Abstract
We aimed to study intestinal immune activation status in juvenile idiopathic arthritis (JIA) by assessing intestinal human leucocyte antigen (HLA) class II expression and the mRNA expression levels of the pro- and anti-inflammatory mediators and pattern recognition receptors. HLA-D-related (HLA-DR) expression was assessed using immunohistochemical staining of frozen sections in 11 children with JIA and 17 controls. The gene expression levels of the anti- and proinflammatory cytokines, lymphocyte recognition receptors and pattern recognition receptors were studied with reverse transcription–polymerase chain reaction (RT–PCR) in 14 children with JIA and 12 controls. All subjects had various gastrointestinal (GI) symptoms indicating endoscopic examinations, but eventually were not diagnosed with GI disease. In JIA patients, the expression of HLA-DR was increased in the crypt epithelial cells and in the epithelial basement membrane of the ileum when compared with the controls. Positive HLA-DR staining in the ileal mucosa was associated with the presence of high clinical disease activity of JIA and low mRNA expression of anti-inflammatory mediators, such as forkhead box protein P3 (FoxP3), glucocorticoid-induced tumour necrosis factor receptor-related protein (GITR) and transforming growth factor (TGF)-beta. Low ileal expression of interleukin (IL)-10, TGF-β, FoxP3, Toll-like receptor 2 (TLR-2) and TLR-4 transcripts correlated significantly with a high clinical disease activity in the JIA patients. The increased HLA-DR expression suggests enhanced intestinal antigen presentation in JIA. A correlation between clinical disease activity and low gene expression of tolerogenic mediators in the ileum supports the hypothesis that a link exists between the gut immune system and JIA.
Keywords: autoimmunity, MHC class II, mucosal immunity, regulatory T cells, rheumatology
Introduction
It is evident that alterations in intestinal immunology are associated with autoimmune diseases [1]. Inflammation in the ileum and colon has been documented in the group of patients with juvenile pauciarthritis; in a new classification, most of the patients would be classified as entesitis-related arthritis [2]. We have recently reported evidence for low-grade intestinal immune activation, such as increased expression of cytotoxic and γ/δ-positive intraepithelial lymphocytes and massive lymphonodular hyperplasia (LNH) in children with juvenile idiopathic arthritis (JIA) and gastrointestinal symptoms [3,4]. In addition, patients with JIA show functional intestinal changes, including increased intestinal permeability [5]. The relation of increased gut permeability and inflammation has also been reported in other autoimmune diseases, such as type 1 diabetes [6]. Inflammation may lead to increased permeability, and conversely increased gut permeability may lead to antigen leakage and an excess immune stimulation of the intestinal mucosa [7] seen, e.g. as high human leucocyte antigen D-related (HLA-DR) expression [8,9]. Also, massive LNH, a characteristic intestinal feature in JIA [3], may be considered to be a result of excessive antigen stimulation, as mucosal lymphoid follicles are the sites of antigen presentation [10].
There are several potential mechanisms for pathogenetic links between intestinal immune response and autoimmune diseases. Researchers have suggested that in type 1 diabetes and in rheumatoid arthritis, immune cells infiltrating the target tissues (Langerhans islets or synovia) originate from inflamed gut [11,12]. This has been explained by the expression of mucosa-associated homing receptors on infiltrating T cells [13,14]. There are also reports on JIA indicating that memory T cells activated in the gut-draining lymph nodes can localize intestinal antigens in synovia and orchestrate synovial inflammation [15,16].
In the current study, we have assessed the presence and mechanisms of intestinal immune activation in JIA. Observations suggesting abnormal intestinal permeability in JIA [5] point to a possible failure in the antigen-driven mucosal activation in JIA patients. Because mucosal epithelial HLA-DR expression is considered to be a marker of antigen-driven mucosal activation [9,17], we first assessed ileal HLA-DR expression in children with JIA and in control children. Our current observation of increased HLA-DR expression in the ileum in JIA prompted us to characterize the activation of mucosal antigen recognition mechanisms and the mechanism regulating inflammation and immunity. Therefore, we studied ileal mucosal samples for gene expression levels of pattern recognition receptors [Toll-like receptor (TLR)-2, TLR-4, TLR-5], proinflammatory [interferon (IFN)-γ, interleukin (IL)-17A, IL-18, IL-6, IL-4, IL-12p40, IL-23] and anti-inflammatory cytokines [IL-10, transforming growth factor (TGF)-β] and regulatory markers [forkhead box protein 3 (FoxP3), cytotoxic T lymphocyte antigen 4 (CTLA4), inducible T cell costimulator (ICOS) and glucocorticoid-induced tumour necrosis factor receptor-related protein (GITR)]. We also analysed the associations of mucosal immune activation markers and the clinical activity of JIA.
