Abstract
One approach to fight against schistosomiasis is to develop an efficient vaccine. Schistosoma mansoni tetraspanning orphan receptor (SmTOR) might be a vaccine candidate, as it is a tegument membrane protein expressed most highly in cercariae. In this study we characterized the recombinant first extracellular domain of SmTOR (rSmTORed1) as having the expected property to bind C2 of complement similarly to a smaller peptide of the same domain, and to produce specific and high-titre antibodies in BALB/c mice immunized using complete Freund's adjuvant/incomplete Freund's adjuvant (CFA/IFA). Immunization was protective against parasite infection, as demonstrated by a significant decrease in worm burden in immunized BALB/c mice versus the control groups over two independent trials [64 and 45% reduction for mean adult worm burden in immunized versus phosphate-bufferd saline (PBS) injected mice]. Interestingly, infection by itself did not lead to the generation of anti-rSmTORed1 antibodies, corresponding to the low frequency of specific anti-rSmTORed1 antibodies detected in the sera of patients infected with S. mansoni (2/20; 10%). These data suggest that, as opposed to the natural infection during which SmTOR induces antibodies only rarely, immunization with its smaller first extracellular domain might be more efficient.
Keywords: complement regulation, Schistosoma mansoni tetraspanning orphan receptor (SmTOR), schistosomiasis, tegument protein, vaccine candidate
Introduction
Schistosomes are parasitic helminths that are able to ensconce themselves in the human host for decades [1]. They were discovered in the mid-19th century [2], but must have infected their human hosts during thousands of years, as calcified eggs had already been discovered in mummies [3]. Their persistent existence over thousands of years might be one of the reasons why, during co-evolution with their human host, schistosomes developed into well-adapted parasites capable of escaping the host immune response and establishing themselves in such an unfriendly environment as the human venous system.
Today, an estimated number of 200 million people are infected with Schistosoma spp., with S. mansoni, S. haematobium and S. japonicum the most important species [4]. Despite many decades of research, praziquantel is the only chemotherapeutic drug available for treatment of schistosomiasis effective against all five schistosome species infecting humans [5]. Concern about the emergence of developing praziquantel resistance [6], the biphasic sensitivity of the parasite to the drug [7], with juvenile worm stages being insensitive to drug treatment [8], and the lack of protection against reinfection [9], are among the major disadvantages of a chemotherapeutic treatment of the infection. Consequently, the development of a schistosomiasis vaccine is highly desirable, although more than 10 years ago it was already stated to be a difficult but achievable goal [10]. This prediction proves true, in so far as no vaccine candidate is currently in the late stages of clinical development [11]. However, since 2009 the S. mansoni genome sequence has been fully available [12]. This remarkable achievement, together with a substantial amount of high-quality data generated by the various other ‘-omics’ disciplines, pave the way for vaccine research against this and other schistosome species [[13,14]]. Some of the most interesting vaccine candidates are transmembrane proteins localized on the S. mansoni tegument, as they are seen immediately by the host immune system [15]. Proteins highly expressed in the early intramammalian stages of S. mansoni, such as schistosomula, are also considered to be favourable vaccine targets [16].
Schistosomes have to defend themselves against the host immune system at various stages in their life cycle [[17–19]]. The initial phase of invasion is characterized by evading innate immunity at the time of skin penetration and migration to small vessels. During this phase the host complement may attack the cercariae [20]. However, schistosomes express several complement regulators/inhibitors on their surface, which apparently block host complement [21]. We have recently described a new putative complement regulator in S. mansoni, termed SmTOR for tetraspanning orphan receptor [22]. This receptor is characterized by a 111 amino acid extracellular domain 1 (SmTORed1) containing the C-terminal H17 motif shown to bind C2 and interfere with its cleavage by C1s, thereby limiting the extent of the complement C3 convertase formation [23]. Its highest expression in cercariae and surface localization in S. mansoni early intramammalian stages would not only suggest a role for the receptor as a complement regulator at an early time-point of infection, but also make it an interesting vaccine target.
In this work, we wanted to define whether recombinant SmTORed1 induces immune responses in mice and confers protection against infection. An additional question was whether or not humans infected with S. mansoni develop specific antibodies.
Materials and methods
Animals
Female C57BL/6 and female BALB/c mice (n = 80, age: 4 weeks, weight: ∼14 g) used for the first round of immunization were purchased from Harlan Laboratories (Horst, the Netherlands). Female BALB/c mice (n = 30, age: 4 weeks, weight: ∼14 g) used for the immunization challenge experiment were purchased from Charles River Laboratories (Sulzfeld, Germany). Animals were kept in groups of five (preliminary experiment) or 10 (immunization infection) in environmentally controlled conditions (temperature: 25°C; humidity: ∼50%; 12-h light/dark cycle) and acclimatized for 1 week. They had free access to water and rodent diet. All experiments were approved by the ethical committees of the Swiss authorities at the Federal Veterinary Department (Bern, Switzerland) and the cantonal veterinary office Basel-Stadt (Switzerland) (permission number: 2346). They were conducted according to local guidelines (Verordnung Veterinäramt Basel-Stadt) and the Swiss animal protection law (TschG) at the Department of Biomedicine at the University Hospital Basel (first round of immunization) and at the Swiss Tropical and Public Health (TPH) Institute (Basel, Switzerland) (immunization challenge experiment).
Recombinant protein expression and purification
SmTORed1 ORF was cloned into the pET15b expression vector (Novagen, Merck Chemicals, Darmstadt, Germany) by the sticky-end polymerase chain reaction (PCR) method [24] using the NdeI and BamHI restriction sites and pCR2·1-TOPO SmTOR [22] as template. Corresponding accession numbers for SmTOR deposited at the databases indicated are: SmTOR mRNA sequence, GenBank ID: JN560697; SmTOR full-length receptor sequence; and UniProt ID: C4QM85.
The primer pairs used were, for PCR 1, 5′-TATGCCGAGACTTATTCCAGAGGATAAT-3′ (forward 1) and 5′-GATCCTTAGTAAGGACTGAAATGCTTTAT-3′ (reverse 1); and for PCR 2, 5′-TGCCGAGACTTATTCCAGAGGATAAT-3′ (forward 2) and 5′-CGATCCTAGTAAGGACTGAAATGCTTTAT-3′ (reverse 2). Sticky-end PCR was performed as follows: two different PCR reactions were performed, reaction products were cleaned up separately by agarose gel electrophoresis and purification of the bands at the expected molecular weight [345 base pairs (bp)] was performed using the QIAquick gel extraction kit (Qiagen, Hombrechtikon, Switzerland). The extracted DNA was then mixed in equimolar ratio (1:1), denatured at 95°C for 5 min and then annealed on ice. pET15b vector was prepared by double digestion with NdeI and BamHI (NEB, Frankfurt, Germany) and purified as described above. Ligation was performed using Quick Ligase (NEB) and the mixture was transformed into TOP 10 bacteria (Invitrogen, Carlsbad, CA, USA) and grown on Luria broth (LB) agar plates containing 100 μg/ml ampicillin (Sigma, St Louis, MO, USA). Clones were analysed by restriction enzyme digestion with SalI (NEB) cutting at position 244 of the insert sequence. Positive clones were sequenced and used for transformation into a bacterial expression strain.
SmTORed1pET15b was transformed into BL21 (DE3) bacteria for protein overexpression. Batch cultures were grown in autoinduction media, as described by Studier [25]. Pre-cultures were grown in MDAG non-inducing medium and main cultures were grown in MDA-5052 autoinducing medium, both supplemented with 100 μg/ml carbenicillin (Sigma). Single clones of SmTORed1pET15b (rSmTORed1 purification) or pET15b (mock transfection, purification of control fraction) transformants were picked and pre-cultures were grown overnight at room temperature (RT) with shaking at 220 rpm and diluted subsequently at 1:50 in MDA-5052 medium for protein expression. Fifty ml main cultures in 250 ml Erlenmeyer flasks were shaken at 250 rpm for 18 h at RT. Bacteria were harvested by centrifugation and pellets frozen at −20°C until required.
Recombinant protein was purified from the insoluble cytoplasmic fraction (inclusion bodies) of SmTORed1pET15b transformed BL21 (DE3) bacteria. Bacteria were lysed using BugBuster bacterial cell lysis detergent (Novagen) supplemented with ethylenediamine tetraacetic acid (EDTA)-free protease inhibitor cocktail (Roche Diagnostics, Basel, Switzerland), and inclusion bodies were purified by repeated centrifugation and washing steps as described in the pET system manual (Novagen). The final inclusion body pellet was resuspended in 10 ml denaturing buffer (buffer A) containing 10 mM imidazole (50 mM NaH2PO4, 500 mM NaCl, 6 M guanidine hydrochloride, pH 8 + 10 mM imidazole). The recombinant protein was then purified by metal affinity chromatography using ÄKTAprime plus system (GE Healthcare Biosciences, Piscataway, NJ, USA). A 5-ml Ni-NTA Superflow Cartridge was equilibrated with buffer A and 5 ml of solubilized sample loaded onto the column (one cleaned-up batch corresponded to 25 ml original bacterial culture). The column was then washed with five bed volumes of buffer A and then eluted with a linear gradient (gradient volume 100 ml) of buffer A to 100% buffer B (50 mM NaH2PO4, 500 mM NaCl, 6 M guanidine hydrochloride, pH 8 + 250 mM imidazole). Fractions containing the main protein peak were pooled and analysed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) (15%). Purified histidine (His)-tagged rSmTORed1 was refolded by stepwise dialysis. First, the sample (in a volume of 20–25 ml buffer A/buffer B) was dialysed into refolding buffer (10 mM Tris-HCl, pH 8, 1 mM EDTA, 400 mM L-arginine) at 4°C overnight using a dialysis membrane of 3500 molecular weight cut-off (Spectrum Laboratories, Breda, The Netherlands). Two additional dialysis steps against 1 × PBS were then performed for 3 h at 4°C each. The purity and concentration of the sample were then evaluated analysing an aliquot by 15% SDS-PAGE and bands visualized by Coomassie staining (Instant Blue; Expedeon, Harson, UK). Molecular weight markers used were Precision Plus Protein standards (Bio-Rad, Munich, Germany) and BenchMark ladder (Invitrogen). Protein concentration in the sample was determined using DC protein assay (Bio-Rad). The Coomassie-stained protein band at the expected molecular weight was excised and analysed by in-gel tryptic digestion and liquid chromatography–mass spectrometry (LC-MS/MS) analysis [26]. An inclusion body control fraction was prepared in Escherichia coli by transformation with pET15b plasmid analogous to the expression and purification of the rSmTORed1 protein.
