Abstract
Accumulating evidence shows that galectins play roles in the initiation and resolution phases of inflammatory responses by promoting anti- or proinflammatory effects. This study investigated the presence of three members of the galectin family (galectin-1, -3 and -9) in induced sputum samples of asthma patients, as well as their possible implication in the immunopathogenesis of human asthma. Levels of interleukin (IL)-5, IL-13, and galectins were determined in leucocytes isolated from induced sputum samples by reverse transcription–polymerase chain reaction (RT–PCR) immunofluorescence and flow cytometry. High levels of IL-5 and IL-13 mRNA were detected in sputum cells from asthma patients. In parallel, immunoregulatory proteins galectin-1 and galectin-9 showed a reduced expression on macrophages from sputum samples compared with cells from healthy donors. In-vitro immunoassays showed that galectin-1 and galectin-9, but not galectin-3, are able to induce the production of IL-10 by peripheral blood mononuclear cells from healthy donors. These findings indicate that macrophages from sputum samples of asthma patients express low levels of galectin-1 and galectin-9, favouring the exacerbated immune response observed in this disease.
Keywords: asthma, galectin-1, galectin-9, immunoregulation, macrophages
Introduction
Asthma is a chronic inflammatory respiratory disease characterized by airway inflammation, airway hyperresponsiveness (AHR) and reversible airway obstruction [1]. In atopic asthma, inhalation of allergens stimulates cells of the innate immune system to secrete cytokines that promote CD4+ T cell antigen recognition, and favouring a T helper type 2 (Th2) response. Recent studies indicate that Th1 and Th17 cells might also play an important role in the pathophysiology of asthma. There is evidence that interferon (IFN)-γ secretion can cause severe airway inflammation [2], while interleukin (IL)-17 is important for neutrophil recruitment; this cytokine has been detected in bronchial biopsies, bronchoalveolar lavage fluid and sputum from asthma patients [3]. The importance of regulatory T cells in controlling these processes, either via contact-dependent suppression or through IL-10 and transforming growth factor (TGF)-β secretion, is now emerging [4–6].
Galectins are a family of β-galactoside-binding animal lectins with functions in a variety of biological processes, including inflammation and allergic pathologies [7]. Galectin-3 (gal-3) has been described mainly as a powerful proinflammatory signal. Deficiency for gal-3 results in less AHR in a model of ovalbumin (OVA)-induced asthma as well as in defects of airways remodelling [8,9]. However, gene therapy with gal-3 has shown beneficial effects in two murine models of asthma through the down-regulation of IL-5 gene expression [10,11] associated with inhibition of suppressor of cytokine signalling (SOCS)1 and SOCS3 expression [12]. In vivo, gal-1 administration has immunosuppressive and anti-inflammatory effects in various experimental animal models of inflammation and autoimmunity [13–15]. Also, gal-9 administration reduces AHR and Th2 cell-associated airway inflammation in a model of asthma [16]. However, in mice with OVA-induced asthma, the blockade of T cell immunoglobulin (Ig) and mucin domain (TIM-3) (gal-9 ligand) has beneficial effects by skewing the Th2 response towards Th1 response, suggesting that its role in airway inflammation may be more complex [17]. In spite of the growing evidence about the immunoregulatory roles of gal-1 and gal-9, our knowledge of their precise role in human inflammatory diseases remains scarce. In this regard, it has been described recently that Langerhans and dendritic cells (DCs) from psoriasis patients express low levels of gal-1 compared to healthy donors [18], as well as higher gal-9 mRNA levels in peripheral blood mononuclear cells (PBMC) of rheumatoid arthritis patients with low disease activity compared to those with high disease activity [19].
To explore the contribution of galectins in human asthma, induced sputum samples were collected from asthma patients and healthy controls. Expression of gal-1, -3 and -9 was analysed by reverse transcription–polymerase chain reaction (RT–PCR), flow cytometry and immunofluorescence.
Material and methods
Subjects
The ethics committee of the Hospital de La Princesa, Madrid, Spain (PI-486) approved the study; all participants gave informed written consent. Twenty-four asthmatic subjects with stable asthma (19 women and five men) without systemic steroids and 18 healthy controls (nine women and nine men) were included. Asthma severity was scored according to the criteria of the Global Strategy for Asthma Management and Prevention (GINA) (http://www.ginasthma.com) based on current therapy. Asthmatic subjects were grouped into atopics and non-atopics based on detection of specific IgE antibodies to house-dust mite, pets or pollen (grass or tree) and on a clinical history suggestive of allergic response to those allergens. Symptoms were measured using the asthma control test (ACT). Prebronchodilator forced expiratory volume in 1 s (FEV1), FEV1 (%), prebronchodilator forced vital capacity (FVC), FVC (%) and ratio FEV1/FVC was measured by spirometry (Jaeger, Wuerzburg, Germany). Exhaled nitric oxide (FeNO) was measured using a NIOX-MINO® monitor (Aerocrine, Solna, Sweden). Patients continued with their usual inhaled corticosteroids (ICS) treatment which was categorized as follows: < 500 μg/day beclomethasone dipropionate (BDP) or equivalent (n = 9), 500–1000 μg/day BDP or equivalent (n = 8) and > 1000 μg/day BDP or equivalent (n = 7). Clinical parameters: age, sex, pulmonary function, asthma severity, atopic status, ACT, FeNO, ICS, number of years since diagnosis and history of smoking, rhinitis and nasal polyps were collected. Clinical parameters are summarized in Table 1.
Table 1.