Methods
Patients and controls
The study included a total of 17 children with JIA and 28 control children without rheumatic disease or gastrointestinal disorders. Additionally, all 45 children included in the study had negative endoscopic examinations based on endoscopic examination and histological analyses of intestinal biopsies taken. Of patients with JIA, 13 were the same as in our earlier study [3]. Indications for endoscopy included abdominal pain (13 of 24 patients and 11 of 28 controls), diarrhoea (five patients and seven controls), constipation (one patient and two controls), melena (two patients and seven controls) and vomiting/dyspepsia (two patients and one control). The exclusion criteria for subjects (patients and controls) was a significant gastrointestinal disorder in the endoscopic or histological examination or in the follow-up (mean 8 years, range 2–10) or other autoimmune disease. One patient with JIA was excluded from the study because of active colitis and one control because of a nephrotic syndrome during follow-up. The determination of JIA type was confirmed by following the patients for several years, as per the International League of Associations for Rheumatology (ILAR) criteria [18]. Finally, for HLA-DR expression analysis in ileum, we studied 11 children with JIA (11 girls; mean age 11 years, range 5–16 years) and 17 children as controls (eight girls, mean age 12 years, range 3–17 years). For reverse transcription–quantitative polymerase chain reaction (RT–qPCR), 14 children with JIA (seven girls; mean age 9·6 years, range 2–16 years) and 12 children as controls (six girls, mean age 9·5 years, range 2–14 years) were analysed. Because of the lack of relevant samples, only 10 subjects were assessed for both mRNA and HLA-DR expression. The clinical characteristics of the patients, including the JIA subtype, are summarized in Table 1.
Table 1.
Clinical characteristics of the patients with juvenile idiopathic arthritis (JIA) in human leucocyte antigen D-related (HLA-DR) and reverse transcription–polymerase chain reaction (RT–PCR) analysis
| Analysis | HLA-DR | RT–PCR | |
|---|---|---|---|
| Number of patients with JIA in analysis | 11 | 14 | |
| JIA subtype* | Oligoarthritis | 5 | 6 |
| Polyarthritis | 2 | 4 | |
| Enthesitis-related arthritis | 2 | 2 | |
| Systemic arthritis | 2 | 2 | |
| HLA-B27 positivity | 4 | 4 | |
| Anti-nuclear antibody positivity (titre > 160) | 2 | 2 | |
| Disease remission on medication** | 5 | 6 | |
| Medication at the time of endoscopy | Only NSAID | 4 | 6 |
| DMARD/s | 7 | 8 | |
| + prednisolone | 3/7 | 3/8 | |
Endoscopic examination and samples
The biopsy specimens for routine analysis were taken from the terminal ileum, caecum, tranversal, descending and sigmoid colon and rectum during colonoscopy under general anaesthesia. Specimens were fixed in buffered formalin, embedded in paraffin, and sections were stained with haematoxylin and eosin. The assessment of presence and severity of LNH on the mucosa of the terminal ileum was based on an endoscopic view, as described previously [19,20]. Ileal samples were frozen with liquid nitrogen immediately after drawing and kept at −70°C until analysis. Histology criteria [21] (T.J.K.), HLA-DR expression (K.L, M.M.) and RT–PCR (H.S., M.V.) of the biopsies were analysed blinded for all clinical information and the subject group (M.A., S.T., P.V.). The ileal mucosal expression of HLA class II was studied using monoclonal antibody (mAb) HLA-DR (Becton Dickinson, Franklin Lakes, NJ, USA) in a dilution of 1:500 on the frozen section, as described previously, using a section thickness of 5 μm. The detection was carried out with Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) with alkaline phosphatase and fast red as a chromogen; the background was stained with haematoxylin [17,22]. HLA-DR staining was performed within a few months after specimens were taken and evaluated from five levels of the mucosa, namely villus epithelial cells, intercellular space in villus, lamina propria, cryptal basement membrane and cryptal cells (Fig. 1). DR expression was graded negative, or weakly, moderately or strongly positive (1+, 2+ or 3+) in villous epithelium and lamina propria. Strong villous epithelial expression of DR or positive expression in the crypt region was considered to be an enhanced expression of DR, and when the crypt region was negative and villous epithelial expression showed slight to a moderate staining, expression was considered normal.
Fig. 1.
