Abstract
Crohn's disease (CD) is characterized by inflammation and an aetiology that is still unknown. Hypertrophy of mesenteric fat is a reflection of disease activity, as this fat covers the entire length of the affected area. Adipocytes synthesize leptin and adiponectin, adipocytokines responsible for pro- and anti-inflammatory effects. Therefore, we evaluated serum levels of adiponectin and leptin, as well as mesenteral expression of adiponectin in active CD and those in remission. Sixteen patients with ileocaecal CD followed at the Outpatient Clinic, Coloproctology Unit of University of Campinas Clinical Hospital, participated in the study. Analysis of serum adiponectin and leptin by enzyme-linked immunosorbent assay was performed in patients with active CD (ACD group), remission CD (RCD group) and in six healthy controls. Ten patients with active ileocaecal CD (FCD group) and eight patients with non-inflammatory disease selected for surgery were also studied. The specimens were snap-frozen and the expression of adiponectin was determined by immunoblot of protein extracts. Serum C-reactive protein levels were higher in the ACD group when compared to the others and no difference of body mass index was observed between the groups. Serum adiponectin was lower in the ACD group when compared to control, but no differences were seen when comparing the ACD and RCD groups. Mesenteric adiponectin expression was lower in the FCD group when compared to the FC group. Serum leptin was similar in all groups. The lower levels of serum and mesenteric adiponectin in active CD suggest a defective regulation of anti-inflammatory pathways in CD pathogenesis.
Keywords: Crohn's disease, cytokines, inflammatory bowel disease, lipid mediators, mesenteric fat tissue
Introduction
Crohn's disease (CD) is an inflammatory disease that can affect any portion of the gastrointestinal tract, most commonly affecting the ileum and colon and often resulting in abdominal pain, diarrhoea, malnutrition and bleeding [1]. Hypertrophy of mesenteric adipose tissue close to the affected area is a common feature in chronic CD with transmural inflammation, and extends from the mesentery, partially covering the circumference with a wrapping of intestinal fat, and may involve the small and large bowels [2].
Although the accumulation of intra-abdominal fat is involved directly in the development of metabolic disorders, such as type 2 diabetes mellitus and coronary artery disease, the hypertrophied mesenteric fat tissue in CD is independent of body mass index (BMI) or the presence of metabolic disorders [3]. The release of adiponectin is significantly higher in CD compared with ulcerative colitis (UC). The increase of this adipocytokine may be related specifically to inflammation and the presence of hypertrophied mesenteric fat tissue [4]. Hypertrophy of mesenteric fat tissue could be understood as a barrier to the inflammatory process or a second factor that maintains the inflammatory process, resulting in the transmural aspect of CD [2,5,6].
Although there is a phenotypic variation between CD patients, some common macroscopic aspects can be observed, especially regarding the thickening of mesenteric fat near the affected area [6,7]. Histological analyses of these studies reveal abnormalities in fat, including infiltration of macrophages and T cells, perivascular inflammation and fibrosis. In addition, there are increased numbers of adipocytes, which have been reported to be smaller and four times higher in number in CD than in controls [7].
Like macrophages and epithelial cells, adipocytes from normal individuals are able to synthesize several proinflammatory and anti-inflammatory cytokines, called adipocytokines, and can express Toll-like receptor (TLR)-4 for the recognition of bacterial antigens [8–11].
Adiponectin is a protein produced by adipose tissue which contains special characteristics, such as increased insulin sensitivity, the prevention of atherosclerosis and fibrosis. Unlike other hormones from adipose tissue, serum levels decrease with increasing adiposity and are correlated inversely with obesity, insulin resistance and metabolic syndrome. In addition, adiponectin has anti-inflammatory effects and inhibits the activity and production of tumour necrosis factor (TNF)-α and interleukin (IL)-6 [2,12–14].