Clinical characteristics of asthma patients and healthy donors
| Asthma | Controls | |
|---|---|---|
| Subjects (n) | 24 | 18 |
| Age | 50·34 (24–75) | 33·88 (26–53) |
| Sex (male/female) | 5/19 | 9/9 |
| Atopic/non-atopic | 12/12 | 0/12 |
| ICS BDP dose: (n) | ||
| • < 500 μg/day | 9 | 0 |
| • 500–1000 μg/day | 8 | 0 |
| • > 1000 μg/day | 7 | 0 |
| Lung function | ||
| • FEV1 (ml) | 2623 (1470–5100)* | 3937 (3350–4550)* |
| • FEV1%pred | 94·5 (65–119)* | 108·5 (65–136)* |
| • FVC (ml) | 3220 (1220–5960)* | 4789 (3850–5730)* |
| • FVC%pred | 100·4 (79–132) | 108·2 (96–128) |
| • FEV1/FVC ratio | 76 (57–105)* | 83·2 (72–88)* |
| FeNO (ppb) | 35 (11–82)* | 20 (14–34)* |
| ACT | 22 (16–25) | 25 |
| Current smokers (%) (yes/no) | 8·3% (2/22) | 44% (8/10) |
| Packs years | 10 | 7 |
| Years of diagnosis | 12·7 (1–55) | |
| Rhinitis (yes/no) | 18/6 | 0/17 |
| Nasal polyps (yes/no) | 8/16 | 0/17 |
Results are expressed as mean (range). ICS BDP dose: inhaled corticosteroid dose μg/day beclomethasone; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FeNO: exhaled nitric oxide; ppb: parts per billion; ACT: asthma control test. Pack years: averaged over the complete life-span. Differences between means were tested for lung function and FeNO by Mann–Whitney U-test,
P ≤ 0·05.
Sputum induction
The sputum induction protocol from Pizzichini was followed, with some modifications [20]. Briefly, before sputum induction all subjects inhaled salbutamol (200 μg) via metered dose inhaler. Sputum was induced by 7-min inhalation of hypertonic saline generated with an Omron Nebulizer (NE-U17-E). Subjects initially inhaled 3% saline, and if sufficient sputum was not produced the procedure was repeated with higher concentrations (4 and 5%). Subjects then expectorated into a sterile specimen cup. FEV1 was measured at baseline, after salbutamol inhalation and after each inhalation period, and the procedure was stopped if FEV1 fell by more than 10% or the patient coughed, wheezed or felt chest pain.
Sputum was weighed, dispersed with 4 volumes of 0·1% dithiothreitol (Calbiochem Corp., San Diego, CA, USA) and incubated in a shaking waterbath at 37°C for 30 min. Cell viability was determined by Trypan blue exclusion. The differential count was obtained by counting 400 cells after Diff-Quik staining. If more than 5 × 105 cells were collected, 50% was frozen immediately for RNA extraction and the remaining 50% used for flow cytometry analysis. When fewer than 5 × 105 cells were collected, the sample was used for just one of these procedures.
Antibodies
Fluorescein isothiocyanate (FITC)-conjugated anti-human CD45, phycoerythrin (PE)-conjugated anti-human HLA-DR, allophycocyanin (APC)-H7-conjugated anti-human CD14, Pacific blue (PB)-conjugated anti-human CD16, Pacific orange (PO)-conjugated anti human CD45 and 7-aminoactinomycin D (7-AAD) were all from BD Biosciences (San Jose, CA, USA). Polyclonal goat anti-human gal-1, gal-3 and gal-9 were from R&D Systems (Minneapolis, MN, USA). Secondary antibodies Alexa Fluor 647-conjugated donkey anti-goat (DAG), Alexa Fluor 568-conjugated goat anti-mouse (GAM) and Alexa Fluor 488-conjugated DAG were from Molecular Probes (Leiden, the Netherlands).
Flow cytometry
Sputum cells from 15 asthma patients and 10 healthy donors were labelled with PO-anti-CD45, PE-anti-HLA-DR, PB-anti-CD16 and APC-H7-anti-CD14. For galectin detection, cells were stained with goat polyclonal anti-gal-1, anti-gal-3 or anti-gal-9 followed by Alexa Fluor 647-DAG. Before antibody incubation, Fc-receptors were blocked with human gamma-globulin. Analyses were performed with a fluorescence activated cell sorter (FACS)Canto II cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Galectin expression was analysed as mean fluorescence intensity (MFI).
Cytokine expression
PBMC were islolated from 15 ml of venous peripheral blood from five healthy donors by density gradient. PBMC were seeded (5 × 105) onto 24-well plates and stimulated with 100 ng/ml lipopolysaccharide (LPS); where indicated, 10 μg/ml human recombinant (h) gal-1 (Prepotech, London UK), gal-3 (ImmunoTools, Friesoythe, Germany) or gal-9 (R&D Systems) were added. After 24 h, cytokine expression was detected at mRNA and protein level using RT–PCR and cytometric bead array (BD Biosciences), respectively. Bead array data were acquired using FACSCanto II cytometer.
In addition, IL-10 and IL-4 production were analysed in peripheral blood lymphocytes (PBLs) from four healthy donors. Briefly, PBMC were depleted of monocytes and PBLs (2 × 106) were seeded onto 24-well plates precoated or not with 0·5 μg/ml anti-CD3 and 1 μg/ml anti-CD28; where indicated, 10 μg/ml h gal-1, h gal-3 or h gal-9 were added. After 24 h of incubation, culture supernatants were collected and quantified by cytometric bead array.
Quantitative real-time PCR
RNA was isolated with Trizol RNA reagent (Invitrogen, Eugene, OR, USA) and RT–PCR was performed from 250 ng of RNA from 16 asthma patients and 11 healthy donors. In the case of PBMC, RNA was isolated from five healthy donors. mRNA levels of IL-5, IL-13, gal-1, gal-3 and gal-9 for sputum samples and IL-10, IL-12A, IL-12B, IL-1β and TNF-α for PBMC were determined in duplicate using Power SYBR Green PCR master mix from Applied Biosystems (Warrington, UK). Expression levels were normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or beta-actin as controls. Primers sequences are shown in Supplementary Table S1.
