ABSTRACT
The transmission of the bacterium Streptococcus pneumoniae (the pneumococcus) marks the first step toward disease development. To date, our ability to prevent pneumococcal transmission has been limited by our lack of understanding regarding the factors which influence the spread of this pathogen. We have previously developed an infant mouse model of pneumococcal transmission which was strictly dependent on influenza A virus (IAV) coinfection of both the experimentally colonized “index mice” and the naive cohoused “contact mice.” Here, we sought to use this model to further elucidate the factors which facilitate S. pneumoniae transmission. In the present report, we demonstrate that increasing the nasopharyngeal load of S. pneumoniae in the colonized index mice (via the depletion of neutrophils) and inducing a proinflammatory response in the naive cohoused contact mice (as demonstrated by cytokine production) facilitates S. pneumoniae transmission. Thus, these data provide the first insights into the factors that help mediate the spread of S. pneumoniae throughout the community.
IMPORTANCE
Streptococcus pneumoniae (the pneumococcus) is a major cause of worldwide morbidity and mortality and is a leading cause of death among children under the age of five years. Transmission of S. pneumoniae marks the first step toward disease development. Therefore, understanding the factors that influence the spread of pneumococci throughout the community plays an essential role in preventing pneumococcal disease. We previously developed the first reproducible infant mouse model for pneumococcal transmission and showed that coinfection with influenza virus facilitates the spread of S. pneumoniae. Here, we show that increasing the bacterial load in the nasal cavity of colonized individuals as well as inducing an inflammatory response in naive “contact cases” facilitates the spread of pneumococci. Therefore, this study helps to identify the factors which must be inhibited in order to successfully prevent pneumococcal disease.
Introduction
Streptococcus pneumoniae (the pneumococcus) is a respiratory pathogen that colonizes the nasopharynx of up to 80% of children (1, 2). Following colonization of the nasopharynx, S. pneumoniae can disseminate to the lung, brain, blood, or middle ear, causing pneumonia, meningitis, sepsis, and otitis media, respectively. Children suffer a large burden of pneumococcal disease, and each year this bacterium kills up to 1 million children under the age of five years (3). Nasopharyngeal colonization with S. pneumoniae marks the first step toward the development of pneumococcal disease (2, 4). Therefore, understanding and preventing the spread of the pneumococcus plays a crucial role in maintaining public health.
Pneumococcal transmission occurs via contact with the respiratory secretions and saliva of colonized individuals either directly via the inhalation of bacteria or indirectly via contact with contaminated surfaces (2, 5). Several studies have provided evidence for the importance of close person-to-person contact (e.g. at day care centers) in the spread of pneumococci (6, 7). A viral infection of the respiratory tract is also associated with the transmission of S. pneumoniae in humans. For example, the horizontal transmission of S. pneumoniae among family members has been linked to a concurrent viral infection of the respiratory tract (8). Similarly, infection with the pandemic 2009 influenza A virus (IAV) was associated with the household transmission of S. pneumoniae (9). The mechanisms by which IAV coinfection may facilitate pneumococcal transmission remain unclear. IAV increases pneumococcal titers in the nasopharynx (10–13), potentially facilitating transmission by then increasing the shedding of this bacterium into the community. Alternatively, IAV is known to suppress specific components of the immune response which may then predispose individuals to acquire S. pneumoniae from colonized individuals. Virus-induced inflammation may also facilitate pneumococcal transmission, as inflammation can upregulate receptors for pneumococcal adherence (14–17).
Thus, while epidemiological studies have provided insights into the factors associated with pneumococcal transmission, few studies have demonstrated the contribution of these factors to the spread of S. pneumoniae in a defined experimental setting. Using infant mice to mimic the underdeveloped immune system of children, we have previously shown that coinfection with IAV facilitated the transmission of S. pneumoniae (13), consistent with subsequent studies in ferrets (18). In this model, influenza virus had an effect on both the “index mice” (i.e., those colonized with S. pneumoniae) and the cohoused, previously naive, “contact mice” (13). Here, we sought to use this model to further elucidate the factors which facilitate pneumococcal transmission from the index to the contact mice.
RESULTS
Multiple influenza virus strains facilitate pneumococcal transmission.
