ABSTRACT
The walls of infectious pathogens, which are essential for transmission, pathogenesis, and diagnosis, contain sugar polymers that are defining structural features, e.g., β-1,3-glucan and chitin in fungi, chitin in Entamoeba cysts, β-1,3-GalNAc in Giardia cysts, and peptidoglycans in bacteria. The goal here was to determine in which of three walled forms of Toxoplasma gondii (oocyst, sporocyst, or tissue cyst) is β-1,3-glucan, the product of glucan synthases and glucan hydrolases predicted by whole-genome sequences of the parasite. The three most important discoveries were as follows. (i) β-1,3-glucan is present in oocyst walls of Toxoplasma and Eimeria (a chicken parasite that is a model for intestinal stages of Toxoplasma) but is absent from sporocyst and tissue cyst walls. (ii) Fibrils of β-1,3-glucan are part of a trabecular scaffold in the inner layer of the oocyst wall, which also includes a glucan hydrolase that has a novel glucan-binding domain. (iii) Echinocandins, which target the glucan synthase and kill fungi, arrest development of the Eimeria oocyst wall and prevent release of the parasites into the intestinal lumen. In summary, β-1,3-glucan, which can be targeted by drugs, is an important component of oocyst walls of Toxoplasma but is not a component of sporocyst and tissue cyst walls.
IMPORTANCE
We show here that walls of Toxoplasma oocysts, the infectious stage shed by cats, contain β-1,3-glucan, a sugar polymer that is a major component of fungal walls. In contrast to fungi, β-1,3-glucan is part of a trabecular scaffold in the inner layer of the oocyst wall that is independent of the permeability barrier formed by the outer layer of the wall. While glucan synthase inhibitors kill fungi, these inhibitors arrest the development of the oocyst walls of Eimeria (an important chicken pathogen that is a surrogate for Toxoplasma) and block release of oocysts into the intestinal lumen. The absence of β-1,3-glucan in tissue cysts of Toxoplasma suggests that drugs targeted at the glucan synthase might be used to treat Eimeria in chickens but not to treat Toxoplasma in people.
Introduction
Toxoplasma gondii is a coccidian parasite that causes disseminated infections in persons lacking cell-mediated immunity (e.g., persons with AIDS and fetuses) (1, 2). It usually causes a transient illness in immunocompetent persons, but the parasite persists for life as tissue cysts in muscles and brain. Toxoplasma has three walled forms: oocysts, sporocysts, and tissue cysts (Fig. 1A) (3). Cats, the only host that contains sexual forms of Toxoplasma, shed oocysts in their feces. The oocyst wall, which is made by the zygote after fusion of gametes, has two distinct layers. In the environment, oocysts sporulate to form two sporocysts, each of which have a wall and contain four sporozoites. Humans and other warm-blooded animals are infected with Toxoplasma when they ingest sporulated oocysts that excyst in the intestine, disseminate, and form tissue cysts. Alternatively, humans and other warm-blooded animals become infected when they ingest tissue cysts present in undercooked meat. Regardless of the route of infection, Toxoplasma can cross the placenta and infect the fetus. Additionally, Toxoplasma, which is dormant in tissue cysts, may disseminate when patients lose cell-mediated immunity by poorly controlled HIV or by chemotherapy for organ transplantation.
FIG 1 .
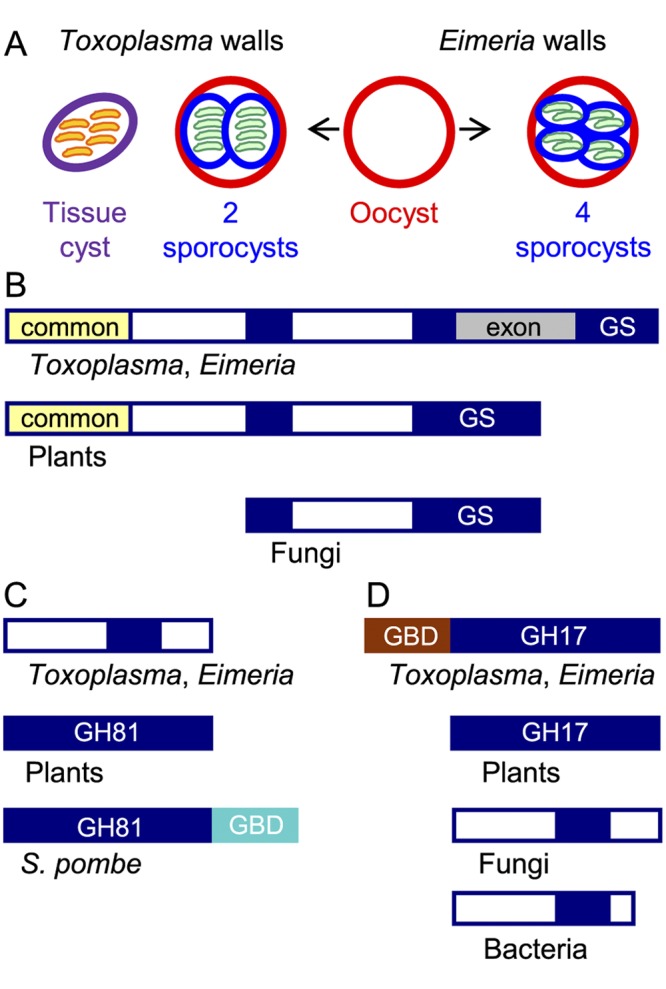
The glucan synthase and one glucan hydrolase of Toxoplasma gondii and Eimeria tenella resemble plant rather than fungal enzymes. (A) Toxoplasma has three walled forms: cat shed oocysts (red wall) in their feces, which sporulate in the environment to form two internal sporocysts (blue wall), each with four sporozoites (green). Tissue cysts (purple wall) in the muscle or brain of any warm-blooded animal contain numerous bradyzoites (orange). Chickens shed Eimeria oocysts in their feces that sporulate to form four sporocysts, each of which contains two sporozoites. (B) Toxoplasma and Eimeria glucan synthases have a conserved glucan synthase (GS) domain that is split into three parts (GS in blue), a domain common to plants, but not to fungi (yellow), and an exon (gray) present only in parasite glucan synthases. The white domains are unique to parasites, plants, or fungi. (C) One Toxoplasma and Eimeria glucan hydrolase contains a portion of the GH81 glycohydrolase domain present in plant and fungal enzymes but does not have a C-terminal glucan-binding domain (GBD in turquoise) present in the Schizosaccharomyces pombe (Eng1) glucan hydrolase. (D) The other Toxoplasma and Eimeria glucan hydrolase contains a GH17 glycohydrolase domain similar to that of plants and different from those of fungi and bacteria. The parasite glucan hydrolase also contains a glucan-binding domain (GBD in brown) between the signal peptide and the GH17 domain. The lectin activity of the TgGBD, which is not phylogenetically related to the SpGBD, is shown in Fig. 2. See Fig. S1 in the supplemental material for sequences of GBD and GH17 domains of the Toxoplasma glucan hydrolase.
