Abstract
Common variable immunodeficiency (CVID), the most frequent symptomatic immunoglobulin primary immunodeficiency, is associated with chronic T cell activation and reduced frequency of CD4+ T cells. The underlying cause of immune activation in CVID is unknown. Microbial translocation indicated by elevated serum levels of lipopolysaccharide and soluble CD14 (sCD14) has been linked previously to systemic immune activation in human immunodeficiency virus/acquired immune deficiency syndrome (HIV-1/AIDS), alcoholic cirrhosis and other conditions. To address the mechanisms of chronic immune activation in CVID, we performed a detailed analysis of immune cell populations and serum levels of sCD14, soluble CD25 (sCD25), lipopolysaccharide and markers of liver function in 35 patients with CVID, 53 patients with selective immunoglobulin (Ig)A deficiency (IgAD) and 63 control healthy subjects. In CVID subjects, the concentration of serum sCD14 was increased significantly and correlated with the level of sCD25, C-reactive protein and the extent of T cell activation. Importantly, no increase in serum lipopolysaccharide concentration was observed in patients with CVID or IgAD. Collectively, the data presented suggest that chronic T cell activation in CVID is associated with elevated levels of sCD14 and sCD25, but not with systemic endotoxaemia, and suggest involvement of lipopolysaccharide-independent mechanisms of induction of sCD14 production.
Keywords: common variable immunodeficiency, endotoxin, IgA deficiency, lymphocyte activation
Introduction
The most frequent form of primary symptomatic hypogammaglobulinaemia, common variable immunodeficiency (CVID), is a group of conditions of unclear pathogenesis characterized by decreased serum immunoglobulin (Ig)G and IgA levels in the presence of variable levels of IgM. CVID patients suffer from clinical manifestation of immunodeficiency that manifests by frequent and severe respiratory tract infections, diarrhoea and autoimmune disorders [1]. Selective IgA deficiency (IgAD) is defined by serum IgA levels below 0·07 g/l in the absence of other disturbances in immunoglobulin production. Although IgAD patients do not usually suffer from symptoms of immunodeficiency, a higher frequency of autoimmune diseases has been documented [2]. Although no common monogenetic defects were observed in both these conditions, a frequent familiar occurrence of both IgAD and CVID and repeatedly reported progression IgAD to CVID suggest a common genetic trait [3] in at least a proportion of the patients. Previous sudies by us and others have demonstrated a number of abnormalities in lymphocyte subpopulations in CVID patients, including chronic T cell activation indicated by an increased expression of activation markers human leucocyte antigen (HLA)-DR and CD38 and decreased numbers of CD4+ T cells and natural killer (NK) cells in the systemic circulation [4–8]. IgAD subjects display a decrease in CD4+ cells [8] and increased expression of CD25 on CD4+ cells [6]. Although the activation of CD8+ cells in cytomegalovirus (CMV)-positive CVID patients has been described recently [9], the mechanisms underlying systemic T cell activation in CVID patients are not well understood.
Elevated levels of lipopolysaccharide (LPS) in the systemic circulation have been linked previously to chronic immune activation in multiple disorders, including chronic active hepatitis, liver cirrhosis, fatty liver disease and Crohn's disease [10–15]. In human immunodeficiency virus/acquired immune deficiency syndrome (HIV-1/AIDS), accumulating evidence suggests that HIV-1 causes extensive damage to the gastrointestinal mucosal surface, alters the epithelial microenvironment and impairs the anti-microbial functions of the mucosal barrier [16–19]. The impairment of mucosal barrier function and the resulting absorption of LPS and other microbial antigens into the systemic compartment is considered to be the major underlying cause of the continuous activation of CD4+ and CD8+ T cells in HIV-1 infection [16,20–23]. A potential role of microbial translocation in CVID, IgAD and other primary immunodeficiencies has not been addressed previously.
Complex interactions between LPS, CD14, myeloid differentiation-2 (MD-2) and Toll-like receptor (TLR)-4 results in an activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signalling pathway and production of multiple inflammatory factors [24–27]. The MD-2/TLR-4/LPS complex induces shedding and secretion of soluble CD14 (sCD14) from myeloid cells [28–30]. Plasma or serum levels of sCD14 are generally accepted as reliable markers of bacterial translocation and endotoxaemia [24,31–34]. Plasma levels of sCD14, rather than LPS itself, predict disease progression independently in hepatitis B and C (HBV, HBC) in HIV-1 infection and in HIV-1/HCV co-infection [14,21,22]. However, alternative sources of sCD14 such as hepatocytes or activated neutrophils have been suggested [35–41]. Induction of sCD14 in conditions that are not associated with direct bacterial exposure, such as crystal-induced arthritis, Sjörgen's syndrome, Kawasaki disease and systemic lupus erythematosus, suggests that the production of sCD14 may be linked to inflammatory processes rather than endotoxaemia or bacteraemia [38,42–44].
In this report we sought to determine whether chronic immune activation in CVID and IgAD is associated with elevated serum levels of sCD14 and LPS. We present data demonstrating an association between T cell activation and serum levels of sCD14, sCD25 and C-reactive protein (CRP) in subjects with CVID.
Materials and methods
Study population
The study comprised 35 patients with CVID (age range 19–78 years, median 45 years, 24 females, 11 males), 53 patients with IgAD (age range 18–63 years, median 33 years, 36 females, 17 males) and 63 control subjects without known immunopathological diseases (age range 18–71 years, median 28 years, 35 females, 28 males). All CVID and IgAD patients fulfilled Pan-American Group for Immunodeficiency/European Society for Immunodeficiencies (PAGID/ESID) diagnostic criteria [45]. Of 35 CVID patients, 27 were on intravenous immunoglobulin (IVIG) at a dose of 170–440 mg/kg/3–4 weeks, five on subcutaneous immunoglobulin at a dose of 60–123 mg /kg/week, one on intramuscular immunoglobulin replacement treatment (40 mg/kg/week) and two were not on immunoglobulin replacement treatment. In the case of patients on IVIG treatment, blood samples were collected before the IVIG infusion. All people included in the study were Caucasians of Moravian origin (eastern part of the Czech Republic). All samples were collected during an apparent infection-free period.
The study was approved by the St Anne's University Hospital Ethics Committee (protocol number 12G/2009); all patients gave informed consent before inclusion into the study and the study was performed according to the Declaration of Helsinki.
Human sCD14 assay
The concentration of sCD14 in serum was determined using an immunoassay kit, according to the manufacturer's protocol (R&D Systems, Minneapolis, MN, USA). Serum samples were diluted 200-fold with sample diluent solution prior to analysis. Optical density was determined using an E-312e plate reader (BioTek Instruments, Winooski, VT, USA) and serum sCD14 concentrations were calculated using the Delta Soft 3 program (BioTek).
Serum sCD25 determination
Serum levels of sCD25 were determined using the 39-plex kit of Milliplex Human Cytokine/Chemokine Panel (Millipore, Billerica, MA, USA). Samples were analysed undiluted using a Bioplex 100 system with Bioplex Manager Software version 5·0 (Biorad, Hercules, CA, USA).
Serum LPS assay
Serum levels of LPS were determined using the limulus amoebocyte lysate (LAL) assay (QCL-1000; Lonza, Walkersville, MD, USA). Serum was diluted 10-fold with LAL reagent water in 0·5 ml siliconized non-pyrogenic tubes and heat-inactivated at 70°C for 10 min. Assay was performed on 96-well non-pyrogenic polystyrene plates (Corning Life Science, NY, USA) strictly at 37°C, according to the manufacturer's protocol. Controls consisting of all components except the LAL reagent were used to minimize the effect of background. Optical density was determined at 405 nm ELx808 reader (BioTek) and analysed using Gen5 software (BioTek).