We previously demonstrated that infection with the IAV strain Udorn/72 facilitated pneumococcal transmission, provided that both the index and contact mice were infected with IAV (13). In order to further assess the factors which contribute to the spread of S. pneumoniae, we determined whether multiple strains of IAV facilitated pneumococcal transmission. Five-day-old index mice were colonized with S. pneumoniae and cohoused with uninfected contact mice. At 14 days of age, the index mice were infected with IAV; 6 days later, the transmission of S. pneumoniae from the index to the contact mice was determined (Fig. 1A). We have previously found that the IAV Udorn/72 strain itself also efficiently transmits between cohoused mice (13). The experimental setup used here was therefore based upon the assumption that, like Udorn/72, other IAV strains would also transmit between mice. Similar to our previous finding (13), there was little to no transmission of pneumococci to the contact mice in the absence of IAV (Fig. 1B; “Mock” treatment group). Infection with HKx31 and Port Chalmers/73 facilitated pneumococcal transmission (Fig. 1B). Accordingly, these virus strains themselves also transmitted between mice (Fig. 1B). In contrast, IAV strains PR8/34 and Brazil/78 did not transmit efficiently to contact animals. Limited pneumococcal transmission also occurred in these mice (Fig. 1B). To investigate whether this was due to an intrinsic defect of these IAV strains with respect to facilitating pneumococcal transmission, we performed experiments in which all mice were infected with PR8/34 or Brazil/78 (Fig. 1C). In these experiments, we observed successful pneumococcal transmission (Fig. 1D), suggesting that multiple different IAV strains facilitate the spread of S. pneumoniae provided that both the index and contact mice are efficiently infected with IAV.
FIG 1 .
Multiple IAV (influenza A virus) strains facilitate pneumococcal transmission. (A and C) The experimental model used to assess pneumococcal transmission. In brief, at 5 days of age, pups were randomly distributed into two groups of equal size (± one mouse). One group, designated index mice, was colonized with S. pneumoniae (indicated by the black mice). The index mice were then returned to, and cohoused in the same cage as, the remaining, noncolonized pups (contact mice; indicated by the white mice). At 14 days of age, either only the index mice (A) or both the index and contact mice (C) were infected with IAV. Six days later, transmission of S. pneumoniae from the index to the contact mice was assessed. (B) Pneumococcal and IAV transmission following infection of index mice with different strains of IAV. (D) Pneumococcal and IAV transmission following infection of both index and contact mice with different strains of IAV. Data were pooled from a minimum of two independent experiments and represent numbers of infected contact mice/total numbers of contact mice.
Increased nasopharyngeal bacterial titers in the index mice facilitated pneumococcal transmission.
In both children and infant mice, IAV increases pneumococcal titers in the nasal cavity (11, 13), and we reasoned that an increased bacterial load may play an important role in pneumococcal transmission. We thus sought to increase the pneumococcal load independently of a viral infection and to assess whether this was sufficient to facilitate pneumococcal transmission. In infant mice, increasing the infectious dose is not sufficient to increase the load of pneumococci in the nasal cavity, as following colonization the bacterial load in the nose rapidly plateaus to the same titer, even when the infectious dose is increased by 1,000-fold (data not shown). Similarly, while intranasal treatment with sialic acid increases pneumococcal colonization in adult mice (19), this treatment did not affect pneumococcal titers in the nose of infant mice (data not shown). Instead, we chose to deplete neutrophils in the index mice, as neutrophils are thought to be important in controlling pneumococcal colonization (20). Intraperitoneal and intranasal treatment with the neutrophil-depleting 1A8 monoclonal antibody (MAb) resulted in an approximately 90% depletion of neutrophils in the nasal turbinate relative to treatment with the control antibody (see Fig. S1 in the supplemental material). Treatment with 1A8 also resulted in a small but significant increase in the numbers of pneumococci in the nasal cavity which was comparable to the increased load detected after infection with the PR8/34, Udorn/72, and HKx31 IAV strains (Fig. 2A and B).
FIG 2 .
