cohort
| Characteristics | Flare |
No Flare (n = 164) | P Value | ||
|---|---|---|---|---|---|
| Host (n = 19) | Virus (n = 17) | Indeterminate (n = 14) | |||
| Demography | |||||
| Mean age, (SD), y | 42 (12.8) | 27 (9.8) | 34 (11.4) | 33.5 (12.4) | .005 |
| Male | 15 (79%) | 14 (82%) | 12 (86%) | 126 (77%) | .85 |
| PEG-IFN monotherapy | 7 (37%) | 3 (18%) | 14 (100%) | 80 (49%) | <.001 |
| Race | .22 | ||||
| Caucasian | 15 (79%) | 14 (82%) | 8 (57%) | 120 (73%) | |
| Asian | 1 (5%) | 1 (6%) | 4 (29%) | 34 (21%) | |
| Other | 3 (16%) | 2 (12%) | 2 (14%) | 10 (6%) | |
| Laboratory results | |||||
| Mean (SD) ALTa | 3.1 (1.3) | 3.2 (1.7) | 2.6 (1.0) | 4.7 (3.3) | .005 |
| Mean (SD) HBV DNA, log c/mL | 9.3 (0.5) | 9.2 (1.1) | 9.3 (0.6) | 9.1 (0.9) | .51 |
| Mean (SD) HBsAg, log IU/mL | 4.6 (0.4) | 4.4 (0.7) | 4.4 (0.5) | 4.4 (0.6) | .38 |
| Mean (SD) HBeAg, log IU/mL | 2.5 (0.9) | 2.2 (0.8) | 2.6 (0.6) | 2.5 (0.7) | .55 |
| HBV genotype | .04 | ||||
| A | 12 (63%) | 2 (12%) | 5 (36%) | 55 (34%) | |
| B | 2 (11%) | 2 (12%) | 0 (0%) | 15 (9%) | |
| C | 0 (0%) | 1 (6%) | 4 (29%) | 24 (15%) | |
| D | 4 (21%) | 12 (71%) | 4 (29%) | 65 (40%) | |
| Other/mixed | 1 (5%) | 0 (0%) | 1 (7%) | 5 (3%) | |
| Previous IFN therapy | 3 (16%) | 5 (29%) | 2 (14%) | 28 (17%) | .62 |
| INNO-LiPA PreCore result | .007 | ||||
| Wild type | 11 (58%) | 1 (6%) | 7 (50%) | 57 (35%) | |
| Non-wild type | 8 (42%) | 16 (94%) | 7 (50%) | 107 (65%) | |
| Response | |||||
| HBeAg loss | 11 (58%) | 2 (12%) | 1 (7%) | 63 (38%) | .003 |
| Combined responseb | 8 (42%) | 0 (0%) | 0 (0%) | 33 (20%) | .003 |
| HBsAg loss | 8 (42%) | 0 (0%) | 0 (0%) | 9 (6%) | <.001 |
Abbreviations: ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IFN, interferon; PEG-IFN, peginterferon; SD, standard deviation.
a Multiples of upper limit of the normal range.
b HBeAg loss and HBV DNA <10 000 copies/mL at week 78.
Relationship Between Type and Timing of Flare and Presence of PC and BCP Mutants
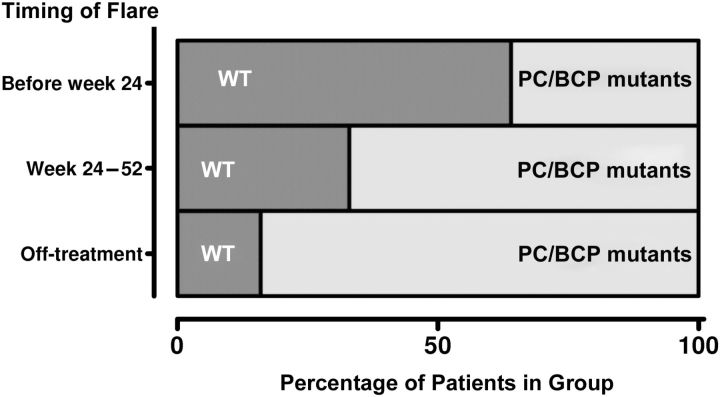
The type of flare differed according to presence of mutants; host flares occurred predominantly in patients with WT virus (58%), whereas virus-induced flares almost always occurred in patients with non-WT virus (16 of 17; 94%). Of the 76 patients with WT virus, 11 (14%) experienced a host-induced flare, compared with 6% of patients with non-WT virus (P = .03). Conversely, 12% of non-WT virus patients experienced a virus-induced flare, compared with only 1 of 76 (1%) of WT virus patients (P = .008). Of the 25 (50%) flares that occurred during treatment, 15 (60%) occurred in patients with WT virus. Interestingly, the timing of the flare was also associated with presence of WT or non-WT virus (P = .004) (Figure 1). Presence of PC and/or BCP mutants at baseline was independently associated with the occurrence of a virus-induced flare; this remained true after adjustment for HBV genotype, patient age, and use of combination therapy (adjusted odds ratio for virus induced flare, 7.04; P = .02).
