Abstract
Successful embryo implantation occurs followed by a local inflammatory/T helper type 1 (Th1) response, subsequently redirected towards a tolerogenic predominant profile. The lack of control of this initial local inflammatory response may be an underlying cause of early pregnancy complications as recurrent spontaneous abortions (RSA). Considering that vasoactive intestinal peptide (VIP) mediates anti-inflammatory and tolerogenic effects in several conditions we hypothesized that VIP might contribute to tolerance towards trophoblast antigens during the early interaction of maternal leucocytes and trophoblast cells. In this study we investigated VIP/VPAC system activity and expression on maternal peripheral blood mononuclear cells (PBMCs) after interaction with immortalized trophoblast cells (Swan-71 cell line) as an in-vitro model of feto–maternal interaction, and we analysed whether it modulates maternal regulatory T cell (Treg)/Th1 responses. We also investigated the contribution of the endogenous VIP/VPAC system to RSA pathogenesis. VIP decreased T-bet expression significantly, reduced monocyte chemotactic protein-1 (MCP-1) and nitrite production in co-cultures of PBMCs from fertile women with trophoblast cells; while it increased the frequency of CD4+CD25+ forkhead box protein 3 (Foxp3)+ cells, transforming growth factor (TGF)-β expression and interleukin (IL)-10 secretion. These effects were prevented by VIP-specific antagonist. Interestingly, PBMCs from RSA patients displayed significantly higher T-bet expression, lower Treg frequency and lower frequency of VIP-producer CD4 lymphocytes after the interaction with trophoblast cells. Moreover, the patients displayed a significantly lower frequency of endometrial CD4+VIP+ cells in comparison with fertile women. VIP showed a Th1-limiting and Treg-promoting response in vitro that would favour early pregnancy outcome. Because RSA patients displayed defects in the VIP/VPAC system, this neuropeptide could be a promising candidate for diagnostic biomarker or surrogate biomarker for recurrent spontaneous abortions.
Keywords: maternal tolerance, recurrent spontaneous abortions, VIP
Introduction
The appropriate generation of a proinflammatory response is thought to be a prerequisite for successful implantation [1,2]. During the first stage, the embryo has to break through the epithelial lining of the uterus to implant, damage the endometrial tissue to invade and replace the endothelium and vascular smooth muscle of the maternal blood vessels. Hence, implantation and placentation in the first trimester of pregnancy require a controlled inflammatory response that will be physiologically limited in their extent and duration by several regulatory and tolerogenic mechanisms [3–5].
Consistent with the need for strict control of the initial local inflammatory profile, enhanced leucocyte infiltration or inappropriate activation may be an underlying cause of pregnancy complications such as recurrent spontaneous abortions (RSA) and implantation failures. An exacerbated inflammatory/T helper type 1 (Th1) response appears to be ultimately responsible for tissue damage and embryo resorption in these conditions [6–8].
Evidence of several regulatory immune mechanisms at the feto–maternal interface involving both the innate and the adaptative response have provided a deeper comprehension of local cross-talk. In particular, the specialized regulatory T cell (Treg) population, essential for maternal tolerance of the conceptus, is stimulated through antigen-specific and antigen non-specific pathways, thus exerting suppressive action in the critical peri-implantation phase of pregnancy [5]. A major role of Treg cells has broadened the classical paradigm of Th1/Th2 to a new overview that can be verified in normal pregnancies, as well as in complicated pregnancies such as RSA [9]. Several leucocyte populations are found at the site of implantation, including T cell subpopulations, uterine natural killer cells, ‘educated’ macrophages and dendritic cells. Also, mediators such as cytokines, chemokines, galectin-1 and neurotransmitters, collectively named BIEFs (blastocyst implantation essential factors), contribute to regulation of this network [10–13].
An emerging topic in the feto–maternal tolerance is the role of neuropeptides, particularly VIP (vasoactive intestinal peptide), as it not only mediates immune and nervous effector functions but is also produced by trophoblast cells, and displays trophic effects in an autocrine manner [14]. In the immune system, it has emerged as a potential effective treatment for inflammatory and autoimmune disorders based on its anti-inflammatory and tolerogenic effects; it down-regulates inflammatory factors and inhibits antigen-specific Th1-driven immune responses, switching the Th1/Th2 balance to Th2 immunity and inducing the generation or expansion of the population of Treg cells [15,16]. As a neurotransmitter, VIP has potent prosecretory and vasodilating effects [17,18]. In addition to its neural and immune regulatory properties, VIP participates in the maternal regulation of embryonic growth in murine pregnancy [19].
In the feto–maternal context, we have shown recently that VIP is present in viable implantation sites of normal mice, where it induces Treg cells [20]. In line with this, lower levels of VIP and forkhead box protein P3 (FoxP3) were found in viable implantation sites of prediabetic non-obese diabetic (NOD) mice, characterized by a Th1 systemic cytokine profile, correlating with a reduction in litter size from 16 weeks of age and increased resorption rates [20]. Interestingly, functional VIP receptors VPAC1 and VPAC2 were expressed at the implantation sites from pregnant BALBc and NOD mice, and a significant increase of FoxP3 expression was induced by VIP in both strains.
Because control of the initial inflammatory response after embryo implantation appears to be crucial for a successful outcome, and considering that VIP mediates anti-inflammatory and tolerogenic immune effects, we hypothesized that VIP may contribute to maternal tolerance towards trophoblast antigens during the early interaction of leucocyte and trophoblast cells. In the present work we investigated the VIP/VPAC system and whether it modulates the maternal Treg/Th1 responses in an in-vitro model of the local interaction between trophoblast cells and maternal leucocytes. We also investigated the putative contribution of the endogenous VIP/VPAC system to the pathogenesis of pregnancy complications associated with recurrent spontaneous abortions.