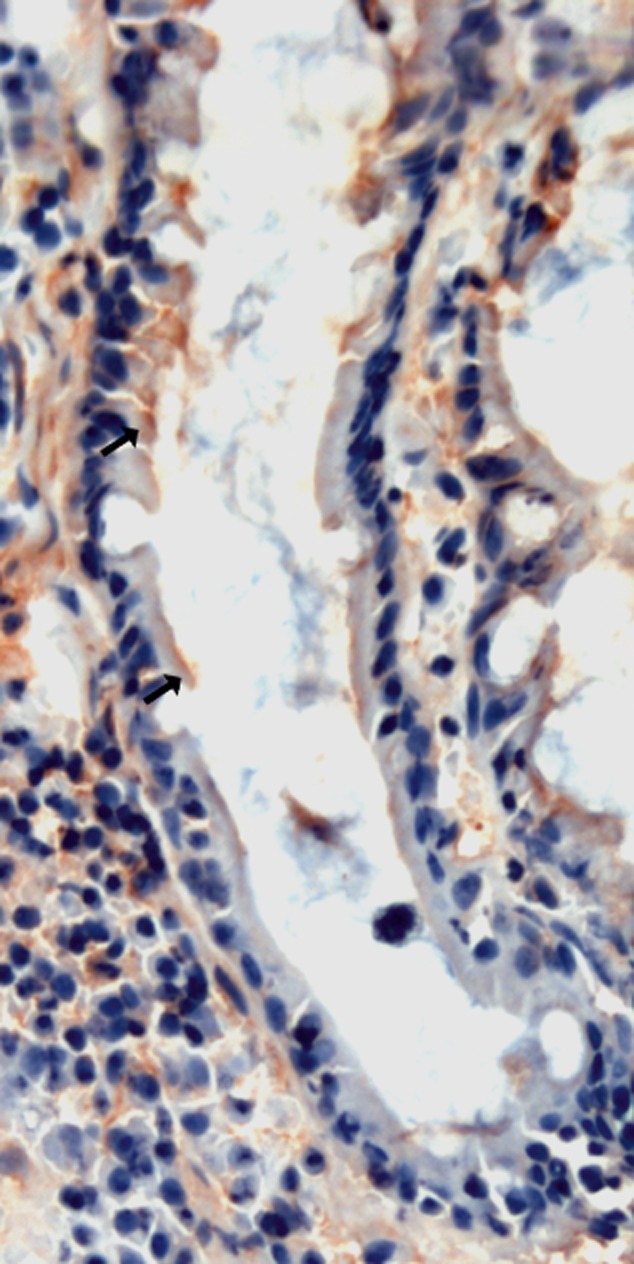
Expression of human leucocyte antigen D-related (HLA-DR) in cryptal epithelial cells and basement membrane in ileum in the patient. Red–brown immunoreaction (arrow) is present in cryptal epithelial cells.
For the assessment of mRNA expression biopsies were melted and homogenized immediately in lysis buffer (Sigma Chemical Co., St Louis, MO, USA) with a pestle. Total RNA was extracted with RNA Total Gen Elute miniprep kit (Sigma) and quantified with a spectrophotometer (Nanodrop, ND-1000). Reverse transcription was performed using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA, USA) with an additional treatment of 200 ng of total RNA with DNAseI (0·01U/l; Roche Diagnostics, Mannheim, Germany) in order to eliminate genomic DNA. Quantitative PCR was run with ABI-Prism 7700 Sequence Detection System (Applied Biosystems) using predesigned FAM-labelled TaqMan Gene Expression Assay reagents (Applied Biosystems) for IFN-γ (Hs00174143_m1), TGF-β (Hs00171257_m1), IL-6 (Hs00174131_m1), IL-4 (Hs00174122_m1), FoxP3 (Hs00203958_m1), IL-17A (Hs00174383_m1), CTLA4 (Hs00175480_m1), ICOS (Hs00359999_m1), GITR (Hs00188346_m1), IL-10 (Hs00174086_m1), IL-18 (Hs00155517_m1), IL-12p40 (Hs00233688_m1), IL-23 (Hs00413259_m1), TLR-2 (Hs00610101_m1), TLR-4 (Hs00152939_m1) and TLR-5 (Hs00152825_m1). Ribosomal 18 s RNA was used as an endogenous control (cat. no. Hs99999901_s1). The quantities of the target gene expression were calculated using a comparative threshold cycle (Ct) method (as recommended by Applied Biosystems). The amount of the target genes is expressed as relative units.
Clinical and laboratory observations
At the time of endoscopy, the disease activity of JIA was assessed retrospectively using the visual analogue scale (VAS 0–100) by a paediatric rheumatologist (P.V.) blinded for the information related to intestinal mucosal changes, including the results of HLA-DR and mRNA analyses. The clinical laboratory tests included serum rheumatoid factor, anti-nuclear antibodies, HLA-B27 serology and erythrocyte sedimentation rate (ESR). We classified the remission on medication in JIA patients according to Wallace criteria [23]: JIA is inactive when there are (1) no active joints, (2) no fever, rash, serositis, splenomegaly or generalized lymphadenopathy attributable to JIA, (3) no active uveitis, (4) normal ESR and/or CRP and (5) when physician's global assessment of disease activity indicates no disease activity. According to Wallace criteria, for clinical remission on medication at least 6 months of inactive disease is required.