Fluorescence microscopy
Cytospin preparations were fixed with 4% paraformaldehyde in PBS, permeabilized with 0·2% Triton X-100. After blocking of Fc-receptors with human gamma-globulin, cytospin preparations were labelled with anti-gal-1, anti-gal-3 or anti-gal-9. Next, Alexa Fluor 488-coupled DAG 1:100 was added. Preparations were blocked with goat serum and incubated with mouse anti-human CD45 followed by Alexa Fluor 568-coupled GAM. Finally, Alexa Fluor 647 anti-MHC-II was added. Nucleus was counterstained with Hoechst 33342. Images were captured with wide-field fluorescence Leica DMIRE2 microscope coupled to a monochromator (Polychrome IV from Till Photonics, Lochhamer Schlag, Germany) and CCD camera (CoolSNAP HQ; Photometrics, Tucson, AZ, USA).
Statistical analysis
Data were analysed with GraphPad Prism (GraphPad Software Inc, San Diego, CA, USA). The Kruskall–Wallis test, Mann–Whitney U-test or Wilcoxon's matched-pairs test were used when appropriate. Differences were considered significant at P < 0·05.
Results
Subjects, lung function and sputum differentials
Sputum samples were obtained from 24 asthma patients and 18 control subjects. The mean FEV1 of the 24 asthma patients was 2623 ml (94·5%) and the mean FVC was 3320 ml (100·4%), while the FEV1/FVC ratio was 76·73. The distribution of asthma according to severity and current therapy using GINA guidelines was as follows: mild intermittent (n = 0), mild persistent (n = 1), moderate persistent (n = 15) and severe persistent (n = 8). Atopy was found in 12 of 24 asthma patients. Two of 24 asthma patients and eight of 18 control subjects had a history of smoking. All healthy controls had normal spirometry and all participants denied clinical symptoms of upper or lower airway disease during the previous 4 weeks and the use of anti-asthma medication in the last 5 years. Clinical characteristics of patients are shown in Table 1.
The quality of induced sputum samples was determined by the presence of < 20% squamous epithelial cells and > 50% cell viability assessed by vital dye 7-AAD exclusion. The samples that did not fulfil quality criteria were excluded from the study. Differential cell count obtained from cytospin preparations are shown in Table 2. FACS analysis of single-cell suspensions stained for cell surface markers detected a predominance of leucocytes (CD45+, 60–90%), most of which were CD16+. Representative flow histograms are shown in Supplementary Fig. S1.
Table 2.
Differential cell counts (cytospins) in induced sputum
| Healthy | Asthma | |
|---|---|---|
| Total cells (105) | 7·20 (1–20) | 7·10 (1–20) |
| Neutrophil % | 43·84 (25–65) | 48·60 (40–57) |
| Macrophage/monocyte % | 53·07 (34–71) | 38·3 (34–41) |
| Lymphocyte % | 0·48 (0·2–0·8) | 0·72 (0·4–1·3) |
| Eosinophil % | 0·97 (0–2) | 1·43 (0·13–2·73) |
| Epithelial cells % | 1·57 (0·5–3·2)* | 9·57 (5·5–12·5)* |
Data are expressed as mean (range) *P < 0·05.
Th2 cytokine and galectin mRNA expression in sputum samples
The expression of gal-1, gal-3 and gal-9 were analysed by RT–PCR in cells isolated of induced sputum samples from asthma patients and healthy control subjects.
Gal-1 and gal-3 mRNA levels in samples from asthma patients [mean ± standard error of the mean (s.e.m.) = 2·6 ± 0·4 and 4·4 ± 1·4, respectively] were lower than those from healthy subjects (4·7 ± 1·2 and 20·0 ± 8·7) (Fig. 1a). In contrast, gal-9 mRNA expression did not vary significantly between the two groups (3·2 ± 1·3 versus 3·3 ± 1·1) (Fig. 1a). As expected, sputum samples from asthma patients contained elevated mRNA levels of the Th2 cytokines IL-5 and IL-13 (P < 0·05, Fig. 1b).
Fig. 1.
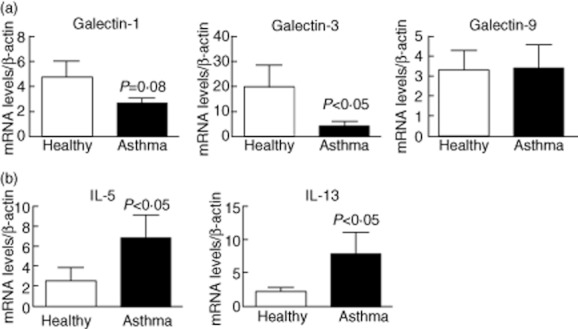
Induced sputum cells of asthma patients show altered mRNA expression of galectins (gal) and Th2 cytokines. Total RNA was isolated from induced sputum of asthma patients (n = 16) and healthy donors (n = 11), and real-time reverse transcription–polymerase chain reaction (RT–PCR) was performed. (a) Gal-1, gal-3 and gal-9 mRNA expression. (b) Interleukin (IL)-5 and IL-13 mRNA expression. mRNA levels are expressed as arbitrary units respect to β-actin expression. Differences between groups were tested by Mann–Whitney U-test. Bars correspond to mean ± standard error of the mean.
The Th17 response has been proposed recently to play an important role during the pathology of allergic asthma [21]. However, the Th17 cytokines IL-17 and IL-23 were undetectable in sputum samples under our experimental conditions (data not shown).
