Abstract
The nature of pathogenic mechanisms associated with the development of multiple sclerosis (MS) have long been debated. However, limited research was conducted to define the interplay between infiltrating lymphocytes and resident cells of the central nervous system (CNS). Data presented in this report describe a novel role for astrocyte-mediated alterations to myelin oligodendrocyte glycoprotein (MOG)35–55-specific lymphocyte responses, elicited during the development of experimental autoimmune encephalitomyelitis (EAE). In-vitro studies demonstrated that astrocytes inhibited the proliferation and interferon (IFN)-γ, interleukin (IL)-4, IL-17 and transforming growth factor (TGF)-β secretion levels of MOG35–55-specific lymphocytes, an effect that could be ameliorated by astrocyte IL-27 neutralization. However, when astrocytes were pretreated with IFN-γ, they could promote the proliferation and secretion levels of MOG35–55-specific lymphocytes, coinciding with apparent expression of major histocompatibility complex (MHC)-II on astrocytes themselves. Quantitative polymerase chain reaction (qPCR) demonstrated that production of IL-27 in the spinal cord was at its highest during the initial phases. Conversely, production of IFN-γ in the spinal cord was highest during the peak phase. Quantitative analysis of MHC-II expression in the spinal cord showed that there was a positive correlation between MHC-II expression and IFN-γ production. In addition, astrocyte MHC-II expression levels correlated positively with IFN-γ production in the spinal cord. These findings suggested that astrocytes might function as both inhibitors and promoters of EAE. Astrocytes prevented MOG35–55-specific lymphocyte function by secreting IL-27 during the initial phases of EAE. Then, in the presence of higher IFN-γ levels in the spinal cord, astrocytes were converted into antigen-presenting cells. This conversion might promote the progression of pathological damage and result in a peak of EAE severity.
Keywords: astrocyte, experimental autoimmune encephalitomyelitis, IL-27, MHC-II, multiple sclerosis
Introduction
Experimental autoimmune encephalitomyelitis (EAE) is a well-described multiple sclerosis animal model, and affects animals presenting with signs similar to multiple sclerosis (MS), including demyelization, axonal damage and paralysis [1–3]. Although still delusory, CD4+ T cells are believed to be the major contributors to autoimmune disease pathogenesis [4], specifically in the context of diseases associated with T helper type 1 (Th1), Th2, Th17 and regulatory T (Treg) cells imbalances mediated by their respective primary signature cytokines interferon (IFN)-γ, interleukin (IL)-4, IL-17 and transforming growth factor (TGF)-β [5–10].
Astrocytes represent the primary cell population in the central nervous system (CNS) and are essential for maintaining CNS homeostasis [11–14]. However, evidence suggests that astrocytes play an important role in CNS inflammatory diseases such as MS [15–19]. Even more poorly defined is the role played by astrocytes in autoimmune diseases; that is, it is suggested by some that astrocytes modulate CNS immune responses in several different ways. Specifically, Meinl et al. have demonstrated that astrocytes inhibit the proliferation of human peripheral blood-derived mononuclear cells by secreting prostaglandins [20], and others have demonstrated that astrocytes inhibit the production of IL-12 by CNS microglia in a model of EAE [21,22]. In addition, astrocytes have been shown to secrete IL-27 [23,24] (a newly heterodimeric cytokine which is composed of two subunits, p28 and EBI3 [25]). IL-27 is associated with suppressors of cytokine signalling (SOCS) with the potential of suppressing IL-2 responses and affecting CD4+ T cell survival [26]. It has been shown that IL-27 could suppress Th17 cells in both active and adoptive transfer models of EAE [27–29].
Conversely, astrocytes have also been shown to hold the potential of promoting the pathogenesis of EAE. Inhibition of glial cell activation ameliorates the severity of experimental autoimmune encephalitomyelitis [30]. Astrocytes hold the potential of secreting IL-12/IL-23 that facilitates the differentiation and survival of Th1 and Th17 cells [31,32]. For example, astrocyte-restricted ablation of IL-17-induced act1-mediated signalling ameliorates autoimmune encephalitomyelitis [33]. These data highlight the fact that MS is not strictly immune cell-mediated, but is also affected significantly by CNS-related factors. Astrocytes are the most abundant cells in the CNS, and their position is closed with the blood–brain barrier (BBB). However, the exact role played by astrocytes during the development of EAE is still debated.
In the present study, we demonstrate that astrocytes are capable of inducing and suppressing lymphocyte functions during different phases of EAE. During the initial phases, astrocytes probably inhibit the activity of myelin oligodendrocyte glycoprotein (MOG)35–55-specific lymphocytes in part by secreting IL-27, which contributes to inhibition of proliferation and lymphocyte secretion. During EAE progression, lymphocyte-derived IFN-γ might induce the up-regulation of major histocompatibility complex (MHC)-II on astrocytes, thereby promoting lymphocyte proliferation and activation and resulting in disease progression. These findings indicate that the changing physiological role of astrocytes is important to EAE development. The study contributes to a clearer understanding of EAE and adds new insights into the field of EAE research.
Materials and methods
Animals
Female C57BL/6 mice (6–8 weeks of age) were purchased from the Beijing Vital River Laboratory Animal Ltd (Beijing, China). All mice were bred and housed in a specific pathogen-free animal facility at the Harbin Medical University. Neonatal C57BL/6 mice aged 1–3 days were used for the isolation of astrocytes. All animal experiments were performed in compliance with the principles and procedures outlined in the Care and Use of Laboratory Animals guidelines, which is published by the China National Institute of Health and approved by the Institutional Animal Care and Use Committee.
Induction and assessment of EAE
C57BL/6 mice were immunized subcutaneously in the axillary fossa with the MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) peptide (200 μg) emulsified in complete Freund's adjuvant (CFA) at a final volume of 100 μl. Mice were then injected intravenously (i.v.) with 200 ng pertussis toxin (PT) on days 0 and 2. The behavioural performance was assessed by a 0–5-point scale as follows: 0, no clinical signs; 1, floppy tail; 2, hind limb weakness; 3, full hind limb paralysis; 4, quadriplegia; and 5, death as described [34].
Isolation of astrocytes
Astrocytes were isolated from newborn mice as described previously [35,36]. Briefly, following removal of the meninges, brains were minced with a Pasteur pipette and passed through a 150 μm nylon filter to remove debris. Cells were then seeded onto 10 μg/ml poly-D-lysine precoated flasks and cultures were incubated at 37°C in 5% CO2. After 72 h, non-adherent cells were removed by changing the media every 3–4 days. When cultures were 70–80% confluent, mixed glia were agitated rigorously for 2 h in an orbital incubator shaker at 0.23 g at 37°C to detach microglia. Cells were then shaken again at 0.23 g at 37°C overnight to ablate oligodendrocytes. Suspended cells were trypsinized [0·25% trypsin and 0·02% ethylenediamine tetraacetic acid (EDTA)] and replated onto flasks. Subcultured astrocytes were 92% positive for glial fibrillary acidic protein (GFAP) by immunofluorescence staining.