Figure 1.
Proportion of patients with wild-type (WT) or precore (PC) and/or basal core promoter (BCP) mutants according to the timing of the flare. Twenty-one patients experienced a flare before week 24, 4 experienced a flare between week 24 and 52, and 25 experienced a flare between week 52 and the end of the study (off-treatment).
Relationship Between Type of Flare, Presence of Mutants, and Response at Week 78
Host-induced flares resulted more frequently in HBeAg loss than virus-induced flares (58% vs 12%; P = .001). HBeAg loss with HBV DNA levels <10 000 copies/mL and HBsAg clearance were achieved in 42% of patients with a host-induced flare, and in none of the patients with virus-induced or indeterminate flares (P < .001). HBV DNA undetectability was observed in 5 (26%) patients with a host-induced flare, compared with zero patients with other types of flares (P = .01). Importantly, in the subgroup of WT virus patients with a host-induced flare (n = 11), HBsAg clearance was achieved in 64% of patients (7 of 11; P < .001).
Monitoring of HBeAg and HBsAg Levels During Flares in Individual Patients
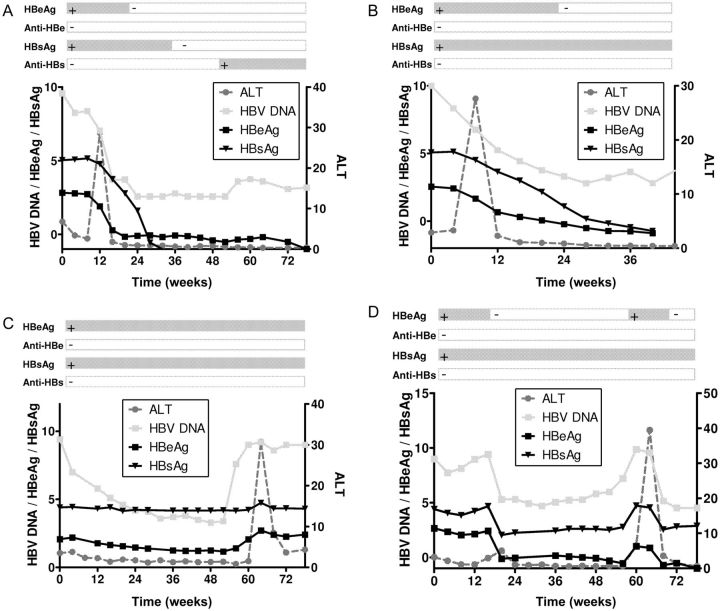
We analyzed individual patients’ changes in ALT, HBV DNA, HBeAg, and HBsAg monthly during PEG-IFN therapy and up to 6 months post-treatment. As shown in Figure 2A and 2B, host-induced flares were characterized by a steep ALT flare, followed by pronounced declines of HBV DNA, HBeAg, and HBsAg. In contrast, virus-induced flares typically showed an increase in HBV DNA before ALT elevation, and serum levels of HBeAg and HBsAg either remained stable (Figure 2C) or temporarily increased (Figure 2D).
Figure 2.