Gal-1, gal-3 and gal-9 expression in leucocytes from induced sputum samples
Surface expression of galectin proteins in sputum cells was determined by flow cytometry. First, using sputum samples from healthy donors we determined whether galectins were expressed differentially in the main subsets of sputum leucocytes observed in cytospin. Differential expression of HLA-DR was used to distinguish macrophages (CD16+DR+) and neutrophils (CD16+DR–) and the expression of galectins was studied in both subpopulations. A low level of eosinophil counts (< 3%) was observed in samples from both asmathic patients and healthy donors (see Table 2). As shown in Fig. 2a, gal-1 and gal-9 were expressed only on macrophages, while gal-3 expression was detected on both macrophages and neutrophils. Differential gal expression by macrophages and neutrophils was also confirmed by immunofluorescence staining of sputum cell samples (Fig. 2b).
Fig. 2.
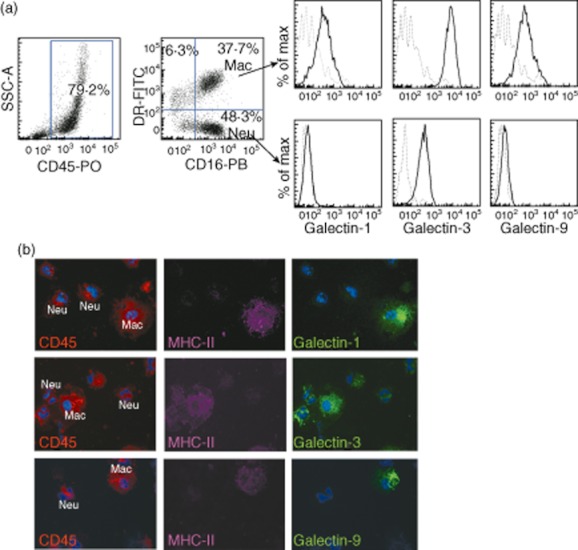
Macrophages are the main sputum cells expressing galectin (gal)-1 and gal-9. (a) Single-cell suspensions of sputum cells from a healthy donor were stained with anti-CD45, anti-CD16, anti-HLA-DR and anti-gal-1, anti-gal-3 or anti-gal-9, and analysed by flow cytometry. Expression of gal-1, gal-3 and gal-9 was analysed on macrophages (CD16+ DR+) and neutrophils (CD16+ DR–). Isotype control (dotted line), gal expression (solid line). (b) Gal-1, gal-3 and gal-9 expression on macrophages detected by triple immunofluorescence in cytospin preparations from sputum cells from a healthy donor. Gal-1, gal-3 and gal-9 (green), major histocompatibility complex (MHC)-class II (magenta) and CD45 (red). Nuclei were stained with Hoescht (blue).
Next, we compared galectin expression between asthma patients and healthy controls. Surface expression of gal-1 and gal-9 was clearly diminished in asthma patients compared with the control group (P < 0·05) (Fig. 3a,b), which is consistent with the reported action of these proteins as negative regulators of the immune responses [22,23]. Surface expression of gal-3 was highly variable, and although it tended to be lower in asthmatic patients, this difference did not reach statistical significance (Fig. 3b).
Fig. 3.
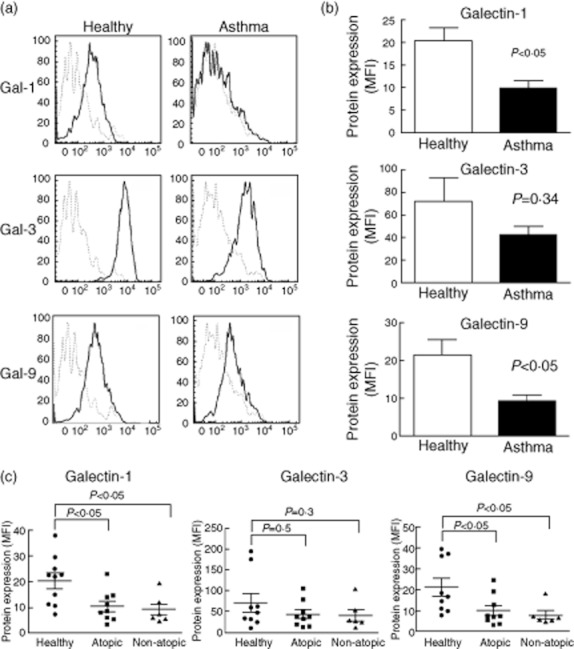
Surface expression of galectin (gal)-1 and gal-9 is reduced in leucocytes from induced sputum of asthma patients. (a) Cells from sputum samples were stained as in Fig. 2 and galectin expression was analysed on macrophages (CD16+ HLA-DR+). Representative histograms from a healthy donor and an asthma patient are shown. Isotype control (dotted line), gal expression (solid line). (b) Gal-1, gal-3 and gal-9 expression on leucocytes from asthma (n = 15) and healthy donors (n = 10). Bars represent mean ± standard error of the mean of mean fluorescence intensity (MFI) of galectins expression. Differences were tested by Mann–Whitney U-test. (c) Gal expression according to allergic state. Differences between atopy and non-atopy against healthy donors were tested by Mann–Whitney U-test.
Gal-1, gal-9 and especially gal-3 have been linked to allergic conditions. However, we did not find any difference in gal expression between atopic and non-atopic asthma patients, indicating that the lower expression of gal-1 and gal-9 is independent of atopic status (Fig. 3c). In addition, no significant differences in galectin expression were observed when patients were classified according to the dose of inhaled corticosteroids (Supplementary Table S2).