Characterization of SmTORed1 by Western blot and enzyme-linked immunosorbent assay (ELISA)
Western blot using anti-ed1 antibody AbyD04644·1 (Serotec) was performed as published previously [22]. Protein samples were run on a standard 15% acrylamide/bisacrylamide gel (Bio-Rad) and transferred to a nitrocellulose membrane. The membrane was blocked in phosphate-buffered saline-0·05% Tween 20 (PBST)/5% milk followed by incubations with primary (AbyD04644·1, 1:1000) and secondary antibody [goat F(ab′)2 anti-human immunoglobulin (Ig)G-horseradish peroxidase (HRP)] (1:3000; Serotec, Martinsried, Germany) in PBST 1% milk, each for 1 h at RT. The blot was developed with SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA).
For enzyme-linked immunosorbent assay (ELISA) measurements, 96-well plates (Maxisorp, Nunc, Rosklide, Denmark) were coated overnight at 4°C with rSmTORed1 (500 nmol/well) in 0·1 M bicarbonate buffer, pH 9·6. Plates were then blocked with 5% bovine serum albumin (BSA) in 1 × PBST for 1 h at RT. After blocking, AbDy04644·1 (2·51 mg/ml stock) diluted in PBST was used at dilutions ranging from 1:1000 to 1:10 000 to detect rSmTORed1 by ELISA. Anti-green fluorescent protein (GFP) AbDy04652·1 (1·14 mg/ml stock) diluted 1:4545 and 1:454 was used as negative control, corresponding to the highest (1:10 000) and lowest dilutions (1:1000) of AbDy04644·1, respectively. The plates were incubated for 45 min at RT with the primary antibody and were then washed five times with PBST. Secondary antibody [goat F(ab′)2 anti-human IgG-HRP; Serotec] diluted 1:10 000 was added for another 30 min at RT, and after washing the ELISA was developed by incubation with a 3,3′,5,5′-tetramethylbenzidine (TMB) peroxidase substrate (BD Pharmingen, Allschwil, Switzerland). The absorbance was measured at 450 nm. For testing C2 binding to rSmTORed1 by ELISA, plates were coated and blocked as described above. Then, purified complement C2 (CompTech, Tyler, TX, USA) was diluted in 20 mM Tris buffer + 1 mM MgCl2 + 1 mM CaCl2 (amounts as indicated in figures) and incubated for 1 h at RT. Plates were washed and incubated with anti-C2 antibody (Calbiochem, Läufelfingen, Switzerland) diluted 1:5000 in PBST for 45 min at RT. After additional washes, plates were incubated with donkey anti-goat HRP, 1:10 000 in 1 × PBS for 30 min at RT. Finally, plates were washed and developed as described previously.
Detection of anti-rSmTORed1 antibodies in human sera by ELISA
Samples of anonymous patient sera were provided from an archived bank of samples collected (at the Swiss TPH, Basel) from known positive S. mansoni-infected individuals. Sera had been tested positively for antibodies against soluble worm antigen (SWA) and soluble egg antigen (SEA) and by immunofluorescence antibody tests (IFAT). Serum samples from uninfected individuals living in Switzerland were obtained from the Blutspendezentrum, Basel.
Nunc Maxisorp 96-well plates were coated with 1 μmol rSmTORed1 per well diluted in 0·1 M bicarbonate buffer, pH 9·6 overnight at 4°C. Plates were washed three times with PBST, and serum samples diluted 1:500 in PBST were added for 45 min, 37°C. After incubation with sera, plates were washed five times with PBST and biotinylated secondary antibody diluted 1:10 000 was added to each well [goat F(ab′)2 anti human IgG; Biosource] for 1 h at RT. This was followed by an additional five washes and 30 min incubation with streptavidin–HRP (Pierce; 1:10 000 in 1 × PBS) at RT. After washing, ELISA was developed using TMB peroxidase substrate and the absorbance at 450 nm was measured.
Cloning of HaloTag fusion constructs
All N-terminal HaloTag fusion constructs were cloned with the primers listed in Table S1 into the bacterial T7 promoter based expression vector pFN18A HaloTag T7® Flexi® Vector (Promega, Madison, WI, USA). The barnase-positive selection cassette was removed by digestion of pFN18A with PvuI and PmeI and replaced by Halofwd and Halorev annealed oligos, as described previously [27]. The construct pFN18A Halo expressing HaloTag only was used as negative control. Halo-SmTORed1 (111 aa) and Halo-C4beta (C4beta: 26 aa corresponding to C4b206–232 peptide stretch) were amplified from pCR2·1-TOPO SmTOR [22] for the SmTORed1 constructs or Hep2G (human hepatoma cell line) cDNA for the C4beta chain peptide. Cloning was performed with the sticky-end PCR method [24] using iProof high-fidelity DNA polymerase (Bio-Rad) and the inserts thus generated were ligated into PvuI/PmeI digested pFN18A vector with Quick Ligase (New England Biolabs, Frankfurt, Germany). Plasmids were propagated in TOP10 E. coli (Invitrogen) and open reading frames verified by sequencing.
Overexpression and purification of Halo-tagged peptides
Plasmids (pFN18A Halo, pFN18A Haloed1 and pFN18A Halo C4 beta) were transformed into chemically competent BL21 (Sigma). Single clones were picked from LB/ampicillin plates and were grown overnight at 37°C (at 220 rpm) in 2 ml LB medium supplemented with 100 μg/ml ampicillin (Sigma). Pre-cultures were diluted 10-fold in LB/ampicillin and when grown to an optical density (OD)600 of >0·6 induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). Aliquots of the cultures were screened for expression of target gene 3–5 h after induction by SDS-PAGE and Coomassie staining. Total cell protein fractions were prepared using PopCulture reagent (Novagen) according to the protocol in the pET system manual (Novagen). Theoretical molecular weights of the fusion constructs were calculated as 35·4 kDa (Halo), 38·5 kDa (Halo C4 beta) and 48·2 kDa (Haloed1).
In order to purify Halo-tagged constructs, bacteria cultures (V = 20 ml) were pelleted, resuspended in BugBuster mix (Novagen) and soluble cytoplasmic fractions were prepared according to the manufacturer's protocol. Protein samples were then prepared for coupling onto magnetic beads (HaloLink, G9311; Promega). Buffer exchange into 1 × PBS was accomplished by using 3000 MWCO Amicon Ultra Centrifugal Filter Devices (Millipore, Volketswil, Switzerland). Halo protein concentrations were estimated in comparison with marker protein bands (BenchMark Protein Ladder; Invitrogen; each protein present at a concentration of approximately 0·1 mg/ml). For the coupling reaction, samples were diluted at the appropriate concentrations in 100 mM TrisHCl, pH 7·5, 150 mM NaCl, 0·01% NP40. HaloTag fusion proteins were immobilized on magnetic beads as described in the instruction manual (G9311; Promega). Magnetic beads were incubated with the HaloTag fusion proteins in excess in order to ensure that equal amounts of protein, i.e. beads, were used in the assay. This was the case because the covalent bond formation between HaloTag and chloralkane linker is specific and highly irreversible [28]. After coupling reactions, beads were washed extensively in PBS and used for competition ELISA or peptide purification. An amount of 40 μg Halo-tagged protein was coupled onto 20-μl beads in gel slurry that was then used in the competition ELISA.
Competition ELISA assay to measure specific anti-rSmTORed1 antibodies in human sera
For competition ELISA, 96-well plates were coated as described above, but with 500 nmol rSmTORed1 per well. Anti-rSmTORed1 antibody measurement in human sera was performed exactly as described above, but sera were first incubated with Halo-tagged fusion constructs, described as follows. Twenty μl washed beads with immobilized Halo or Haloed1 were resuspended in 250 μl PBST. One ml of serum sample (resulting in a serum dilution of 1:250) was added and the mix incubated on a wheel for 20 min at RT. As a control, 1 μl of serum sample was diluted in 250 μl PBST and incubated in parallel without beads. The coated ELISA plate was washed and 50 ml of PBST added per well. Magnetic beads were removed after incubation with the diluted serum and 50 μl of depleted serum (or control sample) was added per well (experiments were performed in triplicate), resulting in a final serum dilution of 1:500. Plates were incubated at 37°C for 45 min and the protocol proceeded as described above.
Vaccination of mice with rSmTORed1 – immunization and immunization challenge schedules
In the first round of immunization, four different mouse strain/adjuvant combinations were tested. As adjuvant, either complete Freund's adjuvant (CFA: first immunization and incomplete Freund's adjuvant (ICF: boosting) (experimental groups 1 and 3) or muramyl dipeptide (MDP: first immunization and boosting) (experimental groups 2 and 4) were used. The four experimental groups were: group 1: BALB/c mice injected with rSmTOR or control injections (see below) using CFA/IFA (n = 20); group 2: BALB/c mice injected with rSmTORed1 or control injections using MDP (n = 20); group 3: C57BL/6 mice injected with rSmTORed1 or control injections using CFA/IFA (n = 20); and group 4: C57BL/6 mice injected with rSmTORed1 or control injections using MDP (n = 20). Within these four groups, there were three subgroups consisting of 10 animals immunized with rSmTORed1 in PBS plus adjuvant (subgroup 1, immunized), five animals injected with inclusion bodies in PBS plus adjuvant (subgroup 2, first control group) and five animals injected with 1 × PBS plus adjuvant (subgroup 3, second control group). Immunization took place on day 0 followed by boosts at days 21 and 42. Ten μg of purified rSmTORed1 in PBS/adjuvant, inclusion bodies in PBS/adjuvant or PBS/adjuvant alone were used per injection at the days indicated. Mice were injected subcutaneously in the neck fold. Bleeding to monitor antibody response was performed at days −7, 14, 35 and 63 via the tail vein. Mice were killed at day 63 and spleens were dissected for generation of spleen cell cultures.
Immunization challenge experiments were performed using BALB/c mice (Charles River, Sulzfeld, Germany) in combination with CFA/IFA only. Groups consisted of mice injected with rSmTORed1 (n = 10, trials 1 and 2), inclusion bodies (n = 10, trial 1) or PBS (n = 10, trials 1 and 2) mixed with adjuvant, as tested in the preliminary round. Mice were infected at day 55 (13 days after the second boost). Cercariae of S. mansoni (Liberian strain) were harvested from infected intermediate host snails (Biomphalaria glabrata) maintained at laboratories at the Swiss TPH after exposure to light for 3 h. Mice were infected subcutaneously with 150 S. mansoni cercariae. At day 105 (7 weeks post-infection) mice were killed and parasite status was assessed by blinded evaluation of the adult worm burden. In trial 2, two mice of the control group (PBS injected) were not infected and were not included in the analysis.