Preparation of mononuclear cells from lymph nodes
Mononuclear cells (MNCs) were obtained from the axillary and inguinal lymph nodes of 7 days post-immunization (dpi) CFA and EAE mice. Cells were washed once with Hanks's balanced salt solution and cultured in Dulbecco's modified Eagle's medium (DMEM) (Hyclone, Logan, UT, USA) supplemented with 5% fetal calf serum (Gibco, Paisley, UK), 1% L-glutamine (Sigma, St Louis, MO, USA), 1% non-essential amino acids (Sigma), 2 × 10−5 M 2-mercaptoethanol (Amresco, Solon, OH, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco). All cells were adjusted to 2 × 106 cells/ml.
Lymphocyte proliferation assays
MNC suspensions (4 × 105) obtained above were seeded in triplicate in 96-well, round-bottomed microtitre plates at different lymphocyte : astrocyte ratios (10:1, 1:1 and 1:5). Cells were stimulated with 25 μg/ml MOG35–55 peptide for 72 h. For anti-CD3/CD28-induced cell proliferation, 96-well culture plates were coated with purified anti-CD3 and anti-CD28 monoclonal antibodies (mAbs) (5 μg/ml each; eBioscience, Ltd, Ireland, UK). ConA (Sigma, St Louis, MO, USA) was used at 5 μg/ml. Proliferation was measured by [3H]-thymidine (specific activity, 60 μCi/mmol; Institute of Atomic Energy, China; 0·5 μCi/well) incorporation after 72 h in complete DMEM medium.
IL-27 neutralization
Astrocytes were cultured at a concentration of 1 × 106 cells/well in 12-well plates, then incubated with 2 μg/ml goat anti-mouse-IL-27 antibody (R&D Systems, Minneapolis, MN, USA) [37] or isotype control immunoglobulin (Ig)G2a in 2 ml medium for 12 h to neutralize IL-27. Astrocytes were co-cultured with MNCs (1 × 107) harvested from the lymph nodes of EAE mice in 2 ml lymphocyte culture medium. The cells were incubated at 37°C, 5% CO2 for 72 h. Supernatants were collected for measurement of the levels of soluble cytokines.
Measurement of cytokine secretion
Astrocytes (1 × 106) were co-cultured with lymph node lymphocytes (1 × 107) harvested from 7 dpi mice in 2 ml lymphocyte culture medium. Where indicated, lymphocytes were also seeded in Transwell™ insert (24-well plates, 3 μm pore size; Corning, NY, USA). Twenty-five μg/ml MOG35–55 peptide was incubated as antigen and the supernatants were collected 72 h later. Measurement of cytokine levels in cell culture supernatants was performed by enzyme-linked immunosorbent assay (ELISA) using commercially available ELISA kits, in accordance with the manufacturer's instructions. IFN-γ, IL-17 and IL-4 ELISA kits were purchased from Peprotech (Rocky Hill, NJ, USA). The TGF-β ELISA kit was obtained from Boster, China. Results are expressed as pg/ml.
RNA extraction and PCR analysis
Total RNA was prepared from spinal cords or lymph node MNCs using TRIzol reagent (Invitrogen). cDNA was synthesized using a reverse transcription–polymerase chain reaction (RT–PCR) kit from TaKaRa (Kyoto, Japan). RT–PCR was used to detect MHC-II genes using the following forward 5′-GATCGGATCCAACCCTGCTGAGGATTCA-3′ and reverse 5′-GATCGGATCCTGTCCTCGGCTGGGAAGA-3′ primers. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was amplified as a reference gene using the following forward 5′-CGGCCGCATCTTCTTGTGCA-3′ and reverse 5′-GCCGTGAGTGAGTCATACT-3′ primers. PCR products were separated on a 1·5% agarose gel and analysed by Image Pro-Plus software (Media Cybernetics, Silver Springs, MD, USA). Real-time PCR was performed by an ABI STEPONE real-time PCR system using the SYBR Green real-time PCR kit (Roche Ltd, Basel, Switzerland). The primers used to amplify IFN-γ [38] (5′-GATGCATTCATGAGTATTGCCAAGT-3′, 5′-GTGGACCACGCGGATGAGCTC-3′), IL-27 p28 [39] (5′-TTCCCAATGTTTCCCTGACTTT-3′, 5′-AAGTGTGGTAGCGAGGAAGCA-3′), IL-27 EBI3 [39] (5′-TGAAACAGCTCTCGTGGCTCTA-3′, 5′-GCCACGGGATACCGAGAA-3′) and MHC-II [40] (5′-GCGACGTGGGCGAGTACC-3′, 5′-CATTCCGGAACCAGCGCA-3′) were used to detect the expression of respective genes. The data were normalized against GAPDH (5′-CGGCCGCATCTTCTTGTGCA-3′, 5′-GCCGTGAGTGAGTCATACT-3′) levels. The amplification of real-time PCR was performed with an initial denaturation of 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Relative gene expression levels were quantified using the comparative ΔCT method. This method normalized CT values of the detected gene to the average of that of the GAPDH and calculated the relative expression values as fold changes of the control, which was set at 1. Melting curve analyses and electrophoresis were performed to verify the specificity of the PCR products.
Immunofluorescence
Frozen spinal cord sections were dually stained with goat anti-mouse GFAP (Santa Cruz Laboratories, Santa Cruz, CA, USA) and rat anti-mouse MHC-II (Santa Cruz Laboratories), followed by incubation with fluorescein isothiocyanate (FITC)-labelled anti-rat and tetramethylrhodamine-5-(and 6)-isothiocyanate (TRITC)-labelled anti-goat secondary antibodies (ZSGB-Bio, Beijing, China). Stained sections were examined and photographed using fluorescence microscopy (Carl Zeiss, Germany) and scanning confocal laser microscopy (Leica, China).
Western blot analysis
Astrocytes were treated with or without 100 U/ml IFN-γ and then co-cultured with lymphocytes obtained from lymph node at a lymphocyte : astrocyte ratio of 10:1 for 72 h. Twenty-five μg/ml MOG35–55 peptide was incubated in the culture as antigen. Astrocytes were lysed in lysis buffer containing protease inhibitors, and cell lysates were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and transferred onto a polyvinylidene difluoride (PVDF) membrane via semidry transfer. Membranes were blocked with 5% non-fat milk for 1 h at room temperature and IL-27 (Santa Cruz, CA, USA) expression was detected. All antibodies were diluted with Tris-buffered saline with 0·1% Tween 20 (TBST).
GAPDH was used as reference genes. The optical density of bands was evaluated using Scion Image Beta version 4·02 (Scion Corporation, Frederick, MD, USA) and statistical comparison was performed with GraphPad Prism version 5 software.