Kinetics of alanine aminotransferase (ALT), hepatitis B virus (HBV) DNA, hepatitis B e antigen (HBeAg), and hepatitis B surface antigen (HBsAg) in 2 patients with a host-induced flare (A and B) and in 2 patients with a virus-induced flare (C and D). HBV DNA is given in log copies/mL; HBeAg and HBsAg are given in log IU/mL; and ALT is given in times upper limit of normal.
Pronounced HBsAg Decline in Patients With a Host-Induced Flare
Only patients with a host-induced flare achieved substantial HBV DNA decline by week 78 (HBV DNA decline from baseline was 4.21, 1.51, and 0.58 log copies/mL in patients with host-induced, virus-induced, or indeterminate flares, respectively; P < .001). Importantly, similar HBeAg reductions were achieved in patients with host- and virus-induced flares, whereas only patients with a host-induced flare achieved a pronounced HBsAg decline at 6 months post-treatment. HBeAg declines were 0.84, 1.02, and 0.25 log IU/mL at week 78 (P = .31) in patients with a host-induced, virus-induced, or indeterminate flare, compared with HBsAg declines of 3.24, 0.25, and 0.31 log IU/mL, respectively (P < .001).
HBsAg Decline After Flare Predicts HBsAg Clearance
Mean HBsAg levels at the peak of ALT during the flare were similar in patients with a host-induced, virus-induced, or indeterminate flare (P = .56). However, pronounced HBsAg declines immediately after the flare were achieved only in patients with a host-induced flare: mean declines were 0.78 and 1.29 log IU/mL at 1 and 2 months, after the ALT peak, respectively, in patients with a host-induced flare. This contrasted strongly with the stable levels of HBsAg observed in patients with a virus-induced or an indeterminate flare (mean increase 2 months after ALT peak was 0.02 and 0.16 log IU/mL, respectively; P = .002). The probability of an HBsAg decline of at least 0.5 log IU/mL was 53% in patients with a host-induced flare, 8% in patients with a virus-induced flare, and 7% in patients with an indeterminate flare (P = .003). Patients who achieved a decline of >0.5 log IU/mL during the first month after the peak of the flare had a probability of HBsAg clearance of 64% (7 of 11), increasing to 75% (6 of 8) in patients with a decline >1 log IU/mL (P < .001 for both).
DISCUSSION
This study shows that host-induced flares occur more frequently in patients with only WT virus and result in pronounced declines of HBsAg levels. Frequent monitoring of serum HBsAg levels during flares may help predict a favorable outcome.
Spontaneous elevations of ALT are frequently encountered in the natural history of chronic hepatitis B and may herald progression from the immunotolerant phase of HBV infection to immune clearance and eventual HBeAg seroconversion [18]. However, flares that occur during or after discontinuation of nucleo(s)tide analogues therapy hardly ever lead to virological remission and may result in hepatic decompensation [19, 20]. Flares that occur after nucleo(s)tide analogue discontinuation do not trigger an adequate immune response [10], suggesting these flares are different from flares that induce disease remission in untreated patients. Flink et al. showed that different types of flares occur during PEG-IFN therapy and showed that where host-induced flares frequently resulted in therapy response, virus-induced flares did not [5]. This study shows that these virus-induced flares occur more frequently in patients where PC and BCP mutants are present before PEG-IFN initiation. Because these mutants have a reduced production of HBeAg, they are less susceptible to a PEG-IFN–induced immune response against HBeAg, which could result in positive selection and a subsequent virus-induced flare not unlike those observed in patients with viral breakthrough during nucleo(s)tide analogue–based therapy [19]. A relationship between type of flare and the host immune system should also be investigated. It has been shown that flares that do not result in viral clearance may result in hepatic decompensation or death and that presence of PC and BCP mutants may more frequently be found in patients suffering from such acute exacerbations [4, 19, 21]. Given that patients with detectable PC and BCP mutants before PEG-IFN initiation are unlikely to achieve a virological response [22] and are more likely to experience virus-induced flares, it appears that patients with PC and BCP mutants are less suitable candidates for PEG-IFN therapy.