Statistics
The data were analysed with spss version 19·1 (SPPS Inc., Chicago, IL, USA). Spearman's correlation, Pearson's χ2, Fisher's exact test, Student's t-test and Mann–Whitney U-test were used to estimate the significance of the difference between the groups, depending on the type of variable and number of cases in each subgroup. Non-parametric tests were used when the distribution was skewed in either group.
Ethical considerations
The protocol was approved by the Ethical Committee for Clinical Science of Oulu University Hospital.
Results
Small intestinal expression of HLA-DR, LNH and histological features in patients with JIA and controls
The positive HLA-DR expression in the ileum (Fig. 1) was found more often in patients than in controls on basement membrane (eight of 11 versus four of 16; P = 0·021, Fisher's exact test) and crypts (eight of 11 versus four of 17; P = 0·019, Fisher's exact test). There was no significant difference in the expression levels of HLA-DR in the villous epithelium or lamina propria (Mann–Whitney U-test) or the presence of expression in the intercellular spaces (patients, 10 of 11 versus controls, 11 of 17) (Pearson's χ2) between patients and controls. Gender and age did not correlate with HLA-DR expression levels.
Patients with positive HLA-DR expression in basement membrane or crypt (n = 8) cells had higher clinical disease activity of JIA than patients with negative HLA-DR expression (n = 3) assessed by a VAS (mean activity 26 versus 3; P = 0·02; Student's t-test) and a trend towards a higher number of clinically actively inflamed joints (4 versus 0, P = 0·06; Student's t-test;) and higher ESR (38 versus 7; P = 0·07; Student's t-test).
LNH in the ileum was more prevalent in JIA (12 of 17) than in the controls (five of 20, P = 0·006, Pearson's χ2) [3]. Interestingly, LNH in the ileum tended to be more prevalent in the subjects (controls or patients) with positive HLA-DR expression in the basement membrane (eight of 10) compared to those with negative HLA-DR expression (four of 11, P = 0·080, Fisher's exact test). Apart from LNH, there were no differences in the endoscopic or histopathological features between patients and controls (data not shown).
Ileal mRNA expression of immunological mediators
There were no significant differences in mRNA expression levels of the studied genes between the patients and the controls (Table 2). Neither gender nor age correlated with the mRNA expression levels, except for the transcripts of IL-10, which correlated negatively with age in the controls (Spearman's correlation, r = −0·63, P = 0·040).
Table 2.
The expression levels of the target gene mRNA in the ileal mucosa as relative units in the patients with juvenile idiopathic arthritis (JIA) and in controls
| Patients | Controls | ||||||
|---|---|---|---|---|---|---|---|
| Target gene | n | Median | Range | n | Median | Range | P |
| Proinflammatory | |||||||
| IFN-γ | 8 | 4 | 0–19 | 5 | 0 | 0–13 | 0·55 |
| IL-4 | 8 | 6 | 6–10 | 5 | 6 | 6–26 | 0·47 |
| IL-6 | 8 | 186 | 1–415 | 5 | 5 | 1–208 | 0·27 |
| IL-18 | 8 | 69 | 20–403 | 5 | 48 | 8–480 | 0·66 |
| IL-23 | 10 | 556 | 5–947 | 12 | 713 | 18–2271 | 0·29 |
| IL-17 | 10 | 28 | 28–576 | 12 | 28 | 28–329 | 0·47 |
| Regulatory proteins | |||||||
| FoxP3 | 11 | 189 | 10–882 | 13 | 206 | 4–1360 | 0·51 |
| CTLA4 | 8 | 202 | 0–651 | 5 | 7 | 0–710 | 0·83 |
| ICOS | 8 | 111 | 0–299 | 5 | 4 | 2–398 | 0·88 |
| GITR | 8 | 23 | 2–110 | 5 | 37 | 5–143 | 0·56 |
| Anti-inflammatory | |||||||
| TGF-β | 12 | 46 | 0–131 | 13 | 53 | 3–223 | 0·45 |
| IL-10 | 12 | 9 | 0–47 | 12 | 24 | 0–70 | 0·20 |
| Pattern recognition | |||||||
| TLR-2 | 7 | 29 | 8–147 | 5 | 7 | 2–78 | 0·37 |
| TLR-4 | 12 | 76 | 5–187 | 12 | 144 | 2–491 | 0·36 |
| TLR-5 | 7 | 733 | 3–1714 | 5 | 109 | 24–1966 | 0·94 |
Groups were compared with Mann–Whitney U-test. GITR: glucocorticoid-induced tumour necrosis factor receptor-related protein; ICOS: inducible T cell costimulator; IFN: interferon; IL: interleukin; FoxP3: forkhead box protein 3; TGF: transforming growth factor; TLR: Toll-like receptor.
