Abstract
B1 B cells represent a unique subset of B lymphocytes distinct from conventional B2 B cells, and are important in the production of natural antibodies. A potential human homologue of murine B1 cells was defined recently as a CD20+CD27+CD43+ cell. Common variable immunodeficiency (CVID) is a group of heterogeneous conditions linked by symptomatic primary antibody failure. In this preliminary report, we examined the potential clinical utility of introducing CD20+CD27+CD43+ B1 cell immunophenotyping as a routine assay in a diagnostic clinical laboratory. Using a whole blood assay, putative B1 B cells in healthy controls and in CVID patients were measured. Peripheral blood from 33 healthy donors and 16 CVID patients were stained with relevant monoclonal antibodies and underwent flow cytometric evaluation. We established a rapid, whole blood flow cytometric assay to investigate putative human B1 B cells. Examination of CD20+CD27+CD43+ cells is complicated by CD3+CD27+CD43hi T cell contamination, even when using stringent CD20 gating. These can be excluded by gating on CD27+CD43lo–int B cells. Although proportions of CD20+CD27–CD43lo–int cells within B cells in CVID patients were decreased by 50% compared to controls (P < 0·01), this was not significant when measured as a percentage of all CD27+ B cells (P = 0·78). Immunophenotypic overlap of this subset with other innate-like B cells described recently in humans is limited. We have shown that putative B1 B cell immunophenotyping can be performed rapidly and reliably using whole blood. CD20+CD27+CD43lo–int cells may represent a distinct B1 cell subset within CD27+ B cells. CVID patients were not significantly different from healthy controls when existing CD27+ B cell deficiencies were taken into account.
Keywords: B cells, common variable immunodeficiency (CVID), diagnostics, immunodeficiency–primary, immunoglobulins
Introduction
B1 cells were first described by Hayakawa et al. in mice as a small population of splenic B cells expressing a pan-T cell marker, CD5, and spontaneously secreting immunoglobulin (Ig)M [1]. They represent a unique subset of B cells ontogenetically and phenotypically and are functionally distinct from conventional B2 cells. B1 cells are generated in liver and bone marrow during the fetal and neonatal period and populate predominantly coelomic cavities and intestinal lamina propria [2–4]. When the peripheral pool is established further de-novo generation is maintained, mainly by self-renewal [5]. One of the characteristic features of B1 cells is the enrichment of their repertoire for poly- and self-reactive specificities. Hayakawa et al. suggested that B1 cells may be positively selected for their auto-antigenic specificity [6].
Although B1 cells present antigens efficiently and can prime T cells, their major role lies in the secretion of natural immunoglobulins in the absence of exogenous antigenic stimulation [7]. These low-affinity polyreactive IgM/IgA antibodies are encoded typically by germline sequences with minimal somatic mutations and non-templated nucleotide insertions [8]. Natural immunoglobulins work not only as an instant defence against invading pathogens, but also as a ‘silent’ non-inflammatory clearance mechanism for apoptotic bodies and other altered self-antigens [9–11].
Most of our current knowledge about the B1 cell role in the immune system is based on experiments in mice. Although much effort has been made to find a human homologue of murine B1 cells, its existence remains controversial. Recently, a ‘novel’ human B1 cell phenotype, CD20+CD27+CD43+CD70–, was proposed as this specific B cell subset showed three key features of B1 cells (spontaneous IgM secretion, tonic intracellular signalling and efficient T cell stimulation) [12]. Subsequently, further division of CD27+ B cells known as memory B cells into ‘true’ memory B cells (CD27+CD43–) and ‘B1’ cells (CD27+CD43+) was suggested according to their CD43 expression [12]. At least two other innate-like B cell subsets have been described in humans, which resemble murine B1 cells both phenotypically and functionally. One of these, termed ‘unswitched’ IgM+IgD+ memory B cells, were demonstrated to be circulating counterparts of splenic marginal zone B cells [13]. The other population comprised CD21lowCD23– CD38lowCD86hi B cells with polyclonal unmutated IgM and IgD, similar to murine B1 cells. These were found to be expanded in peripheral tissues such as the bronchoalveolar space [14]. These cells were described initially in some patients with common variable immunodeficiency (CVID), especially in those with splenomegaly and granulomatous disease [15].
CVID are a group of conditions linked by symptomatic primary antibody failure, resulting in the lack of serum IgG and IgA with variable levels of IgM plus low/absent specific antibody responses [16]. Understanding the aetiopathogenesis of CVID is complicated by the enormous heterogeneity of this syndrome [17]. Defects in B, T and dendritic cell compartments have been reported, but only a very limited correlation with certain clinical phenotypes has been found [18,19]. One of the most common abnormalities seen in CVID is a reduced number of switched memory B cells, indicating abrogated function of the germinal centre. This defect has been associated with splenomegaly and granulomatous disease [20]. The flow cytometric evaluation of CD27+IgM–IgD– switched memory B cells is performed routinely in diagnostic laboratories, and is an important part of B cell immunophenotyping classification systems in CVID [18,21].