Determination of lymphocyte populations and expression of activation markers
The frequency of lymphocyte subsets and expression of activation markers was determined by five-colour flow cytometry on an FC 500 MCL cytometer (Beckman Coulter, Inc., Miami, FL, USA). Briefly, peripheral blood was stained by selected immunofluorescence-dye-conjugated monoclonal antibodies using standard procedures for direct immunofluorescence as described [5]. The following monoclonal antibody–fluorochrome combinations were used: phycoerythrin–cyanin 7 (PC7)-labelled anti-CD4, CD8 and CD19; phycoerythrin–Texas red anti-CD8 and CD45RO; fluorescein isothiocyanate (FITC) anti-CD8 and CD38; phycoerythrin (PE) anti-CD127; phycoerythrin–cyanin 5 (PC5) anti-CD27 and CD25 (Beckman Coulter); FITC–HLA-DR, FITC–CD27, PC5–IgM; PE–CD38, PE–CD21 and PE–IgD (BD Biosciences, San Jose, CA, USA). Absolute counts of lymphocyte subsests were determined by multiplying absolute numbers of leucocytes by the lymphocyte frequency in blood count and by the frequency of the specific lymphocyte subpopulation.
Determination of serum CRP levels
Serum CRP levels were measured by Immage (Beckman Coulter, Inc.) nephelometer using anti-sera Beckman Coulter; sensitivity starting at 1 mg/l.
Data analysis
Data were analysed using Mann–Whitney rank sum and Spearman's rank order correlation tests. In case of multiple group analysis, variables were compared using the Kruskal–Wallis test with post-hoc comparisons of mean ranks of all pairs of groups; P-values were corrected for multiple comparisons using Bonferroni adjustment. Statistical packages GraphPad Prism version 5, SigmaStat version 3·1 and Statistica were used. A standard level of statistical significance α = 0·05 was used. Unless indicated otherwise, the results of descriptive statistics are presented as median (5th–95th percentile).
Results
Clinical and laboratory characteristics of the CVID patients, including the EUROclass classification [46], is given in Table 1. None of the patients had suffered from opportunistic infections typical for late-onset combined immunodeficiency (LOCID) [47]. Patient 6 underwent partial nefrectomy for renal cancer, but no other malignancies were recorded in the patients. Patient 16 was treated by steroids (methylprednisolone 4 mg every other day) for lymphocytic interstitial pneumonia.
Table 1.
Characteristics of common variable immunodeficiency (CVID) patients involved in the study. Presence of bronchiectasis was determined by computerized tomography (CT) scan. Splenomegaly was defined in spleen length >11 cm in abdominal sonography. Natural killer (NK) cells were defined as CD3–CD16/CD56+ cells
| Patient no. | Age (years) | Sex | EUROclass classification | Chronic diarrhoea | Splenomegaly | Granuloma | Bronchiectasis | Dose IgG/kg/week | IgG through level (g/l) | Leucocytes (103/μl) | Lymphocytes (%) | Lymphocytes (103/μl) | CD3+ (103/μl) | CD3+CD4+ (103/μl) | CD3+CD8+ (103/μl) | CD19+ (103/μl) | NK cells (103/μl) | CD4+CD45RO+/CD4+(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 33 | F | smB-21lo | Yes | Yes | Yes | No | 0.12 | 4.2 | 5.2 | 9 | 0.47 | 0.37 | 0.23 | 0.12 | 0.05 | 0.04 | 87 |
| 2 | 78 | F | smB-21lo | No | Yes | No | Yes | 0.12 | 6.66 | 5.1 | 36 | 1.84 | 1.36 | 0.55 | 0.66 | 0.39 | 0.07 | 86 |
| 3 | 59 | F | smB-21lo | No | Yes | No | Yes | 0.12 | 8.3 | 3.3 | 28 | 0.92 | 0.72 | 0.37 | 0.34 | 0.13 | 0.06 | 93 |
| 4 | 45 | F | smB-21lo | No | Yes | No | Yes | 0.08 | 5.32 | 8.3 | 25 | 2.08 | 1.58 | 0.87 | 0.62 | 0.39 | 0.08 | 80 |
| 5 | 68 | F | smB-21lo | Yes | Yes | No | Yes | 0.09 | 5.95 | 4.2 | 27 | 1.13 | 0.90 | 0.43 | 0.43 | 0.09 | 0.15 | 78 |
| 6 | 35 | F | smB-21norm | No | No | No | No | 0.10 | 6.95 | 5.9 | 25 | 1.48 | 1.19 | 0.65 | 0.52 | 0.04 | 0.24 | 47 |
| 7 | 40 | F | ND | No | Yes | No | No | 0.07 | 5.63 | 8.7 | 18 | 1.57 | 1.35 | 0.53 | 0.83 | ND | 0.22 | 85 |
| 8 | 22 | F | smB-21lo | No | Yes | No | Yes | 0.06 | 7.66 | 4.2 | 44 | 1.85 | 1.48 | 0.79 | 0.61 | 0.24 | 0.11 | 55 |
| 9 | 65 | F | smB-21lo | No | No | No | No | 0.10 | 6.61 | 7.4 | 21 | 1.55 | 0.93 | 0.56 | 0.31 | 0.30 | 0.30 | 70 |
| 10 | 55 | F | smB-21lo | No | No | No | Yes | 0.06 | 5.78 | 5.7 | 29 | 1.65 | 1.19 | 0.58 | 0.36 | 0.20 | 0.26 | 88 |
| 11 | 19 | M | smB-21norm | No | No | No | No | 0.09 | 5.2 | 4.4 | 25 | 1.10 | 0.84 | 0.39 | 0.39 | 0.17 | 0.10 | 70 |
| 12 | 58 | F | smB-21lo | Yes | Yes | No | Yes | 0.08 | 6.23 | 5.7 | 23 | 1.31 | 0.88 | 0.43 | 0.41 | 0.18 | 0.25 | 92 |
| 13 | 52 | M | ND | Yes | Yes | Yes | Yes | 0.10 | 6.37 | 15.4 | 4 | 0.62 | 0.49 | 0.26 | 0.19 | ND | 0.11 | 70 |