Worm burden and liver egg burden analysis
Worms were dissected from the mesenteric veins of the mice. Protection values were calculated as described elsewhere [29]. Livers were then weighed and eggs and worms were counted microscopically in pressed livers using a counting grid. The average number of eggs per square was determined by counting eggs in three squares/liver and total liver area, i.e. number of grids per liver, was determined. Number of eggs per gram liver (epg) was calculated by dividing total number of eggs per liver by the liver weight. The spleens were removed in order to generate spleen cell cultures. Serum samples to assess antibody response were taken at days −7, 14, 35 as in the preliminary round (pre-challenge) and between days 105 and 107 (post-challenge).
Mouse serology
Specific antibody titres against rSmTORed1 were measured in individual mouse sera using an ELISA assay. For this, 96-well flat-bottomed microtitre plates (Nunc) were coated overnight at 4°C with 500 nmol rSmTORed1 per well in 100 μl 0·1 M carbonate bicarbonate buffer, pH 6·8. The plates were then blocked in 1 × PBST/5% BSA for 1 h, 37°C. Plates were washed five times with PBST between the different incubation steps. Serum samples were diluted in PBST at the appropriate dilution determined by serial dilutions for individual samples of each of the possible mouse strain adjuvant combinations and the different detection antibodies (described below), and 100 μl were then added per well. Plates were incubated for 1 h at RT, and after washing the biotinylated primary antibody diluted in PBST was then added for another hour at RT. Bound antibody was detected with streptavidin–HRP (Pierce) diluted 1:10 000 in PBS (30 min, RT) and the ELISA was developed by incubation with a TMB peroxidase substrate (BD Pharmingen). The absorbance was measured at 450 nm.
Biotinylated detection antibodies used were: goat anti-mouse IgG+IgM+IgA (ab6005; AbCam, Cambridge, UK) diluted 1:10 000, goat anti-mouse IgG (ab5868; AbCam) diluted 1:10 000, goat anti-mouse IgM (ab5929; AbCam) diluted 1:20 000, goat anti-mouse IgE (no. 1110-08; SouthernBiotech, Birmingham, AL, USA) diluted 1:5000, goat anti-mouse IgA (no. 1040-08; SouthernBiotech) diluted 1:10 000, rat anti-mouse IgG1 (no. 406604; Biolegend, San Diego, CA, USA) and rat anti-mouse IgG2a (no. 407104; Biolegend) both diluted 1:500. Serial dilutions to determine the working serum dilution were performed for the individual mouse strain adjuvant combinations as shown in Supplementary Fig. S3. For Ig serology (IgG + IgM + IgA determination), sera of BALB/c CFA/IFA groups were diluted 1:25 600; C57BL/6 CFA/IFA sera were diluted 1:6400 and sera of MDP vaccinated mice were diluted 1:3200 for BALB/c and C57BL/6 mice. For IgG, IgM, IgE, IgA, IgG1 and IgG2a determination in BALB/c CFA/IFA-vaccinated mice, sera were diluted 1:12 800 for IgG measurement and 1:1600 for all other antibody isotype levels measured, as determined by end-point titrations as well (data not shown).
Spleen cell cultures
Freshly removed spleen from infected and uninfected mice was cut into small pieces and the suspension pressed through a 70-μm nylon mesh (BD Falcon, BD Biosciences, Allschwil, Switzerland; no. 352350) into a 15-ml Falcon tube containing RPMI-1640 medium supplemented with 5% fetal calf serum (FCS) (RPMI/FCS). Cells were pelleted, resuspended in 5 ml lysis buffer (0·15 M NH4Cl, 15 mM KHCO3, 0·1 mM Na2EDTA, pH 7·4) and incubated for 5 min at RT. Cell lysis was stopped by adding 10 ml RPMI/FCS and centrifuged for 10 min, 200 g at 4°C. Cells were resuspended in complete medium (RPMI/FCS + 2 mM L-glutamine, 50 μM beta-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin sulphate + 250 ng/ml amphotericin B + 30 μg/ml polymyxin B) and cell number adjusted to 1 × 107 cells per ml. Cells were cultured at 1 × 106 cells/ml in 200 ml in a 96-well tissue culture plate, either stimulated with 10 μg/ml rSmTORed1 or 5 μg/ml concanavalin A. Cell culture supernatants were collected after 48 h of stimulation for interleukin (IL)-4 analysis and after 72 h for IL-10 and interferon (IFN)-γ. Mouse cytokine ELISA measurements were performed using ELISA sets (BD OptEIA™, BD Biosciences, Allschwil, Siwtzerland). Stimulation with concanavalin A was performed to ensure the responsiveness of the splenocytes to stimulation and was positive for all the cultures and cytokines measured (mean values 8500 pg/ml and 850 pg/ml for IFN-γ and IL-10 for samples shown in Supplementary Fig. S6a and 150 pg/ml for IL-4, not detectable in rSmTORed1 stimulated cultures). The differences in the level of responses between the two sets of experiments (immunization alone, Supplementary Fig. S6a; immunization + challenge, Supplementary Fig. S6b) were due to cytokine kits as indicted by internal controls used in both series of tests.
Immunofluorescence staining and confocal microscopy
S. mansoni schistosomula were prepared as described elsewhere [30]. After incubation in medium for the indicated number of hours and washing in 1 × PBS, schistosomula were either embedded directly in octreotide (OCT) or used for staining of the whole parasite. Nine-μm OCT sections of 3-h schistosomula were fixed in ice-cold methanol for 10 min, blocked in PBST/5% BSA and incubated with anti-rSmTORed1 sera (pooled sera from 10 BALB/c mice immunized with rSmTORed1, bleeding at day 63) or pre-immune serum (pooled sera from BALB/c mice immunized with rSmTORed1 but bled before first injection, bleeding day −7) diluted 1:25 in PBST/3% BSA for 2·5 h at RT. After four washes, slides were incubated for 1 h with Alexa Fluor® 488 rabbit anti-mouse IgG (H + L) (A11059; Molecular Probes, Eugene, OR, USA) diluted 1:150 in PBST/3% BSA and washed again before mounting. Whole parasites (24-h schistosomula) were fixed 1 h in 4% paraformaldehyde/PBS on ice and after washing five times with PBS were blocked and stained as described for OCT sections above. All slides were mounted with Vectashield fluorescence mounting medium (Vector Laboratories, Peterborough, UK) and examined using the LSM 510 META confocal laser scanning microscopy system (Carl Zeiss, Feldbach, Switzerland) with a Zeiss Plan Neofluar 63×/1·25 numeric aperture oil (1/0·17) objective.
Statistical analyses
Student's t-test was used to compare Ig amounts in mouse sera at two different time-points, and to compare experimental and control group (immunization challenge trial 2) on worm burden and liver egg burden. Statistical differences between antibody amounts of Ig at day 35 and differences in worm burden between three different groups (trial 1) were determined by one-way analysis of variance (anova) (Kruskal–Wallis) followed by Dunn's multiple comparison test. Linear correlation between female worm burden and epg liver was evaluated using Spearman's rank correlation analysis.
Results
First, the recombinant SmTORed1 was prepared and characterized, then used in mice to define immunogenicity and protection against infection. Finally, we investigated human sera for the presence of specific anti-SmTORed1 antibodies.
Recombinant SmTORed1, purification and molecular characterization
In order to test the immunogenicity of the SmTOR extracellular domain 1, we produced it as an N-terminally His-tagged fusion protein rSmTORed1 containing only a short linker sequence (Fig. 1a). SmTORed1 ORF was cloned into a pET15b vector where its expression was manageable under the control of the lac repressor [31]. An aliquot of the fast protein liquid chromatography (FPLC)-purified recombinant SmTORed1 dissolved in PBS was analysed by gel electrophoresis before and after the final centrifugation step and a protein band of the calculated molecular weight of 14·7 kDa was detected (Fig. 1b). The protein band was sequenced by mass spectrometry and fragments belonging to recombinant SmTORed1 were identified (Fig. 1a). The protein concentration of total compared to soluble rSmTORed1 was 145 ng/μl and 132 ng/μl, indicating that only a small portion of rSmTORed1 was not soluble under optimized conditions for refolding in a large volume of buffer. Pooled protein fractions analysed before refolding into 1 × PBS showed an additional protein band at an approximate molecular weight of 30 kDa (Fig. 1c). No other bands were detected by Coomassie staining in this or other similarly concentrated samples of purified rSmTORed1. Recombinant SmTORed1, as well as the 30 kDa protein band at the size of a potential peptide dimer, were detected by Western blot using monoclonal anti-ed1 antibody directed against the 27 C-terminal amino acids of rSmTORed1 (Fig. 1c). The same monoclonal antibody recognized plate-bound rSmTORed1 (data not shown), and rSmTORed1 was also shown to bind C2, as expected [[23,32]] (Fig. 1d).
Fig. 1.