Statistical analysis
Data are expressed as means ± standard error of the mean (s.e.m.). Statistical analyses were performed with one-way analysis of variance (anova) followed by the Bonferroni correction for multiple group comparisons. Clinical scores were analysed using the non-parametric Mann–Whitney U-test. The level of significance was set at P < 0·05.
Results
EAE induction
EAE was induced in C57BL/6 mice by immunization with the MOG35–55 peptide in CFA followed by i.v. injection of PT. EAE mice exhibited three disease phases: preclinical, peak and remission phases. Clinical signs (partial limp tail) presented at 7 dpi. Disease then progressed to limp tail, waddling gait and paralysis during the peak phases (at 16 dpi). Finally, mice recovered but still presented with clinical signs during the remission phases (at 28 dpi). CFA mice showed no clinical signs at all (Fig. 1a).
Fig. 1.
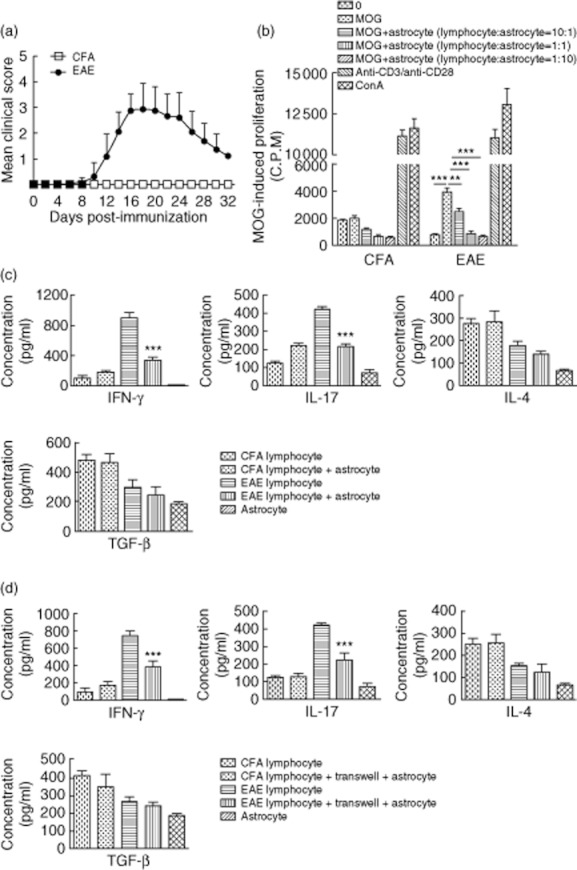
(a) Clinical experimental autoimmune encephalitomyelitis (EAE) scores. EAE was induced in C57BL/6 mice (n = 8 mice/group) following immunization with the myelin oligodendrocyte glycoprotein (MOG)35–55 peptide (200 μg/mouse) emulsified in complete Freund's adjuvant (CFA) followed by pertussis toxin (PT) injection (400 ng/mouse). Control animals were immunized with CFA only. Clinical scores were evaluated every other day. Data are expressed as the mean clinical score ± standard error of the mean (s.e.m.). (b) Proliferation of MOG35–55-specific lymphocytes co-cultured with astrocytes at various lymphocyte : astrocyte ratios. Lymphocytes were isolated from the lymph nodes of 7 days post-immunization (dpi) CFA and EAE mice, and co-cultured with astrocytes at lymphocyte : astrocyte ratios of 10:1, 1:1 or 1:5. Proliferation of MOG35–55 -specific lymphocytes was analysed after 72 h. Values are expressed as mean counts per minute (cpm) ± s.e.m. of three independent experiments. **P < 0·01; ***P < 0·001. (c, d) Cytokine levels resulting from the co-culture of astrocyte and lymphocyte. Lymphocytes were isolated from 7 dpi CFA and EAE mice and co-cultured with or without direct contact with astrocytes at a lymphocyte : astrocyte ratio of 10:1 for 72 h, in the presence of 25 μg/ml MOG35–55 peptide. Supernatants were collected and interferon (IFN)-γ, interleukin (IL)-17, IL-4 and transforming growth factor (TGF)-β concentrations were assessed by enzyme-linked immunosorbent assay (ELISA). Astrocytes cultured alone were used as controls. Data are expressed as mean ± s.e.m. of three experiments. ***P < 0·001.
Astrocytes affected MOG35–55-specific lymphocyte proliferation and cytokine production
Lymph node MNCs were isolated from 7 dpi EAE and CFA mice and then co-cultured with astrocytes at lymphocyte : astrocyte ratios of 10:1, 1:1, and 1:5. At the lymphocyte : astrocyte ratios tested there were no differences in proliferation among cells isolated from the CFA group, with the exception of CD3/CD28 and concanavalin A (ConA)-stimulated cells (Fig. 1b). Conversely, lymphocytes isolated from EAE mice proliferated significantly in response to stimulation with MOG35–55 peptide (P < 0·001). In the EAE lymphocyte : astrocyte co-cultured group, lymphocyte proliferation was inhibited by half at a ratio of 10:1 (P < 0·01) and inhibited completely at ratios of 1:1 and 1:5 (P < 0·001) compared to proliferation observed for MOG35–55 peptide-stimulated EAE lymphocytes alone. These data indicate that the inhibitory effect of astrocytes on MOG35–55-specific lymphocytes is correlated with lymphocyte : astrocyte ratios.
Lymphocytes were then co-cultured with astrocytes at a lymphocyte : astrocyte ratio of 10 : 1. Supernatants were obtained 72 h later and cytokine levels were detected by ELISA. In the supernatants collected from EAE lymphocyte : astrocyte cultures, IFN-γ (P < 0·001) and IL-17 (P < 0·001) levels were decreased significantly; IL-4 and TGF-β levels were also decreased compared to levels observed for EAE lymphocytes. There were no significant differences in cytokine production by cells harvested from mice in the CFA groups. Levels of the above cytokines were lower in the supernatants of astrocytes cultured alone (Fig. 1c).
The suppressing effect of astrocyte on MOG35–55-specific lymphocytes might be mediated by soluble factors as well as cell contact. We cultured astrocyte and MOG35–55-specific lymphocytes without contact between both cells using Transwell plates. Supernatants were taken out to test cytokine levels after 72 h. Results are shown in Fig. 1d. Significant reductions of IFN-γ (P < 0·001) and IL-17 (P < 0·001) levels were also observed at the co-culture group without contact between both cells. These results suggest that cell contact is not required in astrocyte-mediated suppression of lymphocyte secreting, and might be mediated by soluble factors.