Material and methods
Patients
Recurrent spontaneous abortion (RSA) patients were defined as women with a history of three or more consecutive pregnancy losses before week 12 of gestation after excluding any infectious, endocrine or anatomic disease that might have caused the abortion (mean age 33·4 years, range 28–42 years, n = 18). Criteria for exclusion were performed following the Clinical Guidelines Recommendation Committee for Diagnosis and Treatment of Recurrent Spontaneous Abortion performed by the American Society for Reproductive Immunology [21]: (i) the presence of any autoimmunity factors, (ii) the presence of any bacterial or viral infection and (iii) genetic causes such as parental balanced chromosomal translocations.
Control fertile women were defined as non-pregnant women who had had two or more previous normal pregnancies without any miscarriage (mean age 32·6 years range 26–42 years, n = 18). The Investigation and Ethics Committee from the Argentinean Society of Gynecological and Reproductive Endocrinology (SAEGRE) has approved this study and all patients provided their written consent to participate.
Peripheral blood mononuclear cells (PBMCs)
PBMCs from RSA patients and fertile women were isolated from heparinized peripheral blood by density gradient centrifugation on Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden) between days 17 and 26 from the first day of the last regular menstrual period. Cells were washed extensively and resuspended in RPMI-1640 (Life Technologies, Grand Island, NY, USA), supplemented with 10% human serum, glutamine and penicillin–streptomycin.
Endometrial leucocyte isolation
Endometrial samples were obtained between days 17 and 26 (mean 21·6 days) from the first day of the last menstrual period in women with regular, 28-day cycles. To confirm timing in the mid-luteal phase of the menstrual cycle, peripheral blood was obtained from all subjects at the time of endometrial biopsy for measurement of serum oestrogen and progesterone levels. Endometrial samples were obtained using a Novac curette and disrupted mechanically with a tissue homogenizer. The recovered cells were resuspended in RPMI-1640 medium (Life Technologies) supplemented with 10% human serum, 2 mM L-glutamine, 100 U/ml penicillin and 100 U/ml streptomycin. Total endometrial cells were analysed by flow cytometry. The Investigation and Ethics Committee from the Argentinean Society of Gynecological and Reproductive Endocrinology (SAEGRE) approved this study and all patients provided an additional written consent to participate.
Co-cultures
Trophoblast cells (Swan-71 cell line, derived by telomerase-mediated transformation of a 7-week human cytotrophoblast isolate, described by Straszewski-Chavez) [22,23] were cultured in 24-well flat-bottomed polystyrene plates (Becton Dickinson, Franklin Lakes, NJ, USA) in complete Dulbecco's modified Eagle's medium (DMEM) 10% fetal calf serum (FCS) (Gibco, Invitrogen, Buenos Aires, Argentina).
For co-cultures, trophoblast cells at 70% of confluence (2 × 105 cells/well) were cultured in the absence/presence of PBMCs from RSA patients or from fertile women (5 × 105 cells/well) with or without VIP (10−7 M), and VIP antagonist (Peninsula-Bachem Inc., San Carlos, CA, USA; 10−6 M) in several combinations. This peptide, a hybrid of neurotensin (6–11) and VIP (7–28), is a competitive antagonist of VIP receptors [24,25]. After 48 h of culture, supernatants were collected for enzyme-linked immunosorbent assay (ELISA) determinations and maternal PBMCs were recovered and then used for flow cytometry or Western blot analysis.
Cytokine and chemokine quantification
Interleukin (IL)-10 and monocyte chemotactic protein-1 (MCP-1) were assayed by ELISA in supernatant collected from the co-cultures performed in the presence of RSA PBMCs or fertile PBMCs during 48 h. The ELISA test was performed according to the manufacturer's instructions (Becton Dickinson for IL-10 and R&D Systems, Minneapolis, MN, USA for MCP-1 quantification). Results were expressed in ng/ml.
Nitrite determination
Nitrite concentration was determined in supernatants obtained as described above for cytokine and chemokine measurements using the Griess method with N-(1-naphthyl) 9 ethylenediamine dihydrochloride (NEDA) and sulphanilamide [26]. Results were expressed as μmol/l of nitrites synthesized during 48 h in the co-cultures performed in the presence of RSA PBMCs or fertile PBMCs.
Western blot assays
Co-culture recovered cells were analysed by Western blot for FoxP3, transforming growth factor (TGF)-β, and T-bet expression. Cells were washed extensively with phosphate-buffered saline (PBS), then the cell pellet was mixed gently with 1 ml ice-cold lysis buffer [PBS containing 5 mM ethylenediamine tetraacetic acid (EDTA), 1% NP-40, 0·5% sodium deoxycholate, 0·1% sodium dodecyl sulphate (SDS), 142·5 mM KCl, 5 mM MgCl2, 10 mM HEPES, pH 7·2] with freshly added protease inhibitor cocktail [0·2 mM phenylmethanesulphonyl fluoride (PMSF), 0·1% aprotinin, 0·7 μg/ml pepstatin and 1 μg/ml leupeptin] and incubated for 1 h on ice. Samples were finally centrifuged at 12 000 g for 20 min at 4°C and the supernatant fluids, representing the whole cell protein lysates, were stored at −70°C until use. Protein concentration was estimated using the micro-BCA™ Protein Assay reagent kit (Pierce, Rockford, IL, USA). Equal amounts of proteins were diluted in sample buffer and resolved on SDS-polyacrylamide gels (10% for FoxP3 and T-bet or 15% for TGF-β). After electrophoresis, the separated proteins were transferred onto nitrocellulose membranes and probed with a 1:500 anti- FoxP3 Ab (eBioscience, San Diego, CA, USA) or 1:500 TGF-β (R&D Systems) or 1:500 T-bet (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Blots were then incubated with a 1:3000 dilution of a horseradish peroxidase (HRP)-conjugated anti-goat immunoglobulin (Ig)G for FoxP3 and T-bet or anti-rabbit for TGF-β and developed using an enhanced chemoluminiscence detection kit (Amersham). Equal loading and absence of protein degradation were checked by Ponceau S staining (Sigma, St Louis, MO, USA). The immunoreactive protein bands were analysed with a Fotodyne Image Analyzer® (Fotodyne, Inc., Hartland, WI, USA). Results were expressed as relative densitometric values by means of the Image Quant software normalized to β-actin expression.