Adipose tissue can also produce and release leptin, which can be found in other organs such as the stomach, pituitary gland and placenta [14–16]. The main function of this hormone is to provide the homeostasis of body weight, resulting in increased energy expenditure and reduced food intake mediated by the hypothalamus [12,14]. Studies have suggested that leptin is responsible for regulating the immune system by stimulating T cell production [17,18]. Indeed, leptin may be reduced in malnourished patients, resulting in increased susceptibility to infection [19]. Leptin has been shown to increase the secretion of TNF-α, IL-6 and proinflammatory cytokines involved in the pathogenesis of CD, as well as activate nuclear factor kappa B (NF-κB), the main transcription factor of TNF-α, in intestinal tissue of CD patients [20,21]. However, leptin levels have been correlated with protection against colitis induced by dextran sulphate sodium (DSS) and trinitrobenzene sulphonic acid (TNBS) in experimental models of intestinal inflammation [22]. This contradiction between the role of leptin in inflammatory murine colitis and CD in humans requires further investigation.
Thus, this study aimed to evaluate serum levels of adiponectin and leptin in patients with CD, with and without disease activity, as assessed by clinical and laboratory tests, in order to establish the role of adipocytokines in the CD pathogenesis.
Material and methods
Blood samples were taken of 16 patients with ileocaecal CD followed at the Inflammatory Bowel Disease Outpatient Clinic, Coloproctology Unit of University of Campinas Clinical Hospital, eight with CD in activity (ACD group) and eight with CD in remission (RCD group). The control group consisted of six healthy individuals. Patients with CD in locations other than ileocaecal were excluded. Disease activity was scored by colonoscopy examinations, small bowel follow-through, histological aspects and activity index of CD (IADC) [23], with 250 cut-off. Plasma C-reactive protein (CRP) was assessed from all patients and controls to characterize disease activity and BMI of each individual was also measured.
For protein analysis and histopathological study, mucosal biopsies were taken from 10 patients with ileocaecal CD who had undergone surgical resection (FCD group). The presence of disease activity was assessed by colonoscopy 1 day before surgery and all patients had Crohn's disease activity index (CDAI) more than 250 points. Patients with CD in locations other than ileocaecal were excluded. The control groups were composed of eight patients who underwent rectosigmoidectomy for non-inflammatory disease (left megacolon due to Chagas's disease), with normal distal ileum (ileum mesenteric fat tissue control group: FC group).
The study was performed in accordance with the Declaration of Helsinki and was approved by the Ethical Committee of the University of Campinas. All blood samples and mesenteric samples were taken after informed consent from the patients. The study was carried out at the University of Campinas, Coloproctology Unit, and at the Cell Signaling Laboratory of the Department of Internal Medicine.
Enzyme-linked immunosorbent assay (ELISA)
Blood samples were centrifuged for 10 min and blood plasma was stored at −80°C until use. The measurements of adiponectin, leptin and CRP were performed using ELISA with a commercial kit, purchased from Phoenix (Burlingame, CA, USA) and Abcam (Cambridge, MA, USA), respectively, based on the manufacturer's protocol.
Immunoblotting – gel electrophoresis
Biopsies from mesenteric fat tissue near the affected intestinal area were snap-frozen in liquid nitrogen and stored at −80°C until use. For total protein extract preparation, the fragments were homogenized in solubilization buffer at 4°C [1% Triton X-100, 100 mM Tris-HCl (pH 7·4), 100 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM ethylenediamine tetraacetic acid (EDTA), 10 mM sodium orthovanadate, 2·0 mM phenylmethylsulphonyl fluoride (PMSF) and 0·1 mg aprotinin/ml] with a Polytron PTA 20S generator (model PT 10/35; Brinkmann Instruments, Westbury, NY, USA) operated at maximum speed for 30 s. Insoluble material was removed by centrifugation (20 min at 9000 g at 4°C). The protein concentrations of the supernatants were determined by the Bradford dye binding method [24]. Aliquots of the resulting supernatants containing 50 μg total proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes and blotted with anti-adiponectin antibody [25,26].
Reagents for SDS-PAGE and immunoblotting were from Bio-Rad Laboratories (Richmond, CA, USA). PMSF, aprotinin, Triton X-100, Tween 20 and glycerol were from Sigma (St Louis, MO, USA). Nitrocellulose paper (BA85, 0·2 μm) was from Amersham (Aylesbury, UK). The anti-adiponectin (ACRP30, sc-17044, goat polyclonal) antibody was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The protein molecular weight was assessed using the PageRuler™ from Fermentas (Glenburnie, MD, USA). The signal was detected by chemiluminescent reaction (SuperSignal®West Pico Chemiluminescent Substrate from Pierce Biotechnology, Inc., Rockford, IL, USA).