Astrocytes secrete significant levels of IL-27 after co-culture with MOG35–55-specific lymphocytes
Astrocytes were incubated in the presence or absence of IFN-γ and then co-cultured with lymphocytes for 72 h. Proteins of astrocytes were obtained to evaluate IL-27 production by Western blot. IL-27 levels in astrocytes co-cultured with EAE lymphocytes were increased significantly compared to levels produced following culture with lymphocytes isolated from CFA-treated mice or by astrocytes cultured alone (P < 0·05). IFN-γ treated astrocytes showed no significant differences in IL-27 secretion regardless of whether they were cultured alone or in the presence of other cells (Fig. 2a,b).
Fig. 2.
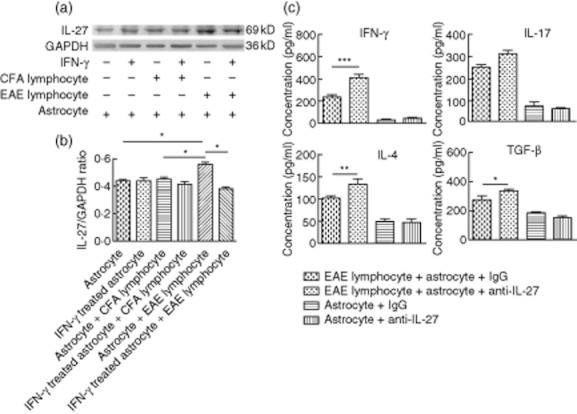
(a, b) Interleukin (IL)-27 production by astrocytes following co-culture with lymphocytes. Astrocytes were pretreated with or without 100 U/ml interferon (IFN)-γ for 24 h. They were then cultured in the presence or absence of lymph node lymphocytes (lymphocyte : astrocyte ratio of 10:1) for 72 h. IL-27 expressed by astrocytes was detected by Western blot. Data are representative of three independent experiments. *P < 0·05. (c) Astrocyte-mediated inhibition of cytokine production by lymphocytes was ameliorated by IL-27 neutralization. Astrocytes were incubated with 2 μg/ml goat-anti-mouse-IL-27 antibody for 12 h prior to co-culture with lymphocytes at a lymphocyte : astrocyte ratio of 10:1. Supernatants were harvested 72 h later to determine cytokine levels. Supernatant of astrocytes cultured alone were used as controls. Data are based on three independent experiments. *P < 0·05; **P < 0·01; ***P < 0·001.
Astrocyte-mediated inhibition of lymphocyte cytokine production was ameliorated following IL-27 neutralization
Production of IFN-γ, IL-17, IL-4 and TGF-β were detected in the supernatants of astrocyte and lymphocyte co-cultures by ELISA (Fig. 1c,d). High levels of astrocyte-derived IL-27 were observed when co-cultured with EAE lymphocytes (Fig. 2a,b). Therefore, we examined what effect of neutralization of IL-27 would have on lymphocyte cytokine production by administration of anti-IL-27 neutralizing antibodies to astrocytes. Lymphocytes from EAE mice were restimulated with astrocytes for 72 h in the absence (astrocytes + anti-IL-27) or presence (astrocytes + goat IgG) of IL-27. Lymphocytes restimulated with astrocytes in the presence of IL-27 neutralizing antibodies expressed significantly elevated IFN-γ (P < 0·001), IL-4 (P < 0·01) and TGF-β (P < 0·001) expression levels compared to lymphocytes restimulated with astrocytes plus control antibody (Fig. 2c).
Assessment of IL-27 and IFN-γ mRNA expression during EAE development
Mice were killed during the course of the different EAE development phases. Spinal cords and draining lymph node MNCs were harvested and the production of IL-27 and IFN-γ were evaluated by real-time PCR. Production of IL-27 p28 and IL-27 EBI3 were increased significantly in spinal cords at 7 dpi compared to levels observed in spinal cords at 16 and 28 dpi (P < 0·001). IL-27 p28 and IL-27 EBI3 levels in lymph nodes were almost undetectable (Fig. 3a,b).
Fig. 3.
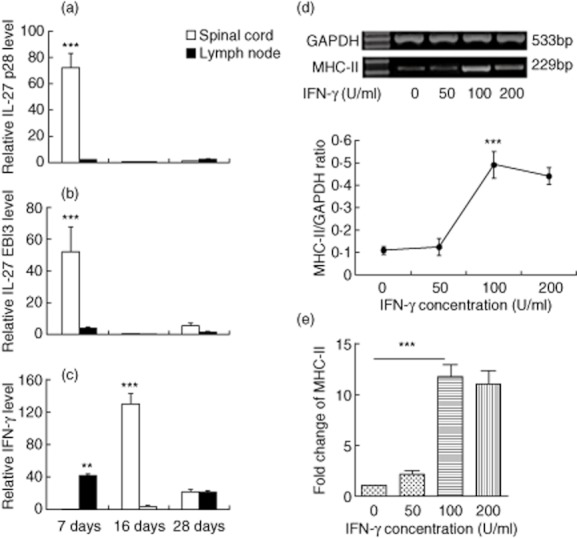
(a,b,c) Detection of interleukin (IL)-27 and interferon (IFN)-γ production in the spinal cord and lymph nodes at different phases of experimental autoimmune encephalitomyelitis (EAE). Spinal cords and lymph node lymphocytes were harvested 7, 16 and 28 days post-immunization (dpi) (n = 8 mice/group). (a) IL-27 p28, (b) IL-27 EBI3 and (c) IFN-γ production were detected by real-time polymerase chain reaction (PCR). The data shown were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression, and the expression levels in the complete Freund's adjuvant (CFA) group were set to 1. **P < 0·01; ***P < 0·001. (d,e) Inducible major histocompatibility complex (MHC)-II expression on astrocytes in vitro. Astrocytes were stimulated in the presence of different concentrations of IFN-γ (0–200 U/ml) for 24 h. Total astrocyte RNA was extracted to assess MHC-II expression by reverse transcription–polymerase chain reaction (RT–PCR) (d) and real-time PCR (e). Data were normalized to GAPDH expression and non-IFN-γ treated group was set to 1 in real-time PCR. Data are based on three independent experiments and data expressed as the mean ± standard error of the mean (s.e.m.). ***P < 0·001.
IFN-γ production in spinal cords peaked at 16 dpi relative to other time-points examined (P < 0·001). In the lymph nodes, IFN-γ production peaked at the beginning of disease (P < 0·001), decreased during the peak phase of EAE and was increased slightly during the remission phase (Fig. 3c).
MHC-II expression on astrocytes was affected by IFN-γ in a dose-dependent manner
Astrocytes in culture were exposed to different concentrations of IFN-γ (ranging from 0 to 200 U/ml) for 24 h. Total RNA was extracted and MHC-II mRNA expression was detected by RT–PCR and real-time PCR. MHC-II expression levels were elevated after stimulation with 100 U/ml IFN-γ, compared to levels observed following culture with either 0 or 50 U/ml IFN-γ (P < 0·001). However, culture in the presence of 200 U/ml IFN-γ down-regulated MHC-II expression levels slightly compared to levels observed following culture with 100 U/ml IFN-γ (Fig. 3d,e).