Eimeria spp. are one of the most common and important parasites of livestock. Unlike Toxoplasma, they are host specific, and mostly, they parasitize the intestine (4, 5). Losses due to coccidiosis amount to billions of dollars worldwide. Eimeria tenella is the most pathogenic coccidium of chickens, and its entire life cycle is confined to the ceca, which are a pair of outpocketings at the junction of the small intestine and the colon. Medication with anticoccidials and immunoprophylaxis with live attenuated oocysts are used to control coccidiosis in poultry (6, 7).
Unlike Toxoplasma oocysts, Eimeria oocysts contain four sporocysts each with two sporozoites (Fig. 1A). Otherwise, oocysts of Toxoplasma and Eimeria are biologically similar. Oocyst and sporocyst walls of Eimeria and Toxoplasma are autofluorescent in UV light based upon the presence of Tyr-rich proteins that form dityrosines (7, 8). Oocyst walls of Toxoplasma also contain Cys- and His-rich proteins that are homologues of the most abundant Cryptosporidium oocyst wall proteins, which are called COWPs (9, 10).
The present studies originated with the prediction from whole-genome sequences of Toxoplasma and Eimeria of a β-1,3-glucan synthase and two glucan hydrolases (11, 12). β-1,3-glucan is a major component of the fungal cell wall that is recognized by Dectin-1, a lectin on the surfaces of macrophages (13, 14). Dectin-1 is a component of the innate immune system and plays an important role in the natural killer cell response to fungi (15). Inhibitors of glucan synthases (caspofungin, micafungin, and anidulafungin) called echinocandins are used to treat human infections with Candida and Aspergillus (16). The goals here were to determine which walls of Toxoplasma and Eimeria contain β-1,3-glucan (oocyst, sporocyst, and/or tissue cyst), identify structures formed by β-1,3-glucan in the parasite walls, and measure the effect, if any, of echinocandins on Eimeria infections of chickens.
RESULTS
The glucan synthases of Toxoplasma and Eimeria resemble plant, rather than fungal, enzymes.
We used reverse transcriptase PCR (RT-PCR) from oocyst RNA to identify the coding sequences of the complete Eimeria glucan synthase gene and most of the Toxoplasma glucan synthase gene. The Eimeria glucan synthase gene is ~12,000 nucleotides long and encodes a 2,687-amino-acid protein coded from 27 exons. The Toxoplasma glucan synthase gene has three extra introns not present in Eimeria and is ~18,000 nucleotides long. The Toxoplasma glucan synthase is nearly the same length as that of Eimeria and shows 53% amino acid identity and 70% similarity with the Eimeria glucan synthase.
The predicted Toxoplasma and Eimeria glucan synthases contain a catalytic domain, which is also present in glucan synthases of fungi and plants (Fig. 1B). This catalytic domain includes numerous transmembrane helices at its C terminus, and there is another set of transmembrane helices that is N terminal to the catalytic domain (17, 18). A domain common to the parasite and plant glucan synthases but absent in fungal glucan synthases is present at the N terminus, suggesting recent common ancestry of the parasite and plant enzymes. In contrast, both parasite glucan synthases contain a stretch of >800 amino acids that is absent in glucan synthases of fungi and plants.
Cryptosporidium parvum and Cryptosporidium hominem, which are coccidian parasites that cause diarrhea in humans and animals, are missing all of the enzymes that make or hydrolyze β-1,3-glucan (19). These observations suggest that the oocyst walls of Cryptosporidium are not homologous to the oocyst walls of Toxoplasma and Eimeria, which contain β-1,3-glucan (see next section).
A Toxoplasma glucan hydrolase has a novel glucan-binding domain near its N terminus and is present in the oocyst wall.