All numerical results are expressed as the mean ± standard error of the mean (s.e.m.) of the indicated number of experiments. The results of blots are presented as direct comparisons of bands in autoradiographs and quantified by densitometry using the Gel-Pro Analyzer 6·0 software (Exon-Intron Inc., Farrell, MD, USA).
Statistical analysis
Comparison of the serum adiponectin, leptin, CRP and BMI between ACD, RCD and control groups was performed using analysis of variance (anova) and the Tukey–Kramer test. Comparison of CDAI between the ACD and RCD groups was analysed using the t-test. The results were reported as means with standard deviations of the indicated number of experiments. The level of significance was set at P < 0·05.
Data from imunoblotting were analysed by t-test. The level of significance was set at P < 0·05.
Results
The groups of samples which were submitted to ELISA did not differ regarding gender, and the mean age was 33·6 (range 16–49) years for patients with active CD, 37·3 (range 24–53) years to those with CD in remission and 31·3 (range 25–48) years for the control group (P > 0·05). The mean disease duration was 84·8 (range 8–192) months for patients with active CD and 112·5 (range 24–204) months for those with CD in remission (P > 0·05). The IADC average was 383·8 (range 283·7–500·8) in patients with active CD, whereas patients with CD in remission had a mean IADC of 82 (range 21·4–141·4) (P < 0·0001).
The CRP (mg/dl) level average was 2·18 (range 0·04–6·32) in patients with active CD, whereas patients with CD in remission had a mean of 0·31 (range 0·05–0·71) and controls had a mean of 0·05 (range 0·02–0·12) (P < 0·0001; Fig. 1).
Fig. 1.
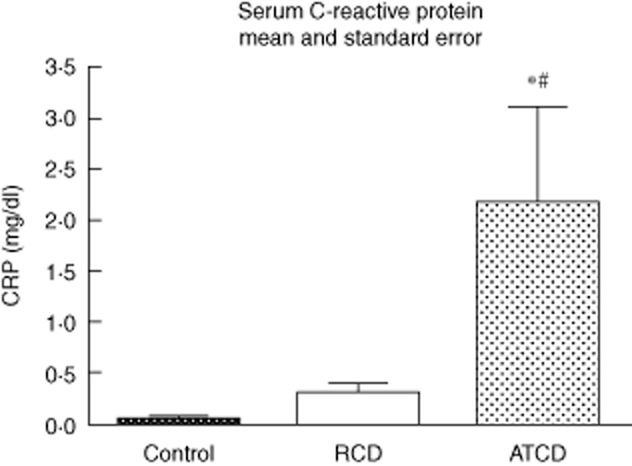
Serum C-reactive protein (mg/dl). Active CD (ACD) group – CD in activity, remission CD (RCD) group – CD in remission, control group. For ACD and RCD groups, n = 8; for control group, n = 6. *P < 0·05 versus control, #P < 0·05 versus CD in remission.
The BMI average was 19·27 (range, 13·1–23·5) in patients with active CD, whereas patients with CD in remission had a mean of 21·26 (range 20·2–22·8) and controls had a mean of 22·36 (range 18·8–26·3) (P > 0·05; Fig. 2).
Fig. 2.
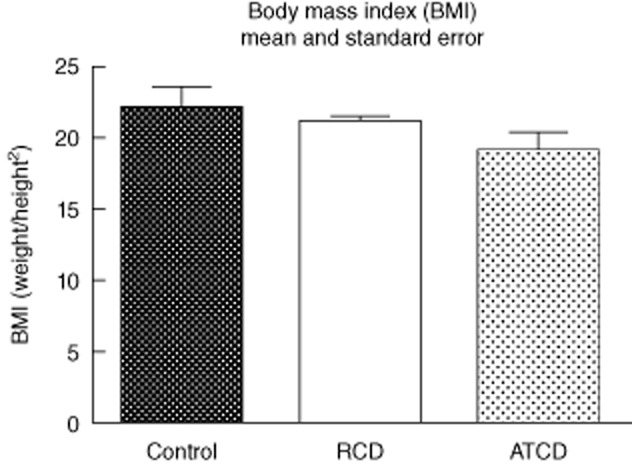
Body mass index (BMI). Active CD (ACD) group – CD in activity, remission CD (RCD) group – CD in remission, control group. For ACD and RCD groups, n = 8; for control group, n = 6. *P < 0·05 versus control, #P < 0·05 versus CD in remission.