Low mRNA expression levels of IL-10, TGF-β, and FoxP3 in the ileum were associated with the high clinical disease activity of JIA, as assessed by a VAS, and the number of active joints (Table 3). Similarly, low mRNA expression of TLR-4, TLR-2 and IL-18 correlated with high clinical disease activity of JIA. Clinical remission of JIA during the colonoscopy (n = 4) associated with a higher mRNA expression of IL-10 in the ileum than active disease (n = 8; 24·1 versus 7·8, P = 0·048; Mann–Whitney U-test). A similar association was seen for mRNA of TGF-β (94·4 versus 38·1; P = 0·048) and TLR-4 (133·8 versus 47·8, P = 0·028). Significant correlations were present even if two patients with the JIA subtype of enthesitis-related arthritis were not included. The presence of LNH [3] did not effect the mRNA levels of all transcripts tested in either the patients or controls. In contrast, IL-17 or IL-23 mRNA levels did not show any correlation with features of JIA (Table 2).
Table 3.
Correlation (Spearman's) between the expression of target gene expression levels and the disease activity of juvenile idiopathic arthritis (JIA) as assessed by the clinician's visual analogue scale (VAS) or the number of active joints
| Activity of JIA (VAS) | No. of active joints | ||||
|---|---|---|---|---|---|
| Target gene | n | r | P | r | P |
| Proinflammatory | |||||
| IL-18 | 8 | −0·87 | 0·005 | −0·67 | 0·048 |
| Regulatory protein | |||||
| FoxP3 | 12 | −0·72 | 0·0012 | −0·574 | 0·065 |
| Anti-inflammatory | |||||
| IL-10 | 12 | −0·82 | <0·001 | −0·696 | 0·012 |
| TGF-β | 12 | −0·74 | 0·006 | −0·724 | 0·011 |
| Pattern recognition | |||||
| TLR-4 | 12 | −0·82 | <0·001 | −0·771 | 0·003 |
| TLR-2 | 7 | −0·88 | 0·008 | −0·711 | 0·073 |
r: Spearman's correlation coefficient; IL: interleukin; FoxP3: forkhead box protein 3; TGF: transforming growth factor; TLR: Toll-like receptor.
To investigate the association between TLR signalling and mediators of tolerance in patients with JIA, we evaluated correlations between mRNA levels of pattern recognition receptors (TLR-2, TLR-4) and tolerogenic mediators (FoxP3, IL-10, TGF-β). TLR-2 mRNA correlated significantly with both FoxP3 mRNA (r = 0·71, P < 0·047, n = 8; Spearman's correlation) and TGF-β mRNA levels (c = 0·79, P = 0·021, n = 8). Similarly, TLR-4 mRNA correlated with FoxP3 (c = 0·853, P < 0·001, n = 12), IL-10 (r = 0·90, P < 0·001, n = 13) and TGF-β mRNA levels (r = 0·87, P < 0·001, n = 10).
Discussion
In the present study, we have demonstrated signs of increased mucosal immune activation in the ileal mucosa of JIA patients. Activation was characterized by aberrant expression of HLA-DR in the basement membrane region and in the crypts. We observed a strong correlation between clinical disease activity of JIA and expression of the mucosal HLA-DR. Furthermore, the high clinical disease activity of JIA associated with down-regulation of mRNA levels for tolerogenic mediators (FoxP3, IL-10, TGF-β). Somewhat unexpectedly, the low expression of pattern recognition receptors (TLR-2, TLR-4) transcripts was associated similarly with active disease. These findings suggest that the excessive antigen stimulation and deficient activation of tolerogenic mechanisms in the ileal mucosa may play a role in modification of the activity of arthritis in JIA. Further studies are necessary to confirm these observations, even in patients with JIA without gastrointestinal symptoms. However, although our patients with JIA presented gastrointestinal symptoms requiring endoscopic investigations, none of them had any histopathological abnormality. This suggests that pathophysiologically important mucosal immune aberration does not necessarily manifest as any structural mucosal abnormality.
In the normal enterocytes of the ileum, HLA-DR is expressed mainly in the brush border of the villous epithelium, and there is no significant expression in crypt epithelial cells or epithelial basement membrane of the crypts [24]. In agreement with this concept, there was no difference in the expression of HLA-DR in the villus epithelial cells in our study. The significance of HLA-DR expression in intercellular spaces or the lamina propria has not been documented. As we did not see any differences between patients and controls in the expression of HLA-DR in these locations, we consider the expression to be physiological. In contrast, HLA-DR expression in the crypts of small intestinal mucosa is considered to be pathological, as it has been documented in children with untreated food allergy [17], coeliac disease or autoimmune enteropathy [25], in patients with Crohn's disease [26] and patients with spondyloarthropathy [27]. Besides being a marker of immunological activation in JIA, aberrant mucosal HLA-DR expression in the intestinal mucosa may contribute to the failure in intestinal tolerance mechanisms because excessive antigen/HLA-DR interaction with CD4+ T cells leads to hyporesponsiveness of CD4+ T cells to tolerogenic signals [28]. IL-10 and TGF-β have been shown to inhibit the antigen-specific proliferative response of T cells in the intestinal mucosa, diminishing the expression of antigen presentation-associated molecules such as major histocompatibility complex class II in intestinal epithelial cells [29,30].