As an important producer of natural IgM and IgA, B1 cells may, hypothetically, be involved in the aetiopathogenesis of CVID. According to their immunophenotype, CD20+CD27+CD43+ B1 cells are part of a heterogeneous CD27+ memory B cell population which is abnormally low in CVID, and should be dissected further [18]. In some CVID B cell classification systems [15] the CD21low B cell subset is often expanded in CVID patients and has been reported recently to have some features similar to B1 cells [14].
In this study we evaluated the ability of a recently described flow cytometric assay [12] to detect CD20+CD27+CD43+ B1 cells and examined its potential flexibility to be adapted for use as a routine diagnostic test. Assessment of this assay revealed a number of technical aspects that needed to be addressed to ensure that accurate measurements of human B1 cells were being ascertained. We have termed the human B1 B cells that our assay measures as ‘putative human B1 cells’. Once modified, a cohort of healthy control samples were analysed to generate a normal range for the proportion of putative human B1 cells present in the B cell compartment. This was then compared to putative human B1 cell proportions analysed in a group of CVID patients and the results discussed.
Methods
Patients and healthy donors
Thirty-three healthy donors were recruited from hospital staff with median age 32 years (range: 23–66) and sex ratio (male : female) 1:2. For the analyses comparing CVID patients with healthy donors, a special subgroup of healthy donors (n = 16) was matched to CVID patients according to their sex and age.
Sixteen patients who met the Pan-American Group for Immunodeficiency/European Society for Immunodeficiencies (PAGID/ESID) diagnostic criteria for CVID participated in this study. Patients' median age was 47 years (range: 25–80), sex ratio (male : female) was 1:1. All patients were on stable immunoglobulin substitution. Patients' past medical histories (including complications and serum IgM/IgA levels) were provided by the Department of Clinical Immunology at the John Radcliffe Hospital, Oxford. The study has been approved by the Central Oxfordshire Research Ethics Committee (05/Q1605/88). Informed consents were obtained from all the enrolled patients and healthy donors.
Preparation of peripheral blood mononuclear cells (PBMCs)
PBMCs were separated from heparinized peripheral blood by density gradient separation using Lymphoprep™ gradient solution (Axis-Schield, Oslo, Norway). The cell suspension was washed twice in sterile phosphate-buffered saline (PBS). For monoclonal antibody staining, the cell concentration was adjusted to 2·5 × 106 per ml (in sterile PBS).
Preparation of whole blood
For the preparation of whole blood lymphocytes, the methodology described by Ferry et al. was used [22].
Monoclonal antibody staining
One hundred μl of the prepared PBMC suspension or washed whole blood was added to the monoclonal antibody cocktail for fluorescence activated cell sorter (FACS) staining. The antibody cocktail included CD20-allophycocyanin-cyanin 7 (APC-Cy7) (Becton Dickinson, Oxford, UK), CD27-fluorescein isothiocyanate (FITC) (Dako, Glostrup, Denmark), CD43-phycoerythrin (PE) (Becton Dickinson), IgM-Cy5 (Jackson Laboratories, Newmarket, UK), CD21-PECy5 (Becton Dickinson) and CD5-PE-Cy7 (Becton Dickinson). Additional flow cytometric analyses were performed using CD3-PE-Cy7, CD27-APC, CD38-PE and IgD-PE obtained from Becton Dickinson; CD19-PE-Cy5 and CD21-FITC from Beckman Coulter (High Wycombe, UK).
FACS analysis
Stained cells were read on the FACS Canto II (Becton Dickinson, Franklin Lakes, NJ, USA) and data analysed using BD FACS Diva software version 6·0. Lymphocytes were examined using forward/side-scatter gating; B cells were identified subsequently as CD19+ or CD20+ cells within the lymphocyte population. Each tube was run until 10 000 events were recorded in the B cell gate or the tube was exhausted. Our gating strategy was based on fluorescence minus one technique (FMO) to determine correctly the positivity in expression of each considered surface marker.
Statistical analysis
Statistical analysis was performed using Microsoft Excel and Prism GraphPad version 5 Software (GraphPad Prism, San Diego, CA, USA). Medians and sample interquartile ranges (IQR) were used to represent the average values and variability unless another data presentation method is stated explicitly. The non-parametric Mann–Whitney U-test was used to determine the significance of differences between patient and control group, unless stated otherwise. For all analyses, P < 0·05 was considered to be statistically significant.