Like fungi and plants, Toxoplasma and Eimeria have a glucan hydrolase with a GH81 glycohydrolase domain (e.g., TgGH81 [Toxoplasma gondii glucan hydrolase with a GH81 glycohydrolase domain]) and a glucan hydrolase with a GH17 domain (e.g., TgGH17) (Fig. 1C and D) (18). While TgGH17 and EtGH17 (Eimeria tenella glucan hydrolase with a GH17 domain) genes have a single exon, the TgGH81 and EtGH81 genes have ~30 exons. The glucanase domain of TgGH81 and EtGH81 is relatively divergent from those of plants and fungi and does not contain a C-terminal glucan-binding domain (GBD) that has been identified in the Schizosaccharomyces pombe Eng1 (SpGBD [Schizosaccharomyces pombe GBD]) (20). The glucanase domain of TgGH17 and EtGH17 closely resembles that of plants rather than that of fungi, again consistent with common ancestry of the parasite and plant enzymes. In contrast, TgGH17 and EtGH17 have a unique Cys-rich domain between the N-terminal signal peptide and the catalytic domain (Fig. 1D; see Fig. S1 in the supplemental material). We speculated that this domain might be a glucan-binding domain (TgGBD [T. gondii GBD] or EtGBD [E. tenella GBD]), which we tested by making a maltose-binding protein (MBP) fusion protein in the periplasm of bacteria (where disulfides are made) (21).
The glucan-binding domain of the Schizosaccharomyces pombe glucan hydrolase that contains a GH81 domain (SpGBD) (Fig. 1C) binds to Saccharomyces cerevisiae walls that were deproteinated using NaOH (Fig. 2A). SpGBD also binds to oocyst walls, but not sporocyst walls, of Toxoplasma (Fig. 2B) and Eimeria (see Fig. S2 in the supplemental material). The putative glucan-binding domain of the Toxoplasma glucan hydrolase that contains a GH17 domain (TgGBD) (Fig. 1D and Fig. S1) also binds to NaOH-deproteinated Saccharomyces walls (Fig. 2A) and to oocyst walls, but not sporocyst walls, of Toxoplasma (Fig. 2B) and Eimeria (Fig. S2).
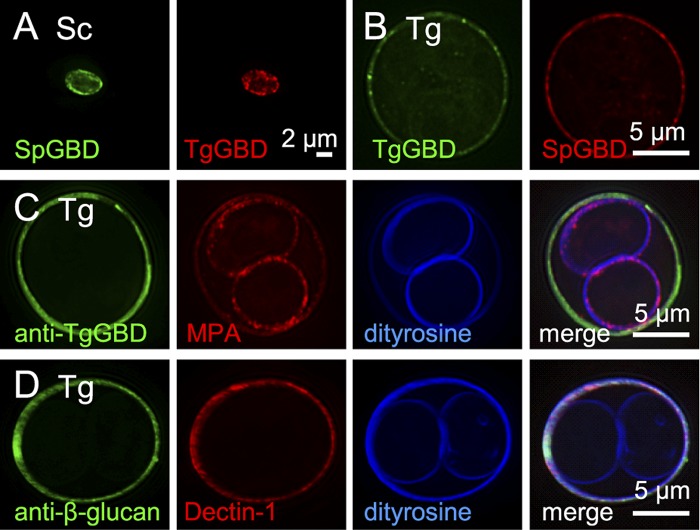
FIG 2 .
Oocyst walls, but not sporocyst walls, of Toxoplasma gondii are labeled with β-1,3-glucan-binding reagents. (A) Walls of Saccharomyces cerevisiae (Sc), deproteinated with NaOH, bind glucan-binding domains from Schizosaccaromyces pombe (SpGBD in green) and Toxoplasma (TgGBD in red) glucan hydrolases, which have been expressed as MBP (maltose-binding protein) fusion proteins and labeled with Alexa Fluor dyes. (B) SpGBD and TgGBD also bind to Toxoplasma gondii (Tg) oocyst walls, but not to sporocyst walls. MBP controls fail to bind to yeast or parasite walls. (C) Antibodies to the Toxoplasma glucan-binding domain (anti-TgGBD in green) bind to oocyst, but not sporocysts, walls of frozen and thawed Toxoplasma. For a control for permeability of sporulated oocysts, the plant lectin MPA (red) that binds to GalNAc labels both the oocyst and sporocyst walls, which are autofluorescent in the UV channel due to the presence of dityrosines. (D) Toxoplasma oocyst walls also are labeled by antibodies to β-1,3-glucan (green) and by the macrophage lectin Dectin-1 (red). See Fig. S2 in the supplemental materialfor fluorescent micrographs of Eimeria oocysts labeled with the same glucan-binding reagents.
Polyclonal rabbit antibodies to the glucan-binding domain of the Toxoplasma glucan hydrolase (anti-TgGBD antibodies) bind to the oocyst walls, but not the sporocyst walls, of sporulated Toxoplasma (Fig. 2C). These oocysts were permeabilized by freezing and thawing, as shown by binding of the GalNAc-binding plant lectin Maclura pomifera agglutinin (MPA), which labels glycoproteins in the oocyst and sporocyst walls of Toxoplasma (Fig. 2C). In contrast, the anti-TgGBD antibodies do not bind to the oocyst walls of Eimeria. The latter result is explained by differences in the primary sequence of the glucan-binding domains of the Toxoplasma and Eimeria glucan hydrolases (see Fig. S1 in the supplemental material).
Dectin-1, which is the macrophage lectin that binds to β-1,3-glucan, binds to oocyst walls, but not sporocyst walls, of both sporulated Toxoplasma (Fig. 2D) and Eimeria (see Fig. S2 in the supplemental material). Antibodies to β-1,3-glucan also bind to oocyst walls, but not sporocyst walls, of both Toxoplasma (Fig. 2D) and Eimeria (see Fig. S2).