Plasma adiponectin levels were lower in the ACD group (active CD) [6828·5 (range 5938·3–8000·9)] when compared to the control group [7911·4 (range 6735·0–8637·3)] (P < 0·01), but not the CD in remission group (RCD) [7246·8 (range 6667·7–7782·5)] (P > 0·05). The levels of adiponectin were similar between the RCD [7246·8 (range 6667·7–7782·5)] and control groups [7911·4 (range 6735·0–8637·3)] (P > 0·05; Fig. 3).
Fig. 3.
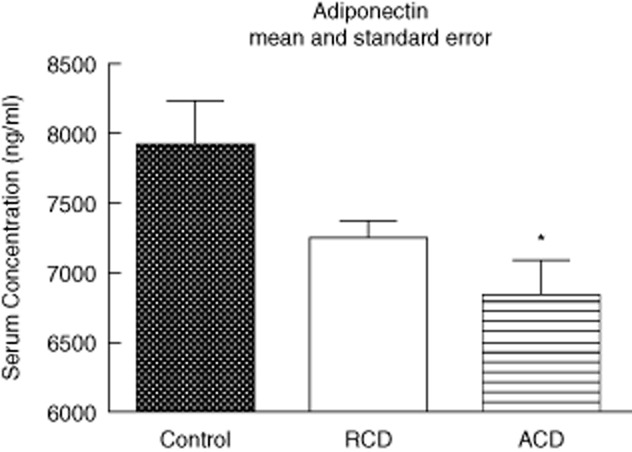
Serum adiponectin (ng/ml). Active CD (ACD) group – CD in activity, remission CD (RCD) group – CD in remission, control group. For ACD and RCD groups, n = 8; for control group, n = 6. *P < 0·05 versus control, #P < 0·05 versus CD in remission.
Serum leptin levels were similar in the ACD [26·3 (range 21·4–34·3)], RCD [30·9 (range 26·9–34·7)] and control groups [28·9 (range 25·9–37·3)] (P > 0·05). Figure 4 shows these results.
Fig. 4.
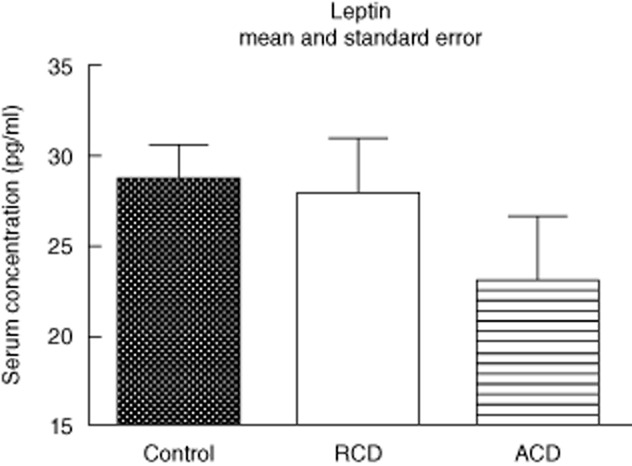
Serum leptin (pg/ml). Active CD (ACD) group – CD in activity, remission CD (RCD) group – CD in remission, control group. For ACD and RCD groups, n = 8; for control group, n = 6. *P < 0·05 versus control, #P < 0·05 versus CD in remission.
The groups in which the samples had undergone protein extraction and imunoblot had the following characteristics: patients with ileocaecal CD [median age 34·9 (range 14–60) years; male 50%; female 50%] and those with normal distal ileum [median age 55·6 (range 39–70) years; male 62·5%; female 37·5%].
Considering the mesenteric fat tissue analysis, protein expression of adiponectin was lower in the FCD group when compared to the control group (P < 0·05; Fig. 5).
Fig. 5.