MHC-II expression in spinal cords during EAE development
The local microenvironment played a critical role in the development of immune responses [16]. CNS antigen presentation is also necessary for pathogenic lymphocytes reactivation and disease progression [41], so we characterized MHC-II expression levels in the spinal cord. mRNA levels were measured by RT–PCR and real-time PCR (Fig. 4). Immunoreactivity to MHC-II expressed on astrocytes was detected by double-labelled immunofluorescence (Fig. 5).
Fig. 4.
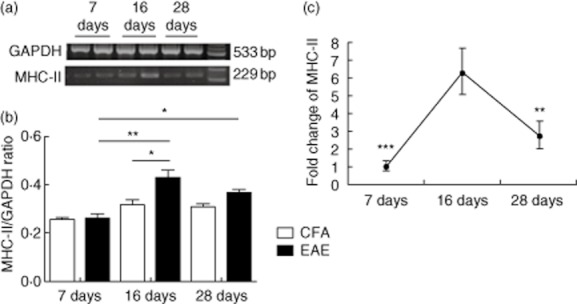
Major histocompatibility complex (MHC)-II expression in the spinal cord at different stages of experimental autoimmune encephalitomyelitis (EAE). Representative levels of MHC-II and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA expression in spinal cords (n = 8 mice/group) of 7, 16 and 28 days post-immunization (dpi) mice were detected by reverse transcription–polymerase chain reaction (RT–PCR) (a,b) and real-time PCR (c). Data were normalized to GAPDH expression and the expression levels in the complete Freund's adjuvant (CFA) group were set to 1 in real-time PCR. Data from three independent experiments are shown and expressed as the mean ± standard error of the mean (s.e.m.). *P < 0·05; **P < 0·01; ***P < 0·001.
Fig. 5.
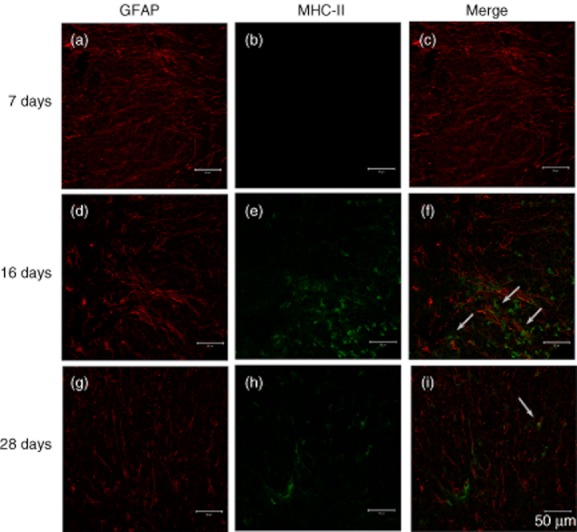
Expression of major histocompatibility complex (MHC)-II on astrocytes at different phases of experimental autoimmune encephalitomyelitis (EAE). Slides are representative sections taken from the spinal cord region of 7, 16 and 28 days post-immunization (dpi) from EAE mice (n = 8 mice/group). Astrocytes were stained with glial fibrillary acidic protein (GFAP) (red) in a, d and g. MHC-II (green) expression levels are shown in b, e, and h. Merge of GFAP and MHC-II is shown in c, f and i. Double-positive cells were indicated by white arrows. Scale bar = 50 μm.
RT–PCR analysis showed significantly elevated MHC-II expression levels in the spinal cords at 16 dpi EAE mice compared to CFA mice (P < 0·05). In the spinal cords of EAE mice, MHC-II expression peaked at 16 dpi compared to levels observed at 7 dpi (P < 0·01) and 28 dpi (P < 0·05) (Fig. 4a,b).
In order to strengthen the observations in RT–PCR, real-time PCR was employed to determine MHC-II mRNA levels in the spinal cord. The data shown were normalized to GAPDH expression, and the expression levels in the CFA group were set to 1. As shown in Fig. 4c, MHC-II mRNA level was promoted significantly in the spinal cords at 16 dpi EAE mice compared to either 7 dpi (P < 0·001) or 28 dpi (P < 0·01). MHC-II expression levels were correlated positively with disease progression and IFN-γ production levels in the spinal cord.
Double-labelled immunofluorescence staining was employed to localize MHC-II expression on astrocytes. Spinal cords harvested from EAE mice presented with undetectable MHC-II expression levels on astrocytes at 7 dpi, peaked at 16 dpi and then expression was down-regulated at 28 dpi (Fig. 5). MHC-II expression could not be detected on astrocytes harvested from mice in the CFA group (data not shown).
IFN-γ treated astrocytes promoted proliferation and cytokine production of MOG35–55-specific lymphocytes
For proliferation assay, astrocytes were treated with different concentrations of IFN-γ ranged from 0 to 200 U/ml for 24 h. They were then co-cultured with lymph node lymphocytes at a lymphocyte : astrocyte ratio of 10:1. Proliferation of lymphocyte was promoted when co-cultured with IFN-γ-treated astrocytes (P < 0·001). These data indicate that IFN-γ-treated astrocytes could promote the proliferation of MOG35–55-specific lymphocytes (Fig. 6a).
Fig. 6.