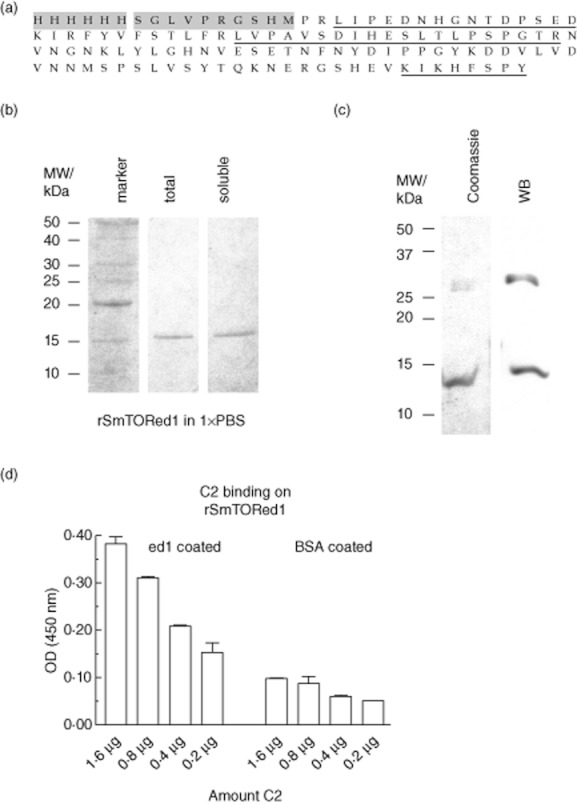
Production of histidine (His)-tagged Schistosoma mansoni tetraspanning orphan receptor extracellular domain 1 (SmTORed1) in Escherichia coli and molecular characterization of purified peptide by mass spectrometry, Western blotting and enzyme-linked immunosorbent assay (ELISA). (a) Recombinant first extracellular domain of rSmTORed1 peptide sequence with the 6 × His-tag and linker sequence (10 aa) originating from the pET15b vector shaded in grey. Fragments identified by mass spectrometry are underlined. (b) Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of purified rSmTORed1 after Ni2+ chromatography (pooled eluates) and refolding into 1 × phosphate-buffered saline (PBS). Total amount of peptide in buffer was analysed before (total, lane 2) and after removal of residual precipitate by centrifugation (soluble, lane 3). Four μl of sample was loaded per lane. Lane 1 (marker): benchmark protein ladder. Protein bands are visualized by Coomassie stain. (c) Pooled fractions of fast protein liquid chromatography (FPLC)-purified peptide analysed before dialysis into 1 × PBS; 20 μl sample was loaded per lane. Peptides detected by Coomassie blue (left) and by Western blot using monoclonal anti-ed1 antibody AbDy04644·1 (right). (d) C2 binding on a rSmTORed1 coated ELISA plate, binding in 20 mM Tris buffer + 1 mM MgCl2 + 1 mM CaCl2. ELISA Results are expressed as means of individual measurements. Error bars indicate standard deviation (s.d.) of the means.
It has been shown previously that complement C2 binds to the β chain of C4, specifically to a peptide sequence in C4β that is homologous to the SmTORed1 peptide [23]. Thus antibodies (human and mouse) that are specific for SmTORed1 might cross-react with C4. We used SmTORed1 peptide and the homologous sequence on the C4β chain peptide aligning with it (termed C4beta) produced as N-terminal Halo-tagged bacterial fusion proteins and cut by digestion with tobacco etch virus (TEV) protease along with rSmTORed1 to demonstrate that there was no cross-reactivity detected by ELISA (Supplementary Fig. S1, S2).
Evaluation of the humoral immune response in BALB/c and C57BL/6 mice immunized with rSmTORed1
To define the immunogenicity of rSmTORed1, we immunized two different mouse strains with rSmTORed1 formulated with CFA/IFA or MDP as adjuvant. After initial immunization, mice were boosted twice at 3-week intervals. After two injections with antigen and the CFA/IFA as adjuvant, BALB/c mice showed a significant increase in Ig levels detectable at high dilution compared to levels in pre-immune sera (Fig. 2a) whereas no antibodies were produced in the inclusion body and PBS-injected mice at the same time-point (Fig. 2a). BALB/c mice responded to the second booster injection with peptide and IFA with sustained production of Ig to rSmTORed1 (data not shown). In fact, dilutions set for Ig analysis had been determined by end-point titrations (Supplementary Fig. S3). Sera were diluted 1:25 600 for Ig analysis of CFA/IFA-immunized BALB/c and specific antibodies in these mice were detected at a dilution as high as 1:1 638 400 compared to pre-immune sera (Supplementary Fig. S3a). CFA/IFA-vaccinated C57BL/6 mice and both strains of MDP-vaccinated mice generated low (Supplementary Fig. S3b) to very low (Supplementary Fig. S3c,d) amounts of anti-rSmTORed1 Ig and sera were therefore tested at low dilutions. Indeed, immunization of C57BL/6 mice with rSmTORed1 in CFA/IFA and BALB/c mice with rSmTORed1 in MDP also resulted in a significant but small increase of Ig levels measured at low dilution (Supplementary Fig. S4a,b). This increase was not uniformly detectable in all animals of the immunized groups and not significant overall compared to the control groups. Finally, no significant Ig levels were detected in C57BL/6 mice immunized with rSmTORed1 in MDP (Supplementary Fig. S4c). No Ig bound on the immobilized inclusion body fraction, and mouse Ig to rSmTORed1 did not cross-react with the TEV cut rC4beta peptide sequence (Supplementary Fig. S2). Control animals, both mice receiving inclusion bodies together with adjuvant and mice receiving adjuvant mixed with buffer only, did not show an increase in anti-rSmTORed1-specific immune response in any set-up.
Fig. 2.
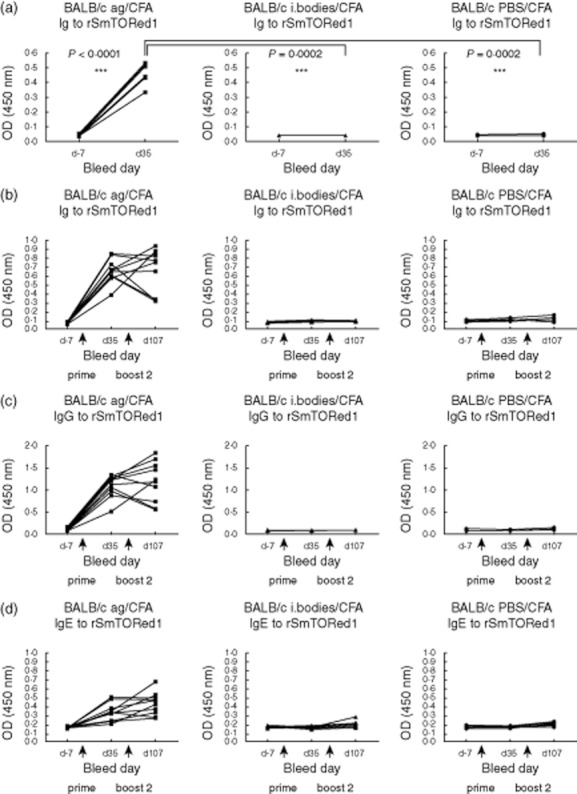
Recombinant first extracellular domain of Schistosoma mansoni tetraspanning orphan receptor (rSmTORed1) complete Freund's adjuvant/incomplete Freund's adjuvant (CFA/IFA)-induced antibody response in BALB/c mice (a) after immunization only, and (b–d) after immunization followed by a challenge with S. mansoni infection. (a) Total immunoglobulin (Ig) amounts in sera of individual BALB/c mice immunized with rSmTORed1/CFA/IFA (antigen, ag; n = 10) and the control groups injected with inclusion bodies/CFA/IFA (i. bodies; n = 5) or phosphate-buffered saline (PBS)/CFA/IFA (PBS; n = 5) measured in sera pre-immunization (day −7) and after the first boost (day 35). Sera for the enzyme-linked immunosorbent assay (ELISA) assay were diluted 1:25 600 as determined by end-point titrations. Statistical analyses used were: Student's t-test to compare titre at days −7 and 35 and one-way analysis of variance (anova) to compare titres at day 35; n.s.: statistically not significant; statistically significant *(P < 0·05), **(P < 0·01), ***(P < 0·001). (b–d) Total Ig (b), IgG (c) and IgE (d) amounts in sera of individual BALB/c mice immunized with rSmTORed1/CFA/IFA (antigen, ag; n = 10) and the control groups injected with inclusion bodies/CFA/IFA (i. bodies; n = 10) or PBS/CFA/IFA (PBS; n = 10) all infected with S. mansoni cercariae at day 55. Sera were sampled at days −7, 35 and 107 and measured at serum dilutions of 1:25 600 (total Ig), 1:12 800 (IgG) and 1:1600 (IgE). Arrows indicate injections at days 0 (prime), 21 (boost 1) and 42 (boost 2).
Vaccination of BALB/c mice and challenge with S. mansoni cercariae
We chose to perform an immunization challenge experiment using BALB/c mice in combination with CFA/IFA to determine whether or not the immune response generated in these mice upon vaccination with rSmTORed1 would have a protective effect against S. mansoni infection. Antibodies generated against rSmTORed1 persisted during the entire duration of the experiment and levels were not altered significantly in most of the animals after infection with S. mansoni (Fig. 2b). Immunized mice produced antigen-specific IgG and IgE (Fig. 2c,d), whereas specific anti-rSmTORed1 IgM and IgA levels were not detected (Supplementary Fig. S5).
Cytokine secretion by spleen cell cultures induced by stimulation with peptide were analysed to assess the T helper cell polarization induced by the immunization of BALB/c mice with rSmTORed1 in CFA/IFA. Spleen cell cultures that were performed 14 days after the second booster with rSmTORed1 produced significant amounts of IFN-γ and IL-10, but no IL-4 when stimulated with the antigen, compared to the spleen cell cultures originating from control animals (Supplementary Fig. S6a). The same cytokine profile was detected in spleen cell cultures of immunized animals after challenge with infection (Supplementary Fig. S6b). Control experiments using concanavalin A confirmed that all spleen cell cultures were capable of producing all three cytokines tested (not shown).
The status of infection in animals immunized with rSmTORed1 and challenged by injection of 150 S. mansoni cercariae was evaluated by analysing total worm burden (7 weeks post-infection). We found that mice immunized with rSmTORed1 showed a significant reduction in worm burden when compared to both control groups (Fig. 3). Vaccination resulted in 57% reduction of worm burden when compared to the mice injected with the inclusion body fraction and a reduction of 64% when compared to the PBS control mice. In a second independent trial, vaccination with rSmTORed1 resulted in 45% reduction of mean adult worm burden and a 50% reduction in mean liver egg burdens when compared to the PBS-injected mice (Table 1). There was a strong correlation between number of female worms and number of eggs per gram liver analysing all values (n = 18) of immunized and control groups (Spearman's r = 0·8589, P < 0·0001).
Fig. 3.