| 14 | 25 | M | smb+21lo | No | No | No | No | NT | 3.51 | 7.8 | 24 | 1.87 | 1.07 | 0.58 | 0.37 | 0.24 | 0.54 | 73 |
| 15 | 30 | F | smB-21lo | Yes | No | No | Yes | 0.07 | 5.56 | 2.7 | 30 | 0.81 | 0.76 | 0.58 | 0.18 | 0.02 | 0.02 | 36 |
| 16 | 51 | M | smB-21lo | Yes | Yes | No | Yes | 0.09 | 3.1 | 4.4 | 35 | 1.54 | 1.43 | 0.83 | 0.57 | 0.05 | 0.06 | 98 |
| 17 | 45 | F | smB-21lo | No | Yes | No | No | 0.10 | 3.07 | 6.4 | 12 | 0.77 | 0.62 | 0.25 | 0.37 | 0.07 | 0.07 | 85 |
| 18 | 29 | F | smB-21norm | No | No | No | Yes | NT | 2.35 | 7.0 | 24 | 1.68 | 0.99 | 0.60 | 0.32 | 0.55 | 0.12 | 68 |
| 19 | 35 | M | smB-21norm | No | Yes | No | No | 0.08 | 5.2 | 5.6 | 31 | 1.74 | 1.48 | 0.49 | 0.95 | 0.17 | 0.09 | 60 |
| 20 | 41 | F | smB-21lo | No | Yes | No | Yes | 0.11 | 6.33 | 7.6 | 28 | 2.13 | 1.66 | 0.83 | 0.66 | 0.32 | 0.13 | 58 |
| 21 | 58 | M | smB-21lo | Yes | Yes | No | Yes | 0.09 | 5.22 | 9.2 | 24 | 2.21 | 1.19 | 0.66 | 0.49 | 0.77 | 0.22 | 68 |
| 22 | 45 | F | smB-21lo | No | No | No | Yes | 0.11 | 6.06 | 5.6 | 45 | 2.52 | 2.24 | 0.86 | 1.21 | 0.20 | 0.03 | 75 |
| 23 | 51 | F | ND | No | Yes | No | Yes | 0.11 | 4.83 | 10.3 | 17 | 1.75 | 1.66 | 0.84 | 0.81 | ND | 0.09 | 91 |
| 24 | 72 | F | smb+21lo | No | Yes | No | No | 0.06 | 4.9 | 8.3 | 38 | 3.15 | 2.40 | 1.58 | 0.91 | 0.69 | 0.03 | 58 |
| 25 | 21 | F | smB-21norm | No | Yes | Yes | Yes | 0.08 | 6.4 | 4.4 | 18 | 0.79 | 0.62 | 0.38 | 0.20 | 0.13 | 0.02 | 86 |
| 26 | 42 | F | smB+21norm | No | No | No | No | 0.07 | 4.7 | 5.7 | 21 | 1.20 | 0.83 | 0.57 | 0.25 | 0.22 | 0.16 | 92 |
| 27 | 38 | M | smB-21lo | No | Yes | Yes | No | 0.09 | 5.73 | 5.2 | 18 | 0.94 | 0.85 | 0.43 | 0.42 | 0.03 | 0.03 | 88 |
| 28 | 51 | F | smB-21lo | No | Yes | No | Yes | 0.14 | 6.12 | 3.3 | 19 | 0.63 | 0.57 | 0.39 | 0.17 | 0.02 | 0.04 | 98 |
| 29 | 60 | M | smB-21norm | Yes | Yes | No | No | 0.06 | 6.26 | 4.9 | 19 | 0.93 | 0.59 | 0.42 | 0.13 | 0.19 | 0.14 | 75 |
| 30 | 41 | F | smB-21lo | Yes | Yes | No | Yes | 0.04 | 2.96 | 3.8 | 29 | 1.10 | 0.94 | 0.37 | 0.53 | 0.12 | 0.04 | 82 |
| 31 | 23 | F | smB-21lo | No | Yes | No | No | 0.06 | 4.5 | 9.0 | 31 | 2.79 | 2.32 | 1.42 | 0.67 | 0.33 | 0.11 | 52 |
| 32 | 51 | M | smB-21lo | No | No | No | Yes | 0.10 | 5.3 | 5.2 | 31 | 1.61 | 1.26 | 0.37 | 0.77 | 0.13 | 0.23 | 77 |
| 33 | 70 | M | smB-21lo | No | Yes | Yes | No | 0.05 | 4.49 | 4.7 | 18 | 0.85 | 0.76 | 0.43 | 0.36 | 0.02 | 0.06 | 98 |
| 34 | 29 | F | smB-21lo | Yes | Yes | No | No | 0.08 | 5.8 | 4.1 | 27 | 1.11 | 0.95 | 0.61 | 0.30 | 0.10 | 0.06 | 63 |
| 35 | 22 | M | smB-21lo | Yes | Yes | No | No | 0.07 | 4.43 | 6.1 | 37 | 2.26 | 1.31 | 0.50 | 0.84 | 0.23 | 0.70 | 81 |
NT: not treated; ND: not determined, the patient had B cells < 1% of total lymphocytes; F: female; M: male.
Serum sCD14 levels in CVID patients correlate with degree of T cell activation
Chronic T cell activation was reported previously to be associated with microbial translocation as indicated by elevated serum levels of LPS and sCD14 in various conditions [10–16,20–23]. The causative mechanisms underlying the chronic immune activation in CVID are not well understood. CVID and, to a lower extent, IgAD patients, exhibit increased levels of T cell activation as indicated by the expression of activation markers HLA-DR on CD4+ and CD8+ T cells (Fig. 1a,c). Importantly, the expression of HLA-DR on both CD4+ and CD8+ lymphocytes and the expression of CD38 on CD8+ lymphocytes in CVID patients correlated significantly with plasma sCD14 levels (Fig. 1b,d,h and Table 2).
Fig. 1.
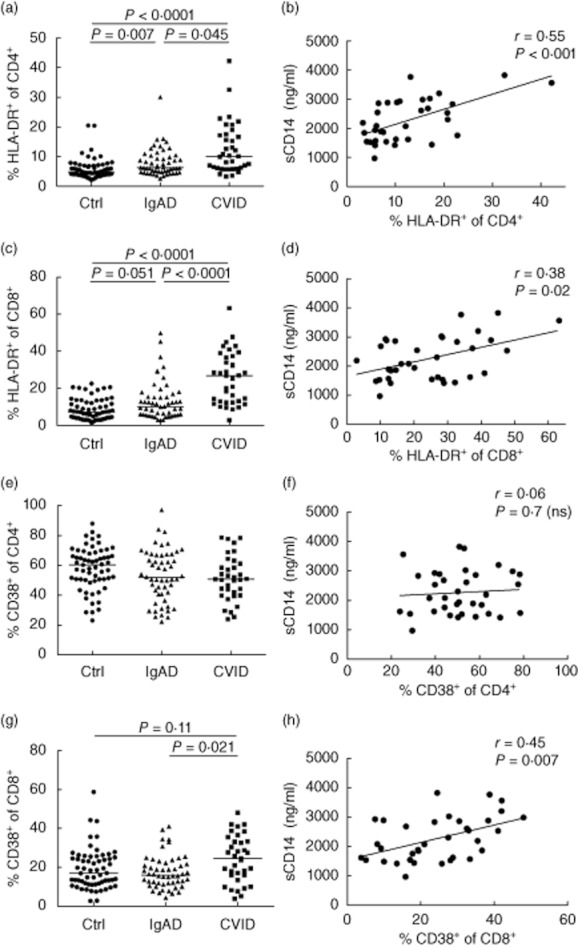
Levels of serum soluble CD14 (sCD14) correlate with the degree of T cell activation in common variable immunodeficiency (CVID) patients. (a,c,e,g) Percentages of cells expressing human leucocyte antigen (HLA)-DR or CD38 out of CD4+ (a,e) or CD8+ (c,g) lymphocyte populations in control subjects (Ctrl) and patients with selective immunoglobulin (Ig) A deficiency (IgAD) and CVID. Horizontal lines indicate group medians; intergroup differences were analysed using Kruskal–Wallis test with post-hoc comparisons of mean ranks of all pairs of groups; P-values were corrected for multiple comparisons using Bonferroni adjustment. (b,d,f,h) Correlations between serum sCD14 and the expression of activation markers (HLA-DR or CD38) on CD4+ or CD8+ lymphocytes in CVID patients. Lines represent linear regression analysis. R and P-values were determined using two-tailed Spearman's rank order correlation test. n.s.: not significant.
Table 2.