In summary, these results show that four different reagents (SpGBD, TgGBD, Dectin-1, and anti-β-glucan antibody), as well as antibodies to TgGBD, bind to oocyst, but not sporocysts, walls. These results also confirm that the unique Cys-rich domain near the N terminus of TgGH17 is a novel glucan-binding domain (TgGBD) and suggest that glucan-binding is the likely mechanism whereby the TgGH17 is targeted to the Toxoplasma oocyst wall.
The inner layer of oocyst walls of Eimeria is a trabecular scaffold.
Transmission electron microscopic studies used unsporulated oocysts of Eimeria (Fig. 1A) that are much cheaper and safer to produce in chickens than are Toxoplasma oocysts in cats (22, 23). As a starting point, we compared the appearance of the oocyst wall of Eimeria with that of Saccharomyces. When ruthenium red is part of the fixative (24), the untreated wall of Saccharomyces shows relatively subtle differences in the granularity of its inner and outer halves (Fig. 3A), while the untreated oocyst wall of Eimeria has two distinct layers (Fig. 3B). The outer layer of the Eimeria oocyst wall is electron dense, while the inner layer is relatively electron lucent. After sonication, the two layers of the oocyst wall of Eimeria often separate from each other.
FIG 3 .
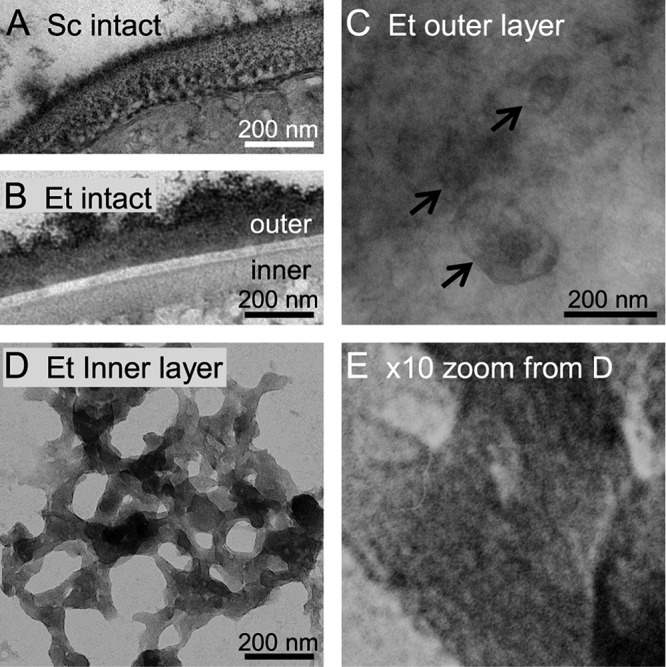
The inner layer of the oocyst wall of Eimeria is a trabecular scaffold. (A) Transmission electron microscopy of cross sections stained with ruthenium red shows that the intact Saccharomyces cerevisiae (Sc) wall is one continuous structure. (B) The Eimeria tenella (Et) oocyst wall is made of an electron-dense outer layer, an electron-lucent interlayer, and a moderately electron-dense inner layer. (C) Negative staining of intact oocysts with ruthenium red shows that the outer layer of the Eimeria oocyst wall is relatively smooth with occasional oval objects that vary in size (arrows). (D) The inner layer of the Eimeria oocyst wall, which is revealed by negatively staining walls that have been mechanically disrupted, is a trabecular scaffold. (E) High-power view of panel D shows putative fibrils of β-1,3-glucan, which are straight and present in parallel arrays, within the trabecular scaffolds. The inner layer of oocyst walls of Toxoplasma are also composed of similar trabecular scaffolds.
Negative staining performed prior to breaking oocyst walls shows that the outer layer is a relatively smooth, continuous structure, upon which oval objects are dispersed (Fig. 3C). In contrast, the inner layer of the oocyst wall is a trabecular scaffold that forms a narrow plane in the absence of sectioning (Fig. 3D). The trabeculae of the inner layer of the oocyst wall are 5 to 20 nm thick and have holes ranging from 5 to 250 nm in diameter. At high magnification, these trabeculae contain fibrils (presumably of β-1,3-glucan), which are for the most part straight and evenly spaced (Fig. 3E). Negative staining of broken Toxoplasma oocyst walls also reveals the presence of trabecular scaffolds, showing that these structures are not specific to Eimeria.
In summary, these results suggest that the continuous outer layer of the oocyst wall forms a permeability barrier, while the inner porous layer of the oocyst wall forms a structural support.
Evidence for β-1,3-glucan in the inner layer of the oocyst wall.
Gas chromatography-mass spectrometry (GC-MS) of deproteinated oocyst walls of Eimeria treated with acid to release monosaccharides reveals glucose as the major sugar present (see Fig. S3 in the supplemental material). Zymolyase, which contains a mixture of β-glucanases and protease from Arthrobacter luteus (a yeast-digesting bacterium first isolated from brewery sewage), completely removes the wall of Saccharomyces to form a spheroplast (Fig. 4A) (25). The inner layer of the Eimeria oocyst wall, which contains fibrils of β-1,3-glucan, is almost completely removed by zymolyase, while the outer layer of the oocyst wall becomes ruffled (Fig. 4B). In addition, the trabecular scaffolds seen by negative staining are dramatically reduced by zymolyase treatment.