Flow cytometric analysis
Intracellular staining for FoxP3 detection
Flow cytometric analysis was performed according to the manufacturer's instructions (human regulatory T cell staining kit; eBioscience). Briefly, 1 × 106 cells were stained with a CD4/CD25 cocktail. After 30 min cells were washed with staining buffer and then incubated with the fixation/permeabilization buffer for 1 h. After washing, unspecific sites were blocked by adding 2 μl (2% final) normal rat serum in approximately 100 μl for 15 min. Cells were then incubated with the anti-human FoxP3 (PCH101) antibody or rat IgG2a isotype control for at least 30 min at 4°C. Finally, cells were washed with permeabilization buffer and analysed.
Intracellular staining for VIP and cytokine detection
Maternal PBMCs were co-cultured with Swan-71 cells for 24 h and incubated with GolgiStop™ in the last 4 h of culture following the manufacturer's instructions (Becton Dickinson), to promote intracellular accumulation. To assess VIP production in endometrial CD4 lymphocytes, cells recovered from endometrium after mechanical disruption were cultured with GolgiStop™ for 4 h in a flat-bottomed plate. In both situations, after washing in PBS, cells were fixed and permeabilized with the Fix/Perm kit (at the manufacturer's recommended concentrations; Becton Dickinson). After washing, permeabilized cells were incubated for 30 min with rabbit anti-VIP antibody (Peninsula-Bachem Inc.), then washed and incubated with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit antibody (Santa Cruz Biotechnology). Cells were then washed with PBS–2% FCS to allow membrane closure and finally surface-stained with phycoerythrin cyanin5 (PECy5)-conjugated anti-CD4 antibody (Becton Dickinson).
Ten thousand events were acquired in a FACS Aria II cytometer® and results were analysed using WinMDI software®. Negative control samples were incubated in parallel with an irrelevant, isotype-matched antibody. Results for positive cells are expressed as a percentage of the respective population and the quadrant was set using irrelevant isotype-specific antibody.
The percentage of CD25+FoxP3+ or VIP+ cells was obtained inside the electronically gated CD4+ cell population using WinMDI software®.
Real-time reverse transcription–polymerase chain reaction (RT–PCR)
Determination of VIP, VPAC1 and VPAC2 expression levels was performed in PBMCs from RSA and fertile women after co-culture with trophoblast cells for 24 h by RT–PCR and real-time RT–PCR. Briefly, maternal PBMC total RNA was isolated with TRIzol reagent (Life Technologies, Grand Island, NY, USA), followed by reverse transcription according to the manufacturer's instructions (Promega). For amplification of the resulting cDNA, 1 or 2 μl of the RT mixture were used. The sample volume was increased to 25 μl with 0·2 mM deoxynucleotide triphosphates (dNTPs), 0·25 uM specific primers, 3 mM MgCl2, 2 U Taq DNA polymerase and 1:30 000 dilution of Sybr Green. Real-time PCR reactions were performed in a DNA Engine Opticon (MJ Research, Inc., Waltham, MA, USA) after a predenaturation step at 95°C for 5 min; we used a denaturation step at 95°C for 30 s, an annealing step at 58°C for 30 s and elongation step at 72°C for 30 s for a total of 40 cycles. An additional extension step at 72°C for 10 min was carried out. PCR products were quantified in Opticon Software® and normalized to endogenous glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primers and thermal profiles were selected with the software Primer-3, as described previously [20]. PCR products were electrophoresed through a 2% ethidium bromide-stained agarose gel, visualized by transillumination and photographed. As a positive control for VIP and VPAC receptors we used human neuronal cell line SH-SY5Y, cultured as described previously [27].
Statistical analysis
The significance of the results was analysed by Student's t-test and Mann–Whitney U-test for non-parametric samples, using GraphPad Prism4 software (GraphPad, San Diego, CA, USA). A value of P < 0·05 was considered significant.
Results
VIP reduces T-bet expression and proinflammatory mediators in an in-vitro model of trophoblast and maternal–leucocyte interaction
To investigate VIP immunoregulatory properties in the materno–placental interface under physiological and pathological conditions, we explored VIP ability to modulate the maternal inflammatory/Th1 effector response using an in-vitro approach, based on the co-culture of trophoblast cells (Swan-71, cell line derived by telomerase-mediated transformation of a 7-week human cytotrophoblast isolate) and maternal PBMCs.
First, we investigated the modulatory effect of VIP on T-bet expression, the main transcription factor involved in Th1 response development. For that purpose, RSA PBMCs or fertile PBMCs were co-cultured with trophoblast cells in the absence or presence of VIP (10−7 M). After 48 h, maternal PBMCs were recovered and T-bet expression was evaluated by Western blot. VIP decreased T-bet expression significantly in maternal PBMCs from both groups after the interaction with Swan-71 cells (Fig. 1a). An interesting point is that PBMCs from RSA patients showed significantly higher levels of T-bet expression in comparison with fertile PBMCs after interaction with trophoblast cells, and could be normalized by VIP.
Fig. 1.

Vasoactive intestinal peptide (VIP) reduces T-bet expression and proinflammatory mediators in an in-vitro model of trophoblast and maternal–leucocyte interaction. Swan 71 cell line was co-cultured with maternal–peripheral blood mononuclear cells (PBMCs) from fertile women or with recurrent spontaneous abortions (RSA), in the absence or presence of VIP (10−7 M). (a) At 48 h of co-culture, the recovered cells were harvested and analysed by Western blot for T-bet expression. Representative immunoreactive bands are shown, and the semiquantification expressed as relative to β-actin in arbitrary units are shown in the bars. Results are representative of three independent experiments using different PBMCs from fertile women (*P < 0·05, Student's t-test). After 48 h of co-culture, supernatants were collected; (b) monocyte chemotactic protein-1 (MCP-1) and (c) nitrites were quantified by enzyme-linked immunosorbent assay (ELISA) and the Griess method, respectively. Results are expressed as mean ± standard error of the mean of at least three independent experiments using different RSA and PBMCs from fertile women (n = 3, *P < 0·05, Student's t-test).