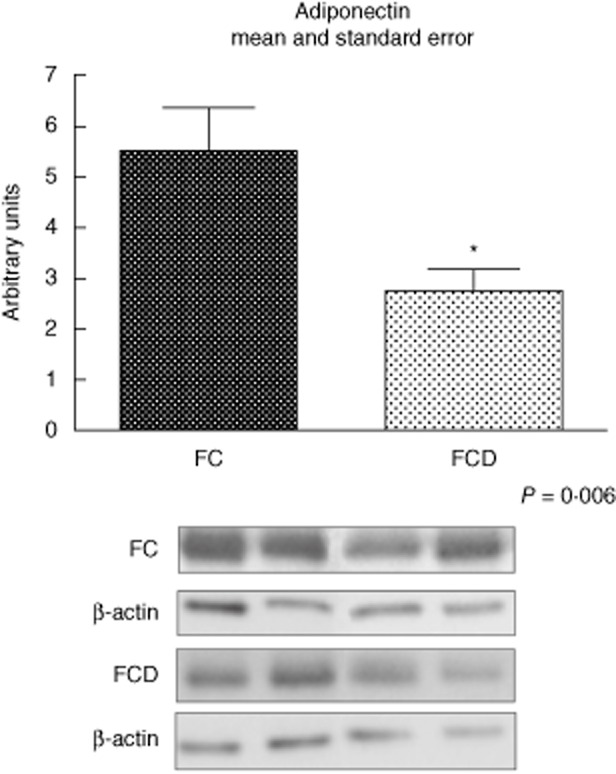
Representative Western blot analyses and determination of adiponectin protein expression in fat tissue of the control (FC) and Crohn's disease (FCD) groups. For illustration purpose each band represents one patient. For FCD group, n = 10; for FC group, n = 8, *P < 0·05 versus control.
The haematoxylin and eosin stains of mesenteric fat tissue in controls and CD are shown in Fig. 6. Adipocytes of the FCD group were smaller than the controls and immune cell infiltration is seen more prolifically in CD patients.
Fig. 6.
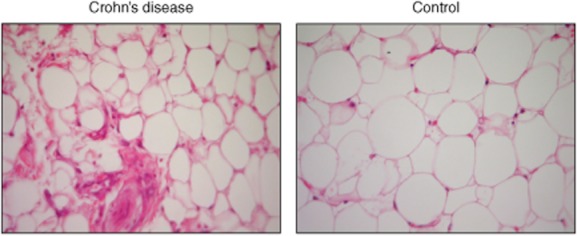
Histopathology of mesenteric fat tissue sections from Crohn's disease (FCD) and fat tissue of the control (FC) groups (×400).
Discussion
Adipose tissue is considered an endocrine organ that may release adipocytokines such as leptin and adiponectin, in addition to participating in energy metabolism and immune processes [27]. Hypertrophy of mesenteric fat tissue is usually shown as a marker of active CD [6,28–30], and a high ratio of areas of visceral to subcutaneous fat is a signal of aggressive disease [31]. An experimental trial noted that hypertrophied mesenteric adipose tissue showed a reduction of triglycerides and adipocytokines (adiponectin and leptin) in mice with TNBS-induced colitis [28].
CRP levels in mesenteric adipose tissue have been shown to demonstrate increased concentrations in patients with CD, compared with UC and control individuals, showing a correlation with the serum CRP concentration and density of the mesenteric adipose tissue in CD [32,33].
The serum levels of adiponectin in IBD and in healthy controls are controversial in the literature. According to Valentini et al., serum adiponectin is reduced in CD and UC compared to controls [34,35]. However, Karmiris et al. showed similar levels of adiponectin in the three groups (CD, UC and control) [36]. The present study showed a lower level of adiponectin in CD compared to controls, but only in active CD and not in CD in remission, suggesting that a defective anti-inflammatory balance may occur in CD.
A negative energy balance, common in active CD patients, could lead to high levels of adiponectin, either locally or systemically. However, the reduction of serum adiponectin is also associated with chronic inflammatory conditions and its release into the bloodstream from adipose tissue depends upon, among other factors, the correct conformation of protein in the endoplasmic reticulum [37]. Low expression of adiponectin in the mesenteric fat tissue of CD patients may show a deficiency in the anti-inflammatory mechanism in intestinal mesenteric fat near the affected intestinal area during the late stages of this chronic disease, and this helps to perpetuate a state of chronic inflammation. Considering this aspect during the late stages of CD, apoptosis of adipocytes could be observed, leading to adipocyte hypertrophy, a positive energy balance and a consequent decrease in adiponectin expression near the affected small bowel area, maintaining chronic inflammation [38]. Indeed, the lower grades of mesenteric adiponectin could reflect lower levels of serum adiponectin.