Results
Correlation of detection of CD20+CD27+CD43+ B cells using PBMCs or whole blood
Although the examination of CD27+CD43+ B cells in human peripheral blood has been based so far on PBMC separation [12], we also examined a parallel whole blood staining method to assess its potential benefits for routine diagnostic testing. Testing of the reproducibility of the whole blood method compared to the standard PBMC method showed a significant correlation in the CD27+CD43+ B cell percentages (r = 1·0, P = 0·02) (Fig. 1). This strong correlation led us to fully adopt a whole blood method for all future B1 cell phenotype analysis.
Fig. 1.

Reproducibility of the whole blood method and initial immunophenotypical analysis. Comparison of the whole blood method (WB) and peripheral blood mononuclear cells (PBMCs) method in measured percentages of CD20+CD27+CD43+ cells within CD27+ B cells.
CD20+CD27+CD43+ cells include an important non-B cell contamination
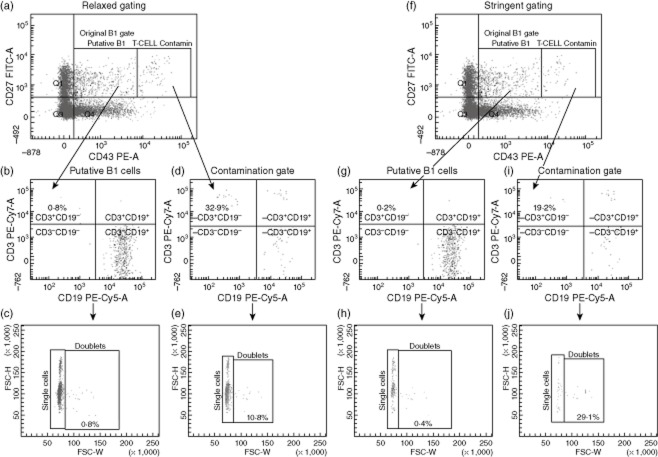
Figure 2a,b shows how the cells were first gated for CD20 and then analysed for CD27 and CD43 expression. It was noted that when B cells were first selected using CD20, it was important that a stringent CD20 gate was set up to prevent an enlarged population of CD27+CD43+hi from appearing (Fig. 2c–f). To assess this further, the CD27+CD43+ quadrant was broken into two smaller regions comprising either CD27+CD43+lo–int cells or CD27+CD43+hi cells (Fig. 2b,d,f). The more stringent the CD20+ gating, the fewer cells that were present in the CD27+CD43hi region (Fig. 2f). This was therefore named the ‘contamination region’, while the CD27+CD43lo–int region was entitled ‘putative B1 cells’ (Fig. 2c,f). We then postulated whether the cells in the contamination region were either T cells expressing CD43 or cell doublets. To examine this further, cells from the pure B1 cell region and the contamination region were analysed for CD3 expression and assessed for size using forward-scatter–pulse width (FSC-W) to indicate the proportion of doublet cells being measured (Fig. 3). Figure 3d,i shows the proportion of contaminating cells that are CD19–CD3+ in both a relaxed and a stringent CD20 gating strategy, respectively. The median proportion of cells within the contamination gate under relaxed CD20 gating that were CD3+CD19– was 31·4% (IQR: 14·5–43·9%), compared to 22·2% (IQR: 17·1–39·7%) CD3+CD19– cells in the contamination gate under stringent CD20 gating (n = 13). More importantly, the median proportion of CD3+CD19– cells present in the ‘putative B1 cell’ with relaxed CD20 gating was 0·6% (IQR: 0·2–1·3%); this was compared to only 0·2% (IQR: 0·0–0·4%) CD3+CD19– cells in the pure B1 cell region with stringent CD20 gating (Fig. 3b,g). These data together indicate that not only is stringent CD20 gating required to help remove contaminants from the CD27+CD43+ B cell compartment but also that CD27+CD43lo–int putative B1 cell gating is required, as the CD27+CD43hi contamination compartment, even with stringent CD20 gating, showed a high percentage of CD3+CD19– cells. Doublet analysis showed a minor contribution to the proportion of contaminated cells compared with single CD3+CD19– cells (Fig. 3e). This was raised slightly in the contamination gate using strict CD20 gating, but was postulated to be due to the reduced number of cells in this region (Fig. 3j). From this point forth all future experiments were carried out using the CD20+CD27+CD43lo–int phenotype as the definition of human putative B1 cells.
Fig. 2.