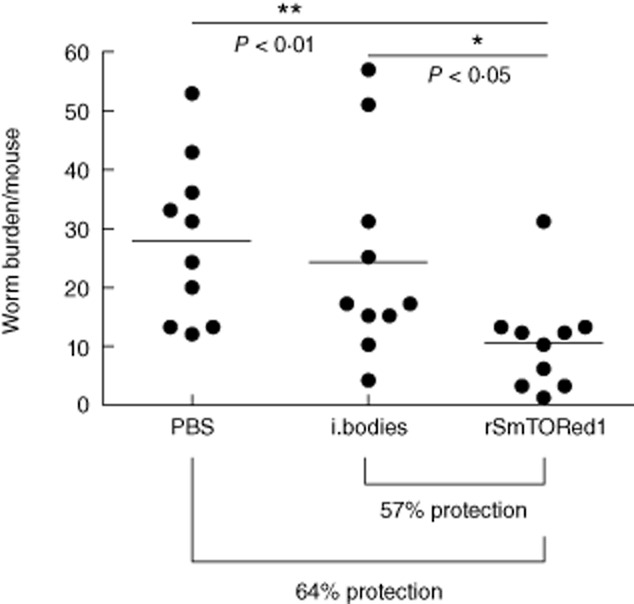
Scattergram of total worm burden of BALB/c mice (trial 1) immunized with complete Freund's adjuvant/incomplete Freund's adjuvant (CFA/IFA) adjuvanted recombinant first extracellular domain of Schistosoma mansoni tetraspanning orphan receptor (rSmTORed1), inclusion bodies (i. bodies) or phosphate-buffered saline (PBS) and challenged with S. mansoni cercariae. The number of animals used per group was n = 10. Mean worm burden ± standard deviation (s.d.) for the different groups were 28·8 ± 14·6 (PBS), 24·2 ± 17·4 (inclusion bodies) and 10·4 ± 8·6 (rSmTORed1). Statistical analyses were performed using one-way analysis of variance (anova) (Kruskis–Wallis) followed by Dunn's multiple comparison test. Statistically significant *(P < 0·05), **(P < 0·01).
Table 1.
Parasitological data of BALB/c mice vaccinated with recombinant first extracellular domain of Schistosoma mansoni tetraspanning orphan receptor (rSmTORed1), inclusion bodies (i. bodies) or phosphate-buffered saline (PBS) all adjuvanted with complete Freund's adjuvant/incomplete Freund's adjuvant (CFA/IFA)
| Mean worm burden ± s.d. % reduction, P-value | Total worm burden Mean ± s.d. % reduction, P-value | Number of eggs per gram liver tissue; epg × 103 Mean ± s.d. % reduction, P-value | ||||
|---|---|---|---|---|---|---|
| Adult worms, range | Male | Female | Pairs | |||
| Trial 1 | ||||||
| Control (PBS) n = 10 | 12–53 | 16·5 ± 8·3 | 12·3 ± 6·7 | 8·7 ± 6 | 28·8 ± 14·6 | ND |
| Control (i. bodies) n = 10 | 4–51 | 14·1 ± 9·8 | 10·1 ± 7·7 | 9·5 ± 7·9 | 24·2 ± 17·4 | ND |
| rSmTORed1 n = 10 | 1–31 | 5·7 ± 4·7 | 4·7 ± 4·2 | 2·9 ± 2·3 | 10·4 ± 8·6 | ND |
| 651/602 % | 621/532 % | 671/692 % | 641/572 % | |||
| P = 0·0131 | P = 0·0038 | P = 0·010 | P = 0·0058 | |||
| Trial 2 | ||||||
| Control (PBS) n = 8 | 12–44 | 10·3 ± 4·7 | 11·3 ± 5·9 | 6·5 ± 4 | 21·5 ± 10·5 | 11·75 ± 5·19 |
| rSmTORed1 n = 10 | 5–19 | 5·5 ± 4·2 | 6·4 ± 3·2 | 4 ± 2·5 | 11·9 ± 5·2 | 5·88 ± 2·99 |
| 47%, P = 0·0138 | 43%, P = 0·0393 | 38%, P = 0·125 | 45%, P = 0·0212 | 50%, P = 0·0082 | ||
Statistical analyses were performed using Student's t-test. P-values are shown for comparisons between the immunized versus both groups of controls taken together (trial 1) and immunized versus PBS/CFA injected mice (trial 2). n: the number of mice per group (from a total of 10) that were included for analysis. Comparison of numbers of parasites was between animals immunized with rSmTORed1 and the correspondent control groups, PBS-injected animals1 (comparison 1) and animals injected with inclusion bodies2 (comparison 2). ND: not determined; i. bodies: inclusion bodies; s.d.: standard deviation.
Detection of SmTOR using sera of immunized BALB/c mice
We wanted to test if antibodies generated in BALB/c mice immunized with rSmTORed1/CFA recognized the antigen on intact S. mansoni parasites. When staining cryosections of 3-h schistosomula with anti-rSmTORed1 serum we detected binding of antibodies in anti-rSmTORed1 serum, but not pre-immune serum, to the parasite surface (Fig. 4a). We also observed binding of anti-rSmTORed1 antibodies to 24-h schistosomula when we stained the whole parasite with anti-sera (Fig. 4b).
Fig. 4.
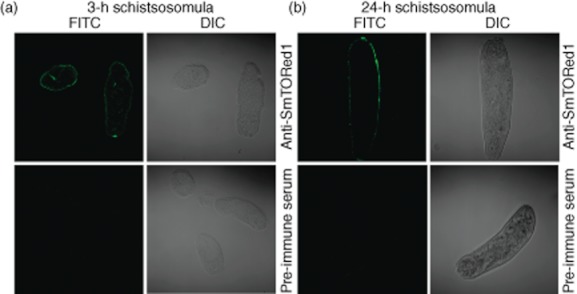
Sera of immunized mice recognizing Schistosoma mansoni tetraspanning orphan receptor (SmTOR) on S. mansoni schistosomula. Immunofluorescence [fluorescein isothiocyanate (FITC)] and differential interface images (DIC) of parasite sections (a) or whole parasite (b) visualized by confocal microscopy (×63 magnification). Three-h (a) and 24-h (b) schistosomula labelled with anti-recombinant first extracellular domain of SmTOR (rSmTORed1) serum are shown in the top panels, the respective control sections labelled with pre-immune serum on the bottom panels.
Furthermore, we tested the binding of sera of BALB/c immunized with irradiated cercariae and found that antibodies recognizing rSmTORed1 were generated even at very low levels compared to levels generated in immunized mice (Supplementary Fig. S7).
Specific anti-rSmTORed1 antibodies are detected in only few S. mansoni infected and uninfected humans
Because SmTOR is a surface-exposed receptor [[22,33]], we tested if S. mansoni-infected patients develop specific antibodies to the SmTORed1 domain. Using ELISA, we evaluated the reactivity of anti-rSmTORed1 antibodies in sera of individuals who were infected with S. mansoni and uninfected controls. Infection was confirmed by the presence of antibodies against SWA/SEA and by immunofluorescence antibody tests (IFAT). IgG antibodies to rSmTORed1 were detected in only five of 20 patients (25%) infected with S. mansoni, but also in four of 40 uninfected individuals (10%) living in Switzerland (Fig. 5a). The positive signal threshold was set arbitrarily considering the relative fold increase values indicated.
Fig. 5.
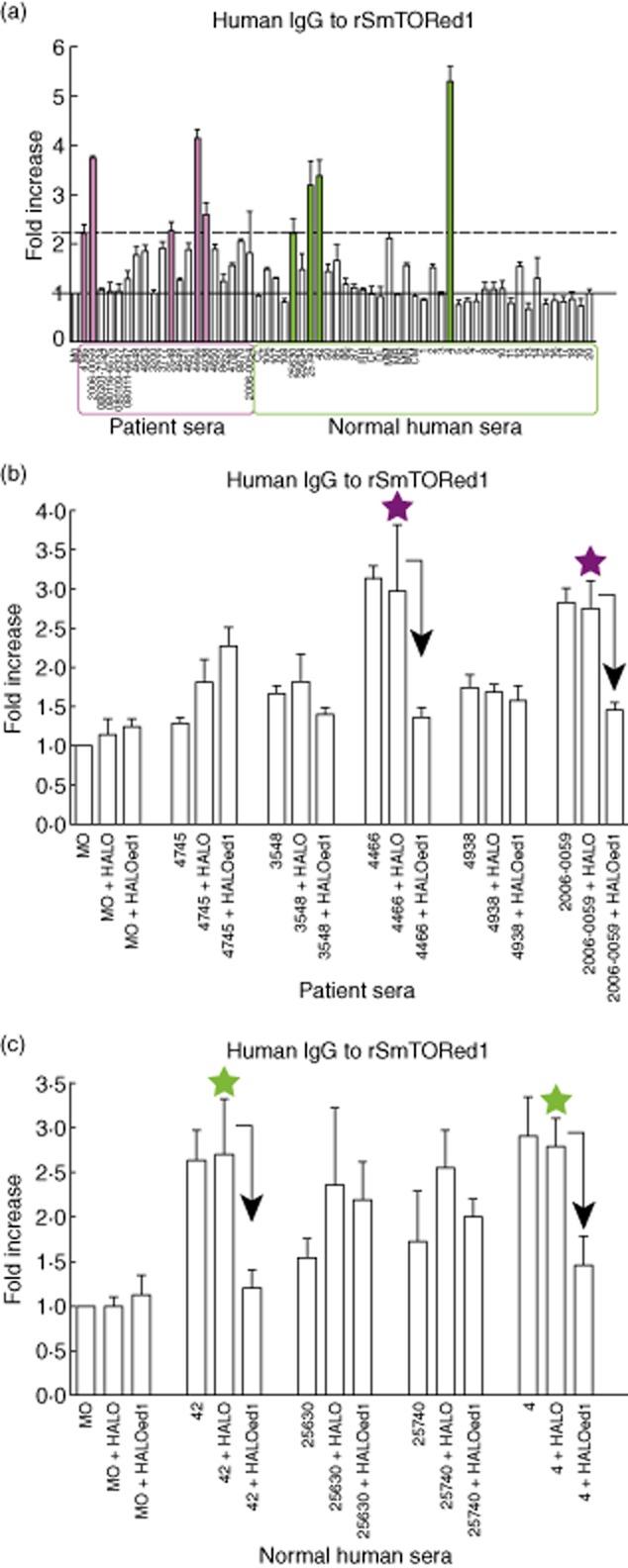
Detection of human immunoglobulin (Ig)G to recombinant first extracellular domain of Schistosoma mansoni tetraspanning orphan receptor (SmTORed1) by enzyme-linked immunosorbent assay (ELISA) and confirmation of specificity by competition ELISA using Halo-tagged bead-bound fusion constructs. (a) Total IgG levels in patients (archived anonymized sera, purple box) and normal human sera (NHS) (green box) normalized to control serum monocytes (MO, grey bar). The black solid line set at 1 corresponds to the mean of absorbance measuring control MO [mean optical density (OD) ± standard deviation (s.d.) (n = 9): OD450 nm = 0·176 ± 0·05], that was set to 1 in order to picture patient and NHS values normalized to one control serum value. The dashed black line was set at an arbitrary threshold and marks the lower limit of a fold-increase value considered as a positive signal. Five (filled purple bars) out of 20 patients and four (filled green bars) out of 40 patients were tested as positive. The results of three different experiments performed in duplicate are shown. Error bars indicate s.d. of the means. (b,c) Measurement of IgG specificity in schistosomiasis patients (b) and NHS (c). OD450 values measured were normalized to mean values recorded for the control sample (MO, OD450 nm = 0·243 ± 0·06, n = 7) and indicated as fold increased values. Prior to IgG measurement, individual sera were preincubated with Halo constructs coupled to magnetic beads: no competition (sample + no beads), with HaloTag alone (sample + Halo) or Halo-SmTORed1 (Haloed1). Stars indicate specific IgG to rSmTORed1 in the corresponding serum, tested by competition ELISA. Bars represent the mean values of three independent experiments. Error bars indicate s.d. of the means. Arrows indicate a significant decrease (>40%) in signal due to depletion of specific antibodies by preincubation with Haloed1.