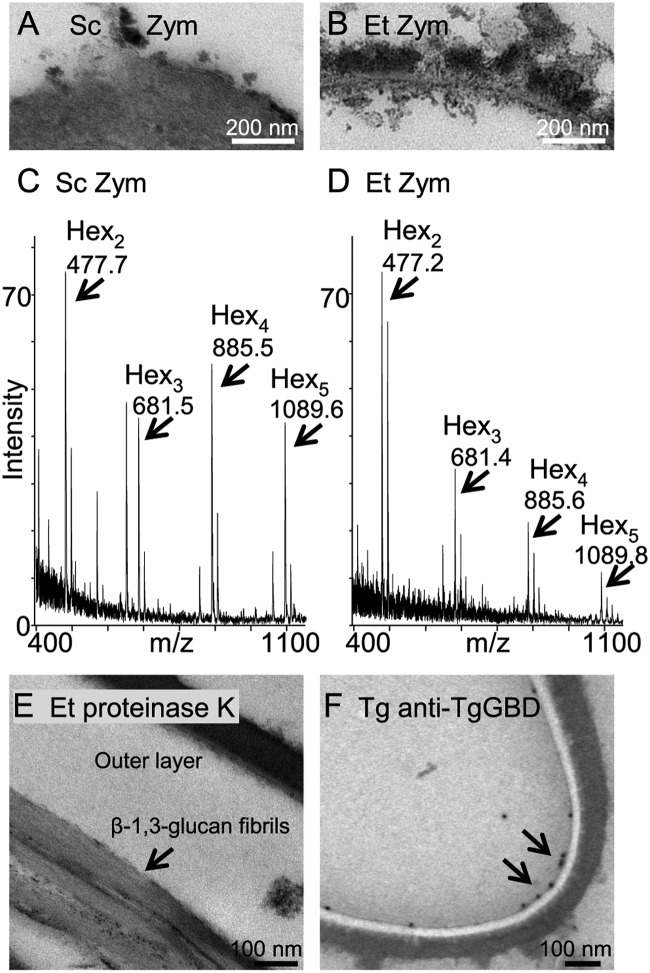
FIG 4 .
Zymolyase releases glucose oligomers from the oocyst walls of Eimeria. (A) Zymolyase (Zym), which is a mixture of β-glucanases and proteases from Arthrobacter luteus, completely removes the walls of Saccharomyces cerevisiae (Sc) to form spheroplasts. (B) In contrast, zymolyase primarily removes the inner layer of oocyst walls of Eimeria. (C) MALDI-TOF MS of glucose oligomers released by zymolyase from Saccharomyces walls. The smaller peaks to the right of each peak marked with arrows are potassium adducts. A peak at 647.6 is unidentified. (D) MALDI-TOF MS of glucose oligomers released by zymolyase from Eimeria oocyst walls are similar to those of Saccharomyces. There are no oligosaccharides present in control samples in which zymolyase was omitted. (E) Proteinase K has little effect on the outer layer of oocyst walls of Eimeria but occasionally releases bundles of fibrils (presumably composed of β-1,3-glucan) (arrow) from the inner layer of the oocyst walls. (F) Antibodies to the Toxoplasma glucan-binding domain (anti-TgGBD antibodies visualized with immunogold) (arrows) bind to the inner layer of the Toxoplasma oocyst wall that is weakly stained in the absence of ruthenium red.
Mass spectrometry shows that hexose oligomers released by zymolyase from walls of Saccharomyces and oocyst walls of Eimeria have similar sizes, while they vary slightly in relative abundance by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) in Fig. 4C and D. These results then confirm the presence of β-hexose in the oocyst wall.
Fibrils of putative β-1,3-glucan are sometimes prominent after oocyst walls are treated with proteinase K, while the outer electron-dense layer is unaffected (Fig. 4E). Anti-TgGBD antibodies bind to the inner layer of Toxoplasma oocyst walls (Fig. 4F).
In summary, the inner layer of the oocyst wall of Eimeria is extensively removed by zymolyase treatment that entirely removes the wall of Saccharomyces to make spheroplasts. While β-1,3-glucan is present throughout the depth of the Saccharomyces walls, β-1,3-glucan appears to be part of a trabecular scaffold present in the inner layers of the oocyst walls of Eimeria and Toxoplasma.
Inhibitors of fungal glucan synthases (echinocandins) markedly decrease recovery of Eimeria oocysts from infected chickens.
Echinocandins are antifungal drugs that target the β-1,3-glucan synthases of Candida and Aspergillus (16). Chickens infected with Eimeria were treated via a wing vein catheter with 10 mg/ml of caspofungin, micafungin, or anidulafungin on day 4 after infection, and animals were euthanized on day 6. While caspofungin has no effect on oocyst recovery from ceca, the number of oocysts recovered is decreased by 71 to 84% by micafungin and by 60 to 86% by anidulafungin (Fig. 5A). We recovered about twice as many oocysts from the ceca of chickens treated with 1 and 3 mg/kg of body weight of micafungin and anidulafungin compared to chickens treated with 10 mg/kg of drug. The oocysts recovered from treated and untreated animals labeled with anti-β-1,3-glucan antibodies and sporulate normally.