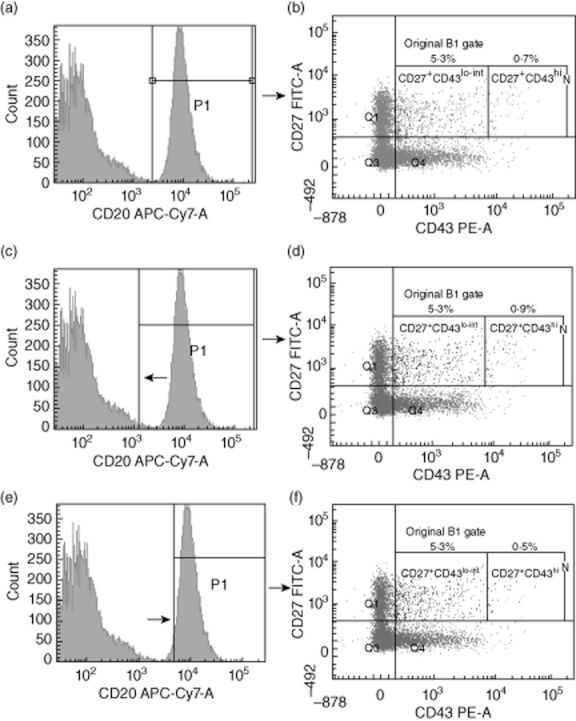
Use of stringent CD20+ gating to reduce the percentage of CD20+CD27+CD43hi cells. Whole blood was stained as described in the Methods. Cells were first selected as lymphocytes using forward/side-scatter (FSC/SSC). These cells were then measured for CD20 expression (a). CD20+ cells were then analysed for CD27 and CD43 expression (b). Relaxed CD20+ gating (c) and stringent CD20+ gating (e) are shown with differences in CD27 and CD43 expression (d,f). A representative sample is shown.
Fig. 3.
Determination of CD20+CD27+CD43lo–int putative B1 cell and CD20+CD27+CD43hi contaminant cell CD3 expression and doublet status. Cells from healthy control samples were gated using either a relaxed CD20 gate (a) or a strict CD20 gate (f). CD20+CD27+CD43lo–int putative B1 cells were analysed in both scenarios for CD3 and CD19 expression (b,g). CD20+CD27+CD43hi contamination cells from both scenarios were also measured for CD3 and CD19 expression (d,i). Percentage doublet cells in each region were measured by forward-scatter–pulse width (FSC-W). The respective doublet plot is shown beneath the CD3/CD19 plot (c,e,h,j). A representative plot is shown (n = 13).
The proportion of CD20+CD27+CD43+ cells but not CD20+CD27+CD43– cells declines with age
Previous reports show that human B1 homologue cells appear to decline with age [12]. The CD20+CD27+CD43lo–int cell percentage within CD20+ and CD27+ B cells was 4·1% (3·3–5·6%) and 18·7% (8·6–23·1%) in the healthy controls [median (IQR)], respectively, with no significant difference between both sexes (P = 0·81) (data not shown). Within CD20+ B cells, we found a moderate negative correlation of the CD20+CD27+CD43lo–int cells proportion with age (r = −0·4, P = 0·02) (data not shown). This correlation became stronger when the proportion of this subpopulation was considered within CD20+CD27+ cells (r = −0·6, P < 0·0001) (data not shown). In contrast, no significant correlation was seen of CD27+CD43– memory B cell percentage with age (data not shown).
CD20+CD27+CD43+ cells show minimal overlap with CD5+ B cells or CD21lo B cells
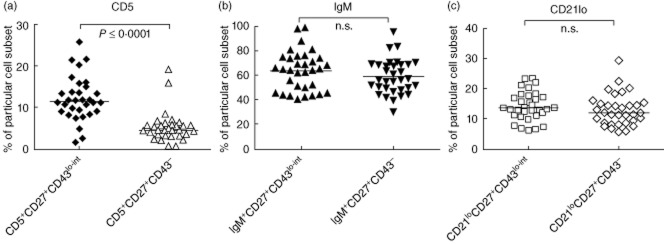
As it has been reported previously that a high percentage of human B1 homologue cells can express IgM or CD5 [12], we examined the expression of surface IgM and CD5 on putative B1 cells using our assay. In addition, we looked at the expression of CD21lo in these cells, as this has been shown to be a potential marker of innate-like B cells [15,23–25].
Investigations using healthy controls (n = 33) revealed that a median of 11·5% (9·0–14·7%) of CD20+CD27+CD43lo–int cells expressed CD5 (Fig. 4a). This proportion was significantly higher compared to the proportion of CD20+CD27+CD43– cells expressing CD5 [4·5% (3·3–5·9%); P = < 0·0001] (Fig. 4a). IgM expression and CD21lo expression were not significantly different in the CD27+CD43lo–int and CD27+CD43– cell populations (P = 0·31 and P = 0·22, respectively) (Fig. 4b,c).
Fig. 4.
Median percentage of CD20+CD27+CD43lo–int or CD20+CD27+CD43– cells expressing either CD5+, CD21lo or IgM+ in healthy controls (n = 33). Whole blood was stained as stated in the Methods. Cells were gated as described in Fig. 2. Scatter graphs show individual percentage values for CD5+ (a), IgM+ (b) or CD21lo (c) cells within the CD20+CD27+CD43lo–int and CD20+CD27+CD43– subpopulations.