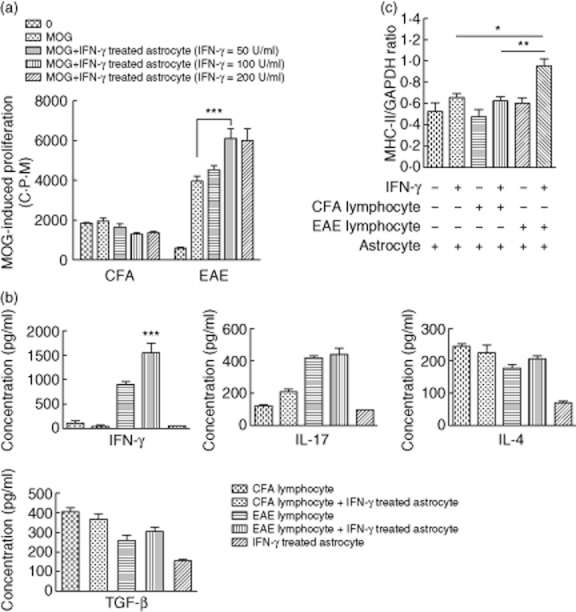
(a) Proliferation of myelin oligodendrocyte glycoprotein (MOG)35–55-specific lymphocytes co-cultured with astrocytes at various interferon (IFN)-γ concentrations. Astrocytes were treated, respectively, with 50, 100 or 200 U/ml IFN-γ for 24 h prior to co-culture with lymph node lymphocytes at a lymphocyte : astrocyte ratio of 10:1. 25 μg/ml MOG35–55 peptide was used as antigen. Proliferation of MOG35–55 -specific lymphocytes were analysed by [3H]-thymidine after 72 h. Values are expressed as mean counts per minute (cpm) ± standard error of the mean (s.e.m.) of three independent experiments. ***P < 0·001. (b) Cytokine levels in the supernatants of co-cultured astrocytes and lymphocytes in the presence of IFN-γ. Lymphocytes were isolated from 7 days post-immunization (dpi) complete Freund's adjuvant (CFA) and experimental autoimmune encephalitomyelitis (EAE) mice. Astrocytes were stimulated with 100 U/ml IFN-γ for 24 h and then co-cultured with lymphocytes at a lymphocyte/astrocyte ratio of 10:1 for 72 h in the presence of the MOG35–55-peptide. Supernatants were collected and IFN-γ, interleukin (IL)-17, IL-4 and transforming growth factor (TGF)-β concentrations were determined by enzyme-linked immunosorbent assay (ELISA). Supernatants of astrocytes cultured alone in the presence of IFN-γ were employed as controls. Data are expressed as mean ± s.e.m. from three experiments. ***P < 0·001. (c) MHC-II mRNA levels in astrocytes after co-culturing with lymphocytes. Astrocytes were treated in the presence or absence of 100 U/ml IFN-γ for 24 h and then co-cultured with lymphocytes at a lymphocyte : astrocyte ratio of 10:1. 72 h later, astrocytes were lysed with TRIzol reagent and major histocompatibility complex (MHC)-II mRNA was evaluated by real-time polymerase chain reaction (PCR). Data were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. Data shown are from three independent experiments. *P < 0·05; **P < 0·01.
For cytokine production assay, astrocytes were treated with 100 U/ml IFN-γ for 24 h. They were then co-cultured with lymph node lymphocytes at a lymphocyte : astrocyte ratio of 10:1. Supernatants were harvested 72 h later and cytokine levels were determined by ELISA. IFN-γ levels in the supernatants of EAE lymphocytes and IFN-γ-treated astrocytes in the co-culture group were elevated significantly (P < 0·001). Levels of IL-4, IL-17 and TGF-β were also elevated compared to levels observed in supernatants from EAE lymphocytes cultured alone. There were no significant differences in cytokine production levels by cells harvested from mice in the CFA group. Levels of the cytokines described above were low in the supernatants of astrocytes cultured (without lymphocytes) in the presence of IFN-γ (Fig. 6b).
IFN-γ treated astrocytes up-regulated MHC-II expression levels after co-culture in the presence of MOG35–55-specific lymphocytes
Astrocytes were treated in the presence or absence of 100 U/ml IFN-γ for 24 h and then co-cultured with lymphocytes at a lymphocyte : astrocyte ratio of 10:1 for 72 h. Total astrocyte RNA was extracted and MHC-II mRNA levels were detected by real-time RT–PCR. IFN-γ-treated astrocytes presented with significantly elevated MHC-II expression levels following co-culture with EAE lymphocytes, compared to levels observed in astrocytes cultured in the presence of CFA lymphocytes (P < 0·01) or astrocytes cultured alone (P < 0·05). Among groups of non-IFN-γ-treated astrocytes, MHC-II expression levels were similar in astrocytes cultured alone or in co-culture (Fig. 6c). The data shown were normalized to GAPDH expression. These indicate that IFN-γ-treated astrocytes might function as antigen-presenting cells by expressing MHC-II.
Discussion
Data presented in this report show that astrocytes hold the potential of either inhibiting or activating MOG35–55-specific lymphocytes during EAE development. We have demonstrated that astrocytes affect both the proliferation and cytokine production of MOG35–55-specific lymphocytes, most probably by secreting IL-27 during the initial phases. Increasing spinal cord levels of IFN-γ contribute to the conversion of astrocytes into antigen-presenting cells, based on their significantly elevated MHC-II expression levels. These alterations may be associated with the reactivation of pathogenic lymphocytes, thus resulting in disease progression. These findings identify two aspects of disease progression that need to be addressed. First, to determine how astrocytes inhibit MOG35–55-specific lymphocytes, and secondly, to define how activated astrocytes promote MOG35–55-specific lymphocytes.
There is a great deal of evidence indicating that astrocytes have the potential of mediating suppressive functions. Gimsa et al. have concluded that astrocytes contribute to the establishment of the immune privileged status of the CNS by suppressing the Th1 and Th2 cell activation, proliferation and effector functions which are mediated mainly by the cytotoxic T lymphocyte antigen (CTLA-4) [42]. Others have shown that astrocytes are capable of inducing T cell unresponsiveness and triggering suppressor activity in T cell in both rat and human lymphocytes [43]. Our research also demonstrates that astrocytes inhibit the proliferative ability of lymphocytes depending on the lymphocyte : astrocyte ratio (Fig. 1b). Further analysis of the lymphocyte cytokine secretion profiles identified that IFN-γ, IL-17, IL-4 and TGF-β are down-regulated when co-cultured with astrocytes, and this effect was mediated probably by soluble factors (Fig. 1c,d). It has been reported that astrocytes could secrete several regulatory cytokines such as IL-27 and IL-10 in a model of experimental autoimmune uveitis (EAU) [44]. IL-27 has also been found to inhibit immune responses, including inhibition of T cell proliferation and differentiation, suppression of proinflammatory cytokine production and attenuation of EAE [45–47]. We therefore determined the amount of IL-27 produced by astrocytes (Fig. 2a). This analysis demonstrated that astrocytes secrete a significantly high dose of IL-27 when treated with EAE lymphocytes. Furthermore, the suppressive effect of astrocytes (on lymphocytes) is ameliorated following incubation with neutralizing anti-IL-27 antibodies (Fig. 2c). These data indicate that astrocytes can inhibit MOG35–55-specific lymphocytes, in part, by secreting IL-27.
The in-vivo studies described in this report demonstrate that spinal cord IL-27 levels are elevated during the initial phases of EAE, but are almost undetectable in the lymph nodes during the disease phases (Fig. 3a,b). These findings suggest that there might be local secretion of IL-27 by resident spinal cord cells (potentially astrocytes) during the early phases. These observations are supported by previous studies which demonstrate that CNS glial cells produce several IL-12 family cytokines (including IL-27) during EAE development [23,24]. Combined with the in-vitro studies described in this report, our data suggest that during the initial phases of EAE, astrocytes might inhibit the proliferation and secretion of invading lymphocytes most probably by secreting IL-27. However, the in-vivo environment is probably more complex and further work will need to be carried out to confirm that astrocytes are the main source of IL-27.