Correlation between soluble CD14 (sCD14), C-reactive protein (CRP) and lymphocyte activation markers in common variable immunodeficiency (CVID) patients. Spearman's rank order correlation test was used for the statistical analysis of data
| sCD14 | CRP | |||
|---|---|---|---|---|
| Activation marker | R | P | R | P |
| % HLA-DR of CD4+ | 0·552 | <0·001 | 0·493 | 0·003 |
| % CD25+ of CD4+ | –0·413 | 0·014 | –0·165 | 0·344 |
| % CD38+ of CD4+ | 0·063 | 0·717 | –0·108 | 0·536 |
| %CD45RO+ of CD4+ | 0·332 | 0·051 | 0·484 | 0·003 |
| % HLA-DR of CD8+ | 0·381 | 0·024 | 0·353 | 0·037 |
| % CD25+ of CD8+ | –0·101 | 0·563 | –0·257 | 0·135 |
| % CD38+ of CD8+ | 0·449 | 0·007 | 0·263 | 0·127 |
| %CD45RO+ of CD8+ | 0·034 | 0·845 | 0·381 | 0·024 |
CVID is associated with decreased frequencies of circulating CD4+ T cells and NK cells, as demonstrated in Fig. 2a,e, and in previous reports [4–7]. A statistically significant inverse correlation was observed between serum sCD14 and absolute number of CD3+CD4+ T cells, CD3–CD16+/CD56+ NK cells and CD19+ B cells (Fig. 2b,f,h). In contrast, the frequency of circulating CD8+ T cells did not differ between CVID and control subjects and no correlation with sCD14 was detected (Fig. 2c,d). In IgAD patients, no significant correlations between major lymphocyte subsets and sCD14 were observed.
Fig. 2.
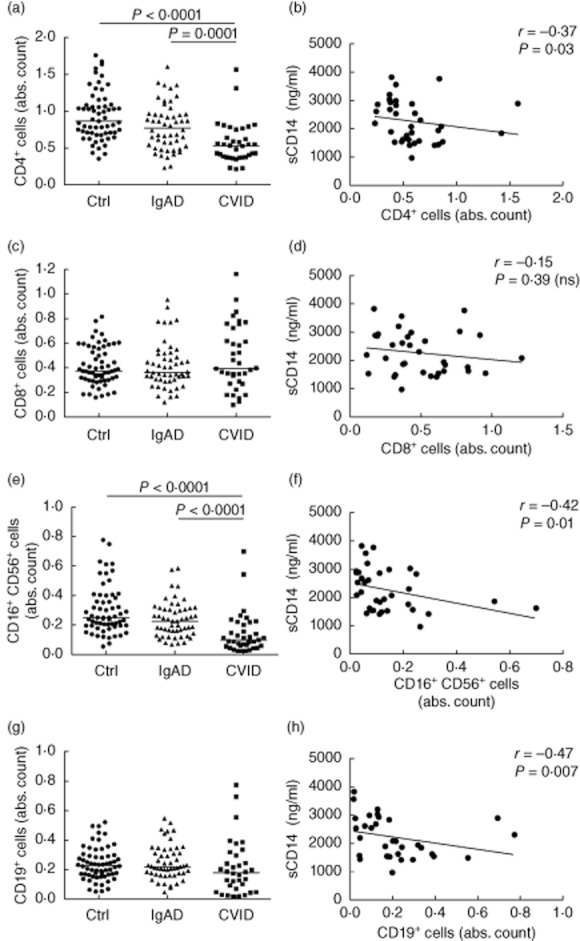
Levels of serum soluble CD14 (sCD14) in common variable immunodeficiency (CVID) patients correlate with decreased numbers of circulating CD4+ T cells, natural killer (NK) cells and B cells but not CD8+ T cells. (a,c,e,g) Absolute counts of specified immune populations are presented as 103 cells/μl. Horizontal lines indicate group medians. Intergroup differences were analysed using Kruskal–Wallis test. (b,d,f,h) Correlations between serum sCD14 and absolute counts of specified immune populations in subjects with CVID. Lines represent linear regression analysis. R and P-values were determined using two-tailed Spearman's rank order correlation test. n.s.: not significant.
Serum levels of sCD14 are elevated in subjects with CVID and correlate directly with the levels of CRP but not with serum LPS
As demonstrated on Fig. 3a, levels of sCD14 in serum were increased significantly in patients with CVID [2080 (1441–3200) ng/ml] compared to control subjects [1790 (1270–2243) ng/ml] or IgAD subjects [1592 (1116–2207) ng/ml] (P = 0·02 and P < 0·001, respectively). In 15 of 35 CVID patients (43%), but in only one of 61 healthy controls (1·6%), serum concentration of sCD14 exceeded 2500 ng/ml. No significant increase in serum sCD14 was observed in IgAD subjects.
Fig. 3.
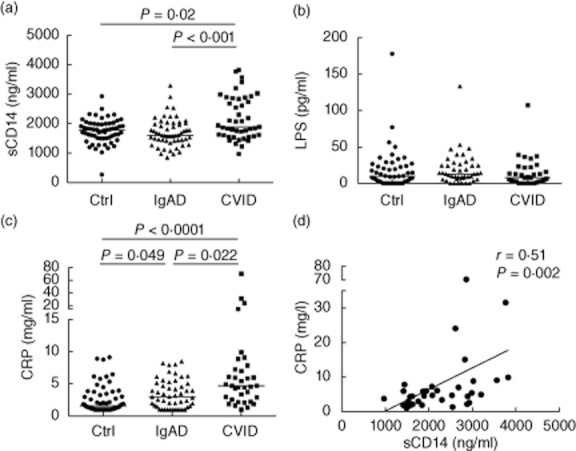
Serum soluble CD14 (sCD14) levels correlate with the concentration of C-reactive protein (CRP) but not lipopolysaccharide (LPS). (a,b,c) Serum levels of sCD14 (a), LPS (b), and CRP (c) in control subjects (Ctrl) and subjects with selective immunoglobulin (Ig) A deficiency (IgAD) or common variable immunodeficiency (CVID). Horizontal lines indicate group medians. Intergroup differences were analysed using Kruskal–Wallis test. (d) Correlation between serum sCD14 and CRP, in CVID patients. Lines represent linear regression. R and P-values were determined using two-tailed Spearman's rank order correlation test.
sCD14 is used frequently as a specific indicator of serum or plasma LPS levels [14,20–22]. Because CVID patients suffer from recurrent bacterial infections, we hypothesized that the elevated level of sCD14 is caused by endotoxaemia as observed in other conditions, and therefore may indicate translocation of microbial antigens into the systemic circulation. Surprisingly, no increase in serum LPS levels was observed in CVID or IgAD subjects (Fig. 3b). A direct correlation between serum sCD14 and LPS levels was observed only in the IgAD group (R = 0·35; P = 0·02), but not in CVID patients (R = −0·17, P = 0·32) or control subjects (R = 0·12, P = 0·39).
Increased plasma sCD14 and endotoxaemia have been linked to production of acute-phase proteins [14,21,22,38,48]. An analysis of the cohort investigated in this study demonstrated that the serum levels of CRP were increased significantly in both IgAD and CVID subjects compared to controls (P = 0·049 and P < 0·0001, respectively; Fig. 3c). A positive correlation between sCD14 and CRP was observed in the CVID group (R = 0·51; P = 0·002; Fig. 3d). In addition, serum CRP levels correlated directly with HLA-DR expression on CD4+ and CD8+ T cells (Table 2). High serum CRP concentration (> 10 mg/l) detected in four CVID patients was not associated with signs of acute infection or acute exacerbation of chronic lung disease. Intergroup differences in sCD14 levels remained significant after exclusion of subjects with CRP >10 mg/l.