Proportions of CD20+CD27+CD43lo–int cells and CD27+CD43– memory B cells in CVID patients are lower compared to controls
A decreased percentage of CD27+ B cells in CVID patients has been described repeatedly, and is one of the key criteria considered in CVID classification systems [18]. We investigated whether this trend is still present after dissecting CD27+ B cells into CD20+CD27+CD43– and CD20+CD27+CD43lo–int cells. The percentages of both these B cell populations in CVID patients were reduced significantly, with less than 50% of the corresponding values in the healthy donors group (P ≤ 0·01) (Fig. 5a,b). Lower CD20+CD27+CD43lo–int cell percentages tended to associate with lower Piqueras categories (Fig. 5c) [21]. To investigate whether the above-reported decrease in the CD20+CD27+CD43lo–int population always corresponds proportionally to the decrease in CD27+CD43– memory B cells in CVID, we also compared their percentages within CD27+ B cells. No significant difference was observed between CVID patients and healthy controls, indicating that decreases seen in CD27+CD43lo–int cell percentages were due probably to an overall decrease in CD20+CD27+ B cells (P = 0·78) (Fig. 5d).
Fig. 5.
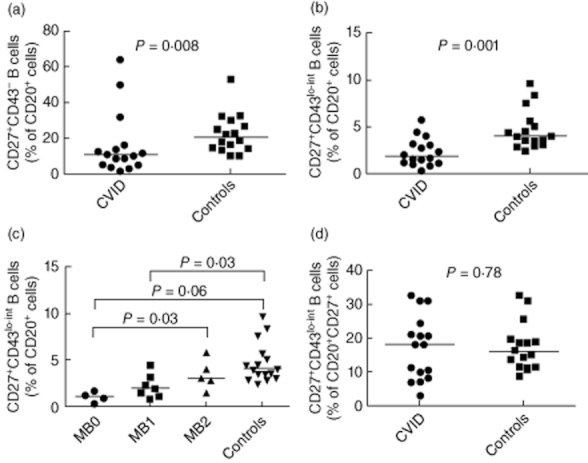
Percentages of CD20+CD27+CD43lo–int cells in common variable immunodeficiency (CVID) patients. Whole blood was stained as stated in the methods. Cells were gated as described in Fig. 2. Percentage of CD27+CD43– cells (a) and CD27+CD43lo–int cells (b) within the CD20+ population were measured in CVID patients (n = 16) and healthy controls (n = 33). (c) Percentage of CD27+CD43lo–int cells within CD20+ cells in CVID patients when stratified into respective Piqueras classifications. (d) Percentage of CD27+CD43lo–int cells within all CD27+ cells in CVID patients and healthy controls.
Expression of CD5, IgM and CD21lo expressing B cells within the CD27+CD43lo–int subpopulation in CVID patients compared to healthy controls
Although the CD5 expression on CD20+CD27+CD43lo–int cells was not significantly different between the healthy control and CVID groups, its variability was higher [7·1% (2·1–15·9%) versus 11·45% (9·0–14·7%), median (IQR); P = 0·09] (Fig. 6a). No association with a specific Piqueras CVID category was observed (data not shown).
Fig. 6.
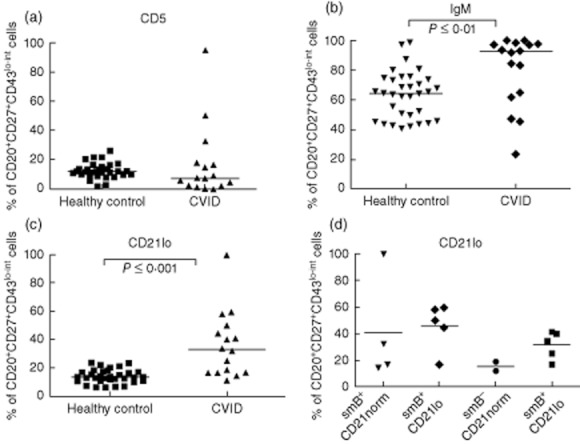
Percentage of CD20+CD27+CD43lo–int cells expressing either CD5, IgM or CD21lo in CVID patients compared to healthy controls. Whole blood was stained as stated in the Methods. Cells were gated as described in Fig. 2. Percentage of CD20+CD27+CD43lo–int cells in common variable immunodeficiency (CVID) patients (n = 16) and healthy controls (n = 33) expressing either CD5 (a), immunoglobulin (Ig)M (b) or CD21lo (c). (d) Percentage of CD20+CD27+CD43lo–int cells expressing CD21lo in CVID patients stratified into respective EUROclass classification groups.