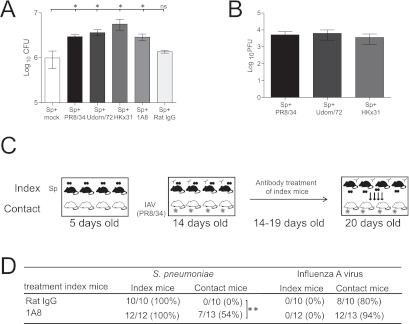
The effect of pneumococcal load in the index mice on S. pneumoniae transmission. (A) Titers of S. pneumoniae (Sp) in the nasal cavity 6 days after mock infection, infection with IAV, or treatment with 1A8 or rat IgG. Statistical significance was determined using a Mann-Whitney U test accompanied by a Bonferroni posttest, and statistical significance is denoted by one asterisk (P < 0.05). Data represent the means ± standard errors of the means (SEM) and are pooled from a minimum of two independent experiments. ns, not statistically significant. (B) Titers of IAV in the nasal cavity 6 days postinfection. Data represent means ± SEM and were pooled from a minimum of two independent experiments. (C) Experimental design to assess the role of increased pneumococcal titers in the index mice in S. pneumoniae transmission. In brief, at 5 days of age, pups were randomly distributed into two groups of equal size (± one mouse). One group, designated index mice, was colonized with S. pneumoniae (indicated by the black mice). The index mice were then returned to, and cohoused in the same cage as, the remaining, noncolonized pups (contact mice; indicated by the white mice). At 14 to 19 days of age, the index mice were treated with rat IgG or the neutrophil-depleting MAb 1A8. At 14 days of age, the contact mice were infected with IAV (strain PR8/34). Six days later, transmission of S. pneumoniae from the index to the contact mice was assessed. (D) Pneumococcal and IAV transmission in mice 6 days postinfection/treatment. Statistical significance was determined using Fisher’s exact test, and statistical significance is denoted by two asterisks (P < 0.01). Data were pooled from a minimum of three independent experiments.
We then investigated if neutrophil depletion and the associated increase in pneumococcal load were sufficient to facilitate pneumococcal transmission to susceptible contact mice. Neutrophils were depleted from 14-day-old S. pneumoniae-colonized index mice by treatment with MAb 1A8. In order to ensure that the contact mice were in an “acquisition-ready” state, 14-day-old contact mice were infected with IAV (Fig. 2C). For these experiments, contact mice were infected with the PR8/34 strain, as the experiments described above demonstrated that this strain does not readily transmit itself between cohoused mice (Fig. 1B). When the index mice were treated with the control antibody, pneumococcal transmission was not observed (Fig. 2C). However, when neutrophils were depleted in the index mice there was a significantly higher transmission rate (53%) compared to the rate seen with mice treated with the control antibody (Fig. 2C; Fischer’s exact test; P < 0.05). This transmission rate was not significantly different from the 78% transmission rate observed when all mice were infected with PR8/34 (Fig. 1D; Fischer’s exact test; P > 0.05). The pneumococcal titers in the nose of the contact mice showed substantial variation (see Fig. S2 in the supplemental material), as the titers depend upon which day the contact mice acquired pneumococci from cohoused index mice. That is, mice that acquired pneumococci early from the index mice would have already reached the “plateau level” at the time of euthanasia, while mice that had just acquired pneumococci at 19 days of age would have had markedly lower titers of S. pneumoniae. Ultimately, however, there was a significantly higher rate of pneumococcal colonization in the contact mice when the index mice were treated with the 1A8 MAb. In summary, these data show that neutrophils play an important role in limiting pneumococcal shedding and, consequently, pneumococcal transmission.
Nasopharyngeal inflammation in contact mice lowered their threshold for acquisition of S. pneumoniae.
In the contact mice, neutrophils may also play an important role in preventing newly acquired S. pneumoniae from attaching to the respiratory epithelium, thereby restricting transmission. To assess this hypothesis, we depleted neutrophils from 14-day-old naive contact mice by treatment with MAb 1A8, while the colonized index mice were coinfected with PR8/34 (Fig. 3A). As in the index mice, intraperitoneal and intranasal treatment of the contact mice with the neutrophil-depleting 1A8 MAb resulted in an approximately 90% depletion of neutrophils in the nasal turbinate (see Fig. S1 in the supplemental material). However, treatment of contact mice with the MAb 1A8 did not significantly increase the rate of pneumococcal transmission compared to contact mice treated with the control antibody (Fig. 3B; Fischer’s exact test; P > 0.05). These data suggest that the absence of neutrophils in the contact mice does not increase their susceptibility to pneumococcal colonization.
FIG 3 .