Several studies have shown that the serum level of HBsAg is a good marker for immune control, both in the natural history and during PEG-IFN therapy [12]. Levels of HBsAg are a proxy for intrahepatic transcriptionally active covalently closed circular DNA, and a reduction in HBsAg levels may signify a decrease in intrahepatic covalently closed circular DNA and induction of immune control over HBV [12]. In this study, host-induced flares resulted in pronounced HBsAg declines that were sustained during post-treatment follow-up. Importantly, close monitoring of serum HBsAg levels during flares revealed that the effect of host-induced flares in reducing serum HBsAg levels was apparent even on the individual patient level. It was also clear that virus-induced flares do not result in a reduction of serum HBsAg levels, which is in line with the low response rates observed in these patients. Furthermore, the induction of a decline in serum HBsAg levels during a flare appears to herald HBsAg clearance, suggesting that monitoring of HBsAg levels in patients experiencing flares during PEG-IFN therapy may give valuable information on a patient's probability of treatment response. We propose to use frequent monitoring of serum HBsAg because it is relatively inexpensive, may provide information on response to PEG-IFN therapy [3, 14], and can help predict outcome of flares. A decline in HBsAg levels during the first months after the flare may identify patients with a very high likelihood of HBsAg loss.
Importantly, our findings were also valid when extending the window for HBV DNA increase or decline from 4 to 6 months before or after the flare and when increasing the ALT level from 3 times to >4 times the level at baseline.
In conclusion, host-induced flares during PEG-IFN therapy may result in HBsAg decline and subsequent clearance, whereas virus-induced flares do not. Monitoring of serum HBsAg levels in patients with flares may help identify patients with a high likelihood of subsequent HBsAg clearance.
Notes
Acknowledgments. M. S. and H. F. were responsible for study coordination and design, data collection, data analysis, writing of manuscript, and approval of final version. H. L. A. J. was responsible for study coordination and design, data collection, critical review of the manuscript, and approval of final version. B. E. H. was responsible for statistical analysis, critical review of the manuscript, and approval of final version. R. Z. was responsible for writing of manuscript, critical review of the manuscript, and approval of final version. H. L. A. J. and M. S. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimers. The funding source did not have influence on study design, data collection, analysis and interpretation of the data, writing of the report, or the decision to submit for publication.
Financial support. This work was supported by the Foundation for Liver and Gastrointestinal Research (SLO), Rotterdam, the Netherlands.
Potential conflicts of interest. M. J. S. has received speaker's fee from Roche. H. L. A. J. received grants from and is a consultant for Bristol Myers Squibb, Gilead Sciences, Novartis, Roche, Schering Plough, and Innogenetics. R. Z. has received speaker's fee from Bristol Myers Squibb. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Janssen HL, van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123–9. doi: 10.1016/S0140-6736(05)17701-0. [DOI] [PubMed] [Google Scholar]
- 2.Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–95. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 3.Liaw YF, Jia JD, Chan HL, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology. 2011;54:1591–9. doi: 10.1002/hep.24555. [DOI] [PubMed] [Google Scholar]
- 4.Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001;120:1009–22. doi: 10.1053/gast.2001.22461. [DOI] [PubMed] [Google Scholar]
- 5.Flink HJ, Sprengers D, Hansen BE, et al. Flares in chronic hepatitis B patients induced by the host or the virus? Relation to treatment response during Peg-interferon {alpha}-2b therapy. Gut. 2005;54:1604–9. doi: 10.1136/gut.2004.062208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nair S, Perrillo RP. Serum alanine aminotransferase flares during interferon treatment of chronic hepatitis B: is sustained clearance of HBV DNA dependent on levels of pretreatment viremia? Hepatology. 2001;34:1021–6. doi: 10.1053/jhep.2001.28459. [DOI] [PubMed] [Google Scholar]