A significantly increased proportion of CD20+CD27+CD43lo–int cells with high expression of surface IgM was seen in the CVID group compared to the healthy controls (P ≤ 0·01) (Fig. 6b). This difference was based on the presence of a distinct subgroup of patients with a lack of switched ‘memory’ (CD27+) B cells. After separation of this subgroup, no significant difference was observed between the remaining CVID patients and their matched controls (data not shown).
An increased percentage (> 20%) of CD21lo cells within CD20+CD27+CD43lo–int cells was found in 10 patients (Fig. 6c). The percentage of CD21lo cells in the CD20+CD27+CD43lo–int subpopulation was not raised significantly compared to the percentage of CD21lo cells in the CD27+CD43– memory cell subpopulation (P = 0·6 data not shown). The percentage of CD21lo expression B cells is a classification criterion used for both the Freiberg and EUROclass classifications of CVID. To analyse the data further, patients were stratified by their EUROclass classification and then compared for CD21lo expression within the CD27+CD43lo–int subpopulation (Fig. 6d). No significant differences could be seen between the difference classification groups, indicating further that the CD21lo expressing B cells within the putative B1 cell subpopulation are probably no more relevant than CD21lo expressing B cells in other B cell compartments.
Discussion
The discovery and subsequent examination of the human counterparts of murine B1 B cells has been complicated by a lack of reliable discriminatory surface markers. Recent identification of a potential human B1 cell phenotype (CD20+CD27+CD43+) provided an opportunity to identify this population rapidly in peripheral blood by flow cytometry for use in a routine diagnostic laboratory [12].
In this study, we established a whole blood method to investigate these putative B1 cells in humans. In clinical work it is well recognized that, where possible, whole blood analysis is the method of choice as it requires minimal blood volumes and minimizes ex-vivo manipulations of clinical specimens, and allows the most accurate quantitation of absolute numbers of B cells (and T cells) in patients' blood [22].
We then examined the technical challenges of using the immunophenotype CD20+CD27+CD43+ as a potential B1 cell signature in peripheral blood. We measured putative B1 B cells in a cohort of healthy controls and a small cohort of patients with CVID, a disease often associated with abnormalities in the CD20+CD27+ population and IgM/IgA production.
The first difficulty complicating examination and accurate measurement of a CD20+CD27+CD43+ putative B1 B cell population was the identification of non-B cell contamination. Initial observations showed that positioning of the CD20 gate for detecting B cells impacted upon the percentage of the CD20+CD27+CD43hi cells, with stringent gating of CD20 B cells resulting in a reduction of these cells in our putative B1 B cell subpopulation. Further analysis showed that a third of CD20+CD27+CD43hi cells expressed CD3 but were negative for CD19. These findings were consistent with previous observations that normal and neoplastic B cells express significantly lower levels of CD43 compared to T cells [26]. In addition, while one study reported the existence of a small population of normal T cells expressing CD20 [27], others claim that this population is a flow cytometry artefact caused by T–B cell doublets [28]. Our analysis identified doublets which were present in higher percentages in the CD27+CD43hi populations compared to CD27+CD43lo–int populations, although this was due probably, in part, to the decreased amount of cells in CD27+CD43hi population. A proportion of the CD20+CD27–CD43hi cells were CD3–CD19+; this is in line with other, more recent reports showing that some CD20+CD27+CD43hi cells could be plasmablasts [29]. Finally, previous work has addressed the possibility that activated conventional memory B2 cells could also up-regulate CD43, and thus further contaminate the B1 population by analysing expression of activation markers such as CD69 and CD70 on the population [12]. Work in this study agreed with previous findings, with < 3% of the CD27+CD43lo–int subpopulation expressing CD69 on their surface (data not shown).
Controversy exists regarding the measurement of the CD20+CD27+CD43lo–int cell subset percentage in the peripheral blood of healthy controls. This study found a median value of 4·1% of all CD20+ B cells and 18·7% of all CD27+ B cells to be CD20+CD27+CD43lo–int. This value differs from the previous reported values of 12·7% of all CD20+ cells and approximately 20% of all CD27+ B cells in healthy controls [12]. However, the age range of these controls is unknown. A subsequent report gave a range of 1–9% of all CD20+ cells to be putative B1 B cells in a further cohort healthy controls although, again, no median age was given for this cohort [30]. This is similar to other groups who reported a value of 2·2% of all CD20+ B cells and a range of 1–25·5% of all CD20+ cells to be human B1 B cells [29,31]. All these values indicate that in the periphery, putative human B1 B cells appear to make up a minor proportion of the circulating B cell population, suggesting that they may behave similarly to murine B1 cells which are predominantly resident in peripheral tissues, in particular the peritoneal cavity [32]. A moderate correlation of CD27+CD43lo–int cell percentage with age was found in this study, with older individuals possessing a smaller percentage compared to younger individuals, correlating with previous reports that also report a decline with age [12,29,31]. These findings highlight the necessity for median age statistics to be known for any given cohort, as this can have an impact on discrepancies seen between study groups.