To ensure antibody specificity, the five schistosomiasis patients and four uninfected individuals considered to have antibodies against rSmTORed1 (Fig. 5a) were tested in a competition-type ELISA. The specificity of antibody binding against SmTORed1 was evaluated by pre-incubation of diluted patient sera with soluble peptide in the form of Halo-tagged ed1 or HaloTag alone (Supplementary Fig. S1) coupled to magnetic beads via a chloralkane linker [28]. Competition ELISA results show that two out of five schistosomiasis patients (Fig. 5b) and two out of four uninfected individuals (Fig. 5c) have specific anti-rSmTORed1 IgG antibodies whose binding on the peptide could be abolished by pre-incubation of diluted sera with HALOed1, but not with HaloTag alone. This decrease in signal (indicated by an arrow) was significant for the patient sera 4466 and 2006-0059 (Fig. 5b) and normal human sera 42 and 4 (Fig. 5c). These four are the sera in which we had measured the highest positive values for anti-rSmTORed1 antibodies. None of these four sera reacted with C4beta (not shown). All sera from the patients, including the two with anti-rSmTORed1 antibodies, were positive for anti-S. mansoni antibodies (anti-SWA and anti-SEA antibodies), whereas this was not the case for the two positive normal sera, indicating that they were not infected by S. mansoni.
Discussion
The treatment of S. mansoni infection is limited by the availability of only one drug (Praziquantel), and the fact that constant reinfections take place [[5,9]]. An efficient immune response to antigens that would reduce the infection burden and rate is highly desirable. Here we found SmTOR to be a putative vaccine target in view of the protective effects in mice. Particularly interesting is that this antigen seems not to be recognized in most individuals and mice infected with S. mansoni, but was evidently a target for a protective immune response in vaccinated mice. Thus, this antigen might provide a novel approach to reduce infection.
We initially started with the hypothesis that SmTOR might be a good vaccine target, as it is localized in the schistosome tegument membrane. In addition, SmTOR is expressed most highly in S. mansoni larvae that are in first contact with the host immune system [22]. Given that its secondary structure contains a relatively large 111 aa surface-exposed extracellular domain, we wanted to see if we could find antibodies directed against it in humans, and if this domain would be a target for an efficient immune response in a mouse model.
Hence, we produced SmTORed1 recombinantly in E. coli and purified rSmTORed1 by affinity chromatography. We encountered specific difficulties; namely, first, that a highly concentrated SmTORed1 solution led to protein precipitation during denaturant removal, followed by dimerization, as shown on SDS-PAGE. Others have observed this type of reaction for the vaccine candidate Sm14, where protein dimerization and subsequent aggregation led to a reduction in vaccine efficacy [34]. We circumvented this problem by optimizing peptide purification and refolding into 1 × PBS diluting rSmTORed1, which yielded reasonable amounts of soluble protein. As basal expression of rSmTORed1 was observed to have detrimental effects on bacterial viability, bacteria producing protein under the control of the lac repressor [31] were grown in autoinduction media [25], thus circumventing this issue [[25,31]].
We found that specific humoral and cellular (T cell) responses were generated in BALB/c mice when immunized with rSmTORed1 using CFA/IFA as adjuvant. The infection with S. mansoni did not alter the total amount of antibody to rSmTORed1 generated in BALB/c mice and did not alter the cytokine profile elicited by stimulation of the spleen cell cultures with rSmTORed1. Thus, vaccination with rSmTORed1 using CFA/IFA as adjuvant induced a strong immune response in mice that was not altered after infection; however, the infection alone did not elicit any response against SmTORed1, as if this peptide – present on cercaria surface – had not been recognized. Also important is that the same immunization protocol in C57BL/6 mice led to only a minimal antibody response, re-emphasizing that the genetic background is also relevant for the presentation/processing of the peptide. Interestingly, the two mouse strains used in this study have a known different genetic background, being either prototypical T helper 1 (Th1) (C57BL/6) or Th2 (BALB/c) types [35]. It is known that a schistosome infection, as opposed to immunization, induces Th1 cytokines in C57BL/6 mice at the time of parasite residency in the lung, whereas in BALB/c mice, however, Th2 cytokines are induced with no measurable antibody production [[36,37]]. Interestingly, exogenous administration of IL-1beta in the days after infection led to the production of specific antibodies against parasite antigens in BALB/c mice associated with a significant reduction in worm burden. By contrast, the same treatment did not induce an antibody response in C57BL/6 mice and even increased the worm burden [36]. An additional example of opposing effects is that immunization with recombinant 3-P dehydrogenase emulsified in CFA, which protected BALB/c but not C57BL/6 mice from infection with S. mansoni [38]. Our data point in the same direction.
The vaccination protocol protected the mice significantly against a challenge with S. mansoni that were injected subcutaneously in order to obtain a controlled number of cercariae [39]. The worm burden was reduced significantly by 64 and 45% in two independent trials, which meets the criteria defined by the Research and Training in Tropical Diseases/World Health Organization (TDR/WHO) of a 40% reduction of infection [40]. We were able to detect specific IgG to rSmTORed1 in immunized BALB/c mice, and furthermore IgG1 and IgG2a subclasses were both generated to equivalent levels (data not shown). We did not observe a decreasing IgG1/IgG2a ratio, meaning no increase of cytophilic antibodies during the course of the experiment. However, in addition to IgG, we detected specific IgE to rSmTORed1 in response to immunization. In humans the occurrence of schistosome-specific IgE can be associated with protection. An age-dependent increase in production of IgE correlates with resistance to reinfection [41], and there are specific IgE levels detected in adolescents with high resistance to infection [42]. IgE can activate macrophages leading to killing of schistosomula [[43,44]] and can be involved in eosinophil-, neutrophil- and basophil-mediated killing of schistosomula [[45–47]]. Antibodies of both subclasses IgG and IgE might target SmTOR immediately, which is expressed in the infectious larvae/schistosomula migrating to the blood vessels. That we were able to detect binding of sera from immunized mice to in-vitro-transformed schistosomula adds some value to this hypothesis. Coating cercariae with antibody may prolong the dwell time in the skin and favour attack by complement. For SmTOR, a non-cytophilic antibody might, in addition, block the natural function of SmTORed1, which is to bind C2 and inhibit complement [41]. Whether the specific antibodies generated or the T cell response were central for the protective effect was not studied further, but it is likely that both participated efficiently in the immune defence.
Several other examples of vaccine candidates meet the target of at least 40% protection in the mouse model [40]. This threshold of protection had been defined in comparison with the radiation-attenuated (RA) cercariae vaccination model that has been shown to be successful in various animal models and induce protection levels of up to 80% [[9,48]]. This is a strong argument that the development of a vaccine against schistosomiasis is possible. Other arguments include the existence of so-called putatively resistant individuals and the fact that the development of other anti-helminth vaccines has been successful [9]. Unfortunately, RA cercariae are not applicable to humans, but with the development of more sophisticated adjuvants and the discovery of new vaccine candidates there is a realistic chance to achieve the desired goal of an anti-schistosomiasis vaccination for humans [49]. Recombinant Sm14/FABP, a well-established vaccine candidate member, that had been proposed originally by the WHO [40], is the only candidate that is now in clinical trial (http://clinicaltrials.gov/) [50]. Sm23 did not confer protection as a recombinant protein [51], but only when delivered as a DNA vaccine [52]. Sm23 is a member of the so-called tetraspanin family that is present on eukaryotic cells and is also abundant in the tegument of S. mansoni [15]. Braschi et al. confirmed the presence of four members of tetraspanins on the adult worm surface by proteomic analysis, including Sm23 [17]; all are considered as new vaccine targets. Two of them, TSP-1 and TSP-2, were tested in CBA mice and were shown to have a protective effect of 52 and 64% [53]. In addition, other membrane proteins have been tested in mice. Sm29 [29], and most recently SmStoLP-2 [54], reduce worm burden by 51 and 32%, respectively, in C57BL/6 mice; both vaccines were formulated with CFA/IFA. Both proteins are not only detectable in the tegument of adult worms, but also in skin-stage and lung-stage schistosomula (Sm29) and in 7-day-old in-vitro-transformed schistosomula (SmStoLP-2), thus accessible as immune targets at an early time-point after infection. However, the general feeling is that it is unlikely that there is one magic bullet that can induce high levels of protection akin to the one provoked by immunization with RA cercariae [[48,55]]. A mixture of different peptides may be necessary to obtain an efficient anti-schistosomal vaccine [56]. In addition, the right adjuvant has to be found as the CFA used in the present study has, as yet, no equivalent for humans.
Very puzzling was the presence of specific antibodies in two normal controls living in Switzerland, who did not have antibodies against other antigens of S. mansoni and thus were highly unlikely to have been in contact with S. mansoni. A possible explanation might be an exposure to bird schistosomes. Trichobilharzia spp. are members of the schistosome genera usually infecting birds. They presumably die in human skin after penetration of the non-specific host, causing an inflammatory skin reaction also known as swimmer's itch [57]. Such an infection induces anti-cercaria antibodies recognizing a broad range of antigens in more than 80% of the infected patients [58]. The prevalence of Trichobilharizia infection in healthy bathers in the Lake of Geneva is 27% [59]. Whether bird schistosomes possess a TOR homologue to SmTOR that would evoke an immune reaction and be recognized upon infection has not yet been investigated.