The effect of inflammation in the contact mice on pneumococcal transmission. (A) Experimental design to assess the neutrophil depletion in the contact mice in S. pneumoniae transmission. In brief, at 5 days of age, pups were randomly distributed into two groups of equal size (± one mouse). One group, designated index mice, was colonized with S. pneumoniae (indicated by the black mice). The index mice were then returned to, and cohoused in the same cage as, the remaining, noncolonized pups (contact mice; indicated by the white mice). At 14 to 19 days of age, the contact mice were treated with rat IgG or the neutrophil-depleting MAb 1A8. At 14 days of age, the index mice were infected with IAV (strain PR8/34). Six days later, transmission of S. pneumoniae from the index to the contact mice was assessed. (B and E) Pneumococcal and IAV transmission in mice 6 days postinfection/treatment. Statistical significance was determined using Fisher’s exact test, and statistical significance is denoted by two asterisks (P < 0.01). Data were pooled from a minimum of three independent experiments. N.S, not statistically significant. (C) Experimental design to assess the role of inflammation in the contact mice in S. pneumoniae transmission. In brief, at 5 days of age, mice were randomly distributed into two groups of equal size (± one mouse). One group, designated index mice, was colonized with S. pneumoniae (indicated by the black mice). The index mice were then returned to, and cohoused in the same cage as, the remaining, noncolonized mice (contact mice; indicated by the white mice). At 14 to 19 days of age, the contact mice were treated with PBS or LPS. At 14 days of age, the index mice were infected with IAV (strain PR8/34). Six days later, transmission of S. pneumoniae from the index to the contact mice was assessed. (D) Cytokine levels in the nasal cavity 6 days postinfection/treatment. These experiments were performed using mice not colonized with S. pneumoniae to reflect the inflammatory response that occurs in the contact mice. Therefore, 14-day-old mice were (i) infected with PR8/34, Udorn/72, or HKx31 or (ii) treated daily with LPS or PBS from day 14 to day 19. At day 20, mice were culled and cytokine levels were determined in clarified homogenates from nasal cavity tissues. Data shown represent means ± SEM. Statistical significance (relative to naive mice) was determined using a Mann-Whitney U test accompanied by a Bonferroni posttest, and statistical significance is denoted by one asterisk (P < 0.05), two asterisks (P < 0.01), or three asterisks (P < 0.001).
Recent studies have shown that inflammation plays an important role in the development of pneumococcal pneumonia by facilitating bacterial adhesion to epithelial cells (15). Thus, we next examined whether the induction of a proinflammatory response increases the susceptibility of contact mice to S. pneumoniae transmission. To induce inflammation in the contact mice, mice were treated with lipopolysaccharide (LPS), a strong proinflammatory ligand (Fig. 3C). As with IAV infection, treatment with LPS induced a proinflammatory response in the nasal cavity of contact mice (as demonstrated by the production of selected proinflammatory cytokines) (Fig. 3D). In contrast, few to no proinflammatory cytokines were detected in the nasal cavities of mice treated with phosphate-buffered saline (PBS) or untreated mice (Fig. 3D). We then assessed if a proinflammatory response in the nose of the contact mice was sufficient to mediate pneumococcal transmission. Contact mice treated with LPS had a 75% rate of pneumococcal transmission, significantly higher than that observed in the mice treated with PBS (Fig. 3D; Fischer’s exact test; P < 0.01; see also Fig. S3 in the supplemental material) and effectively equivalent to the 78% transmission rate observed when all mice were infected with PR8/34 (Fig. 1D). Together, these data suggest that modulation of airway inflammation in the contact mice may represent an essential aspect of pneumococcal transmission.
DISCUSSION
The transmission of bacterial respiratory pathogens is a complex process which depends on a variety of host, environmental, and microbial factors. Here, we have used our recently developed mouse model (13) to provide novel insights into the factors which contribute to the spread of pneumococci.
Using a panel of different IAV strains, we showed that pneumococcal transmission was not restricted to a particular IAV subtype and that both H1N1 and H3N2 strains facilitated bacterial spread. Interestingly, we also observed that not all IAV stains were able to transmit between cohoused mice. The factors which determine the transmission of IAV remain an important area for future study. However, the extrapolation of these data suggests that the transmission of S. pneumoniae is likely to increase during any future IAV epidemic/pandemic, regardless of the viral strain involved.
In the index mice, we demonstrated that a small but significant increase in pneumococcal load in the nasal cavity was sufficient to mediate bacterial transmission to susceptible mice. In the contact mice, an inflammatory response in the nasal cavity also facilitated pneumococcal transmission. Given that coinfection with IAV also increases the pneumococcal load in index mice and induces inflammation in the contact mice, it is therefore tempting to speculate that these represent the mechanisms by which IAV facilitates S. pneumoniae transmission. Of course, IAV may induce additional changes in the index and contact mice, other than those shown here, that may impact upon pneumococcal transmission. For example, a viral infection in mice may induce changes such as increased nasal secretions and/or altering the way in which mice interact with their cohoused littermates. The complex and multifaceted nature of transmission makes it difficult to elucidate the contribution of these additional factors in an experimental setting. However, given that increasing the nasopharyngeal bacterial load and inducing inflammation resulted in equivalent transmission rates for a viral infection, our data would suggest that these are the predominant effects of IAV in this model. It is also likely that any other virus/condition that allows increased pneumococcal shedding and/or increased inflammation in susceptible hosts may also favor pneumococcal spread.