Of the CD20+CD27+CD43lo–int cells, 11·5% expressing CD5 were observed in this study. This conflicts with previous work which describes 75% of ‘B1 cells’ to be CD5-positive [12]. Although such a high CD5 positivity could be caused by a potential T cell contamination, further data provided by their study showed that this is unlikely, as their ‘B1 cell’ population co-expressed almost exclusively other typical B cell markers (CD19), as shown by confocal microscopy [30].
We found a median surface IgM expression percentage of 64·4% in the CD27+CD43lo–int putative B1 B cell subpopulation in healthy controls. This probably indicates that some cells in this population have undergone class switching [33]. Although the original human B1 homologue cell report demonstrated spontaneous IgM secretion in the CD27+CD43+ subpopulation, quantification of IgM-expressing cells was not reported. Further studies also reported the existence of IgM– cells in CD27+CD43lo–int subpopulations, with one report noting that IgD– cells were more prevalent with increasing age [29,31]. Further analysis of IgM+ cells within the CD27+CD43lo–int subpopulation showed there to be a proportion of IgMhi cells (data not shown). As high expression of surface IgM is one of the discriminatory criteria for murine B1 cells [3], we re-ran our previous immunophenotyping analysis to distinguish between IgMhigh and IgMlo CD20+CD27+CD43lo–int cells. We found a ninefold higher proportion of CD5+ cells within the IgMhigh subset compared to their IgMlow counterparts, which might indicate a closer phenotypic approximation to the ‘B1 cell’ population described previously [12] (data not shown). Nevertheless, discrepancies in the CD20+CD27+CD43+ cell immunophenotype we reported raised the need for a functional study which would match with our FACS results and reconfirm the functional B1 status of these putative B1 cells.
The percentage and immunophenotype differences found in the CD20+CD27+CD43lo–int cell subpopulation in CVID patients compared to healthy controls appeared not to be specific for this B cell subpopulation, but rather reflected a more general immune dysregulation in CVID. This could, potentially, be due to a lack of analysis using absolute counts of cells rather than percentages, which provides a much more accurate measure of difference [34]. We acknowledge this as a limitation of our study. A significantly increased percentage of CD21lo B cells within the CD20+CD27+CD43lo–int subset in CVID patients compared to controls was observed. Although CD21lo B cells are known to have some innate-like features similar to murine B1 cells [14], our analysis showed that the proportion of CD21lo cells in the CD20+CD27+CD43lo–int was not significantly different when compared with the proportion of CD21lo cells found in the CD20+CD27+CD43– cell subpopulation of the same patients. In addition, there was an observed lack of correlation with existing EUROclass classifications on CD21lo B cells; it is therefore likely that B1 cells and CD21lo innate-like B cells are not the same population. Further work investigating CVID and putative B1 B cells should focus on the functional aspects of B1 B cells, as any potential functional abnormalities have yet to be elucidated.
In conclusion, our study showed that it is possible to use a rapid whole blood flow cytometric method to identify and analyse putative human B1 B cells. We demonstrated that CD20+CD27+CD43lo–int cells most probably represent a distinct subset within CD27+ B cells. Our study also revealed the importance of careful gating when analysing flow cytometry results, including the application of stringent CD20 gating and an exclusion gate of CD20+CD27+CD43hi cells for potential T cell, doublet and plasmablast contamination. Work comparing CVID patients with a cohort of healthy controls showed only minor differences in CD20+CD27+CD43lo–int cell numbers when existing CD27+ B cell deficiencies were taken into account. Further work including absolute cell count measurements and functional assays is required with CVID patients to ascertain what role, if any, this B cell subset plays in the pathogenesis of this disease.
Acknowledgments
We would like to thank the patients and controls for their time and generosity. We would also like to thank staff members of the Clinical Immunology Laboratory for their help in this study.
Disclosure
There are no disclosures associated with this work.