B cell activation/differentiation stages in CVID patients do not reflect LPS or sCD14 levels
A detailed analysis of B lymphocyte differentiation stages was performed [naive (CD19+IgD+CD27–), transitional (CD19+IgM++CD38++), ‘CD21lowCD38low B cells’, switched memory (CD19+IgD–CD27+), marginal zone-like B cells (CD19+IgD+CD27+) and plasmablasts (CD19+CD38+++IgM–)]. With the exception of the correlation of serum LPS with switched memory B cells in CVID patients (R = 0·44, P = 0·01), no significant correlations between sCD14 and the frequency of these subpopulations were observed in controls, CVID or IgAD subjects (data not shown). There were no differences in serum sCD14 or LPS levels in CVID subgroups defined by the EUROclass (P = 0·16 and P = 0·26 respectively, Kruskal–Wallis test).
Increased serum sCD14 does not correlate with markers of hepatic dysfunction, presence of splenomegaly, bronchiectasis or granuloma
To test the possibility that increased serum sCD14 in CVID patients is associated with hepatopathy, as reported in other conditions [14,49], we determined the correlations of sCD14 with markers of liver synthetic function [aspartate aminotrasnferase (AST), alanine aminotransferase (ALT), bilirubin and albumin serum levels] and indirect marker of portal hypertension (platelet count). sCD14 correlated inversely with serum direct bilirubin (R = −0·03; P = 0·04) and albumin (R = −0·37; P = 0·03). No significant correlations between the concentrations of sCD14 and platelet count (R = −0·24; P = 0·17), AST (R = 0·03; P = 0·58) or ALT (R = −0·03; P = 0·85) levels were detected.
Higher levels of sCD14 were detected in 11 CVID patients suffering from chronic diarrhoea [2545 (2528–2880) ng/ml] compared to 24 patients without diarrhoea [1915 (1419–3561) ng/ml]; however, the observed difference was not significant (P = 0·58, Mann–Whitney U-test, Fig. 4). No differences in sCD14 were observed comparing 19 patients with and 16 patients without bronchiectasis (P = 0·27), 25 patients with and 10 patients without splenomegaly (P = 0·15) and five patients with and 30 patients without granuloma (P = 0·08), although a trend to higher sCD14 levels was observed in patients with these clinical complications (see Fig. 4).
Fig. 4.
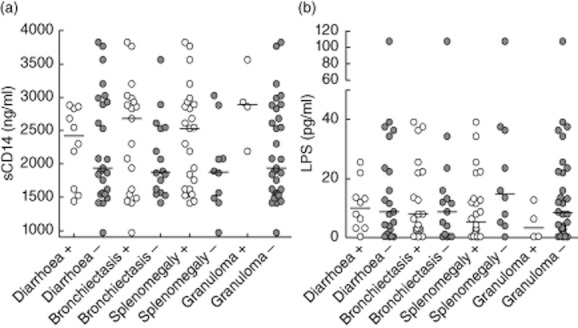
Serum soluble CD14 (sCD14) and lipopolysaccharide (LPS) levels do not reflect clinical complications of common variable immunodeficiency (CVID) patients. Levels of sCD14 (a) and LPS (b) in CVID patients with and without chronic diarrhoea, bronchiectasis, splenomegaly and granuloma. Horizontal lines indicate group medians. Data were analysed using Mann–Whitney rank sum test. No statistically significant differences were observed.
Serum levels of sCD25 are elevated in CVID but not IgAD patients
Elevated plasma or serum concentration of sCD25 is used frequently as a biomarker of immune activation [50–52]. Here we show that CVID, but not IgAD, is associated with a significant rise in serum levels of sCD25 (Fig. 5a). A strong correlation between serum sCD14 and sCD25 concentration was observed in the CVID group (R = 0·58; P < 0·001; Fig. 5b). In contrast, the correlation between sCD25 and CRP was less pronounced (R = 0·35; P = 0·05). Serum sCD25 correlated directly with the expression of HLA-DR on CD4+ T cells (R = 0·38; P = 0·03; Fig. 5c); however, correlation with HLA-DR on CD8+ T cells did not reach statistical significance. Interestingly, an elevated concentration of sCD25 was associated with decreased absolute numbers of CD4+CD25+ T cells (Fig. 5d), but no correlation between sCD25 and the frequency of regulatory T cells (Treg) determined as CD4+ CD25++CD127– was detected (data not shown).
Fig. 5.
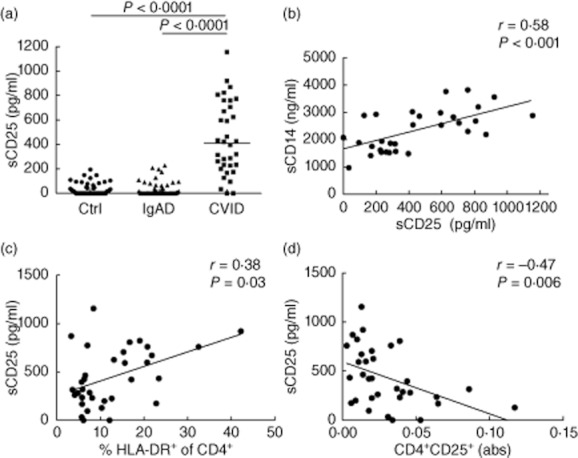
Common variable immunodeficiency (CVID) patients exhibit elevated concentration of serum soluble CD25 (sCD25). (a) Levels of sCD25 (sIL-2Rα) in control subjects (Ctrl) and patients with selective immunoglobulin (Ig) A deficiency (IgAD) and CVID. Intergroup differences were analysed using Kruskal–Wallis test. (b) Correlation between serum soluble CD14 (sCD14) and sCD25. (c) Correlation between serum sCD25 and the expression of human leucocyte antigen D-related (HLA-DR) on CD4+ T cells in CVID subjects. (d) Negative correlation between serum sCD25 and the absolute count of CD4+ CD25+ T cells in CVID subjects. Lines represent linear regression. R and P-values were determined using two-tailed Spearma's rank order correlation test.
Discussion
In this study, we tested a hypothesis that systemic T cell activation in CVID and IgAD patients is associated with elevated serum levels sCD14 and LPS. We present data demonstrating increased levels of serum sCD14 in patients with CVID and a significant correlation between sCD14 and the extent of T cell activation. Importantly, although serum sCD14 is frequently considered a specific and sensitive marker of LPS bioactivity in blood [14,21,24,31–34], we observed no differences in LPS levels between the CVID and IgAD patients and control subjects.
Several potential mechanisms can be involved to explain the obtained results. It is feasible that sCD14 is induced by a limited microbial translocation and elevated LPS concentration confined to the mucosal tissues. It is also possible that the level of serum LPS in CVID is restricted by augmented LPS clearance mechanisms. CVID is characterized by frequent or chronic infections of the respiratory and gastrointestinal tracts and localized monocyte activation may occur in the lamina propria of the associated mucosal tissues. Colitis with clinical and histological features of inflammatory bowel disease is observed in patients with CVID, with variable frequency ranging from 2 to 13% [53]. A new clinical entity termed CVID colitis has been introduced recently due to its abnormal histological profile [54]. This disease is difficult to control in many patients and frequently remains in an active state [55]. Previous studies have demonstrated a number of abnormalities within the gastrointestinal tract, including distortion of crypt architecture, villus blunting and nodular lymphoid hyperplasia [53]. In our cohort, 11 of 35 of CVID patients suffering from chronic diarrhoea displayed higher sCD14 concentrations, although the difference to subjects without diarrhoea was not statistically significant. Increased serum levels of sCD14 and LPS were observed previously in patients with active stages of ulcerous colitis and Crohn's disease compared with inactive stages of the diseases, due probably to increased absorption of macromolecules [15,56].