IFN-γ is a classic inflammatory cytokine associated with autoimmune diseases [48]. Many pathogenic immune cells such as Th1, Tc1 and natural killer (NK) cells are characterized by IFN-γ production [49]. IFN-γ can induce MHC-II expression on antigen-presenting cells [50–52]. Microglial cells are well-described CNS antigen-presenting cells [53]; conversely, astrocytes (the most abundant cells in the CNS) have rarely been examined in the context of antigen presentation. Our study demonstrates a dose-dependent relationship between IFN-γ concentrations and MHC-II expression on astrocytes (Fig. 3d,e). When astrocytes are pretreated with IFN-γ, they can promote the proliferation and secretion of IFN-γ, IL-17, IL-4 and TGF-β by MOG35–55-specific lymphocytes (Fig. 6a,b) and astrocytes, in turn, express elevated levels of MHC-II (Fig. 6c). Unfortunately, astrocytes still secrete few IL-27 (Fig. 2a). Due to the fact that IL-27 mediates a strong limitation on IL-17-producing cells [29,46,47,54], the promotion of IL-17 levels is not as significant as IFN-γ. These indicate that IFN-γ-treated astrocytes might turn into antigen-presenting cells with lymphocyte activating potential.
In vivo, we have demonstrated that IFN-γ production in the spinal cord and lymph nodes could also be detected, supporting previously published observations [55]. The highest levels of IFN-γ production are observed in the spinal cord during the peak phases of EAE (Fig. 3c). Under these conditions, resident CNS cells are activated and converted into antigen-presenting cells [51]. Quantitative analysis of MHC-II expression in the spinal cord shows a positive correlation with IFN-γ production (Fig. 4). Because the observed up-regulation in MHC-II expression may be due to activation of macrophages and/or microglia [56], as well as astrocytes, we focused on determining the level of MHC-II expression on astrocytes. The results show that astrocyte MHC-II expression is similar to expression levels of MHC-II observed in spinal cords (Fig. 5). Taken together, these data suggest that astrocytes might be able to develop into antigen-presenting cells during the late phase of EAE, thereby contributing to lymphocyte-mediated disease pathogenesis and resulting in severe presentation of disease.
CNS factors have been shown to contribute equally (with immune cells) to MS disease progression [4]. Data presented in this report demonstrate that astrocytes act both as suppressors and promoters of MOG35–55-specific lymphocyte responses; these are associated closely with the disease stage and the local microenvironment. Therefore, targeting of astrocytes during the optimal time-points in the course of disease progression may be used to develop novel EAE therapeutic strategies.
Acknowledgments
This research was supported by the Master Innovation Research Foundation of Hei Longjiang Province (YJSCX2011-325HLJ), the Natural Science Foundation for Youth of China (30901330; 81000512; 81100883), the Natural Science Foundation of China (81171121), the Doctoral Fund of the Ministry of Education of China (20102307110013) and Open project of key laboratory of Myocardical Ischemia Mechanism and Treatment (KF201013).
Disclosure
The authors have declared that no competing interests exist.
References
- 1.Linker RA, Lee DH. Models of autoimmune demyelination in the central nervous system: on the way to translational medicine. Exp Transl Stroke Med. 2009;1:5. doi: 10.1186/2040-7378-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 3.Recks MS, Addicks K, Kuerten S. Spinal cord histopathology of MOG peptide 35–55-induced experimental autoimmune encephalomyelitis is time- and score-dependent. Neurosci Lett. 2011;494:227–231. doi: 10.1016/j.neulet.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 5.Aranami T, Yamamura T. Th17 cells and autoimmune encephalomyelitis (EAE/MS) Allergol Int. 2008;57:115–120. doi: 10.2332/allergolint.R-07-159. [DOI] [PubMed] [Google Scholar]
- 6.Leipe J, Grunke M, Dechant C, et al. Th17 cells in autoimmune arthritis. Arthritis Rheum. 2010;62:2876–2885. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 7.Mu L, Sun B, Kong Q, et al. Disequilibrium of T helper type 1, 2 and 17 cells and regulatory T cells during the development of experimental autoimmune myasthenia gravis. Immunology. 2009;128(Suppl):e826–836. doi: 10.1111/j.1365-2567.2009.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayne CG, Spanier JA, Relland LM, Williams CB, Hayes CE. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur J Immunol. 2011;41:822–832. doi: 10.1002/eji.201040632. [DOI] [PubMed] [Google Scholar]
- 9.Axtell RC, de Jong BA, Boniface K, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang E, Cho ML, Oh HJ, Youn J. Deficiency of foxp3 regulatory T cells exacerbates autoimmune arthritis by altering the synovial proportions of CD4 T cells and dendritic cells. Immune Netw. 2011;11:299–306. doi: 10.4110/in.2011.11.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbott NJ, Ronnback L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 12.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwaninger M, Sallmann S, Petersen N, et al. Bradykinin induces interleukin-6 expression in astrocytes through activation of nuclear factor-kappaB. J Neurochem. 1999;73:1461–1466. doi: 10.1046/j.1471-4159.1999.0731461.x. [DOI] [PubMed] [Google Scholar]
- 14.Didier N, Romero IA, Creminon C, Wijkhuisen A, Grassi J, Mabondzo A. Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem. 2003;86:246–254. doi: 10.1046/j.1471-4159.2003.01829.x. [DOI] [PubMed] [Google Scholar]
- 15.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 16.Nair A, Frederick TJ, Miller SD. Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci. 2008;65:2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saikali P, Antel JP, Pittet CL, Newcombe J, Arbour N. Contribution of astrocyte-derived IL-15 to CD8 T cell effector functions in multiple sclerosis. J Immunol. 2010;185:5693–5703. doi: 10.4049/jimmunol.1002188. [DOI] [PubMed] [Google Scholar]
- 18.Argaw AT, Asp L, Zhang J, et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122:2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- 20.Meinl E, Aloisi F, Ertl B, et al. Multiple sclerosis. Immunomodulatory effects of human astrocytes on T cells. Brain. 1994;117(Pt 6):1323–1332. doi: 10.1093/brain/117.6.1323. [DOI] [PubMed] [Google Scholar]
- 21.Aloisi F, Penna G, Cerase J, Menendez Iglesias B, Adorini L. IL-12 production by central nervous system microglia is inhibited by astrocytes. J Immunol. 1997;159:1604–1612. [PubMed] [Google Scholar]