In the same cultures, we also evaluated the modulation of inflammatory mediators relevant in the early stages of implantation; in particular MCP-1, a chemokine that is responsible for recruiting macrophages during the pro-implantatory response, accompanies tissue damage at high levels [28], and nitrites as an indicator of the induction of nitric oxide synthase which is related to the maintenance of the uterine quiescence [29] and, at high levels, to local proinflammatory profiles. As shown in Fig. 1b,c, VIP significantly decreased MCP-1 secretion quantified by ELISA and nitrite production as determined by the Griess method in the co-cultures performed with RSA and fertile PBMCs. It is noteworthy that those co-cultures performed with RSA PBMCs displayed significantly higher levels of nitrites after interaction with the trophoblast in comparison with fertile PBMCs.
Taken together, these data suggest that VIP has the ability to down-modulate Th1-type responses in early trophoblast–maternal leucocyte cross-talk.
VIP increases Treg population and anti-inflammatory mediators during the in-vitro model of trophoblast and maternal–leucocyte interaction
Human CD4+CD25+FoxP3+ regulatory T cells mediate feto–maternal tolerance and it has been demonstrated clearly that a reduction in their frequency or function is associated with recurrent spontaneous abortions [30,31]. As previous evidence, obtained mainly in vivo, suggests that VIP induces de-novo generation of peripheral CD4+CD25+ IL-10-secreting T cells from the CD4+CD25+ repertoire, and also induces alloantigen-specific human CD4+CD25+FoxP3+ cells [32,33], in this study we investigated if VIP has the ability to expand Treg cells within maternal PBMCs after trophoblast interaction.
We performed co-cultures of trophoblast cells and PBMCs from RSA or fertile women in the absence or presence of VIP (10−7 M); PBMCs were recovered after 48 h and FoxP3 expression was evaluated by Western blot. As depicted in Fig. 2a, VIP displayed a slight increase of FoxP3 expression in maternal PBMCs from both groups of women under study. Interestingly, we observed a significant decrease in FoxP3 expression levels in RSA PBMCs after interaction with trophoblast cells in comparison with that observed in fertile PBMCs.
Fig. 2.

Vasoactive intestinal peptide (VIP) increases regulatory T cell (Treg) population and anti-inflammatory mediators during the in-vitro model of trophoblast and maternal–leucocyte interaction. Swan 71 cell line was co-cultured with maternal–peripheral blood mononuclear cells (PBMCs) from fertile women or with recurrent spontaneous abortions (RSA), in the absence or presence of VIP (10−7 M). (a) At 48 h of co-culture, the suspension cells were recovered, harvested and analysed by Western blot for forkhead box protein P3 (FoxP3) expression and (b) transforming growth factor (TGF)-β expression. Representative immunoreactive bands are shown, and the semiquantification expressed as relative to β-actin in arbitrary units are shown in the bars. (c) In parallel, after 48 h of co-culture, supernatants were collected and interleukin (IL)-10 was quantified by enzyme-linked immunosorbent assay (ELISA). Results are expressed as mean ± standard error of the mean of at least three independent experiments using different fertile and RSA PBMCs (n = 3, *P < 0·05, Student's t-test)
FoxP3 modulation was also accompanied by a significant increase in TGF-β expression and IL-10 secretion, the main anti-inflammatory cytokines, in co-cultures performed with PBMCS from both groups of women (see Fig. 2b,c). In particular, even though the co-cultures performed with RSA PBMCs displayed significantly lower IL-10 secretion after trophoblast interaction, VIP was able to normalize this level to that observed in the cultures with fertile PBMCs.
To confirm these results using a more specific technique, we performed co-cultures in the absence or presence of VIP along with its specific antagonist (10−6 M), and Treg frequency was evaluated by FACS. As shown in Fig. 3a, VIP significantly increased the frequency of CD4+CD25+FoxP3+ cells in maternal PBMCs and the specific VIP antagonist prevented this modulation in both groups of women.
Fig. 3.
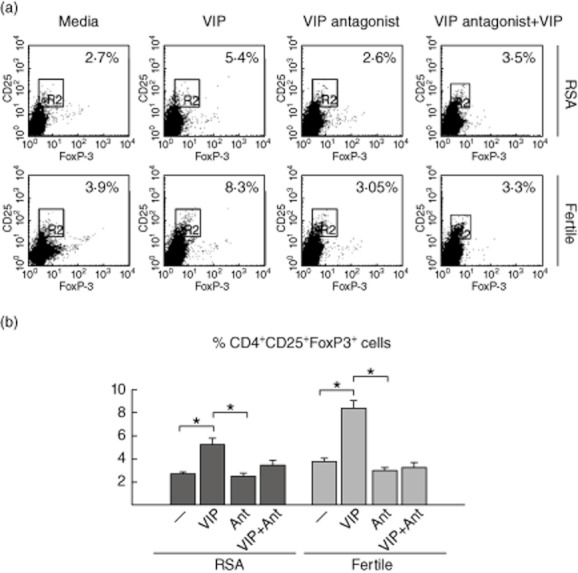
Vasoactive intestinal peptide (VIP) specifically increases the frequency of regulatory T cells (Treg) during the early trophoblast–maternal dialogue. Swan 71 cell line was co-cultured with maternal–peripheral blood mononuclear cells (PBMCs) from recurrent spontaneous abortions (RSA) or fertile women, in the absence or presence of VIP (10−7 M) or VIP-antagonist (10−5 M). (a) After 48 h of culture, cells in suspension were recovered and triple-stained for surface CD4, CD25 and intranuclear forkhead box protein P3 (FoxP3) markers and analysed by fluorescence activated cell sorter (FACS). The upper panel shows representative dot-plots and the frequency of the CD4+CD25+FoxP3+ population expressed as a percentage of CD4+ cells. (b) Lower panel shows mean ± standard error of the mean of three independent PBMC preparations (n = 3, *P < 0·05, Student's t-test).