While this represents the first study to demonstrate that increasing the load of pneumococci in the nasal cavity facilitates pneumococcal transmission, this was not an entirely unexpected finding. These data are consistent with previous studies demonstrating that the titers in the index animal are important for the transmission of other respiratory pathogens (21, 22). It is likely that these increased titers result in increased shedding of S. pneumoniae to other mice and into the environment, where the bacteria can survive for up to 15 h (13, 23). In the present study, depletion of neutrophils was sufficient to increase the load of pneumococci in the nasal cavity, suggesting an important role for neutrophils in controlling pneumococcal transmission. This is in contrast to previous observations that neutrophil depletion using the RB6 antibody did not affect pneumococcal density in the nasal cavity 3 days postcolonization (24). However, it must be emphasized that, in contrast to the present study, those studies were performed in adult mice. As infant mice (like human children) display different colonization dynamics compared to adult mice, it is likely that the factors which control pneumococci in the nose also show age-dependent variation. It is interesting to speculate on the mechanisms by which neutrophils control pneumococcal density. One potential explanation is that neutrophils kill S. pneumoniae directly following opsonophagocytosis, e.g., through serine proteases. Identifying the exact roles of neutrophils in controlling the mucosal density of S. pneumoniae remains an important area of future research.
In the contact mice, the depletion of neutrophils did not affect the rate of pneumococcal transmission. This most likely reflects the fact that neutrophils enter the lumen of the nasal cavity in large amounts only from 3 days post-pneumococcal colonization (2). Therefore, it is likely that neutrophils play an important role in controlling pneumococci in the period postcolonization, rather than serving to prevent the initial colonization event. Instead, we showed that a proinflammatory response in the nasal cavity was sufficient to mediate pneumococcal transmission. There is now a growing body of evidence to suggest that, in contrast to many other bacterial pathogens, an inflammatory response can facilitate both pneumococcal colonization and disease (15, 16, 25, 26). It has been suggested that this is due to the ability of an inflammatory response to upregulate receptors for pneumococci (such as the platelet activating factor receptor [PAF-R]) (16, 17) or to expose components of the basement membrane for pneumococcal adherence (27). These data thus support the growing body of evidence that virus-induced inflammation may play an important role in the development of pneumococcal disease. Interestingly, treatment of the index mice with LPS did not increase pneumococcal titers or facilitate pneumococcal transmission (data not shown), suggesting that the role of inflammation in pneumococcal transmission is restricted to the contact mice.
In summary, while extensive research has been performed on the factors which contribute to the development of pneumococcal disease, these data provide the first experimental evidence that changes in bacterial load and inflammation in the nasal cavity facilitate pneumococcal transmission. Given that blocking S. pneumoniae transmission is the most effective way to prevent pneumococcal disease in the community, our data suggest that these factors must be assessed when evaluating the clinical efficacy of novel antipneumococcus therapies and vaccines.
MATERIALS AND METHODS
Bacterial and viral strains.
The S. pneumoniae EF3030 strain (type 19F) was used in all experiments. The preparation and storage of pneumococci prior to infection are described elsewhere (28). The panel of different IAV strains used in this study is shown in Table 1. Virus stocks were prepared in embryonated eggs, and titers of infectious virus were determined by three independent plaque assays on Madin-Darby canine kidney (MDCK) cells as described previously (28).
TABLE 1 .
Viral strains used
| Virus name | Description |
|---|---|
| Mt. Sinai strain of A/PR/8/34 (H1N1; PR8/34) | Wild-type virus |
| A/Udorn/307/72 (H3N2; Udorn/72) | Wild-type virus |
| A/Port Chalmers/1/73 (H3N2; Pt. Chalmers/73) | Wild-type virus |
| A/Brazil/11/78 (H1N1; Brazil/78) | Wild-type virus |
| HKx31 (H3N2) | A high-yielding reassortant of A/PR/8/34 (H1N1) and A/Aichi/2/68 (H3N2) |
Infection of mice.