Paul et al. studied adipose tissue specimens of 10 CD patients; these authors showed that adiponectin levels were up-regulated in CD compared with the other groups, as measured by ELISA [39]. However, BMI was different among the groups, which could lead to a different release of adiponectin to the bloodstream. Indeed, the control groups used were patients who had undergone surgery with diverticulitis or cancer, which may have involved inflammatory pathways. According to Yamamoto et al. [2], adiponectin expression in mesenteric fat of humans is increased in CD and is correlated with the inflammation intensity. However, similarly to the described previous study, controls were patients with colon cancer or ulcerative colitis. Our study compared groups with similar BMI (P ≤ 0·05), and the control group was composed of individuals without inflammatory disease. These aspects could lead to contrasting results.
The role of adiponectin in adipose tissue hypertrophy in CD is not well established, and most of the studies were carried out in animal models [4]. An experimental study with adiponectin ‘knock-out’ animals showed protection against chemically induced colitis [40]; in contrast, other studies have shown that increased expression of adiponectin leads to reduced expression of proinflammatory cytokines such as TNF-α and IL-1β [41].
Adiponectin blood levels of patients with CD in remission did not differ from controls in the present study, showing a balance of this adipocytokine in the non-inflammatory stage. Moreover, adiponectin can reduce secretion of TNF-α from monocytes/macrophages and attenuate the biological effects caused by TNF-α, helping in disease remission [42].
Mesenteric fat tissue can have an important role in the maintenance of CD, as the altered balance between proinflammatory and anti-inflammatory factors in adipocytes could lead to activation of the innate immune system cells, such as dendritic cells, and also the adaptive immune system. It has been estimated that the percentage of macrophages in adipose tissue ranges from 10 to 50%, suggesting a high cellular plasticity of adipose tissue [43,44]. The observation that adipocytes can function as macrophage-like cells by expressing and secreting molecules related to inflammation and innate immunity brings mesenteric adipose tissue within the focus of mesenteric diseases [28]. Macrophages may be more numerous than adipocytes in the late stages of CD, which can lead to decreased secretion of adiponectin by adipocytes. These results were verified in experimental colitis by Olivier et al. [28], suggesting that creeping fat occurs only in severe colonic inflammation and shows an inflammatory and fibrous, but not an adipose, pattern. These authors verified few adipocytes and low expression of adipocytokines in mouse TNBS-induced colitis.
In the present study, no difference was observed in serum adiponectin levels between the ACD and RCD groups. This could be explained because patients without clinical activity and endoscopic disease may also have fat mesenteric inflammation that is not detected by blood tests performed in this study, but could be assessed by abdominal magnetic resonance imaging (MRI). We used two different groups of patients to analyse serum and tissue protein, which could be a limitation of the study; however, this was performed because is impossible to obtain mesenteric tissue samples of patients with disease remission.
With regard to leptin, the results of this study showed no difference in serum levels between the groups, showing that this adipocytokine may not be involved in the pathogenesis of CD. The study by Valentini et al. [34], which compared CD, UC and control individuals, also identified similar serum concentrations of leptin between CD patients and controls, whereas the findings in UC showed an increase in serum leptin levels in the case of active disease.
Thus, adiponectin levels in mesenteric fat tissue close to the area affected by CD, and the integrity of the release mechanisms of the adiponectin into the bloodstream, having been shown to be relevant, play an important role in the inflammatory process. The presence of mesenteric hypertrophy at the onset of the disease, the release of active molecules from local adipocytes and the association between connective tissue changes and transmural inflammation support the role of adipose tissue in the pathogenesis of CD. Mesenteric adipose tissue may be an important factor in the balance of local inflammation in these patients. Further studies are needed to elucidate its role in CD development.
Acknowledgments
We thank FAEPEX (Fundo de Apoio ao Ensino, à Pesquisa e à Extensão) and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for financial support. We thank Dr Russell Cohen for his careful review and editing of our manuscript and Dr Eugene Chang for the suggestions on the analysis of the results.
Disclosure
None.