In the present study, ileal LNH was more prevalent in the patients with JIA than controls [3], and LNH tended to be more prevalent in subjects with positive HLA-DR expression in the basement membrane. Our findings of LNH and the up-regulation of HLA-DR in JIA may be consequences of the increased intestinal permeability, which has also been reported in JIA [5]. We have reported previously [3] that the presence of massive LNH in any part of the GI tract is associated with the high disease activity of JIA as assessed by clinician's VAS or ESR [3]. LNH has been associated with food allergy and is considered to reflect antigen-driven mucosal immune activation [20]. We speculate that increased permeability could allow leakage of excess luminal antigens, food or microbiota-derived antigens, which contribute to the abnormal activation of antigen presentation in the intestinal mucosa in JIA, and thus cause a simultaneous increase in the presence of LNH and HLA-DR.
A low mRNA expression of pattern recognition receptors (TLR-2, TLR-4) and regulatory transmitters (FoxP3, IL-10, TGF-β) in the ileum was associated with active disease in JIA, and their mRNA levels showed a significant positive correlation. These findings suggest that activation of TLR signalling and regulatory mechanism in the ileal mucosa have a functional association, and that impaired activation contributes to the clinical activity of JIA. In a recent report, specific bacterial species of the normal flora induced FoxP3+ regulatory T cells through TLR-2 signalling and promoted immunological intestinal tolerance in mice [31]. Collectively, these findings support the idea that pattern recognition receptors and anti-inflammatory cytokines form an important network in the maintenance of the mucosal tolerance [31].
Our present findings of intestinal mucosa in JIA patients should be taken into account in the context of mucosal immunomodulatory therapies in JIA. Immunomodulation with mucosally administered heat shock protein (HSP) peptides can induce systemically beneficial T cell responses in rheumatoid arthritis [32], and also potentially in JIA [33,34]. However, pre-existent mucosal inflammation may modify the response to immunomodulation into an unwanted, proinflammatory direction [35]. Accordingly, although collective evidence favours the therapeutic potential of mucosal immunomodulatory therapies in JIA, it seems important to characterize mucosal factors affecting the response to immunomodulatory peptides before clinical trials. In particular, this is underscored by our finding of low TLR mRNA levels in active JIA, as a low mucosal TLR expression may result in the insufficient induction of the HSP-specific T cells [36].
In patients with high clinical activity of JIA in the intestinal mucosa, we observed low mRNA levels of regulatory markers (FoxP3, IL-10, TGF-β) and high expression of cryptal HLA-DR. These associations support the importance of the gut–joint axis in the pathophysiology of JIA. Although the mechanisms linking gut and joint remain speculative, there are some interesting observations to be reintroduced. An unbalanced or excessive immune stimulation of intestinal mucosa is suspected to induce abnormal heterogeneous expression of adhesion molecules in mucosal T lymphocytes [37,38], allowing these cells to also home into the joints [38]. Furthermore, lymphoproliferative responses induced by intestinal pathogens have been detected in enthesitis-related arthritis, a subtype of JIA [16]. In our study, association of intestinal immune activation markers and JIA activity was present even if cases with enthesitis-related arthritis were excluded, also supporting the importance of intestinal immune activations status in other types of JIA.
In summary, we have demonstrated aberrant ileal HLA-DR expression in patients with JIA linked to the clinical activity of JIA. Moreover, the low expression of intestinal regulatory markers, tolerogenic mediators (FoxP3, IL-10, TGF-β) and pattern recognition receptors (TLR-2, TLR-4) in the ileal mucosa correlated with the clinical activity of JIA. However, more extensive case series would be necessary to confirm these findings and to investigate the association of intestinal changes with the subtypes of JIA. Nevertheless, our findings favour the idea of a link between intestinal alterations and the pathogenesis of JIA [39], and even application of intestinal immunomodulatory therapies [40] in JIA.
Acknowledgments
This study was largely initiated by paediatric gastroenterologist Dr Jorma Kokkonen, who conducted most of the endoscopies. Dr Kokkonen died in 2005. Mrs Anneli Suomela, Mrs Mirja Vahera, Mrs Erja Tomperi, Mrs Mervi Himanka and Mr Hannu Wäänänen are acknowledged for their technical help. This work was supported by Maire Lisko Foundation and grant from Oulu University Hospital, Finland.
Disclosure
Authors have no competing interests.