The low number of patients having anti-rSmTORed1 antibodies was of particular interest: Schistosoma mansoni infection had induced a series of specific antibodies in all these patients, but not anti-rSmTORed1, i.e. 18 of 20 were negative. It might be worthwhile to mention that mice infected with S. mansoni without prior immunization did not develop antibodies against SmTOR, suggesting that SmTOR is not recognized at the time of infection. One possible reason for these findings is that S. mansoni cercariae, as opposed to Trichobilharzia, do not stay in human skin for a long enough period to render a local immune response possible, but move rapidly into the blood vessels. Similarly, cercariae might be transformed rapidly into schistosomula following subcutaneous application in mice, and hence do not evoke an immune response. The absence of specific antibodies in most patients with S. mansoni infection suggests that SmTOR might be a very good target for vaccination, as it induces something that natural infection cannot do and that, at least in one mouse model, is efficient.
In conclusion, we found that, despite all the drawbacks discussed, rSmTORed1 might be a new vaccine target against S. mansoni infection. The next steps will include testing its vaccine efficacy in an outbred mouse strain and determine whether using other adjuvants acceptable for human use would be sufficient to produce a protective immune response.
Acknowledgments
This work was supported by the Swiss Life Jubiläumsstiftung and by the Swiss National Science Foundation (grant 330030–131008211 (C.L. and J.A.S.) and PPOOP3-135170 (J.K.). We are grateful to Mireille Vargas, Augustine Corfu and Theresia Manneck for expert help with the in-vivo studies. We thank Daniela Vinzenz and Dr Christian Ostermeier for advice on protein production by auto-induction and Professor Marten Trendelenburg for advice on animal experiments. We thank the Blutspendezentrum SRK Beider Basel for the supply of normal human sera and we also thank Professor Alexandar Tzankov and his team for preparation of frozen sections of parasites.
Disclosure
None of the authors has conflicts of interest to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Production of HaloTag, Halo-tagged C4beta and Halo-tagged ed1 as bacterial fusion proteins used covalently coupled to magnetic beads for competition enzyme-linked immunosorbent assay (ELISA). (a) Schematic representation of HaloTag fusion peptides generated. The HaloTag protein (boxed) is attached N-terminally to the sequences of interest via a 16 aa linker containing a tobacco etch virus (TEV) protease cleavage site. The six C-terminal amino acids of the linker sequence remaining attached to the peptides after cleavage are shown (underlined). HaloTag protein linked to Schistosoma mansoni tetraspanning orphan receptor (rSmTORed1) loop is denoted as HALOed1 and HaloTag fused to a 26 aa C4β chain stretch is termed HALOC4beta (human C4β chain peptide S206). Local sequence alignment over the C-terminal 27 aa was performed with ClustalW2 and then edited manually (identical amino acids in bold letters, similar amino acids shaded in grey). (b) Protein samples extracted from BL21 4·5 h after induction with isopropyl β-D-1-thiogalactopyranoside (IPTG). HaloTag (HALO) and Halo-tagged (HALOC4 beta, HALOed1) peptides in crude Escherichia coli extracts visualized by Coomassie stain (left) and detected by Western blot probed with monoclonal anti-ed1 antibody AbDy04644·1 (right).
Fig. S2. Antibodies against recombinant first extracellular domain of Schistosoma mansoni tetraspanning orphan receptor (rSmTORed1) generated in mice do not recognize the homologous stretch on the complement C4 β chain. Total immunoglobulin (Ig) detection in sera of individual BALB/c mice immunized with rSmTORed1 (n = 3; mice 1·1–1·3) and a control mouse injected with phosphate-buffered saline (PBS) only (mouse 3·1). rSmTORed1 = purified histidine (HIS)-tagged SmTORed1; rSmTORed1/C4beta/HALO control tobacco etch virus (TEV) cut = SmTORed1, human C4β chain peptide S206 (C4beta) produced as HALO-tagged peptides or HALO-tag alone purified by TEV protease digestion.
Fig. S3. Titration of sera from individual mice before and after immunization analysing anti-recombinant first extracellular domain of Schistosoma mansoni tetraspanning orphan receptor (rSmTORed1) immunoglobulin (Ig) (IgG, IgM, IgA) responses. Open and filled symbols denote Ig levels determined at days −7 and 35, respectively. Data points for the three mice (n = 3) of the strain indicated immunized with antigen and (a,b) complete Freund's adjuvant/incomplete Freund's adjuvant (CFA/IFA) or (c,d) muramyl dipeptide (MDP) adjuvant are shown in the left panel; values for the control mice (n = 3) immunized with adujvant/phosphate-buffered saline (PBS) only are shown in the right panel. The dilution determined for further screening of sera in the different settings is boxed. It was set according to optical density (OD) values at 450 nm still within but near the boarder of the plateau reached at low serum dilutions.
Fig. S4. Total immunoglobulin (Ig) amounts in sera of individual mice immunized with recombinant first extracellular domain of Schistosoma mansoni tetraspanning orphan receptor (rSmTORed1) and the control groups injected with inclusion bodies measured in sera pre-immunization and after the first boost. Ig levels in mice immunized with rSmTORed1 (antigen, ag; n = 10), inclusion bodies (i. bodies; n = 5) or PBS (n = 5) in complete Freund's adjuvant/incomplete Freund's adjuvant (CFA/IFA) were monitored at days −7 and 35 of the trial. Sera for the enzyme-linked immunosorbent assay (ELISA) assay were diluted as determined by end-point titrations for the different mouse strain adjuvant combinations that was (a) 1:6400 for C57BL/6 mice injected with antigen in CFA/IFA and (b,c) 1:3200 for BALB/c and C57BL/6 mice immunized with muramyl dipeptide (MDP). Statistical analyses used were: Student's t-test to compare titre at days −7 and 35 and one-way analysis of variance (anova) to compare titres at day 35; n.s.: statistically not significant; statistically significant *(P < 0·05), **(P < 0·01), ***(P < 0·001).
Fig. S5. Recombinant first extracellular domain of Schistosoma mansoni tetraspanning orphan receptor (rSm-TORed1) complete Freund's adjuvant/incomplete Freund's adjuvant (CFA/IFA)-induced antibody response in BALB/c mice after challenge with Schistosoma mansoni infection. Immunoglobulin (Ig)M (a) and IgA (b) serology in mouse sera sampled at days −7, 35 and 107 (serum dilution both 1:1600). BALB/c mice were immunized with rSmTORed1 (antigen, ag) or control groups immunized with inclusion bodies (i. bodies) or buffer only [phosphate-buffered saline (PBS)] using complete CFA/IFA as adjuvant. All groups were infected with S. mansoni cercariae at day 55. Groups consisted of n = 10 animals. Arrows indicate injections at days 0 (prime), 21 (boost 1) and 42 (boost 2).
Fig. S6. The cytokine secretion profile of spleen cell cultures stimulated with antigen of immunized BALB/c mice was maintained after the infection challenge. Measurement of cytokines in the supernatant of spleen cell cultures after stimulation with recombinant first extracellular domain of Schistosoma mansoni tetraspanning orphan receptor (rSmTORed1) (a) 14 days after the second boost or (b) after immunization challenge experiment (trial 1) after 8 weeks (day 114) of infection. Number of spleens analysed: (a) n = 3/2 (rSmTORed1/PBS), (b) n = 2/2 (rSmTORed1/PBS). The bars represent the mean values ± standard deviation (s.d.) of either three or two (rSmTORed1/PBS) different samples each measured in triplicate. Splenocytes of all mice were confirmed to have the capacity for release of cytokines in response to stimulation with concanavalin A.
Fig. S7. Detection of anti-recombinant first extracellular domain of Schistosoma mansoni tetraspanning orphan receptor (rSmTORed1)-specific antibodies in sera from BALB/c mice (n = 5) immunized with radiation-attenuated cercariae. Total immunoglobulin (Ig) to rSmTORed1 was measured in pre-immune sera (day −7), after the first (day 35) and second boost (day 63). Results for the same sera diluted 1:3200 and 1:6400 are shown.
Table S1. Primer list used for cloning of Halo-tagged fusion constructs.