Regulation of cytokine expression by galectins
Next, we explored the role of gal-1, gal-3 and gal-9 in the cytokine production induced by LPS. PBMC were stimulated with LPS in the absence or presence of gal-1, gal-3 and gal-9 during 24 h. RT–PCR assays showed that gal-3 reduced the expression of IL-12A induced by LPS (Fig. 4a). When samples were matched it was observed that the reduction of IL-12A levels occurred in four of five samples tested; however, statistical analysis did not show any significant differences (Supplementary Fig. S2a). Gal-9 also caused a mild inhibition of IL-12B in four of five samples included (Fig. 4a and Supplementary Fig. S2b). In addition, we observed a slight increment of TNF-α expression in PBMC stimulated with LPS in the presence of gal-9. However, analysis of matched samples showed that this effect occurs in only three of five samples (Fig. 4a and Supplementary Fig. S2c). Regarding IL-1β, we did not detect any significant difference among treatments (Fig. 4a). Conversely, both gal-1 and gal-9 were able to increase the expression of LPS-induced IL-10 mRNA; in both cases the induction of IL-10 expression was observed in all samples tested (P = 0·01 and P = 0·03, respectively; Fig. 4b and Supplementary Fig. S2d). Moreover, gal-1 and gal-9 in the absence of additional stimulus induced a high expression of IL-10 (Supplementary Fig. S2e). Gal-1, gal-3 and gal-9 were also explored by their effect on anti-CD3/anti-CD28-induced cytokines in peripheral T lymphocytes. Lymphocytes were stimulated during 24 h with anti-CD3 and anti-CD28 in the presence or not of gal-1, gal-3 and gal-9 as indicated in Material and methods. Cytokine production was determined using a bead-based immunoassay. Our results showed that the presence of gal-1 during T cell receptor (TCR) stimulation induces a high production of IL-10, P = 0·02 (Fig. 4c). An augmented IL-4 production was also observed in those lymphocytes co-incubated with gal-3 and anti-CD3/anti-CD28; however, this difference was not statistically significant (data not shown).
Fig. 4.
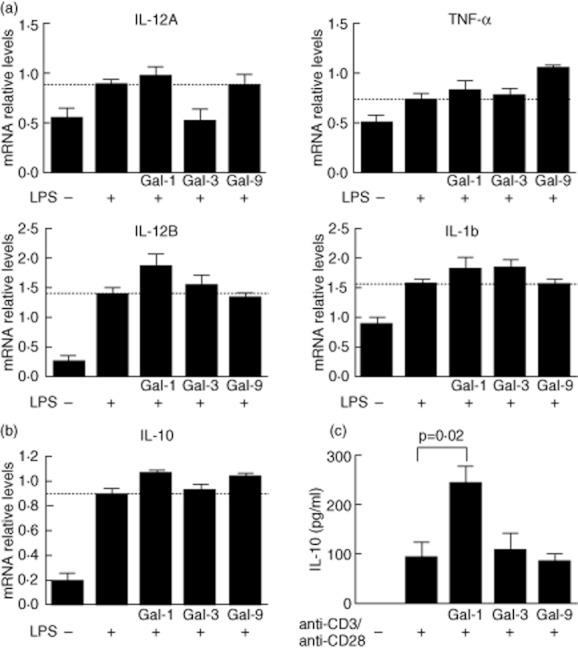
Galectin (gal)-1 and gal-9 induce interleukin (IL)-10 in human peripheral blood mononuclear cells of healthy donors. (a) Peripheral blood mononuclear cells (PBMC) (5 × 105) were incubated on p24 plates in the presence or not of 100 ng/ul lipopolysaccharide (LPS) plus 10 μg/ml gal-1, gal-3 or gal-9. After 24 h of culture, IL-12A, IL-12B, tumour necrosis factor (TNF)-α and IL-1b expression were analysed by reverse transcription–polymerase chain reaction (RT–PCR). Bars correspond to mean ± standard error of the mean (s.e.m.) from five independent experiments. Dashed lines indicate LPS-induced cytokine expression. Differences among groups were tested by one-way analysis of variance (anova) test. mRNA levels are expressed as arbitrary units respect to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression (b). mRNA IL-10 expression of PBMC treated as in (a), bars correspond to mean ± s.e.m. from five independent experiments. Dashed line indicates LPS-induced IL-10 expression. (c) IL-10 secretion by peripheral blood lymphocytes (PBL) stimulated with anti-CD3/anti-C28 in the presence or absence of gal-1, gal-3 or gal-9. PBLs (2 × 106/ml) were incubated on p24 plates precoated with anti-CD3/anti-CD28, where indicated 10 μg/ml of gal-1, gal-3 or gal-9 were added. IL-10 production was tested using bead-based immunoassay by flow cytometry. Bars correspond to mean ± s.e.m. from four independent experiments. Differences among groups were tested by one-way anova test.
Discussion
Most published studies on the immunopathogenesis of asthma and other inflammatory diseases focus on proinflammatory mediators. However, in recent years the study of cells and molecules with immunoregulatory activity has begun to gain importance. The data presented here show that airway cells obtained from induced sputum samples of asthma patients express lower levels of gal-1 and gal-9 and higher levels of IL-5 and IL-13 compared with cells from healthy subjects. In addition, we have identified macrophages as the cells from sputum expressing gal-1 and gal-9. A recent study analysed the presence of galectin-bound proteins in broncoalveolar lavage (BAL) from patients with mild asthma, and a different profile of galectin-bound proteins was observed between patients and healthy subjects. In parallel, authors describe that BAL contains galectins at low concentrations, suggesting that functional interactions with galectins occur at sites where airway cells are present [24].