References
- 1.Vaarala O, Atkinson MA, Neu J. The ‘perfect storm’ for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–2562. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mielants H, Veys EM, Cuvelier C, et al. Gut inflammation in children with late onset pauciarticular juvenile chronic arthritis and evolution to adult spondyloarthropathy – a prospective study. J Rheumatol. 1993;20:1567–1572. [PubMed] [Google Scholar]
- 3.Kokkonen J, Arvonen M, Vahasalo P, Karttunen TJ. Intestinal immune activation in juvenile idiopathic arthritis and connective tissue disease. Scand J Rheumatol. 2007;36:386–389. doi: 10.1080/03009740701394005. [DOI] [PubMed] [Google Scholar]
- 4.Arvonen M, Ikni L, Augustin M, Karttunen TJ, Vahasalo P. Increase of duodenal and ileal mucosal cytotoxic lymphocytes in juvenile idiopathic arthritis. Clin Exp Rheumatol. 2010;28:128–134. [PubMed] [Google Scholar]
- 5.Picco P, Gattorno M, Marchese N, et al. Increased gut permeability in juvenile chronic arthritides. A multivariate analysis of the diagnostic parameters. Clin Exp Rheumatol. 2000;18:773–778. [PubMed] [Google Scholar]
- 6.Vaarala O. The gut as a regulator of early inflammation in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2011;18:241–247. doi: 10.1097/MED.0b013e3283488218. [DOI] [PubMed] [Google Scholar]
- 7.Vaarala O. Leaking gut in type 1 diabetes. Curr Opin Gastroenterol. 2008;24:701–706. doi: 10.1097/MOG.0b013e32830e6d98. [DOI] [PubMed] [Google Scholar]
- 8.Savilahti E, Ormala T, Saukkonen T, et al. Jejuna of patients with insulin-dependent diabetes mellitus (IDDM) show signs of immune activation. Clin Exp Immunol. 1999;116:70–77. doi: 10.1046/j.1365-2249.1999.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westerholm-Ormio M, Vaarala O, Pihkala P, Ilonen J, Savilahti E. Immunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes. 2003;52:2287–2295. doi: 10.2337/diabetes.52.9.2287. [DOI] [PubMed] [Google Scholar]
- 10.Fujimura Y, Hosobe M, Kihara T. Ultrastructural study of M cells from colonic lymphoid nodules obtained by colonoscopic biopsy. Dig Dis Sci. 1992;37:1089–1098. doi: 10.1007/BF01300292. [DOI] [PubMed] [Google Scholar]
- 11.Turley SJ, Lee JW, Dutton-Swain N, Mathis D, Benoist C. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci USA. 2005;102:17729–17733. doi: 10.1073/pnas.0509006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandtzaeg P. Review article: homing of mucosal immune cells – a possible connection between intestinal and articular inflammation. Aliment Pharmacol Ther. 1997;11(Suppl. 3):24–37. doi: 10.1111/j.1365-2036.1997.tb00806.x. [DOI] [PubMed] [Google Scholar]
- 13.Schmutz C, Cartwright A, Williams H, et al. Monocytes/macrophages express chemokine receptor CCR9 in rheumatoid arthritis and CCL25 stimulates their differentiation. Arthritis Res Ther. 2010;12:R161. doi: 10.1186/ar3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu B, Cook RE, Michie SA. Alpha4beta7 integrin/MAdCAM-1 adhesion pathway is crucial for B cell migration into pancreatic lymph nodes in nonobese diabetic mice. J Autoimmun. 2010;35:124–129. doi: 10.1016/j.jaut.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black AP, Bhayani H, Ryder CA, Pugh MT, Gardner-Medwin JM, Southwood TR. An association between the acute phase response and patterns of antigen induced T cell proliferation in juvenile idiopathic arthritis. Arthritis Res Ther. 2003;5:R277–284. doi: 10.1186/ar791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena N, Misra R, Aggarwal A. Is the enthesitis-related arthritis subtype of juvenile idiopathic arthritis a form of chronic reactive arthritis? Rheumatology (Oxf) 2006;45:1129–1132. doi: 10.1093/rheumatology/kel056. [DOI] [PubMed] [Google Scholar]
- 17.Kokkonen J, Holm K, Karttunen TJ, Maki M. Children with untreated food allergy express a relative increment in the density of duodenal gammadelta+ T cells. Scand J Gastroenterol. 2000;35:1137–1142. doi: 10.1080/003655200750056592. [DOI] [PubMed] [Google Scholar]
- 18.Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 19.Kokkonen J. Lymphoid nodular hyperplasia. In: Guandalini S, editor. Textbook of pediatric gastroenterology and nutrition. London: Taylor & Francis; 2004. pp. 479–488. [Google Scholar]
- 20.Kokkonen J, Ruuska T, Karttunen TJ, Maki M. Lymphonodular hyperplasia of the terminal ileum associated with colitis shows an increase gammadelta+ T-cell density in children. Am J Gastroenterol. 2002;97:667–672. doi: 10.1111/j.1572-0241.2002.05547.x. [DOI] [PubMed] [Google Scholar]