References
- 1.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The ‘Ly-1 B’ cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 3.Hayakawa K, Hardy RR, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroese FG, Butcher EC, Stall AM, Lalor PA, Adams S, Herzenberg LA. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol. 1989;1:75–84. doi: 10.1093/intimm/1.1.75. [DOI] [PubMed] [Google Scholar]
- 5.Lalor PA, Herzenberg LA, Adams S, Stall AM. Feedback regulation of murine Ly-1 B cell development. Eur J Immunol. 1989;19:507–513. doi: 10.1002/eji.1830190315. [DOI] [PubMed] [Google Scholar]
- 6.Hayakawa K, Asano M, Shinton SA, et al. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 7.Zhong X, Gao W, Degauque N, et al. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur J Immunol. 2007;37:2400–2404. doi: 10.1002/eji.200737296. [DOI] [PubMed] [Google Scholar]
- 8.Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J Immunol. 1997;158:1175–1186. [PubMed] [Google Scholar]
- 9.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 10.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci USA. 2000;97:1184–1189. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weller S, Braun MC, Tan BK, et al. Human blood IgM ‘memory’ B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakhmanov M, Keller B, Gutenberger S, et al. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci USA. 2009;106:13451–13456. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–1551. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 16.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93:190–197. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 17.Orange JS, Glessner JT, Resnick E, et al. Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127:1360–1367. doi: 10.1016/j.jaci.2011.02.039. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warnatz K, Schlesier M. Flowcytometric phenotyping of common variable immunodeficiency. Cytometry B Clin Cytom. 2008;74:261–271. doi: 10.1002/cyto.b.20432. [DOI] [PubMed] [Google Scholar]
- 19.Mouillot G, Carmagnat M, Gerard L, et al. B-cell and T-cell phenotypes in CVID patients correlate with the clinical phenotype of the disease. J Clin Immunol. 2010;30:746–755. doi: 10.1007/s10875-010-9424-3. [DOI] [PubMed] [Google Scholar]
- 20.Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 21.Piqueras B, Lavenu-Bombled C, Galicier L, et al. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J Clin Immunol. 2003;23:385–400. doi: 10.1023/a:1025373601374. [DOI] [PubMed] [Google Scholar]
- 22.Ferry BL, Jones J, Bateman EA, et al. Measurement of peripheral B cell subpopulations in common variable immunodeficiency (CVID) using a whole blood method. Clin Exp Immunol. 2005;140:532–539. doi: 10.1111/j.1365-2249.2005.02793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasaian MT, Ikematsu H, Casali P. Identification and analysis of a novel human surface CD5- B lymphocyte subset producing natural antibodies. J Immunol. 1992;148:2690–2702. [PMC free article] [PubMed] [Google Scholar]
- 24.Weller S, Mamani-Matsuda M, Picard C, et al. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J Exp Med. 2008;205:1331–1342. doi: 10.1084/jem.20071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackenzie LE, Youinou PY, Hicks R, Yuksel B, Mageed RA, Lydyard PM. Auto- and polyreactivity of IgM from CD5+ and CD5– cord blood B cells. Scand J Immunol. 1991;33:329–335. doi: 10.1111/j.1365-3083.1991.tb01778.x. [DOI] [PubMed] [Google Scholar]
- 26.Lynch EF, Jones PA, Swerdlow SH. CD43 and CD5 antibodies define four normal and neoplastic B-cell subsets: a three-color flow cytometric study. Cytometry. 1995;22:223–231. doi: 10.1002/cyto.990220310. [DOI] [PubMed] [Google Scholar]
- 27.Wilk E, Witte T, Marquardt N, et al. Depletion of functionally active CD20+ T cells by rituximab treatment. Arthritis Rheum. 2009;60:3563–3571. doi: 10.1002/art.24998. [DOI] [PubMed] [Google Scholar]
- 28.Henry C, Ramadan A, Montcuquet N, et al. CD3+CD20+ cells may be an artifact of flow cytometry: comment on the article by Wilk et al. Arthritis Rheum. 2010;62:2561–2563. doi: 10.1002/art.27527. author reply 3–5. [DOI] [PubMed] [Google Scholar]
- 29.Descatoire MWJ, Reynaud CA, Weller S. A human equivalent of mouse B-1 cells? J Exp Med. 2011;208:2563–2564. doi: 10.1084/jem.20112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells are CD3–: a reply to ‘A human equivalent of mouse B-1 cells?’ and ‘The nature of circulating CD27+CD43+ B cells’. J Exp Med. 2011;208:2566–2569. doi: 10.1084/jem.20111761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peres-Andres M, Grosserichter-Wagener C, Teodosio C, van Dongen JJ, Orfao A, van Zelm MC. The nature of circulating CD27+CD43+ B cells. J Exp Med. 2011;208:2565–2569. doi: 10.1084/jem.20112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nisitani S, Murakami M, Akamizu T, et al. Preferential localization of human CD5+ B cells in the peritoneal cavity. Scand J Immunol. 1997;46:541–545. doi: 10.1046/j.1365-3083.1997.d01-166.x. [DOI] [PubMed] [Google Scholar]
- 33.Kaminski DA, Stavnezer J. Enhanced IgA class switching in marginal zone and B1 B cells relative to follicular/B2 B cells. J Immunol. 2006;177:6025–6029. doi: 10.4049/jimmunol.177.9.6025. [DOI] [PubMed] [Google Scholar]
- 34.Bateman EAL, Ayers L, Sadler R, et al. T cell phenotypes in patients with common variable immunodeficiency disorders: associations with clinical phenotypes in comparison with other groups with recurrent infections. Clin Exp Immunol. 2012 doi: 10.1111/j.1365-2249.2012.04643.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]