Appendix S1. Supplementary materials and methods.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 2.Bilharz T, Von Siebold CT. A contribution to human helminthology. Zeitsch Wissensch Zool. 1852;4:53–76. –53. [Google Scholar]
- 3.Jordan P. From katayama to the Dakhla Oasis: the beginning of epidemiology and control of bilharzia. Acta Trop. 2000;77:9–40. doi: 10.1016/s0001-706x(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 4.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 5.Abdul-Ghani R, Loutfy N, el-Sahn A, Hassan A. Current chemotherapy arsenal for Schistosomiasis mansoni: alternatives and challenges. Parasitol Res. 2009;104:955–965. doi: 10.1007/s00436-009-1371-7. [DOI] [PubMed] [Google Scholar]
- 6.Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008;21:659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- 7.Caffrey CR. Chemotherapy of schistosomiasis: present and future. Curr Opin Chem Biol. 2007;11:433–439. doi: 10.1016/j.cbpa.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Schistosoma mansoni: chemotherapy of infections of different ages. Exp Parasitol. 1986;61:294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- 9.McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21:225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McManus DP. The search for a vaccine against schistosomiasis – a difficult path but an achievable goal. Immunol Rev. 1999;171:149–161. doi: 10.1111/j.1600-065x.1999.tb01346.x. [DOI] [PubMed] [Google Scholar]
- 11.Hagan P. Schistosomiasis – a rich vein of research. Parasitology. 2009;136:1611–1619. doi: 10.1017/S003118200999093X. [DOI] [PubMed] [Google Scholar]
- 12.Berriman M, Haas BJ, LoVerde PT, et al. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hokke CH, Fitzpatrick JM, Hoffmann KF. Integrating transcriptome, proteome and glycome analyses of Schistosoma biology. Trends Parasitol. 2007;23:165–174. doi: 10.1016/j.pt.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RA, Ashton PD, Braschi S, Dillon GP, Berriman M, Ivens A. ‘Oming in on schistosomes: prospects and limitations for post-genomics. Trends Parasitol. 2007;23:14–20. doi: 10.1016/j.pt.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Loukas A, Tran M, Pearson MS. Schistosome membrane proteins as vaccines. Int J Parasitol. 2007;37:257–263. doi: 10.1016/j.ijpara.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RA, Coulson PS. Schistosome vaccines: a critical appraisal. Mem Inst Oswaldo Cruz. 2006;101(Suppl. 1):13–20. doi: 10.1590/s0074-02762006000900004. [DOI] [PubMed] [Google Scholar]
- 17.Braschi S, Borges WC, Wilson RA. Proteomic analysis of the shistosome tegument and its surface membranes. Mem Inst Oswaldo Cruz. 2006;101:205–212. doi: 10.1590/s0074-02762006000900032. [DOI] [PubMed] [Google Scholar]
- 18.Braschi S, Curwen RS, Ashton PD, Verjovski-Almeida S, Wilson A. The tegument surface membranes of the human blood parasite Schistosoma mansoni: a proteomic analysis after differential extraction. Proteomics. 2006;6:1471–1482. doi: 10.1002/pmic.200500368. [DOI] [PubMed] [Google Scholar]
- 19.Braschi S, Wilson RA. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol Cell Proteomics. 2006;5:347–356. doi: 10.1074/mcp.M500287-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Hansell E, Braschi S, Medzihradszky KF, et al. Proteomic analysis of skin invasion by blood fluke larvae. PLoS Negl Trop Dis. 2008;2:e262. doi: 10.1371/journal.pntd.0000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skelly PJ. Immunoparasitology series: intravascular schistosomes and complement. Trends Parasitol. 2004;20:370–374. doi: 10.1016/j.pt.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Lochmatter C, Schifferli JA, Martin PJ. Schistosoma mansoni TOR is a tetraspanning orphan receptor on the parasite surface. Parasitology. 2009;136:487–498. doi: 10.1017/S0031182009005757. [DOI] [PubMed] [Google Scholar]
- 23.Inal JM, Schifferli JA. Complement C2 receptor inhibitor trispanning and the beta-chain of C4 share a binding site for complement C2. J Immunol. 2002;168:5213–5221. doi: 10.4049/jimmunol.168.10.5213. [DOI] [PubMed] [Google Scholar]
- 24.Zeng G. Sticky-end PCR: new method for subcloning. Biotechniques. 1998;25:206–208. doi: 10.2144/98252bm05. [DOI] [PubMed] [Google Scholar]
- 25.Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Exp Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Soulard A, Cremonesi A, Moes S, Schutz F, Jeno P, Hall MN. The rapamycin-sensitive phosphoproteome reveals that TOR controls protein kinase A toward some but not all substrates. Mol Biol Cell. 2010;21:3475–3486. doi: 10.1091/mbc.E10-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurst R, Hook B, Slater MR, Hartnett J, Storts DR, Nath N. Protein–protein interaction studies on protein arrays: effect of detection strategies on signal-to-background ratios. Anal Biochem. 2009;392:45–53. doi: 10.1016/j.ab.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 28.Los GV, Encell LP, McDougall MG, et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol. 2008;3:373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 29.Cardoso FC, Macedo GC, Gava E, et al. Schistosoma mansoni tegument protein Sm29 is able to induce a Th1-type of immune response and protection against parasite infection. PLoS Negl Trop Dis. 2008;2:e308. doi: 10.1371/journal.pntd.0000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manneck T, Haggenmüller Y, Keiser J. Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Parasitology. 2010;137:85–98. doi: 10.1017/S0031182009990965. [DOI] [PubMed] [Google Scholar]
- 31.Dubendorff JW, Studier FW. Controlling basal expression in an inducible T7 expression system by blocking the target T7 promoter with lac repressor. J Mol Biol. 1991;219:45–59. doi: 10.1016/0022-2836(91)90856-2. [DOI] [PubMed] [Google Scholar]
- 32.Inal JM, Sim RB. A Schistosoma protein, Sh-TOR, is a novel inhibitor of complement which binds human C2. FEBS Lett. 2000;470:131–134. doi: 10.1016/s0014-5793(00)01304-1. [DOI] [PubMed] [Google Scholar]
- 33.Inal JM. Schistosoma TOR (trispanning orphan receptor), a novel, antigenic surface receptor of the blood-dwelling, Schistosoma parasite. Biochim Biophys Acta. 1999;1445:283–298. doi: 10.1016/s0167-4781(99)00051-2. [DOI] [PubMed] [Google Scholar]
- 34.Ramos CR, Spisni A, Oyama S, Jr, et al. Stability improvement of the fatty acid binding protein Sm14 from S. mansoni by Cys replacement: structural and functional characterization of a vaccine candidate. Biochim Biophys Acta. 2009;1794:655–662. doi: 10.1016/j.bbapap.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004;22:460–466. doi: 10.1097/01.shk.0000142249.08135.e9. [DOI] [PubMed] [Google Scholar]
- 36.El Ridi R, Wagih A, Salem R, Mahana N, El Demellawy M, Tallima H. Impact of interleukin-1 and interleukin-6 in murine primary schistosomiasis. Int Immunopharmacol. 2006;6:1100–1108. doi: 10.1016/j.intimp.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 37.El Ridi R, Salem R, Wagih A, Mahana N, El Demellawy M, Tallima H. Influence of interleukin-2 and interferon-gamma in murine schistosomiasis. Cytokine. 2006;33:281–288. doi: 10.1016/j.cyto.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 38.El Ridi R, Mahrous A, Afifi A, Montash M, Velek J, Jezek J. Human and murine humoral immune recognition of multiple peptides from Schistosoma mansoni glyceraldehyde 3-P dehydrogenase is associated with resistance to Schistosomiasis. Scand J Immunol. 2001;54:477–485. doi: 10.1046/j.1365-3083.2001.00992.x. [DOI] [PubMed] [Google Scholar]
- 39.Hota-Mitchell S, Siddiqui AA, Dekaban GA, Smith J, Tognon C, Podesta RB. Protection against Schistosoma mansoni infection with a recombinant baculovirus-expressed subunit of calpain. Vaccine. 1997;15:1631–1640. doi: 10.1016/s0264-410x(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 40.Bergquist NR, Colley DG. Schistosomiasis vaccine:research to development. Parasitol Today. 1998;14:99–104. doi: 10.1016/s0169-4758(97)01207-6. [DOI] [PubMed] [Google Scholar]
- 41.Pinot de Moira A, Fulford AJ, Kabatereine NB, Ouma JH, Booth M, Dunne DW. Analysis of complex patterns of human exposure and immunity to Schistosomiasis mansoni: the influence of age, sex, ethnicity and IgE. PLoS Negl Trop Dis. 2010;4:e820. doi: 10.1371/journal.pntd.0000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capron A, Dessaint JP. Immunologic aspects of schistosomiasis. Annu Rev Med. 1992;43:209–218. doi: 10.1146/annurev.me.43.020192.001233. [DOI] [PubMed] [Google Scholar]
- 43.Moser G, Sher A. Studies of the antibody-dependent killing of schistosomula of Schistosoma mansoni employing haptenic target antigens. II. In vitro killing of TNP-schistosomula by human eosinophils and neutrophils. J Immunol. 1981;126:1025–1029. [PubMed] [Google Scholar]
- 44.Horowitz S, Tarrab-Hazdai R, Eshhar Z, Arnon R. Anti-schistosome monoclonal antibodies of different isotypes – correlation with cytotoxicity. EMBO J. 1983;2:193–198. doi: 10.1002/j.1460-2075.1983.tb01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gounni AS, Lamkhioued B, Ochiai K, et al. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–186. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- 46.Moqbel R, MacDonald AJ, Kay AB. Enhancement of human eosinophil- and neutrophil-mediated killing of schistosomula of Schistosoma mansoni by reversed type (IgE-mediated) anaphylaxis in vitro. Clin Exp Immunol. 1985;59:577–586. [PMC free article] [PubMed] [Google Scholar]
- 47.Kazura JW, Fanning MM, Blumer JL, Mahmoud AA. Role of cell-generated hydrogen peroxide in granulocyte-mediated killing of schistosomula of Schistosoma mansoni in vitro. J Clin Invest. 1981;67:93–102. doi: 10.1172/JCI110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minard P, Dean DA, Jacobson RH, Vannier WE, Murrell KD. Immunization of mice with Co-60 irradiated Schistosoma mansoni cercariae. Am J Trop Med Hyg. 1978;27:76–86. doi: 10.4269/ajtmh.1978.27.76. [DOI] [PubMed] [Google Scholar]
- 49.Bergquist NR, Leonardo LR, Mitchell GF. Vaccine-linked chemotherapy: can schistosomiasis control benefit from an integrated approach? Trends Parasitol. 2005;21:112–117. doi: 10.1016/j.pt.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Tendler M, Simpson AJ. The biotechnology-value chain: development of Sm14 as a schistosomiasis vaccine. Acta Trop. 2008;108:263–266. doi: 10.1016/j.actatropica.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Da'Dara AA, Skelly PJ, Walker CM, Harn DA. A DNA-prime/protein-boost vaccination regimen enhances Th2 immune responses but not protection following Schistosoma mansoni infection. Parasite Immunol. 2003;25:429–437. doi: 10.1111/j.1365-3024.2003.00651.x. [DOI] [PubMed] [Google Scholar]
- 52.Da'dara AA, Skelly PJ, Wang MM, Harn DA. Immunization with plasmid DNA encoding the integral membrane protein, Sm23, elicits a protective immune response against schistosome infection in mice. Vaccine. 2001;20:359–369. doi: 10.1016/s0264-410x(01)00374-7. [DOI] [PubMed] [Google Scholar]
- 53.Tran MH, Pearson MS, Jeffrey MB, et al. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med. 2006;12:835–840. doi: 10.1038/nm1430. [DOI] [PubMed] [Google Scholar]
- 54.Farias LP, Cardoso FC, Miyasato PA, et al. Schistosoma mansoni stomatin like protein-2 is located in the tegument and induces partial protection against challenge infection. PLoS Negl Trop Dis. 2010;4:e597. doi: 10.1371/journal.pntd.0000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kariuki TM, Farah IO, Yole DS, et al. Parameters of the attenuated schistosome vaccine evaluated in the olive baboon. Infect Immun. 2004;72:5526–5529. doi: 10.1128/IAI.72.9.5526-5529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson RA, Coulson PS. Immune effector mechanisms against schistosomiasis: looking for a chink in the parasite's armour. Trends Parasitol. 2009;25:423–431. doi: 10.1016/j.pt.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horak P, Kolarova L. Bird schistosomes: do they die in mammalian skin? Trends Parasitol. 2001;17:66–69. doi: 10.1016/s1471-4922(00)01770-0. [DOI] [PubMed] [Google Scholar]
- 58.Lichtenbergova L, Kolbekova P, Kourilova P, et al. Antibody responses induced by Trichobilharzia regenti antigens in murine and human hosts exhibiting cercarial dermatitis. Parasite Immunol. 2008;30:585–595. doi: 10.1111/j.1365-3024.2008.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chamot E, Toscani L, Rougemont A. Public health importance and risk factors for cercarial dermatitis associated with swimming in Lake Leman at Geneva, Switzerland. Epidemiol Infect. 1998;120:305–314. doi: 10.1017/s0950268898008826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