C57BL/6 mice were bred and housed under conventional conditions at the animal facility of the Department of Microbiology and Immunology at the University of Melbourne, Melbourne, Australia, and were held under specific pathogen-free conditions. All animal experiments were approved by the University of Melbourne Animal Ethics and Experimentation Committee and complied with the Prevention of Cruelty to Animals Act (1986) and the National Health and Medical Research Council (NHMRC) Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (1997). At 5 days of age, pups were randomly distributed into two groups of equal size (± one mouse). One group, designated index mice, was colonized intranasally (i.n.) with 2 × 103 CFU of S. pneumoniae EF3030 in a volume of 3 µl. The index mice were then returned to, and cohoused in the same cage as, the remaining, noncolonized pups (contact mice) and dam. At 14 days of age, pups were infected i.n. with 20 PFU (PR8/34), 104 PFU (Brazil/78), or 102.5 PFU (all other strains) of IAV in 3 µl PBS. Six days later, mice were euthanized and organs were collected for analysis. Where relevant, mice were treated daily (days 14 to 19) i.n. with 50 µg Salmonella enterica serovar Typhimurium LPS (Sigma-Aldrich) in 10 µl PBS.
Enumeration of bacterial and viral loads.
Quantification of bacterial and viral loads was performed as described previously (29). We defined pneumococcal transmission as a contact mouse having ≥222 CFU/ml detectable in the nasal cavity. Similarly, we defined viral transmission as having occurred when ≥5 PFU/ml was detected in the nasal cavity or lungs of contact mice.
Depletion of neutrophils.
Neutrophils were depleted using purified anti-Ly6G rat MAb (clone 1A8; a gift from Thomas Malek, University of Miami). MAb 1A8 was administered intraperitoneally (i.p.) (0.3 mg) and i.n. (0.1 mg) to mice 14 to 19 days of age. Control mice received an equivalent dose of nonspecific rat IgG (Jackson Laboratories). To assess the efficacy of the depletion, a series of separate experiments were performed in which 5-day-old mice were colonized with 2 × 103 CFU of S. pneumoniae EF3030 in a volume of 3 µl of PBS. The relevant antibody was then administered intraperitoneally (i.p.) (0.3 mg) and i.n. (0.1 mg) daily to mice 14 to 19 days of age. When mice were 20 days of age, blood was collected in lithium heparin tubes (BD Biosciences) and neutrophil levels were analyzed using an Advia hematology system (Bayer). Successful depletion of neutrophils in the nose was confirmed using fluorescence-activated cell sorter (FACS) analysis. Briefly, the nasal turbinate was taken from each mouse and treated in RPMI medium (Gibco Invitrogen Corporation) containing 200 µg/ml collagenase D (Roche, Switzerland) and 40 µg/ml DNase (Roche) for 30 min at 37°C. Single-cell suspensions were prepared and stained for CD45 (APC-Cy7; clone 30-F11; BD Pharmigen), CD3 (PE-Cy7; clone 145-2C11; BD Pharmigen), CD11b (fluorescein isothiocyanate [FITC]; clone M1/70; BD Pharmigen), and Ly6G (phycoerythrin [PE]; clone IA8; BD Pharmigen). Each sample was pooled from the nasal turbinates of ≥2 mice. Data were acquired on a FACSCanto II flow cytometer (BD Biosciences) immediately after propidium iodide (PI; Sigma-Aldrich) addition. Collected cells were analyzed with FlowJo, version 8.8.7 (TreeStar, Inc.). Neutrophils were defined as single cells which were PI negative (PI−) CD45+ Ly6G+ CD3− (30). The expression of CD11b was also confirmed on PI− CD45+ Ly6G+ CD3− cells. In each transmission experiment, depletion efficiency was confirmed by Advia analysis of the blood and FACS analysis of the spleen (as the nose was used for analysis of bacterial and viral titers).
Cytokine analysis.
Levels of cytokines in nasal cavity homogenates were measured using a mouse cytometric bead array (CBA) Flex Set (BD Biosciences).
SUPPLEMENTAL MATERIAL
The efficiency of MAb 1A8 treatment on neutrophil numbers in the nasal cavity (A) and blood (B) of mice colonized with S. pneumoniae (“colonized”) or uninfected mice (“naïve”). Mice were infected with S. pneumoniae or mock infected at 5 days of age. From 14 to 19 days of age mice, were treated i.n. and i.p. with MAb 1A8 or rat IgG. Neutrophil numbers were then assessed in 20-day-old mice. (A) Neutrophil numbers in the nasal cavity were determined by FACs analysis of the nasal turbinate. Data are expressed as the percentages of single PI+ CD45+ cells which were Ly6G+ CD3−. Each sample was pooled from the nasal turbinate of ≥2 mice. (B) Neutrophil numbers in the blood were determined by Advia analysis of whole blood. Data shown represent means ± SEM and were pooled from two independent experiments. Download Figure S1, EPS file, 1 MB.