- 22.Aloisi F, Ria F, Adorini L. Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today. 2000;21:141–147. doi: 10.1016/s0167-5699(99)01512-1. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-alpha agonist fenofibrate regulates IL-12 family cytokine expression in the CNS: relevance to multiple sclerosis. J Neurochem. 2007;103:1801–1810. doi: 10.1111/j.1471-4159.2007.04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists suppress the production of IL-12 family cytokines by activated glia. J Immunol. 2007;178:1904–1913. doi: 10.4049/jimmunol.178.3.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 26.Owaki T, Asakawa M, Kamiya S, et al. IL-27 suppresses CD28-mediated [correction of medicated] IL-2 production through suppressor of cytokine signaling 3. J Immunol. 2006;176:2773–2780. doi: 10.4049/jimmunol.176.5.2773. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald DC, Ciric B, Touil T, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 28.Stumhofer JS, Laurence A, Wilson EH, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 29.Batten M, Li J, Yi S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 30.Guo X, Nakamura K, Kohyama K, et al. Inhibition of glial cell activation ameliorates the severity of experimental autoimmune encephalomyelitis. Neurosci Res. 2007;59:457–466. doi: 10.1016/j.neures.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Constantinescu CS, Tani M, Ransohoff RM, et al. Astrocytes as antigen-presenting cells: expression of IL-12/IL-23. J Neurochem. 2005;95:331–340. doi: 10.1111/j.1471-4159.2005.03368.x. [DOI] [PubMed] [Google Scholar]
- 32.Miljkovic D, Momcilovic M, Stojanovic I, Stosic-Grujicic S, Ramic Z, Mostarica-Stojkovic M. Astrocytes stimulate interleukin-17 and interferon-gamma production in vitro. J Neurosci Res. 2007;85:3598–3606. doi: 10.1002/jnr.21453. [DOI] [PubMed] [Google Scholar]
- 33.Kang Z, Altuntas CZ, Gulen MF, et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414–425. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang ZW, Wang P, Lin FH, et al. Early-life exposure to lipopolysaccharide reduces the severity of experimental autoimmune encephalomyelitis in adulthood and correlated with increased urine corticosterone and apoptotic CD4+ T cells. Neuroscience. 2011;193:283–290. doi: 10.1016/j.neuroscience.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Sonobe Y, Akahori T, et al. IL-9 promotes Th17 cell migration into the central nervous system via CC chemokine ligand-20 produced by astrocytes. J Immunol. 2011;186:4415–4421. doi: 10.4049/jimmunol.1003307. [DOI] [PubMed] [Google Scholar]
- 36.Fraczek LA, Martin CB, Martin BK. c-Jun and c-Fos regulate the complement factor H promoter in murine astrocytes. Mol Immunol. 2011;49:201–210. doi: 10.1016/j.molimm.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bender H, Wiesinger MY, Nordhoff C, et al. Interleukin-27 displays interferon-gamma-like functions in human hepatoma cells and hepatocytes. Hepatology. 2009;50:585–591. doi: 10.1002/hep.22988. [DOI] [PubMed] [Google Scholar]
- 38.Yang XO, Panopoulos AD, Nurieva R, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 39.Sonobe Y, Yawata I, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res. 2005;1040:202–207. doi: 10.1016/j.brainres.2005.01.100. [DOI] [PubMed] [Google Scholar]
- 40.Pai RK, Askew D, Boom WH, Harding CV. Regulation of class II MHC expression in APCs: roles of types I, III, and IV class II transactivator. J Immunol. 2002;169:1326–1333. doi: 10.4049/jimmunol.169.3.1326. [DOI] [PubMed] [Google Scholar]
- 41.Chastain EM, Duncan DS, Rodgers JM, Miller SD. The role of antigen presenting cells in multiple sclerosis. Biochim Biophys Acta. 2010;1812:265–274. doi: 10.1016/j.bbadis.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gimsa U, ØRen A, Pandiyan P, et al. Astrocytes protect the CNS: antigen-specific T helper cell responses are inhibited by astrocyte-induced upregulation of CTLA-4 (CD152) J Mol Med (Berl) 2004;82:364–372. doi: 10.1007/s00109-004-0531-6. [DOI] [PubMed] [Google Scholar]
- 43.Trajkovic V, Vuckovic O, Stosic-Grujicic S, et al. Astrocyte-induced regulatory T cells mitigate CNS autoimmunity. Glia. 2004;47:168–179. doi: 10.1002/glia.20046. [DOI] [PubMed] [Google Scholar]
- 44.Lee YS, Amadi-Obi A, Yu CR, Egwuagu CE. Retinal cells suppress intraocular inflammation (uveitis) through production of interleukin-27 and interleukin-10. Immunology. 2011;132:492–502. doi: 10.1111/j.1365-2567.2010.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Wang G, Sun B, et al. Interleukin-27 suppresses experimental autoimmune encephalomyelitis during bone marrow stromal cell treatment. J Autoimmun. 2008;30:222–229. doi: 10.1016/j.jaut.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Sweeney CM, Lonergan R, Basdeo SA, et al. IL-27 mediates the response to IFN-beta therapy in multiple sclerosis patients by inhibiting Th17 cells. Brain Behav Immun. 2011;25:1170–1181. doi: 10.1016/j.bbi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleinschnitz C, Meuth SG, Kieseier BC, Wiendl H. Immunotherapeutic approaches in MS: update on pathophysiology and emerging agents or strategies 2006. Endocr Metab Immune Disord Drug Targets. 2007;7:35–63. doi: 10.2174/187153007780059414. [DOI] [PubMed] [Google Scholar]
- 49.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 50.De Keyser J, Laureys G, Demol F, Wilczak N, Mostert J, Clinckers R. Astrocytes as potential targets to suppress inflammatory demyelinating lesions in multiple sclerosis. Neurochem Int. 2010;57:446–450. doi: 10.1016/j.neuint.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Sun D, Newman TA, Perry VH, Weller RO. Cytokine-induced enhancement of autoimmune inflammation in the brain and spinal cord: implications for multiple sclerosis. Neuropathol Appl Neurobiol. 2004;30:374–384. doi: 10.1111/j.1365-2990.2003.00546.x. [DOI] [PubMed] [Google Scholar]
- 52.Stoll G, Muller S, Schmidt B, et al. Localization of interferon-gamma and Ia-antigen in T cell line-mediated experimental autoimmune encephalomyelitis. Am J Pathol. 1993;142:1866–1875. [PMC free article] [PubMed] [Google Scholar]
- 53.Crocker SJ, Whitmire JK, Frausto RF, et al. Persistent macrophage/microglial activation and myelin disruption after experimental autoimmune encephalomyelitis in tissue inhibitor of metalloproteinase-1-deficient mice. Am J Pathol. 2006;169:2104–2116. doi: 10.2353/ajpath.2006.060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 55.Lee E, Chanamara S, Pleasure D, Soulika AM. IFN-gamma signaling in the central nervous system controls the course of experimental autoimmune encephalomyelitis independently of the localization and composition of inflammatory foci. J Neuroinflammation. 2012;9:7. doi: 10.1186/1742-2094-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merkler D, Boscke R, Schmelting B, et al. Differential macrophage/microglia activation in neocortical EAE lesions in the marmoset monkey. Brain Pathol. 2006;16:117–123. doi: 10.1111/j.1750-3639.2006.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