The observed elevated levels of sCD14 in an absence of overt endotoxaemia in CVID patients can be explained by other mechanisms. sCD14 production may be induced by LPS-independent pathways. High quantities of sCD14 are released following stimulation of monocytes or neutrophils with tumour necrosis factor (TNF)-α in the absence of LPS (Z.H., unpublished observation). Previous studies show that large quantities of sCD14 are released from intracellular store granules of activated granulocytes [35–37]. The intracellular origin of sCD14 is supported further by the fact that sCD14 level in serum exceeds by 1–2 logs that of the cell membrane-bound receptor [57]. Normalized per population frequency, neutrophils may represent the principal sCD14-producing population in blood. Thus, the levels of sCD14 may be a reflection of neutrophil and monocyte activation, rather than an indication of endotoxaemia. This is supported further by the fact that higher sCD14 levels are found in patients with crystal-induced arthritis than those with infection-mediated arthritis, and elevated sCD14 levels are observed in conditions that are not associated with direct bacterial exposure [38,42–44].
Increased serum sCD14 levels have been reported previously to be associated with altered liver function, inflammation and hepatopathy [10–14]. Although this phenomenon has not been elucidated fully, various mechanisms, including bacterial overgrowth, local immune alterations, increased small intestinal permeability and portal hypertension, have been suggested [58,59]. Altered liver function is relatively common in CVID. Ward et al. reported abnormal liver function in 47 of 108 patients with CVID, nodular regenerative hyperplasia (NRH) being the most frequent histopathological finding in patients who underwent liver biopsy [60]. Signs of portal hypertension such as ascites or variceal haemorrhages were observed in five of 13 patients with NRH [60]. In a different CVID cohort, portal hypertension was observed in 17 of 34 (50%) of patients with hepatopathy [61]. In our cohort, the inverse correlation between sCD14 and the number of thrombocytes, a commonly used indicator of portal hypertension [14,49], did not reach statistical significance. There was no correlation between sCD14 and AST or ALT levels in CVID patients. All these exclude disturbed liver function as a significant mechanism leading to the increased sCD14 level. The observed negative correlation of serum albumin with sCD14 may be related to the fact that albumin behaves as a negative acute-phase protein [62]. It has been suggested that sCD14 is an acute-phase protein produced by hepatocytes following stimulation with interleukin (IL)-6 [38–41,63]. However, sCD14 production by hepatocytes is relatively low and is not likely to account for the high sCD14 concentration present in serum. In contrast, Kupffer cells produce sCD14, and local LPS-mediated activation of Kupffer cells may account for the release of sCD14 to circulation [64–66].
Elevated levels of sCD25 (sIL-2Rα) have also been reported previously in patients with autoimmune disease, cancer, inflammation and infection [50–52,67], such as in patients with CVID [68]. As demonstrated in Fig. 4, we observed a highly significant correlation between sCD14 and sCD25 in CVID subjects. sCD25 is produced by activated T cells and is released by a proteolytic cleavage of surface CD25 by enzymes referred to collectively as ‘sheddases’, including neutrophil-derived elastase and metalloproteinase-9 [69,70]. It is feasible that the sustained activation of blood myeloid populations may result in the release of sheddases and cleavage of CD25 from the surface of T cells. sCD25 may act as a reservoir for IL-2 in circulation leading to a prolonged persistence of IL-2 signalling.
Both T [4–8] and B [5,46,71] cell abnormalities are frequent in CVID patients. Searching for abnormalities of sCD14 levels and T cell subpopulations, we observed correlations between the decreased frequency of CD4+ cells and T cell activation (expression of HLA-DR on both CD4+ and CD8+ cells and expression of CD38 on CD8+ cells), suggesting a potential link between these abnormalities. In contrast, there was no correlation between the frequencies of B cell differentiation stages and sCD14 and, more importantly, there were no differences in serum sCD14 levels between groups of patients stratified according to EUROclass classification, suggesting a lack of relationship between B cell abnormalities in CVID and the presence of stimuli leading to an increase of sCD14.
The results of this study may have important implications for our understanding of the pathogenesis of other conditions, such as HIV-1/AIDS, characterized by chronic immune activation. In HIV-1 and CVID patients, a decrease in the absolute numbers of circulating CD4+ T cells but not CD8+ T cells is observed, and the activation profile of CD4+ and CD8+ T cells is strikingly similar. Importantly, the decrease in the frequency of CD4+ T cells in CVID occurs in an absence of a virus specifically targeting CD4+ T cells. Although it remains to be established whether chronic T cell activation is the major cause of CD4+ T cell lymphopenia in CVID, our findings provide a clue for the hypothesis that the depletion of CD4+ T cells in HIV-1-infected individuals may be a consequence of chronic T cell activation and high T cell turnover, rather than virus-mediated depletion of CD4+ T cells. CD8+ T cells are less sensitive to the effect of chronic activation than CD4+ T cells due to the differences in their regenerative capacity [72], as seen in chemotherapy-induced depletion or stem cell transplantation [73,74].
In conclusion, the data presented here suggest that sCD14 production in CVID is driven by inflammatory processes, independent of LPS stimulation, although a limited microbial translocation associated with altered liver function cannot be excluded. Although a trend towards higher serum sCD14 levels was observed in patients with diarrhoea, bronchiectasis, splenomegaly and granuloma, we could not document any correlation of sCD14 serum levels with these clinical characteristics or the EUROclass classification of CVID patients in our cohort. The fact that no significant change of sCD14 concentration was observed in IgAD subjects suggests that disturbed IgA-mediated protection of colon mucosa does not play a significant role in the stimulation of sCD14 production in IgAD or, most probably, in CVID patients. Further studies with larger cohort of well-defined CVID patients, mainly in respect of bowel pathology and other inflammatory conditions, may help to characterize precisely the mechanisms of T cell activation and regulation of sCD14 levels in IgAD and CVID.
Acknowledgments
This study was supported by grants awarded by the Czech Ministry of Health, Czech Republic (10398-3, 11414-5; J.L., M.V.) and National Institutes of Health, USA (AI074438; Z.H.). The authors thank Dr Jiri Mestecky and Dr Charles Elson for their comments and a critical reading of this manuscript. UAB Center for AIDS Research (CFAR) flow cytometry core was instrumental in the analysis of samples by the MILLIPLEX MAP assay.