References
- 1.Chuo JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nature. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto K, Kiyohara T, Murayama Y, et al. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn's disease. Gut. 2005;54:789–796. doi: 10.1136/gut.2004.046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schäffler A, Schölmerich J, Büchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue-emerging role in intestinal and mesenteric diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:103–111. doi: 10.1038/ncpgasthep0090. [DOI] [PubMed] [Google Scholar]
- 4.Schäffler A, Schölmerich J. The role of adiponectin in inflammatory gastrointestinal diseases. Gut. 2009;58:317–322. doi: 10.1136/gut.2008.159210. [DOI] [PubMed] [Google Scholar]
- 5.Karmiris K, Koutroubakis IE, Kouroumalis EA. Leptin, adiponectin, resistin, and ghrelin – implications for inflammatory bowel disease. Mol Nutr Food Res. 2008;52:855–866. doi: 10.1002/mnfr.200700050. [DOI] [PubMed] [Google Scholar]
- 6.Zulian A, Cancello R, Micheletto G, et al. Visceral adipocytes: old actors in obesity and new protagonists in Crohn's disease? Gut. 2012;61:86–94. doi: 10.1136/gutjnl-2011-300391. [DOI] [PubMed] [Google Scholar]
- 7.Sheehan AL, Warren BF, Gear MW, Shepherd NA. Fat-wrapping in Crohn's disease: pathological basis and relevance to surgical practice. Br J Surg. 1992;79:955–958. doi: 10.1002/bjs.1800790934. [DOI] [PubMed] [Google Scholar]
- 8.Vettor R, Milan G, Rossato M, Federspil G. Review article: adipocytokines and insulin resistance. Aliment Pharmacol Ther. 2005;22:3–10. doi: 10.1111/j.1365-2036.2005.02587.x. [DOI] [PubMed] [Google Scholar]
- 9.Lin Y, Lee H, Berg AH, Shapiro L, Scherer PE. The lipopolysaccharide-activated Toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J Biol Chem. 2000;275:24255–24263. doi: 10.1074/jbc.M002137200. [DOI] [PubMed] [Google Scholar]
- 10.Pietsch J, Batra A, Stroh T, et al. Toll-like receptor expression and response to specific stimulation in adipocytes and preadipocytes: on the role of fat in inflammation. Ann NY Acad Sci. 2006;1072:407–409. doi: 10.1196/annals.1326.021. [DOI] [PubMed] [Google Scholar]
- 11.Gay J, Tachon M, Neut C, et al. Mesenteric adipose tissue is colonized by bacterial flora and express pathogen recognition receptors in Crohn's disease. Gastroenterology. 2005;128:A503. [abstract] [Google Scholar]
- 12.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone. Diabetes Care. 2003;26:2442–2450. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 13.Drew JE, Farquharson AJ, Padidar S, et al. Insulin, leptin and adiponectin receptors in colon: regulation relative to differing body adiposity independent of diet and in response to dimethylhydrazine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G682–G691. doi: 10.1152/ajpgi.00231.2007. [DOI] [PubMed] [Google Scholar]
- 14.Yarandi SS, Hebbar G, Sauer CG, Cole CR, Ziegler TR. Review: diverse roles of leptin in the gastrointestinal tract: modulation of motility, absorption, growth, and inflammation. Nutrition. 2011;27:269–275. doi: 10.1016/j.nut.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishi Y, Isomoto H, Ueno H, et al. Plasma leptin and ghrelin concentrations in patients with Crohn's disease. World J Gastroenterol. 2005;11:7314–7317. doi: 10.3748/wjg.v11.i46.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobhani I, Bado A, Vissuzaine C, et al. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47:178–183. doi: 10.1136/gut.47.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batra A, Zeitz M, Siegmund B. Adipokine signaling in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1897–1905. doi: 10.1002/ibd.20937. [DOI] [PubMed] [Google Scholar]
- 18.Batra A, Stroh T, Siegmund B. Extraluminal factors contributing to inflammatory bowel disease. World J Gastroenterol. 2011;17:572–577. doi: 10.3748/wjg.v17.i5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fantuzzi G. Adipose tissue, adipokines and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Barbier M, Vidal H, Desreumax P, et al. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroenterol Clin Biol. 2005;27:1–5. [PubMed] [Google Scholar]