Numerous studies have highlighted the immunomodulatory properties of galectins [7]. The anti-inflammatory properties of gal-1 have been evaluated in animal models of chronic inflammation [13,25–27]. However, the role of gal-1 in asthma has not been explored previously. Published data highlight the ability of gal-1 to counteract Th1 and Th17-mediated responses through a number of anti-inflammatory mechanisms. One reported mechanism is a skewing of the balance from Th1 towards Th2 polarized immune responses, mainly through the induction of Th1 cell apoptosis. The numerous anti-inflammatory effects of gal-1 include induction of IL-10 release [28,29], down-regulation of the secretion of TNF-α and IFN-γ [30,31] and inhibition of transendothelial migration as well as chemotaxis of neutrophils [32]. Disruption of all these processes could contribute to exacerbated inflammatory responses in an environment with defective expression of this lectin. In the context of asthma, IL-10 plays a key role in the control of inflammatory process, able to down-modulate the Th2 response [33–35]. Decreased IL-10 expression has been linked recently to the impaired ability of natural regulatory T cells from allergic asthma patients to induce a tolerogenic phenotype in dendritic cells [36]. In this regard, our data show that the presence of gal-1 during LPS stimulation augments the IL-10 expression by PBMCs; this effect was also observed in lymphocytes stimulated through the TCR. Expression of gal-1 is induced by budesonide in an in-vitro assay and may account for its immunosuppressive efficacy. The increased gal-1 expression appears to translate into a marked decrease in the migration of eosinophils, the predominant inflammatory cell type in this condition [37].
Gal-3, the most studied galectin in relation to asthma, has been described as a molecule that might contribute to allergic airway inflammation and AHR. We found lower gal-3 gene expression in sputum samples from asthma patients compared with healthy controls; however, differences in surface gal-3 protein were not statistically significant, due possibly to the high variability among subjects.
Gal-9 has a variety of biological activities but is known mainly for its chemotactic activity towards eosinophils [38]. Gal-9 has also been described as a negative regulator of Th1 cells [39], but its role in allergic inflammation is controversial. Administration of gal-9 inhibits allergic airway inflammation and Th2 cytokine expression [16]. However, it has been described that blockade of the ligand of gal-9 (TIM-3) results in ameliorated OVA-induced asthma [17]. Our data show that macrophages of induced sputum samples of asthma patients present low levels of membrane surface-expressed gal-9; however, data obtained from RT–PCR assays did not show any difference in mRNA expression. The gal-9 expressed on the cellular surface corresponds mainly with that produced by the own cell; however, we cannot rule out that, to a certain extent, gal-9 detected on the macrophages could be derived from bystander cells; in addition, post-transcriptional regulation of gal-9 could also account for such differences. Our data show that gal-9 is able to induce IL-10 production by human mononuclear cells, an effect that could be associated with its negative role on the immune response. In this sense, macrophages from mice treated with exogenous gal-9 produced less TNF-α and IL-1β but more IL-10 than PBS-treated mice in a model of acute lung injury, in which gal-9 administration resulted in an ameliorated disease [40].
It has been described that galectins might be modified by corticosteroids either inducing or inhibiting their expression [41,42]. However, when asthma patients were classified according to the doses of corticosteroids (< 500 μg/day and > 1000 μg/day) no significant differences were detected between groups.
In this study we have also explored the possible regulation of additional LPS-induced cytokines, as IL-1β, IL-12 and TNF-α by gal-1, -3 and -9. Our results reveal that gal-3 and gal-9 were able to reduce the LPS-induced expression of IL-12A and IL-12B in four of five subjects tested. Accordingly, splenocytes from gal-3-deficient mice secreted more IL-12 compared with wild-type mice in a model of atopic dermatitis [43]. Under our experimental conditions we have not observed inhibition of IL-1β and TNF-α. Moreover, our results show an increase in TNF-α expression in response to LPS plus gal-9. It is important to note that the doses employed in this study are very low (< 1 μM) and higher doses of galectins could be necessary to down-modulate cytokine expression. In addition, in-vitro gal-9 has shown to induce human monocyte-derived DCs activation [44] as well as TNF-α production [45].
While animal models are extremely useful tools for investigating the role of molecules in the immunopathogenesis of inflammatory diseases, in many situations functions described in animals cannot be extrapolated to humans. Studies of immune parameters in asthma patients are, however, hampered by the restricted availability of lung tissue or bronchoalveolar samples because of the risks and contraindications to obtaining these samples. Sputum induction is thus a valuable, non-invasive means of obtaining viable cells from the lower airway for evaluation of airway inflammation. Using this method to obtain airway cells, we have detected defective expression of gal-1 and gal-9 in asthma patients. The balance of pro- and anti-inflammatory signals determines the final outcome of the immune response, and the low levels of the negative regulators as gal-1 and gal-9 in human asthma may contribute to the inflammatory response present in this disease.
Acknowledgments
We thank the asthmatic patients and healthy subjects for their participation in this study and S. Bartlett for English editing of the manuscript. Supported in part by EU–Mexico FONCICYT-C002-2009-1 ALA/127249, SAF-2008–02635 and SAF-2011–25834 from the Spanish Ministry of Science and Innovation, INDISNET (Redes Moleculares y Celulares en Enfermedades Inflamatorias) S2011/BMD-2332, MEICA (Molecular and Cellular Mechanisms in Chronic Inflammatory and Autoimmune Diseases, Genoma España) and SEPAR (Sociedad Española de Patología Respiratoria).
Disclosure
Authors declare that they have no conflicts of interest.
Supporting information
Additional supporting information may be found in the online version of this article.
Fig. S1. Immunophenotype of induced sputum cells. Single-cell suspensions were prepared from sputum samples and stained with anti-CD45, anti-CD16 and anti-CD3 or anti-CD14. Vital dye 7-aminoactinomycin D (7-AAD) was used to exclude dead cells. Representative flow histogram of an asthmatic patient is shown. Numbers inside dot-plots indicate the percentage of each subpopulation.