The titers of pneumococci in the nasal cavity of contact mice following treatment of the index mice with MAb 1A8 or rat IgG. Data were pooled from a minimum of three independent experiments, and the geometric mean is shown. Download Figure S2, EPS file, 0.6 MB.
The titers of pneumococci in the nasal cavity of contact mice following treatment of the contact mice with LPS or PBS. Data were pooled from a minimum of three independent experiments, and the geometric mean is shown. Download Figure S3, EPS file, 0.6 MB.
ACKNOWLEDGMENTS
K.R.S. is supported by a GlaxoSmithKline postgraduate support grant and the Elizabeth and Vernon Puzey postgraduate research scholarship. D.A.D. is supported by the 7th Framework Programme of the European Commission (ETB grant 08010). O.L.W. is supported by a Career Development Fellowship (RD Wright Fellowship) from the Australian National Health and Medical Research Council and a Pfizer-funded Robert Austrian Award. The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health and Ageing.
Footnotes
Citation Short KR, Reading PC, Wang N, Diavatopoulos DA, Wijburg OL. 2012. Increased nasopharyngeal bacterial titers and local inflammation facilitate transmission of Streptococcus pneumoniae. mBio 3(5):e00255-12. doi:10.1128/mBio.00255-12.
REFERENCES
- 1. Adegbola RA, Obaro SK, Biney E, Greenwood BM. 2001. Evaluation of Binax NOW Streptococcus pneumoniae urinary antigen test in children in a community with a high carriage rate of pneumococcus. Pediatr. Infect. Dis. J. 20:718–719 [DOI] [PubMed] [Google Scholar]
- 2. Kadioglu A, Weiser JN, Paton JC, Andrew PW. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6:288–301 [DOI] [PubMed] [Google Scholar]
- 3. WHO 2003. Pneumococcal vaccines. Wkly. Epidemiol. Rec. 78:110–119 [Google Scholar]
- 4. Gray BM, Converse GM, III, Dillon HC., Jr. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923–933 [DOI] [PubMed] [Google Scholar]
- 5. Levine H, et al. 2012. Transmission of Streptococcus pneumoniae in adults may occur through saliva. Epidemiol. Infect. 140:561–565 [DOI] [PubMed] [Google Scholar]
- 6. Sá-Leão R, et al. 2008. High rates of transmission of and colonization by Streptococcus pneumoniae and Haemophilus influenzae within a day care center revealed in a longitudinal study. J. Clin. Microbiol. 46:225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yagupsky P, et al. 1998. Acquisition, carriage, and transmission of pneumococci with decreased antibiotic susceptibility in young children attending a day care facility in southern Israel. J. Infect. Dis. 177:1003–1012 [DOI] [PubMed] [Google Scholar]
- 8. Gwaltney JM, Jr, Sande MA, Austrian R, Hendley JO. 1975. Spread of Streptococcus pneumoniae in families. II. Relation of transfer of S. pneumoniae to incidence of colds and serum antibody. J. Infect. Dis. 132:62–68 [DOI] [PubMed] [Google Scholar]
- 9. Ujiie M, et al. 2010. Household transmission of pneumococcal pneumonia associated with pandemic influenza (H1N1) 2009. Nihon Kokyuki Gakkai Zasshi 48:322–327 (In Japanese) [PubMed] [Google Scholar]
- 10. Speshock JL, Doyon-Reale N, Rabah R, Neely MN, Roberts PC. 2007. Filamentous influenza A virus infection predisposes mice to fatal septicemia following superinfection with Streptococcus pneumoniae serotype 3. Infect. Immun. 75:3102–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vu HT, et al. 2011. Association between nasopharyngeal load of Streptococcus pneumoniae, viral coinfection, and radiologically confirmed pneumonia in Vietnamese children. Pediatr. Infect. Dis. J. 30:11–18 [DOI] [PubMed] [Google Scholar]
- 12. Wadowsky RM, Mietzner SM, Skoner DP, Doyle WJ, Fireman P. 1995. Effect of experimental influenza A virus infection on isolation of Streptococcus pneumoniae and other aerobic bacteria from the oropharynges of allergic and nonallergic adult subjects. Infect. Immun. 63:1153–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diavatopoulos DA, et al. 2010. Influenza A virus facilitates Streptococcus pneumoniae transmission and disease. FASEB J. 24:1789–1798 [DOI] [PubMed] [Google Scholar]