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Chapel H, Cunningham-Rundles C. Update in understanding common variable immunodeficiency disorders (CVIDs) and the management of patients with these conditions. Br J Haematol. 2009;145:709–727. doi: 10.1111/j.1365-2141.2009.07669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latiff AH, Kerr MA. The clinical significance of immunoglobulin A deficiency. Ann Clin Biochem. 2007;44:131–139. doi: 10.1258/000456307780117993. [DOI] [PubMed] [Google Scholar]
- 3.Hammarström L, Vorechovsky I, Webster D. Selective IgA deficiency (SIgAD) and common variable immunodeficiency (CVID) Clin Exp Immunol. 2000;120:225–231. doi: 10.1046/j.1365-2249.2000.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guazzi V, Aiuti F, Mezzaroma I, et al. Assessment of thymic output in common variable immunodeficiency patients by evaluation of T cell receptor excision circles. Clin Exp Immunol. 2002;129:346–353. doi: 10.1046/j.1365-2249.2002.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlková M, Thon V, Sárfyová M, et al. Age dependency and mutual relations in T and B lymphocyte abnormalities in common variable immunodeficiency patients. Clin Exp Immunol. 2006;143:373–379. doi: 10.1111/j.1365-2249.2006.02999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litzman J, Vlkova M, Pikulova Z, Stikarovska D, Lokaj J. T and B lymphocyte subpopulations and activation/differentiation markers in patients with selective IgA deficiency. Clin Exp Immunol. 2007;147:249–254. doi: 10.1111/j.1365-2249.2006.03274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aspalter RM, Sewell WA, Dolman K, Farrant J, Webster AD. Deficiency in circulating natural killer (NK) cell subsets in common variable immunodeficiency and X-linked agammaglobulinaemia. Clin Exp Immunol. 2000;121:506–514. doi: 10.1046/j.1365-2249.2000.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nechvatalova J, Pikulova Z, Stikarovska D, Pesak S, Vlkova M, Litzman J. B-lymphocyte subpopulations in patients with selective IgA deficiency. J Clin Immunol. 2012;32:441–448. doi: 10.1007/s10875-012-9655-6. [DOI] [PubMed] [Google Scholar]
- 9.Marashi SM, Raeiszadeh M, Workman S, et al. Inflammation in common variable immunodeficiency is associated with a distinct CD8(+) response to cytomegalovirus. J Allergy Clin Immunol. 2011;127:1385–1393. doi: 10.1016/j.jaci.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jirillo E, Caccavo D, Magrone T, et al. The role of the liver in the response to LPS: experimental and clinical findings. J Endotoxin Res. 2002;8:319–327. doi: 10.1179/096805102125000641. [DOI] [PubMed] [Google Scholar]
- 11.Urbaschek R, McCuskey RS, Rudi V, et al. Endotoxin, endotoxin-neutralizing-capacity, sCD14, sICAM-1, and cytokines in patients with various degrees of alcoholic liver disease. Alcohol Clin Exp Res. 2001;25:261–268. [PubMed] [Google Scholar]
- 12.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- 13.Harte AL, da Silva NF, Creely SJ, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandler NG, Koh C, Roque A, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220–1230. doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastor Rojo O, López San Román A, Albéniz Arbizu E, de la Hera Martínez A, Ripoll Sevillano E, Albillos Martínez A. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:269–277. doi: 10.1002/ibd.20019. [DOI] [PubMed] [Google Scholar]
- 16.Estes JD, Harris LD, Klatt NR, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010;6:e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotler DP. HIV infection and the gastrointestinal tract. AIDS. 2005;19:107–117. doi: 10.1097/00002030-200501280-00002. [DOI] [PubMed] [Google Scholar]
- 18.Sharpstone D, Neild P, Crane R, et al. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut. 1999;45:70–76. doi: 10.1136/gut.45.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George MD, Reay E, Sankaran S, Dandekar S. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J Virol. 2005;79:2709–2719. doi: 10.1128/JVI.79.5.2709-2719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 21.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hel Z, McGhee JR, Mestecky J. HIV infection: first battle decides the war. Trends Immunol. 2006;27:274–281. doi: 10.1016/j.it.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS–host cell interactions. J Endotoxin Res. 2005;11:225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 25.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–2925. [PubMed] [Google Scholar]
- 26.Gioannini TL, Weiss JP. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol Res. 2007;39:249–260. doi: 10.1007/s12026-007-0069-0. [DOI] [PubMed] [Google Scholar]
- 27.Miyake K. Roles for accessory molecules in microbial recognition by Toll-like receptors. J Endotoxin Res. 2006;12:195–204. doi: 10.1179/096805106X118807. [DOI] [PubMed] [Google Scholar]
- 28.Bazil V, Strominger JL. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991;147:1567–1574. [PubMed] [Google Scholar]
- 29.Hiki N, Berger D, Prigl C, et al. Endotoxin binding and elimination by monocytes: secretion of soluble CD14 represents an inducible mechanism counteracting reduced expression of membrane CD14 in patients with sepsis and in a patient with paroxysmal nocturnal hemoglobinuria. Infect Immun. 1998;66:1135–1141. doi: 10.1128/iai.66.3.1135-1141.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landmann R, Knopf HP, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64:1762–1769. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cross AS, Opal SM, Warren HS, et al. Active immunization with a detoxified Escherichia coli J5 lipopolysaccharide group B meningococcal outer membrane protein complex vaccine protects animals from experimental sepsis. J Infect Dis. 2001;183:1079–1086. doi: 10.1086/319297. [DOI] [PubMed] [Google Scholar]
- 32.Kitchens RL, Thompson PA, Viriyakosol S, O'Keefe GE, Munford RS. Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. J Clin Invest. 2001;108:485–493. doi: 10.1172/JCI13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ketchum PA, Novitsky TJ. Assay of endotoxin by limulus amebocyte lysate. Methods Mol Med. 2000;36:3–12. doi: 10.1385/1-59259-216-3:3. [DOI] [PubMed] [Google Scholar]
- 34.Eichbaum EB, Harris HW, Kane JP, Rapp JH. Chylomicrons can inhibit endotoxin activity in vitro. J Surg Res. 1991;51:413–416. doi: 10.1016/0022-4804(91)90143-a. [DOI] [PubMed] [Google Scholar]
- 35.Sohn EJ, Paape MJ, Bannerman DD, Connor EE, Fetterer RH, Peters RR. Shedding of sCD14 by bovine neutrophils following activation with bacterial lipopolysaccharide results in down-regulation of IL-8. Vet Res. 2007;38:95–108. doi: 10.1051/vetres:2006052. [DOI] [PubMed] [Google Scholar]
- 36.Rodeberg DA, Morris RE, Babcock GF. Azurophilic granules of human neutrophils contain CD14. Infect Immun. 1997;65:4747–4753. doi: 10.1128/iai.65.11.4747-4753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Detmers PA, Zhou D, Powell D, Lichenstein H, Kelley M, Pironkova R. Endotoxin receptors (CD14) are found with CD16 (Fc gamma RIII) in an intracellular compartment of neutrophils that contains alkaline phosphatase. J Immunol. 1995;155:2085–2095. [PubMed] [Google Scholar]
- 38.Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C. CD14 is an acute-phase protein. J Immunol. 2004;172:4470–4479. doi: 10.4049/jimmunol.172.7.4470. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Khemlani LS, Shapiro RA, et al. Expression of CD14 by hepatocytes: upregulation by cytokines during endotoxemia. Infect Immun. 1998;66:5089–5098. doi: 10.1128/iai.66.11.5089-5098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su GL, Dorko K, Strom SC, Nussler AK, Wang SC. CD14 expression and production by human hepatocytes. J Hepatol. 1999;31:435–442. doi: 10.1016/s0168-8278(99)80034-8. [DOI] [PubMed] [Google Scholar]