Fig. S2. Effect of galectins (gal) on cytokine expression. (a–c). Effect of gal-3 on lipopolysaccharide (LPS)-induced interleukin (IL)-12A (a) and of gal-9 on LPS-induced IL-12B and TNF-α (b,c) expression on peripheral blood mononuclear cells (PBMC) from healthy subjects. PBMC (5 × 105) were incubated on p24 plates with 100 ng/μl LPS in the presence or not of gal-3 or gal-9 (10 μg/ml). After 24 h culture, cytokine expression was analysed by reverse transcription–polymerase chain reaction (RT–PCR). Data correspond to five independent experiments. Difference between treatments was analysed by Wilcoxon's matched-pairs test. (d) Effect of gal-1 and gal-9 on LPS-induced IL-10 expression on peripheral blood mononuclear cells (PBMC). Cells were treated and analysed as in (a–c). (e) Gal-1 and gal-9 induce the expression of IL-10 in PBMC. Mononuclear cells (5 × 105) were incubated on p24 plates in the presence of 10 μg/ml gal-1, gal-3 and gal-9 during 24 h, and then IL-10 expression was determined by RT–PCR. LPS (100 ng/ml) was used as positive control. Data correspond to mean ± standard error of the mean of five independent experiments. Differences among treatment were tested by one-way analysis of variance test, *P < 0·05.
Table S1. Sequence of primers used for reverse transcription–polymerase chain reaction (RT–PCR).
Table S2. Relation between beclomethasone (BDP) dose and levels of protein expression [mean fluorescence intensity (MFI)] by flow cytometry.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Cui J, Pazdziorko S, Miyashiro JS, et al. TH1-mediated airway hyperresponsiveness independent of neutrophilic inflammation. J Allergy Clin Immunol. 2005;115:309–315. doi: 10.1016/j.jaci.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 3.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97:726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 4.McGee HS, Agrawal DK. Naturally occurring and inducible T-regulatory cells modulating immune response in allergic asthma. Am J Respir Crit Care Med. 2009;180:211–225. doi: 10.1164/rccm.200809-1505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Presser K, Schwinge D, Wegmann M, et al. Coexpression of TGF-beta1 and IL-10 enables regulatory T cells to completely suppress airway hyperreactivity. J Immunol. 2008;181:7751–7758. doi: 10.4049/jimmunol.181.11.7751. [DOI] [PubMed] [Google Scholar]
- 6.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 7.Rabinovich GA, Croci DO. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity. 2012;36:322–335. doi: 10.1016/j.immuni.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Zuberi RI, Hsu DK, Kalayci O, et al. Critical role for galectin-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. Am J Pathol. 2004;165:2045–2053. doi: 10.1016/S0002-9440(10)63255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge XN, Bahaie NS, Kang BN, et al. Allergen-induced airway remodeling is impaired in galectin-3-deficient mice. J Immunol. 2010;185:1205–1214. doi: 10.4049/jimmunol.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Pozo V, Rojo M, Rubio ML, et al. Gene therapy with galectin-3 inhibits bronchial obstruction and inflammation in antigen-challenged rats through interleukin-5 gene downregulation. Am J Respir Crit Care Med. 2002;166:732–737. doi: 10.1164/rccm.2111031. [DOI] [PubMed] [Google Scholar]
- 11.Lopez E, del Pozo V, Miguel T, et al. Inhibition of chronic airway inflammation and remodeling by galectin-3 gene therapy in a murine model. J Immunol. 2006;176:1943–1950. doi: 10.4049/jimmunol.176.3.1943. [DOI] [PubMed] [Google Scholar]
- 12.Lopez E, Zafra MP, Sastre B, Gamez C, Lahoz C, del Pozo V. Gene expression profiling in lungs of chronic asthmatic mice treated with galectin-3: downregulation of inflammatory and regulatory genes. Mediat Inflamm. 2011;2011 doi: 10.1155/2011/823279. doi: 10.1155/2011/823279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perone MJ, Bertera S, Shufesky WJ, et al. Suppression of autoimmune diabetes by soluble galectin-1. J Immunol. 2009;182:2641–2653. doi: 10.4049/jimmunol.0800839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cedeno-Laurent F, Barthel SR, Opperman MJ, Lee DM, Clark RA, Dimitroff CJ. Development of a nascent galectin-1 chimeric molecule for studying the role of leukocyte galectin-1 ligands and immune disease modulation. J Immunol. 2010;185:4659–4672. doi: 10.4049/jimmunol.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CR, Shiau AL, Chen SY, et al. Intra-articular lentivirus-mediated delivery of galectin-3 shRNA and galectin-1 gene ameliorates collagen-induced arthritis. Gene Ther. 2010;17:1225–1233. doi: 10.1038/gt.2010.78. [DOI] [PubMed] [Google Scholar]
- 16.Katoh S, Ishii N, Nobumoto A, et al. Galectin-9 inhibits CD44-hyaluronan interaction and suppresses a murine model of allergic asthma. Am J Respir Crit Care Med. 2007;176:27–35. doi: 10.1164/rccm.200608-1243OC. [DOI] [PubMed] [Google Scholar]
- 17.Kearley J, McMillan SJ, Lloyd CM. Th2-driven, allergen-induced airway inflammation is reduced after treatment with anti-Tim-3 antibody in vivo. J Exp Med. 2007;204:1289–1294. doi: 10.1084/jem.20062093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Fuente H, Perez-Gala S, Bonay P, et al. Psoriasis in humans is associated with down-regulation of galectins in dendritic cells. J Pathol. 2012;228:193–203. doi: 10.1002/path.3996. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Oh JM, Hwang J, et al. Expression of human TIM-3 and its correlation with disease activity in rheumatoid arthritis. Scand J Rheumatol. 2011;40:334–340. doi: 10.3109/03009742.2010.547871. [DOI] [PubMed] [Google Scholar]