- 14. Nakamura S, Davis KM, Weiser JN. 2011. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J. Clin. Invest. 121:3657–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shivshankar P, Boyd AR, Le Saux CJ, Yeh IT, Orihuela CJ. 2011. Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell 10:798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Sluijs KF, et al. 2006. Involvement of the platelet-activating factor receptor in host defense against Streptococcus pneumoniae during postinfluenza pneumonia. Am. J. Physiol. Lung Cell Mol. Physiol. 290:194–199 [DOI] [PubMed] [Google Scholar]
- 17. McCullers JA, Rehg JE. 2002. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis. 186:341–350 [DOI] [PubMed] [Google Scholar]
- 18. McCullers JA, et al. 2010. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J. Infect. Dis. 202:1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trappetti C, et al. 2009. Sialic acid: a preventable signal for pneumococcal biofilm formation, colonization, and invasion of the host. J. Infect. Dis. 199:1497–1505 [DOI] [PubMed] [Google Scholar]
- 20. Lu YJ, et al. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4 e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lowen AC, Mubareka S, Tumpey TM, García-Sastre A, Palese P. 2006. The guinea pig as a transmission model for human influenza viruses. Proc. Natl. Acad. Sci. USA 103:9988–9992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mubareka S, et al. 2009. Transmission of influenza virus via aerosols and fomites in the guinea pig model. J. Infect. Dis. 199:858–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith-Vaughan H, Crichton F, Beissbarth J, Morris PS, Leach AJ. 2008. Survival of pneumococcus on hands and fomites. BMC Res. Notes 1:112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matthias KA, Roche AM, Standish AJ, Shchepetov M, Weiser JN. 2008. Neutrophil-toxin interactions promote antigen delivery and mucosal clearance of Streptococcus pneumoniae. J. Immunol. 180:6246–6254 [DOI] [PubMed] [Google Scholar]
- 25. Hinojosa E, Boyd AR, Orihuela CJ. 2009. Age-associated inflammation and Toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J. Infect. Dis. 200:546–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karlström A, Boyd KL, English BK, McCullers JA. 2009. Treatment with protein synthesis inhibitors improves outcomes of secondary bacterial pneumonia after influenza. J. Infect. Dis. 199:311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harford CG, Leidler V, Hara M. 1949. Effect of the lesion due to influenza virus on the resistance of mice to inhaled pneumococci. J. Exp. Med. 89:53–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Short KR, et al. 2011. Using bioluminescent imaging to investigate synergism between Streptococcus pneumoniae and influenza A virus in infant mice. J. Vis. Exp.:e2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Short KR, et al. 2011. Influenza virus induces bacterial and nonbacterial otitis media. J. Infect. Dis. 204:1857–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fleming TJ, Fleming ML, Malek TR. 1993. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 MAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 151:2399–2408 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The efficiency of MAb 1A8 treatment on neutrophil numbers in the nasal cavity (A) and blood (B) of mice colonized with S. pneumoniae (“colonized”) or uninfected mice (“naïve”). Mice were infected with S. pneumoniae or mock infected at 5 days of age. From 14 to 19 days of age mice, were treated i.n. and i.p. with MAb 1A8 or rat IgG. Neutrophil numbers were then assessed in 20-day-old mice. (A) Neutrophil numbers in the nasal cavity were determined by FACs analysis of the nasal turbinate. Data are expressed as the percentages of single PI+ CD45+ cells which were Ly6G+ CD3−. Each sample was pooled from the nasal turbinate of ≥2 mice. (B) Neutrophil numbers in the blood were determined by Advia analysis of whole blood. Data shown represent means ± SEM and were pooled from two independent experiments. Download Figure S1, EPS file, 1 MB.
The titers of pneumococci in the nasal cavity of contact mice following treatment of the index mice with MAb 1A8 or rat IgG. Data were pooled from a minimum of three independent experiments, and the geometric mean is shown. Download Figure S2, EPS file, 0.6 MB.
The titers of pneumococci in the nasal cavity of contact mice following treatment of the contact mice with LPS or PBS. Data were pooled from a minimum of three independent experiments, and the geometric mean is shown. Download Figure S3, EPS file, 0.6 MB.