- 41.Pan Z, Zhou L, Hetherington CJ, Zhang DE. Hepatocytes contribute to soluble CD14 production, and CD14 expression is differentially regulated in hepatocytes and monocytes. J Biol Chem. 2000;275:36430–36435. doi: 10.1074/jbc.M003192200. [DOI] [PubMed] [Google Scholar]
- 42.Egerer K, Feist E, Rohr U, Pruss A, Burmester GR, Dorner T. Increased serum soluble CD14, ICAM-1 and E-selectin correlate with disease activity and prognosis in systemic lupus erythematosus. Lupus. 2000;9:614–621. doi: 10.1191/096120300678828749. [DOI] [PubMed] [Google Scholar]
- 43.Takeshita S, Nakatani K, Tsujimoto H, Kawamura Y, Kawase H, Sekine I. Increased levels of circulating soluble CD14 in Kawasaki disease. Clin Exp Immunol. 2000;119:376–381. doi: 10.1046/j.1365-2249.2000.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nockher WA, Wigand R, Schoeppe W, Scherberich JE. Elevated levels of soluble CD14 in serum of patients with systemic lupus erythematosus. Clin Exp Immunol. 1994;96:15–19. doi: 10.1111/j.1365-2249.1994.tb06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93:190–197. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 46.Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 47.Malphettes M, Gérard L, Carmagnat M, et al. Late-onset combined immune deficiency: a subset of common variable immunodeficiency with severe T cell defect. Clin Infect Dis. 2009;49:1329–1338. doi: 10.1086/606059. [DOI] [PubMed] [Google Scholar]
- 48.Lakatos PL, Kiss LS, Palatka K, et al. Serum lipopolysaccharide-binding protein and soluble CD14 are markers of disease activity in patients with Crohn's disease. Inflamm Bowel Dis. 2011;17:767–777. doi: 10.1002/ibd.21402. [DOI] [PubMed] [Google Scholar]
- 49.Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000–1007. doi: 10.1016/j.jhep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Greenberg SJ, Marcon L, Hurwitz BJ, Waldmann TA, Nelson DL. Elevated levels of soluble interleukin-2 receptors in multiple sclerosis. N Engl J Med. 1988;319:1019–1020. doi: 10.1056/NEJM198810133191517. [DOI] [PubMed] [Google Scholar]
- 51.Makis AC, Galanakis E, Hatzimichael EC, Papadopoulou ZL, Siamopoulou A, Bourantas KL. Serum levels of soluble interleukin-2 receptor alpha (sIL-2Ralpha) as a predictor of outcome in brucellosis. J Infect. 2005;51:206–210. doi: 10.1016/j.jinf.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 52.Giordano C, Galluzzo A, Marco A, et al. Increased soluble interleukin-2 receptor levels in the sera of type 1 diabetic patients. Diabetes Res. 1988;8:135–138. [PubMed] [Google Scholar]
- 53.Agarwal S, Smereka P, Harpaz N, Cunningham-Rundles C, Mayer L. Characterization of immunologic defects in patients with common variable immunodeficiency (CVID) with intestinal disease. Inflamm Bowel Dis. 2011;17:251–259. doi: 10.1002/ibd.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalha I, Sellin JH. Common variable immunodeficiency and the gastrointestinal tract. Curr Gastroenterol Rep. 2004;6:377–383. doi: 10.1007/s11894-004-0053-y. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal S, Mayer L. Gastrointestinal manifestations in primary immune disorders. Inflamm Bowel Dis. 2010;16:703–711. doi: 10.1002/ibd.21040. [DOI] [PubMed] [Google Scholar]
- 56.Miki K, Moore DJ, Butler RN, Southcott E, Couper RT, Davidson GP. The sugar permeability test reflects disease activity in children and adolescents with inflammatory bowel disease. J Pediatr. 1998;133:750–754. doi: 10.1016/s0022-3476(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 57.Arias MA, Rey Nores JE, Vita N, et al. Cutting edge: human B cell function is regulated by interaction with soluble CD14: opposite effects on IgG1 and IgE production. J Immunol. 2000;164:3480–3486. doi: 10.4049/jimmunol.164.7.3480. [DOI] [PubMed] [Google Scholar]
- 58.Almeida J, Galhenage S, Yu J, Kurtovic J, Riordan SM. Gut flora and bacterial translocation in chronic liver disease. World J Gastroenterol. 2006;12:1493–1502. doi: 10.3748/wjg.v12.i10.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Tsao G, Wiest R. Gut microflora in the pathogenesis of the complications of cirrhosis. Best Pract Res Clin Gastroenterol. 2004;18:353–372. doi: 10.1016/j.bpg.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 60.Ward C, Lucas M, Piris J, Collier J, Chapel H. Abnormal liver function in common variable immunodeficiency disorders due to nodular regenerative hyperplasia. Clin Exp Immunol. 2008;153:331–337. doi: 10.1111/j.1365-2249.2008.03711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malamut G, Ziol M, Suarez F, et al. Nodular regenerative hyperplasia: the main liver disease in patients with primary hypogammaglobulinemia and hepatic abnormalities. J Hepatol. 2008;48:74–82. doi: 10.1016/j.jhep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 62.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 63.Meuleman P, Steyaert S, Libbrecht L, et al. Human hepatocytes secrete soluble CD14, a process not directly influenced by HBV and HCV infection. Clin Chim Acta. 2006;366:156–162. doi: 10.1016/j.cca.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 64.Matsuura K, Ishida T, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Upregulation of mouse CD14 expression in Kupffer cells by lipopolysaccharide. J Exp Med. 1994;179:1671–1676. doi: 10.1084/jem.179.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomita M, Yamamoto K, Kobashi H, Ohmoto M, Tsuji T. Immunohistochemical phenotyping of liver macrophages in normal and diseased human liver. Hepatology. 1994;20:317–325. [PubMed] [Google Scholar]
- 66.Sandler NG, Koh C, Roque A, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220–1230. doi: 10.1053/j.gastro.2011.06.063. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Witkowska AM. On the role of sIL-2R measurements in rheumatoid arthritis and cancers. Mediators Inflamm. 2005;2005:121–130. doi: 10.1155/MI.2005.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.North ME, Spickett GP, Webster AD, Farrant J. Raised serum levels of CD8, CD25 and beta 2-microglobulin in common variable immunodeficiency. Clin Exp Immunol. 1991;86:252–255. doi: 10.1111/j.1365-2249.1991.tb05805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bank U, Reinhold D, Schneemilch C, Kunz D, Synowitz HJ, Ansorge S. Selective proteolytic cleavage of IL-2 receptor and IL-6 receptor ligand binding chains by neutrophil-derived serine proteases at foci of inflammation. J Interferon Cytokine Res. 1999;19:1277–1287. doi: 10.1089/107999099312957. [DOI] [PubMed] [Google Scholar]
- 70.Sheu BC, Hsu SM, Ho HN, Lien HC, Huang SC, Lin RHA. novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res. 2001;61:237–242. [PubMed] [Google Scholar]
- 71.Warnatz K, Schlesier M. Flowcytometric phenotyping of common variable immunodeficiency. Cytometry B Clin Cytom. 2008;74:261–271. doi: 10.1002/cyto.b.20432. [DOI] [PubMed] [Google Scholar]
- 72.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 73.Atkinson K, Hansen JA, Storb R, Goehle S, Goldstein G, Thomas ED. T-cell subpopulations identified by monoclonal antibodies after human marrow transplantation. I. Helper-inducer and cytotoxic-suppressor subsets. Blood. 1982;59:1292–1298. [PubMed] [Google Scholar]
- 74.Watanabe N, De Rosa SC, Cmelak A, Hoppe R, Herzenberg LA, Roederer M. Long-term depletion of naive T cells in patients treated for Hodgkin's disease. Blood. 1997;90:3662–3672. [PubMed] [Google Scholar]