- 20.Pizzichini MM, Popov TA, Efthimiadis A, et al. Spontaneous and induced sputum to measure indices of airway inflammation in asthma. Am J Respir Crit Care Med. 1996;154:866–869. doi: 10.1164/ajrccm.154.4.8887576. [DOI] [PubMed] [Google Scholar]
- 21.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 22.Toscano MA, Bianco GA, Ilarregui JM, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 23.Oomizu S, Arikawa T, Niki T, et al. Galectin-9 suppresses Th17 cell development in an IL-2-dependent but Tim-3-independent manner. Clin Immunol. 2012;143:51–58. doi: 10.1016/j.clim.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Cederfur C, Malmstrom J, Nihlberg K, et al. Glycoproteomic identification of galectin-3 and -8 ligands in bronchoalveolar lavage of mild asthmatics and healthy subjects. Biochim Biophys Acta. 2012;1820:1429–1436. doi: 10.1016/j.bbagen.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Tourino I, Sanchez-Espinel C, Hernandez-Fernandez A, et al. Galectin-1 synthesis in type 1 diabetes by different immune cell types: reduced synthesis by monocytes and Th1 cells. Cell Immunol. 2011;271:319–328. doi: 10.1016/j.cellimm.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Ilarregui JM, Croci DO, Bianco GA, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 27.Toscano MA, Commodaro AG, Ilarregui JM, et al. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J Immunol. 2006;176:6323–6332. doi: 10.4049/jimmunol.176.10.6323. [DOI] [PubMed] [Google Scholar]
- 28.van der Leij J, van den Berg A, Blokzijl T, et al. Dimeric galectin-1 induces IL-10 production in T-lymphocytes: an important tool in the regulation of the immune response. J Pathol. 2004;204:511–518. doi: 10.1002/path.1671. [DOI] [PubMed] [Google Scholar]
- 29.Cedeno-Laurent F, Opperman M, Barthel SR, Kuchroo VK, Dimitroff CJ. Galectin-1 triggers an immunoregulatory signature in Th cells functionally defined by IL-10 expression. J Immunol. 2012;188:3127–3137. doi: 10.4049/jimmunol.1103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stowell SR, Qian Y, Karmakar S, et al. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol. 2008;180:3091–3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- 31.Gil CD, Gullo CE, Oliani SM. Effect of exogenous galectin-1 on leukocyte migration: modulation of cytokine levels and adhesion molecules. Int J Clin Exp Pathol. 2010;4:74–84. [PMC free article] [PubMed] [Google Scholar]
- 32.La M, Cao TV, Cerchiaro G, et al. A novel biological activity for galectin-1: inhibition of leukocyte–endothelial cell interactions in experimental inflammation. Am J Pathol. 2003;163:1505–1515. doi: 10.1016/s0002-9440(10)63507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogawa Y, Duru EA, Ameredes BT. Role of IL-10 in the resolution of airway inflammation. Curr Mol Med. 2008;8:437–445. doi: 10.2174/156652408785160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faith A, Singh N, Farooque S, et al. T cells producing the anti-inflammatory cytokine IL-10 regulate allergen-specific Th2 responses in human airways. Allergy. 2012;67:1007–1013. doi: 10.1111/j.1398-9995.2012.02852.x. [DOI] [PubMed] [Google Scholar]
- 35.Xystrakis E, Kusumakar S, Boswell S, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen KD, Vanichsarn C, Nadeau KC. Impaired IL-10-dependent induction of tolerogenic dendritic cells by CD4+CD25hiCD127lo/– natural regulatory T cells in human allergic asthma. Am J Respir Crit Care Med. 2009;180:823–833. doi: 10.1164/rccm.200905-0761OC. [DOI] [PubMed] [Google Scholar]
- 37.Delbrouck C, Doyen I, Belot N, et al. Galectin-1 is overexpressed in nasal polyps under budesonide and inhibits eosinophil migration. Lab Invest. 2002;82:147–158. doi: 10.1038/labinvest.3780407. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto R, Matsumoto H, Seki M, et al. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J Biol Chem. 1998;273:16976–16984. doi: 10.1074/jbc.273.27.16976. [DOI] [PubMed] [Google Scholar]
- 39.Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 40.Kojima K, Arikawa T, Saita N, et al. Galectin-9 attenuates acute lung injury by expanding CD14– plasmacytoid dendritic cell-like macrophages. Am J Respir Crit Care Med. 2011;184:328–339. doi: 10.1164/rccm.201010-1566OC. [DOI] [PubMed] [Google Scholar]
- 41.Gong YB, Huang YF, Li Y, et al. Experimental study of the mechanism of tolerance induction in dexamethasone-treated dendritic cells. Med Sci Monit. 2011;17:BR125–131. doi: 10.12659/MSM.881758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maldonado CA, Sundblad V, Salatino M, et al. Cell-type specific regulation of galectin-3 expression by glucocorticoids in lung Clara cells and macrophages. Histol Histopathol. 2011;26:747–759. doi: 10.14670/HH-26.747. [DOI] [PubMed] [Google Scholar]
- 43.Saegusa J, Hsu DK, Chen HY, et al. Galectin-3 is critical for the development of the allergic inflammatory response in a mouse model of atopic dermatitis. Am J Pathol. 2009;174:922–931. doi: 10.2353/ajpath.2009.080500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai SY, Nakagawa R, Itoh A, et al. Galectin-9 induces maturation of human monocyte-derived dendritic cells. J Immunol. 2005;175:2974–2981. doi: 10.4049/jimmunol.175.5.2974. [DOI] [PubMed] [Google Scholar]
- 45.Kanzaki M, Wada J, Sugiyama K, et al. Galectin-9 and T cell immunoglobulin mucin-3 pathway is a therapeutic target for type 1 diabetes. Endocrinology. 2012;153:612–620. doi: 10.1210/en.2011-1579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