- 7.Perrillo R, Tamburro C, Regenstein F, et al. Low-dose, titratable interferon alfa in decompensated liver disease caused by chronic infection with hepatitis B virus. Gastroenterology. 1995;109:908–16. doi: 10.1016/0016-5085(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 8.ter Borg MJ, Hansen BE, Bigot G, Haagmans BL, Janssen HL. ALT and viral load decline during PEG-IFN alpha-2b treatment for HBeAg-positive chronic hepatitis B. J Clin Virol. 2008;42:160–4. doi: 10.1016/j.jcv.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Kusumoto K, Yatsuhashi H, Nakao R, et al. Detection of HBV core promoter and precore mutations helps distinguish flares of chronic hepatitis from acute hepatitis B. J Gastroenterol Hepatol. 2008;23:790–3. doi: 10.1111/j.1440-1746.2008.05391.x. [DOI] [PubMed] [Google Scholar]
- 10.Tan AT, Koh S, Goh W, et al. A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J Hepatol. 2010;52:330–9. doi: 10.1016/j.jhep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Sonneveld MJ, Rijckborst V, Cakaloglu Y, et al. Durable hepatitis B surface antigen decline in hepatitis B e antigen-positive chronic hepatitis B patients treated with pegylated interferon-α2b: relation to response and HBV genotype. Antivir Ther. 2012;17:9–17. doi: 10.3851/IMP1887. [DOI] [PubMed] [Google Scholar]
- 12.Janssen HL, Sonneveld MJ, Brunetto MR. Quantification of serum hepatitis B surface antigen: is it useful for the management of chronic hepatitis B? Gut. 2012;61:641–5. doi: 10.1136/gutjnl-2011-301096. [DOI] [PubMed] [Google Scholar]
- 13.Sonneveld MJ, Zoutendijk R, Janssen HL. Hepatitis B surface antigen monitoring and management of chronic hepatitis B. J Viral Hepat. 2011;18:449–57. doi: 10.1111/j.1365-2893.2011.01465.x. [DOI] [PubMed] [Google Scholar]
- 14.Sonneveld MJ, Rijckborst V, Boucher CA, Hansen BE, Janssen HL. Prediction of sustained response to peginterferon alfa-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology. 2010;52:1251–7. doi: 10.1002/hep.23844. [DOI] [PubMed] [Google Scholar]
- 15.Buster EH, Flink HJ, Cakaloglu Y, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology. 2008;135:459–67. doi: 10.1053/j.gastro.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Hussain M, Chu CJ, Sablon E, Lok AS. Rapid and sensitive assays for determination of hepatitis B virus (HBV) genotypes and detection of HBV precore and core promoter variants. J Clin Microbiol. 2003;41:3699–705. doi: 10.1128/JCM.41.8.3699-3705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pas SD, Fries E, De Man RA, Osterhaus AD, Niesters HG. Development of a quantitative real-time detection assay for hepatitis B virus DNA and comparison with two commercial assays. J Clin Microbiol. 2000;38:2897–901. doi: 10.1128/jcm.38.8.2897-2901.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuen MF, Yuan HJ, Hui CK, et al. A large population study of spontaneous HBeAg seroconversion and acute exacerbation of chronic hepatitis B infection: implications for antiviral therapy. Gut. 2003;52:416–9. doi: 10.1136/gut.52.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang NP, Reijnders JG, Perquin M, Hansen BE, Janssen HL. Frequency and clinical outcomes of flares related to nucleos(t)ide analogue therapy in patients with chronic hepatitis B. J Viral Hepat. 2011;18:e252–7. doi: 10.1111/j.1365-2893.2011.01448.x. [DOI] [PubMed] [Google Scholar]
- 20.Rijckborst V, Sonneveld MJ, Janssen HL. Review article: chronic hepatitis B - anti-viral or immunomodulatory therapy? Aliment Pharmacol Ther. 2011;33:501–13. doi: 10.1111/j.1365-2036.2010.04555.x. [DOI] [PubMed] [Google Scholar]
- 21.Aritomi T, Yatsuhashi H, Fujino T, et al. Association of mutations in the core promoter and precore region of hepatitis virus with fulminant and severe acute hepatitis in Japan. J Gastroenterol Hepatol. 1998;13:1125–32. doi: 10.1111/j.1440-1746.1998.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 22.Sonneveld MJ, Rijckborst V, Zeuzem S, et al. Presence of precore and core promoter mutants limits the probability of response to peginterferon in HBeAg-positive chronic hepatitis B. Hepatology. 2012;56:67–75. doi: 10.1002/hep.25636. [DOI] [PubMed] [Google Scholar]