The present results suggest that after the interaction with trophoblast cells, VIP specifically shifted the maternal Th1/Treg balance towards a tolerogenic response, increasing the frequency of CD4+CD25+FoxP3+ cells, TGF-β and IL-10 production. In addition, RSA PBMCs displayed an exacerbated Th1 effector response and decreased frequency of Treg cells after interaction with trophoblast cells.
VIP/VPAC system in maternal PBMCs after interaction with trophoblast cells
Because VIP added exogenously promoted a tolerogenic profile towards trophoblast antigens, and RSA PBMCs displayed alterations in the Th1/Treg effector responses, we investigated the VIP/VPAC system under physiological and pathological conditions.
First, we evaluated VIP receptor expression, VPAC1 and VPAC2, in maternal PBMCs from RSA and fertile women after the interaction with trophoblast cells. Real-time RT–PCR showed that VPAC1 is expressed constitutively in both groups of women; however, RSA PBMCs displayed significantly lower expression compared with fertile PBMCs (Fig. 4a). Conversely, VPAC2 was not detected by RT–PCR in maternal PBMCs after culture with trophoblast cells (data not shown).
Fig. 4.
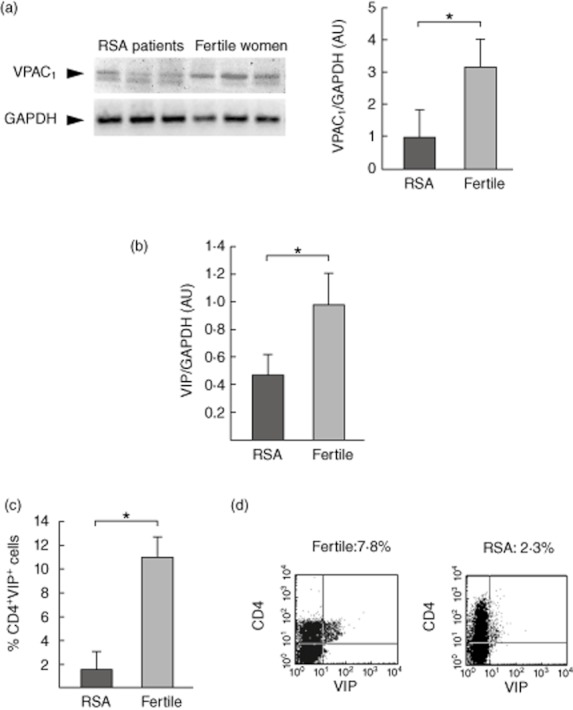
Vasoactive intestinal peptide (VIP)/VPAC system in maternal–peripheral blood mononuclear cells (PBMCs) after interaction with trophoblast cells. Swan 71 cell line was co-cultured with maternal PBMCs from recurrent spontaneous abortions (RSA) or fertile women, and after 48 h of culture, cells in suspension were recovered, cDNA was obtained and (a) VPAC1 and (b) VIP expression was quantified by real-time reverse transcription–polymerase chain reaction (RT–PCR). Results shown are representative of six RSA patients and six fertile women (*P < 0·05, Mann–Whitney U-test). (c) Suspension cells were stained for surface CD4 and intracellular VIP markers and analysed by fluorescence activated cell sorter (FACS). (d) Figure shows representative dot-plots and the frequency of the CD4+VIP+ cells. Results are expressed as mean ± standard error of the mean of three different RSA and three fertile women (*P < 0·05, Student's t-test).
We then quantified VIP production by real-time RT–PCR and, as shown in Fig. 4b, RSA PBMCs presented a significant reduction of VIP expression after culture with trophoblast cells in comparison with fertile PBMCs. To confirm this result, we quantified VIP production by maternal CD4+ cells after trophoblast stimulation by means of intracellular staining and FACS analysis. We observed a significant decrease in the frequency of CD4+VIP+ cells in co-cultures performed with RSA PBMCs in comparison with those performed with fertile PBMCs (Fig. 4c). Figure 4d shows representative dot-plots with the frequency of CD4+VIP+ cells from a culture performed with RSA or fertile PBMCs after the interaction with trophoblast cells.
Taken together, the present results indicate that PBMCs from RSA patients show a decreased expression of VIP after interaction with trophoblast cells that might be related to an imbalance of Th1/Treg immune responses observed in these patients.
Contribution of endogenous VIP to induce a maternal tolerogenic microenvironment
To confirm the contribution of endogenous VIP to the interaction between trophoblast cells and maternal leucocytes, we performed co-cultures in the presence of the specific VIP antagonist. As shown in Fig. 5a, the frequency of CD4+CD25+FoxP3+ cells from fertile PBMCs decreased significantly in the presence of the VIP antagonist, similar to that observed in RSA PBMCs after co-culture with trophoblast cells. Moreover, IL-10 secretion quantified by ELISA in the co-cultures performed with fertile PBMCs was also reduced significantly in the presence of VIP antagonist (Fig. 5b); however, these levels were not as low as those observed in the cultures with RSA PBMCs, suggesting that other mechanisms might be affected in RSA patients.
Fig. 5.

Immunomodulatory effects of the endogenous vasoactive intestinal peptide (VIP). Swan 71 cell line was co-cultured with peripheral blood mononuclear cells (PBMCs) from recurrent spontaneous abortions (RSA) or fertile women in the absence or presence of VIP antagonist (10−5 M). After 48 h of culture, cells and supernatants were collected and (a) regulatory T cells (Tregs) were quantified by fluorescence activated cell sorter (FACS) analysis and (b) interleukin (IL)-10 by enzyme-linked immunosorbent assay (ELISA). Results are expressed as mean ± standard error of the mean of at least three independent experiments with three different RSA and PBMCs from fertile women (*P < 0·05, Student's t-test).