- 21.Turunen S, Karttunen TJ, Kokkonen J. Lymphoid nodular hyperplasia and cow's milk hypersensitivity in children with chronic constipation. J Pediatr. 2004;145:606–611. doi: 10.1016/j.jpeds.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 22.Holm K, Savilahti E, Koskimies S, Lipsanen V, Maki M. Immunohistochemical changes in the jejunum in first degree relatives of patients with coeliac disease and the coeliac disease marker DQ genes. HLA class II antigen expression, interleukin-2 receptor positive cells and dividing crypt cells. Gut. 1994;35:55–60. doi: 10.1136/gut.35.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace CA, Ruperto N, Giannini E Childhood Arthritis and Rheumatology Research Alliance, Pediatric Rheumatology International Trials Organization, Pediatric Rheumatology Collaborative Study Group. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290–2294. [PubMed] [Google Scholar]
- 24.Scott H, Solheim BG, Brandtzaeg P, Thorsby E. HLA-DR-like antigens in the epithelium of the human small intestine. Scand J Immunol. 1980;12:77–82. doi: 10.1111/j.1365-3083.1980.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 25.Mirakian R, Hill S, Richardson A, Milla PJ, Walker-Smith JA, Bottazzo GF. HLA product expression and lymphocyte subpopulations in jejunum biopsies of children with idiopathic protracted diarrhoea and enterocyte autoantibodies. J Autoimmun. 1988;1:263–277. doi: 10.1016/0896-8411(88)90032-7. [DOI] [PubMed] [Google Scholar]
- 26.Hirata I, Austin LL, Blackwell WH, Weber JR, Dobbins WO., 3rd Immunoelectron microscopic localization of HLA-DR antigen in control small intestine and colon and in inflammatory bowel disease. Dig Dis Sci. 1986;31:1317–1330. doi: 10.1007/BF01299810. [DOI] [PubMed] [Google Scholar]
- 27.Cuvelier C, Mielants H, De Vos M, Veys E, Roels H. Major histocompatibility complex class II antigen (HLA-DR) expression by ileal epithelial cells in patients with seronegative spondylarthropathy. Gut. 1990;31:545–549. doi: 10.1136/gut.31.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD4(+)CD25(+high) regulatory T cell function. J Immunol. 2002;169:6210–6217. doi: 10.4049/jimmunol.169.11.6210. [DOI] [PubMed] [Google Scholar]
- 29.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donnet-Hughes A, Schiffrin EJ, Huggett AC. Expression of MHC antigens by intestinal epithelial cells. Effect of transforming growth factor-beta 2 (TGF-beta 2) Clin Exp Immunol. 1995;99:240–244. doi: 10.1111/j.1365-2249.1995.tb05539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Round JL, Lee SM, Li J, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koffeman EC, Genovese M, Amox D, et al. Epitope-specific immunotherapy of rheumatoid arthritis: clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double-blind, placebo-controlled, pilot phase II trial. Arthritis Rheum. 2009;60:3207–3216. doi: 10.1002/art.24916. [DOI] [PubMed] [Google Scholar]
- 33.de Kleer IM, Wedderburn LR, Taams LS, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172:6435–6443. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- 34.Kamphuis S, Albani S, Prakken BJ. Heat-shock protein 60 as a tool for novel therapeutic strategies that target the induction of regulatory T cells in human arthritis. Expert Opin Biol Ther. 2006;6:579–589. doi: 10.1517/14712598.6.6.579. [DOI] [PubMed] [Google Scholar]
- 35.Puga Yung GL, Fidler M, Albani E, et al. Heat shock protein-derived T-cell epitopes contribute to autoimmune inflammation in pediatric Crohn's disease. PLoS ONE. 2009;4:e7714. doi: 10.1371/journal.pone.0007714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vercoulen Y, van Teijlingen NH, de Kleer IM, Kamphuis S, Albani S, Prakken BJ. Heat shock protein 60 reactive T cells in juvenile idiopathic arthritis: what is new? Arthritis Res Ther. 2009;11:231. doi: 10.1186/ar2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salmi M, Jalkanen S. Endothelial ligands and homing of mucosal leukocytes in extraintestinal manifestations of IBD. Inflamm Bowel Dis. 1998;4:149–156. doi: 10.1002/ibd.3780040210. [DOI] [PubMed] [Google Scholar]
- 38.Fantini MC, Pallone F, Monteleone G. Common immunologic mechanisms in inflammatory bowel disease and spondylarthropathies. World J Gastroenterol. 2009;15:2472–2478. doi: 10.3748/wjg.15.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu HJ, Ivanov II, Darce J, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albani S, Koffeman EC, Prakken B. Induction of immune tolerance in the treatment of rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:272–281. doi: 10.1038/nrrheum.2011.36. [DOI] [PubMed] [Google Scholar]