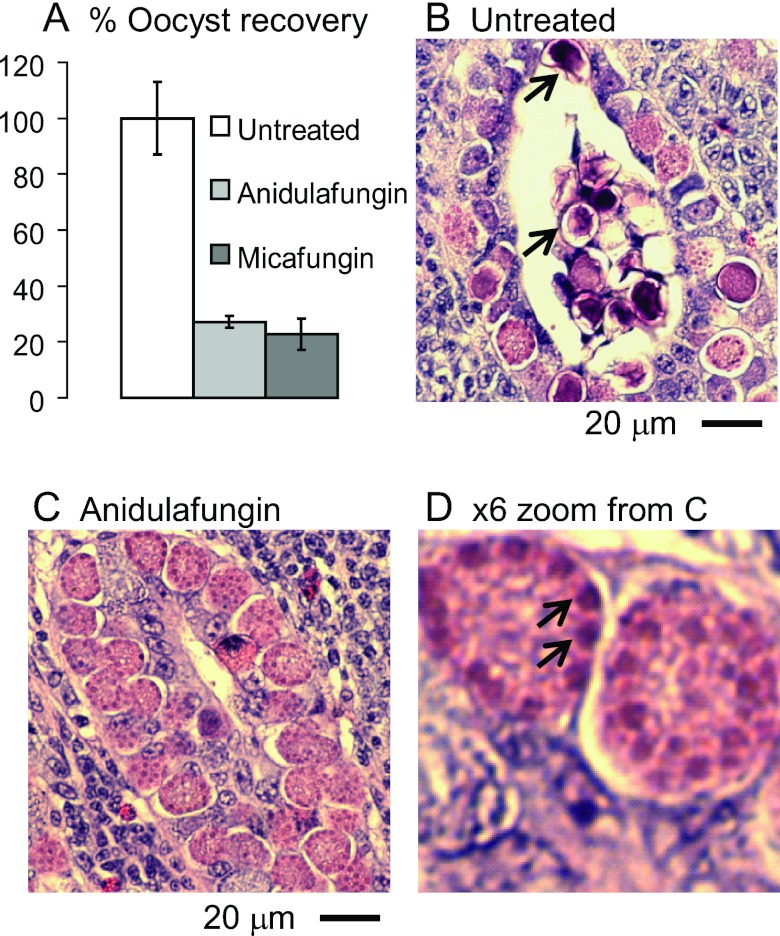
FIG 5 .
Echinocandins arrest the development of oocyst walls of Eimeria in the ceca of infected chickens. (A) Oocyst recoveries are markedly reduced versus untreated chickens in chickens treated with 10 mg/kg anidulafungin or 10 mg/kg micafungin. Error bars show standard deviations of the oocyst counts in two experiments in which there were two chickens per group. (B) Hematoxylin-and-eosin-stained section of the cecum of an untreated chicken infected with Eimeria shows zygotes in numerous developmental stages, including some with small secretory vesicles (early), large secretory vesicles near the periphery (later), and darkly stained walls (fully developed and often released into the lumen of the crypt) (arrows). (C) A cecum from a chicken treated with anidulafungin shows numerous zygotes that are arrested, so few walled forms are present. Similar results were observed with micafungin. (D) High-power view of panel C shows large purple secretory vesicles at the periphery of developing oocysts arrested by anidulafungin (arrows).
The mechanism for how echinocandins decrease oocyst recovery was suggested by histological examination of infected ceca. While Eimeria oocysts with stained walls are frequent in the mucosa and in crypt lumens of untreated chickens (Fig. 5B), walled oocysts are more difficult to find in echinocandin-treated ceca. In ceca of drug-treated chickens, there is also an increase in the number of Eimeria oocysts with large, purple-stained vesicles at their periphery (Fig. 5C and D). These large purple-stained vesicles likely are the same as wall-forming bodies that contain Tyr-rich proteins and other proteins, which are secreted onto the surfaces of zygotes to form the oocyst wall (4, 5).
These results suggest that the glucan synthase of Eimeria, like those of fungi, can be targeted by drugs and demonstrate the importance of β-1,3-glucan in oocyst wall formation. While fungi are killed by echinocandins, these drugs appear to arrest the development of oocyst walls of Eimeria, so that immature cysts remain in the mucosa and fail to be released into the crypt lumen.
Knockout experiments show that glucan synthase is not essential for tissue cyst formation by Toxoplasma in vitro.
Tissue cysts that contain bradyzoites (slowly dividing forms) can be labeled with Dolichos biflorus lectin (DBL) (walls) and anti-SAG2Y antibodies (bradyzoites) (Fig. 6B) (26–28). In contrast, in vitro tissue cysts and brain cysts failed to be labeled with anti-β-1,3-glucan antibodies and with antibodies to the glucan-binding domain of the Toxoplasma glucan hydrolase (anti-TgGBD antibodies), each of which intensely labels the oocyst wall of Toxoplasma (Fig. 2). RT-PCR with glucan synthase primers with Toxoplasma tachyzoite and bradyzoite mRNA led to few products, many of which contained unspliced introns.
FIG 6 .