RSA patients have a deficiency in VIP production by T endometrial cells
Finally, we investigated VIP production in CD4+ lymphocytes infiltrated in endometrial samples from RSA and fertile women. We obtained endometrial biopsies during the secretory phase of the menstrual cycle from RSA and fertile women, and the cells recovered after mechanical disruption of biopsies were analysed by flow cytometry for intracellular VIP detection into CD4+ cells. As shown in Fig. 6a, there was a significantly lower frequency of infiltrated CD4+VIP+ cells in endometrium of RSA patients in comparison with fertile women (9·6 ± 3·8% versus 29 ± 4·5%, respectively). Figure 6b shows representative histograms of endometrial samples from a fertile woman and an RSA patient with the percentages of VIP producer cells inside the CD4+ gated cells. These results support the idea that a lower frequency of VIP-producing endometrial T cells might precondition RSA patients to an imbalance of the immune response.
Fig. 6.

Patients with recurrent spontaneous abortions have a deficiency in vasoactive intestinal peptide (VIP) production. (a) Endometrial biopsies from fertile and recurrent spontaneous abortion (RSA) patients were disrupted mechanically and the cells recovered were stained for intracellular VIP detection in CD4+ cells and analysed by flow cytometry. Results are expressed as the mean ± standard error of the mean from three fertile women and three RSA patients and shows the percentages of VIP producer cells inside the CD4+ gated cells (*P < 0·05 Mann–Whitney U-test). (b) Histograms show the percentages of VIP producer cells inside the CD4+ gated cells from a fertile woman and from an RSA patient.
Discussion
Several reports have proposed that pregnancy evolves through different immunological stages with a predominantly pro- or anti-inflammatory profile depending on the stage of gestation analysed [34,35]. While the appropriate generation of a proinflammatory response is a prerequisite for successful implantation [1,2], and immune cells are critical for decidual and trophoblast development in an early inflammatory environment, a switch to an anti-inflammatory and tolerogenic profile is needed later until delivery where, again, a proinflammatory response is predominant. Multiple regulatory mechanisms and check-points are required to balance such a variety of immune mediators and for the fine tuning of the local immune–trophoblast interaction throughout gestation [36].
The results presented herein provide experimental evidence that the neuropeptide VIP contributes to the induction of a physiological maternal tolerogenic microenvironment. Using an in-vitro model representing the trophoblast–maternal leucocyte interaction, we demonstrated that VIP contributes to the immunoprivilege at the feto–maternal interface through multiple strategies as an increase of maternal CD4+CD25+FoxP3+ cells, an increase of TGF-β and IL-10 production, a decrease in T-bet expression and a decrease in inflammatory mediators. In fact, the immunomodulatory effects of VIP were prevented by a VIP antagonist, indicating the endogenous VIP contribution.
Therefore, VIP might act as a tolerogenic factor modulating the Th1/Treg effector responses and the production of pro/anti-inflammatory mediators promoting an overall balance that favours tolerance towards trophoblast antigens.
The role of VIP in the maintenance of immune tolerance by expansion of the Treg population has been demonstrated [32,33]. In fact, VIP was able to modulate the Treg subpopulation in several acute and chronic inflammatory processes [37–41]. Previously, in line with this, we have demonstrated Treg cell modulation by VIP through the up-regulation of FoxP3 and TGF-β in pancreas of diabetic NOD mice, which may lead to the restoration of tolerance to pancreatic autoantigens [17].
VIP expression was detected only in decidua and trophoblast cells, with a peak at day 8 of gestation in the murine model [19]. However, when extra-embryo tissues were separated from the embryo, the main source of VIP production was from maternal lymphocytes. This transient VIP expression correlates with the critical period of VIP effects as an embryo growth regulator and a neural growth factor [19,42,43].
Consistent with a strict regulation of the immune response during pregnancy, thrombotic/inflammatory processes are often observed at the maternal–fetal interface as the final pathological assault of pregnancy losses in many cases, including those of unexplained aetiologies. Tissue damage and embryo resorption is associated with the failure of several immunological mechanisms, such as an exacerbated inflammatory/Th1 response, ultimately responsible for cytotoxic natural killer activation and reflected by elevated leucocyte infiltration [9,44] or limited maternal repertoire of killer inhibitory receptors and lack of fetal human leucocyte antigen Cw (HLA-Cw) molecules on trophoblast cells [30], among others [6,8].
In this study, we evaluated the role of immunomodulatory VIP in the trophoblast–maternal cell interaction under normal and pathological conditions, using maternal PBMCs from fertile or RSA women. Our results showed clearly that RSA PBMCs displayed an exacerbated proinflammatory and Th1 immune response after interaction with trophoblast cells, reflected by an increase in T-bet expression level and nitrite production. Conversely, we observed a significant decrease in the frequency of Treg cells in these co-cultures with lower levels of TGF-β and IL-10 secretion.
Various factors contribute to sustaining tolerance at the maternal–fetal interface [31], and trophoblast cells play an active role in the recruitment and differentiation of maternal Treg [45]; however, reproductive failures in women with RSA might result from the inability of Treg to expand during the pre-implantatory phase combined with their lower functional capacity [30,46–48].
On this basis, we hypothesized that RSA patients might present deficiencies in the VIP/VPAC system among other factors required for a suitable homeostasis control at the interface. Certainly, the reduction of VPAC1 and VIP expression in maternal PBMCs after trophoblast interaction observed only in RSA patients might underlie failures in VIP-activated pathways. In this sense, RSA patients displayed a significantly lower frequency of CD4+VIP+ endometrial cells in comparison with fertile women, suggesting a negative precondition of endometrium before embryo implantation.