- 21.Sitaraman S, Liu X, Charrier L, et al. Colonic leptin: source of a novel pro-inflammatory cytokine involved in inflammatory bowel disease. J FASEB. 2004;18:696–698. doi: 10.1096/fj.03-0422fje. [DOI] [PubMed] [Google Scholar]
- 22.Otero M, Lago R, Lago F, et al. Review: leptin from fat to inflammation: old questions and new insights. FEBS Lett. 2005;579:295–301. doi: 10.1016/j.febslet.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 24.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 25.Velloso LA, Folli F, Sun XJ, White MF, Saad MJ, Kahn CR. Cross-talk between the insulin and angiotensin signaling systems. Proc Natl Acad Sci USA. 1996;93:12490–12495. doi: 10.1073/pnas.93.22.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araújo EP, De Souza CT, Gasparetti AL, et al. Short-term in vivo inhibition of insulin receptor substrate-1 expression leads to insulin resistance, hyperinsulinemia, and increased adiposity. Endocrinology. 2005;146:1428–1437. doi: 10.1210/en.2004-0778. [DOI] [PubMed] [Google Scholar]
- 27.Karmiris K, Koutrobakis IE, Kouroumalis EA. The emerging role of adipocytokines as inflammatory mediators in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:847–855. doi: 10.1097/01.mib.0000178915.54264.8f. [DOI] [PubMed] [Google Scholar]
- 28.Olivier I, Theodorou V, Valet P, et al. Is Crohn's creeping fat an adipose tissue? Inflamm Bowel Dis. 2011;17:747–757. doi: 10.1002/ibd.21413. [DOI] [PubMed] [Google Scholar]
- 29.Schaffler A, Herfarth H. Creeping fat in Crohn's disease: travelling in a creeper lane of research. Gut. 2005;54:742–744. doi: 10.1136/gut.2004.061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westcott EDA, Mattacks CA, Windsor ACJ, Knight SC, Pond CM. Perinodal adipose tissue and fatty acid composition of lymphoid tissues in patients with and without Crohn's disease and their implications for the etiology and treatment of Crohn's disease. Ann NY Acad Sci. 2006;1072:395–400. doi: 10.1196/annals.1326.034. [DOI] [PubMed] [Google Scholar]
- 31.Erhayiem B, Dhingsa R, Hawkey CJ, Subramanian V. Ratio of visceral to subcutaneous fat area is a biomarker of complicated Crohn's disease. Clin Gastroenterol Hepatol. 2011;9:684–687. doi: 10.1016/j.cgh.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Biroulet LP, Chamaillard M, Gonzalez F, et al. Mesenteric fat in Crohn's disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577–583. doi: 10.1136/gut.2005.082925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen K, Li F, Li J, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;12:425–432. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- 34.Valentini L, Wirth EK, Schweizer U, et al. Circulating adipokines and the protective effects of hyperinsulinemia in inflammatory bowel disease. Nutrition. 2009;25:172–181. doi: 10.1016/j.nut.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Weigert J, Obermeier F, Neumeier M, et al. Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn's disease. Inflamm Bowel Dis. 2010;16:630–637. doi: 10.1002/ibd.21091. [DOI] [PubMed] [Google Scholar]
- 36.Karmiris K, Koutroubakis LE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100–105. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L, Liu M, Zhang J, Chen H, Dong LQ, Liu F. DsbA-L alleviates endoplasmic reticulum stress-induced adiponectin downregulation. Diabetes. 2010;59:2809–2816. doi: 10.2337/db10-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121:326–330. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Paul G, Schaffler A, Neumeier M, et al. Profiling adipocytokine secretion from creeping fat in Crohn's disease. Inflamm Bowel Dis. 2006;12:47–477. doi: 10.1097/00054725-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Fayad R, Pini M, Sennello JA, et al. Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology. 2007;132:601–614. doi: 10.1053/j.gastro.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 41.Arsenescu V, Meena L, Narasimhan TH, et al. Adiponectin and plant-derived mammalian adiponectin homolog exert a protective effect in murine colitis. Dig Dis Sci. 2011;56:2818–2832. doi: 10.1007/s10620-011-1692-0. [DOI] [PubMed] [Google Scholar]
- 42.Ouchi N, Kihara S, Funahashi T, et al. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curat CA, Miranville A, Sengenes C, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]