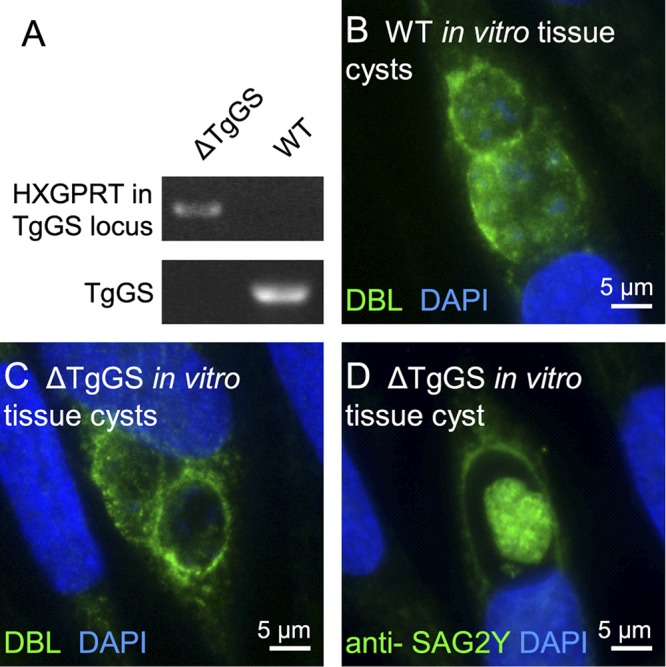
Knockout experiments show that glucan synthase is not essential for tissue cyst formation by Toxoplasma in vitro. (A) PCR products for the HXGPRT gene in the glucan synthase locus are present in the glucan synthase knockout (ΔTgGS), but not in nontransformed Toxoplasma (wild type [WT]). Conversely, the Toxoplasma glucan synthase gene (TgGS) is present in nontransformed parasites, but not in the ΔTgGS parasites. (B) In vitro tissue cysts from nontransformed Toxoplasma are labeled with DBL (green walls), while nuclei are labeled with DAPI (blue). (C) ΔTgGS in vitro tissue cysts showlabeling of walls with DBL similar to that of nontransformed tissue cysts shown in panel B, (D) ΔTgGS bradyzoites within an in vitro tissue cyst are la beled with anti-SAG2Y antibody similarly to nontransformed bradyzoites.
To rule out the possibility that there may be cryptic glucan synthase activity in bradyzoites in vitro contributing to the tissue cyst wall of Toxoplasma, we knocked out the glucan synthase gene in an RH strain of Toxoplasma in which both the Ku80 and the hypoxanthine-xanthine-guanine phosphoribosyltransferase genes have been knocked out (RH ΔHGPRT ΔKu80) (Fig. 6A) (29). These glucan synthase knockouts (ΔTgGS) form tissue cysts in vitro that are labeled with DBL and anti-SAG2Y antibodies in the same way as nontransformed Toxoplasma (Fig. 6C and D). These results strongly suggest that β-1,3-glucan is not a component of the tissue cyst wall, as has also been shown for sporocysts (Fig. 2). Because Toxoplasma loses its sexual competence quickly when it is passed in culture, we were not able to knock out the glucan synthase gene in a strain of Toxoplasma that infects cats and forms oocysts.
DISCUSSION
With regard to β-1,3-glucan, Eimeria oocysts appear to be a good model for Toxoplasma, because Eimeria contains similar glucan synthase and glucan hydrolases as Toxoplasma, Eimeria oocyst walls can be labeled with the same set of probes for β-1,3-glucan, and the inner layers of oocyst walls of Eimeria and Toxoplasma are each composed of trabecular scaffolds. We were able to use Eimeria to demonstrate release of glucose oligomers by zymolyase from purified oocyst walls and to show that echinocandins markedly reduce oocyst release from ceca from infected chickens.
The inner layer of the oocyst wall has a trabecular appearance, which suggests that it is a scaffold that supports the outer wall and is not part of the permeability barrier that is formed by the outer layer of the oocyst wall. While a scaffold function for β-1,3-glucan is also important in fungal walls, the trabecular appearance of the inner layer of the oocyst wall is distinct. The importance of β-1,3-glucan to the formation of the oocyst wall is shown by inhibitor studies with micafungin and anidulafungin that blocked oocyst release from chickens infected with Eimeria. While we expected that echinocandin treatment would result in the release of oocysts with defective walls, we found instead that oocysts without a complete wall remained within the intestinal epithelium. Tyr-rich proteins that form dityrosine bonds likely contribute to the stability of oocyst walls, as shown for the spore walls of Saccharomyces (7, 8, 30).
Some negative results here have important implications for our understanding of these parasites and their potential control. The absence of β-1,3-glucan in the walls of sporocysts and tissue cysts suggests the presence of another yet-to-be-identified component in the walls of infectious stages of these Toxoplasma and Eimeria that make them hard, impenetrable, and resistant to stomach acids and intestinal proteases. The absence of β-1,3-glucan in tissue cysts suggests that (i) Dectin-1 is not involved in human innate and acquired immune responses to Toxoplasma and (ii) drugs targeting glucan synthase cannot be used to remove residual cysts from persons previously infected with Toxoplasma. The apparent resistance of Eimeria to one echinocandin (caspofungin) that inhibits fungal glucan synthases suggests the possibility that a glucan synthase inhibitor might be found to prevent or treat Eimeria infections in chickens that would not induce resistance by fungi to echinocandins.
MATERIALS AND METHODS
Parasites and animals.
All animal work was approved by Institutional Animal Care and Use Committee at Boston University and the USDA. Three-week-old Sexsal chickens (USDA) or 6-week-old chickens (Boston University) were infected with 75,000 sporulated oocysts of Eimeria tenella. Eimeria oocysts were recovered using a blender from ceca of euthanized chickens, filtered through gauze cloth, purified by flotation on saturated NaCl solution three times, washed three times in water, resuspended in phosphate-buffered saline (PBS), and stored at 4°C in PBS for short periods or in 1% K2Cr2O7 in PBS for long periods (22). Some oocysts were sporulated by incubation at 30°C for 48 to 72 h with agitation and then stored at 4°C or used for infections.