To our knowledge, this is the first report showing that deficiencies in VIP production could be associated with recurrent pregnancy loss. In line with this, in NOD mice, which show pregnancy complications and an increased rate of embryo resorption at the prediabetic stage, the local expression of VIP mRNA was diminished at viable implantation sites compared with control mice [20].
Given the action of VIP in the development of Treg and the efficacy of these cells to control inflammatory processes, this peptide could arise as a promising candidate as a diagnostic or surrogate biomarker in current treatment of early pregnancy losses as recurrent spontaneous abortions.
Research in the past few years has provided a clearer understanding of the molecular mechanisms leading to immune tolerance and homeostasis, but the definitive cellular and molecular interactions underlying the embryo–uterine cross-talk remain to be resolved. Although further studies are required to assess the clinical, diagnostic and therapeutic applications of VIP in the human maternal–fetal interface, these observations might contribute to the design of novel therapeutic strategies to prevent fetal rejection.
Acknowledgments
This study was supported by grants to R.R. (CONICET PIP 2659, UBACyT 2010–2012) and C.P.L. (UBACyT 2011–2014 and PICT 2011-0144 from ANPCyT). We thank Dr Gil Mor, who kindly gave us the Swan 71 cell line. We also thank Dr E. Lombardi and PROEGRE (Research Program from the Argentinean Society of Gynecological and Endocrinological Reproduction) for continuous support.
Disclosure
The authors have no financial conflict of interest.
References
- 1.Abrahams V, Visitin I, Aldo P, Guller S, Romero R, Mor G. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol. 2005;176:8096–8104. doi: 10.4049/jimmunol.175.12.8096. [DOI] [PubMed] [Google Scholar]
- 2.Rugeles MT, Shearer GM. Alloantigen recognition in utero: dual advantage for the fetus? Trends Immunol. 2004;25:348–352. doi: 10.1016/j.it.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Terness P, Kallikourdis M, Betz AG, Rabinovich GA, Saito S, Clark DA. Tolerance signalling molecules and pregnancy: IDO, galectins, and the renaissance of regulatory T cells. Am J Reprod Immunol. 2007;58:238–254. doi: 10.1111/j.1600-0897.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 4.Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in reproductive disorders. Reprod Sci. 2009;16:216–229. doi: 10.1177/1933719108330087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. 2009;15:517–535. doi: 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaouat G. The Th1/Th2 paradigm: still important in pregnancy? Semin Immunopathol. 2007;29:95–113. doi: 10.1007/s00281-007-0069-0. [DOI] [PubMed] [Google Scholar]
- 7.Chaouat G, Petibarat M, Dubanchet S, Rahmati M, Leedée N. Tolerance to fetal allograft? Am J Reprod Immunol. 2010;62:624–636. doi: 10.1111/j.1600-0897.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 8.Paria B, Reese J, Das S, Dey S. Deciphering the cross talk of implantation: advances and challenges. Science. 2002;296:2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- 9.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;62:601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 10.Aluvihare V, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 11.Blois SM, Ilarregui JM, Tometten M, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 12.Molvarec A, Blois SM, Stenczer B, et al. Peripheral blood galectin-1-expressing T and natural killer cells in normal pregnancy and preeclampsia. Clin Immunol. 2011;139:48–56. doi: 10.1016/j.clim.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Yosinaga K . Research on blastoyst implantation essential factors (BIEFs) Am J Reprod Immunol. 2010;62:624–636. doi: 10.1111/j.1600-0897.2010.00853.x. [DOI] [PubMed] [Google Scholar]
- 14.Fraccaroli L, Alfieri J, Larocca L, et al. VIP modulates the pro-inflammatory maternal response, inducing tolerance to trophoblast cells. Br J Pharmacol. 2009;156:116–126. doi: 10.1111/j.1476-5381.2008.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leceta J, Gomariz RP, Martinez C, Carrión M, Arranz A, Juarranz Y. Vasoactive intestinal peptide regulates Th17 function in autoimmune inflammation. Neuroimmunomodulation. 2007;14:134–138. doi: 10.1159/000110636. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Rey E, Anderson P, Delgado M. Emerging roles of vasoactive intestinal peptide: a new approach for autoimmune therapy. Ann Rheum Dis. 2007;66:70–76. doi: 10.1136/ard.2007.078519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosignoli F, Torroba M, Juarranz J, et al. VIP and tolerance induction in autoimmunity. Ann NY Acad Sci. 2006;1070:525–530. doi: 10.1196/annals.1317.073. [DOI] [PubMed] [Google Scholar]
- 18.Ekström J, Mansson B, Tobin G. Vasoactive intestinal peptide evokes secretion of fluid and protein from rat salivary glands and the development of supersensitivity. Acta Physiol Scand. 1983;119:169–175. doi: 10.1111/j.1748-1716.1983.tb07322.x. [DOI] [PubMed] [Google Scholar]
- 19.Spong C, Lee S, Mcune S, et al. Maternal regulation of embryonic growth: the role of vasoactive intestinal peptide. Endocrinology. 1999;140:917–924. doi: 10.1210/endo.140.2.6481. [DOI] [PubMed] [Google Scholar]
- 20.Roca V, Calafat M, Larocca L, et al. Potential immunomodulatory role of VIP in the implantation sites of prediabetic nonobese diabetic mice. Reproduction. 2009;138:733–742. doi: 10.1530/REP-09-0171. [DOI] [PubMed] [Google Scholar]
- 21.Coulam CB, Clark DA, Kutteh WH, Kwak S, Stephenson M. Current clinical options for diagnosis and treatment of recurrent spontaneous abortions. Am J Reprod Immunol. 1997;38:57–74. doi: 10.1111/j.1600-0897.1997.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 22.Straszewski-Chavez SL, Abrahams VM, Alvero AB, et al. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta. 2009;30:939–948. doi: 10.1016/j.placenta.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aplin JD, Straszewski-Chavez SL, Kalions B, et al. Trophoblast differentiation: progenitor cells, fusion and migration. A workshop report. Placenta. 2006;27(Suppl A):S141–143. doi: 10.1016/j.placenta.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Gozes I, Brenneman DE. VIP: molecular biology and neurobiological function. Mol Neurobiol. 1989;3:201–236. doi: 10.1007/BF02740606. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler S, Eardley JE, McNulty KF, Sutcliffe CP, Morrison JD. An investigation into the relative merits of pituitary adenylate cyclase-activating polypeptide (PACAP-27) and vasoactive intestinal polypeptide as vagal neuro-transmitters in exocrine pancreas of rats. Exp Physiol. 1997;82:729–747. doi: 10.1113/expphysiol.1997.sp004061. [DOI] [PubMed] [Google Scholar]
- 26.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2404–2412. [PubMed] [Google Scholar]
- 27.Pregi N, Vittori D, Pérez G, Pérez Leirós C, Nesse A. Effect of erythropoietin on staurosporine-induced apoptosis and differentiation of SH-SY5Y neuroblastoma cells. Biochim Biophys Acta. 2006;1763:238–246. doi: 10.1016/j.bbamcr.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Huang SJ, Schatz F, Masch R, et al. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol. 2006;72:60–73. doi: 10.1016/j.jri.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Lunghi L, Ferretti ME, Medici S, Biondi C, Vesce F. Control of human trophoblast function. Reprod Biol Endocrinol. 2007;8:5–6. doi: 10.1186/1477-7827-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwak-Kim J, Yang KM, Gilman-Sachs A. Recurrent pregnancy loss: a disease of inflammation and coagulation. J Obstet Gynaecol Res. 2009;35:609–622. doi: 10.1111/j.1447-0756.2009.01079.x. [DOI] [PubMed] [Google Scholar]
- 31.Leber A, Teles A, Zenclussen AC. Regulatory T cells and their role in pregnancy. Am J Reprod Immunol. 2010;63:445–459. doi: 10.1111/j.1600-0897.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 32.Pozo D, Anderson P, Gonzalez-Rey E. Regulatory cells by vasoactive intestinal peptide. J Immunol. 2009;183:4346–4359. doi: 10.4049/jimmunol.0900400. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Rey E, Ganea D, Delgado M. Neuropeptides: keeping the balance between pathogen immunity and immune tolerance. Curr Opin Pharmacol. 2010;10:473–481. doi: 10.1016/j.coph.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mor G. Inflammation and pregnancy the role of Toll-like receptors in trophoblast–immune interaction. Ann NY Acad Sci. 2008;1127:121–128. doi: 10.1196/annals.1434.006. [DOI] [PubMed] [Google Scholar]
- 35.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warning JC, McCracken SA, Morris JM. A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction. 2011;141:715–724. doi: 10.1530/REP-10-0360. [DOI] [PubMed] [Google Scholar]
- 37.Abad C, Gomariz RP, Waschek JA. Neuropeptide mimetics and antagonists in the treatment of inflammatory disease: focus on VIP and PACAP. Curr Top Med Chem. 2006;6:151–163. doi: 10.2174/156802606775270288. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong BD, Hu Z, Abad C, et al. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosc Res. 2003;15:240–247. doi: 10.1002/jnr.10750. [DOI] [PubMed] [Google Scholar]
- 39.Delgado M, Abad C, Martinez C, Juarranz MG. Vasoactive intestinal peptide in the immune system: potential therapeutic role in inflammatory and autoimmune diseases. J Mol Med. 2002;80:16–24. doi: 10.1007/s00109-001-0291-5. [DOI] [PubMed] [Google Scholar]
- 40.Gomariz RP, Martinez C, Abad C, Leceta J, Delgado M. Immunology of VIP: a review and therapeutical perspectives. Curr Pharm Des. 2001;7:89–111. doi: 10.2174/1381612013398374. [DOI] [PubMed] [Google Scholar]
- 41.Onoue S, Misaka S, Aoki Y, et al. Inhalable powder formulation of vasoactive intestinal peptide derivative, [R15,20,21, L17]-VIP-GRR, attenuated neutrophilic airway inflammation in cigarette smoke-exposed rats. Eur J Pharm Sci. 2010;41:508–514. doi: 10.1016/j.ejps.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Girard BA, Lelievre V, Braas KM, et al. Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. J Neurochem. 2006;99:499–513. doi: 10.1111/j.1471-4159.2006.04112.x. [DOI] [PubMed] [Google Scholar]
- 43.Hill JM, Cuasay K, Abebe DT. Vasoactive intestinal peptide antagonist treatment during mouse embryogenesis impairs social behavior and cognitive function of adult male offspring. Exp Neurol. 2007;206:101–113. doi: 10.1016/j.expneurol.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Ledee-Bataille N, Dubanchet S, Coulomb-L'hermine A, Durand-Gasselin I, Frydman R, Chaouat G. A new role for natural killer cells, interleukin (IL)-12, and IL-18 in repeated implantation failure after in vitro fertilization. Fertil Steril. 2004;81:59–65. doi: 10.1016/j.fertnstert.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Ramhorst R, Fraccaroli L, Aldo P, et al. Modulation and recruitment of inducible regulatory T cells by first trimester trophoblast cells. Am J Reprod Immunol. 2012;67:17–27. doi: 10.1111/j.1600-0897.2011.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178:2572–2578. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- 47.Baek KH, Lee EJ, Kim YS. Recurrent pregnancy loss: the key potential mechanisms. Trends Mol Med. 2007;13:310–317. doi: 10.1016/j.molmed.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Toth B, Jeschkec U, Rogenhoferb N, et al. Recurrent miscarriage: current concepts in diagnosis and treatment. J Reprod Immunol. 2010;85:25–32. doi: 10.1016/j.jri.2009.12.006. [DOI] [PubMed] [Google Scholar]