To determine whether echinocandins had any effect on oocyst production, we treated two or three chickens each on day 4 after Eimeria infection with 10 mg of caspofungin, micafungin, or anidulafungin per kg of body weight in 500 µl of saline that was injected via a catheter into a wing vein. On day 6 (2 days after drug treatment), the chickens were euthanized and Eimeria oocysts were recovered as described above, counted, and examined by fluorescence and transmission electron microscopy. Two birds each were also treated with 1 and 3 mg/kg micafungin and anidulafungin.
Toxoplasma gondii oocysts were obtained from the feces of parasite-free, 10- to 16-week-old kittens that were infected at the USDA with tissue cysts from the brains of Swiss Webster mice infected with the VEG strain of Toxoplasma gondii (23). The oocysts were separated from cat feces by flotation in sucrose solution. The oocysts were incubated at room temperature in 2% sulfuric acid for 1 week, and sporulated oocysts were stored at 4°C. To obtain unsporulated oocysts, we euthanized infected kittens, and unsporulated oocysts were recovered from rectal contents, floated in cold sucrose solution, and stored at 4°C in 2% sulfuric acid to prevent sporulation. The oocysts were transported cold from the USDA to Boston.
Tissue cysts of Pru strain of Toxoplasma in mouse brains of C57BL/6 mice were a generous gift from Jeroen Saeij of the Massachusetts Institute of Technology.
Molecular biology and biochemistry.
A Toxoplasma gene encoding putative glucan synthase (TGVEG_028810) was downloaded from ToxoDB and compared with an Eimeria gene encoding glucan synthase (ETH_00000330) using TBLASTX that highlights amino acid sequences common to each parasite protein (11, 12). Reverse transcriptase PCR (RT-PCR) with numerous primers that spanned groups of predicted exons were used to amplify coding sequences for glucan synthases from mRNA from unsporulated oocysts of Toxoplasma and Eimeria. Predicted glucan synthases were compared with each other and with glucan synthases of fungi and plants, conserved domains and transmembrane helices were identified (17, 18), and sequences common to the parasite and plant enzymes were determined by multiple sequence alignments.
The predicted glucan hydrolase of Toxoplasma that contained a single exon product and a GH17 glucan hydrolase domain (TgGH17) (TGVEG_018140) was compared to the Eimeria glucan hydrolase (Supercontig_1 19950 to 198146). A putative Cys-rich, glucan-binding domain of TgGH17 (TgGBD), which was between the signal peptide and the catalytic domain (see Fig. S1 in the supplemental material), was cloned into the pMAL-p5X (New England Biolabs, Ipswich, MA) vector for expression in the bacterial periplasm (21). A maltose-binding protein (MBP) fusion protein (MBP-TgGBD) was expressed in the periplasm of NEB Express Escherichia coli (New England Biolabs). MBP-TgGBD was conjugated to Alexa Fluor dyes (Invitrogen, Grand Island, NY) and used to label fungal and oocyst walls and to make a polyclonal rabbit antibody (anti-TgGBD) (Strategic Diagnostics, Inc., Newark, DE). The Schizosaccharomyces pombe Eng1 glucan-binding domain (SpGBD) was cloned, expressed, and used for fluorescence microscopy in the same way. A plasmid encoding Dectin-1 received from Gerald Fink of the Massachusetts Institute of Technology was transformed into E. coli. Dectin-1, which formed an inclusion body in the bacterial cytosol, was renatured and labeled with Alexa Fluor dye.
The second glucan hydrolase of Toxoplasma, which contains a portion of a GH81 glycohydrolase domain (TgGH81), was roughly predicted by comparing syntenic sequences of Toxoplasma and Eimeria chromosomes using TBLASTX.
Toxoplasma glucan synthase gene knockout (ΔTgGS) and in vitro tissue cysts.
T. gondii of the RH strain, which has a deletion of hypoxanthine-xanthine-guanine phosphoribosyltransferase (HXGPRT) (30) for use as a selectable marker and a deletion of Ku80 (29) to prevent nonhomologous DNA recombination (RH ΔHGPRT ΔKu80), were used as parental strains for glucan synthase knockouts (ΔTgGS). A knockout construct, which consists of an HXGPRT gene flanked by a 500-nucleotide (nt) sequence upstream of the Toxoplasma glucan synthase gene and 500-nt sequence downstream of it, was cloned into a gateway pTKO2 destination vector (a generous gift of Jeroen Saiej of the Massachusetts Institute of Technology) using LR clonase (Invitrogen) (31). Upstream and downstream sequences were cloned into pDONR vectors using BP clonase (Invitrogen). Five-hundred-nucleotide sequences from the 5′ untranslated region (5′ UTR) and 3′ UTR of TgGS were amplified by PCR. Twenty-five micrograms of the knockout construct was transfected into freshly lysed parasites as previously described (32). Selection began one day after transfection, using 25 µg/ml mycophenolic acid and 50 µg/ml xanthine. After 10 to 14 days, parasites were tested for the absence of the glucan synthase gene by PCR using a sense primer upstream of the deleted area and an antisense primer within the HXGPRT marker and sets of gene-specific primers. Parasites were cloned in 384-well plates, and glucan synthase gene knockout was confirmed as described above. A single cell clone of the ΔTgGS knockout was used in subsequent experiments.
Differentiation of tachyzoites to bradyzoites and tissue cyst production in human foreskin fibroblasts were induced by making the medium alkaline in the absence of CO2 (26). The formation of tissue cysts was confirmed by light microscopy and by fluorescence microscopy using Dolichos biflorus lectin (DBL) and anti-SAG2Y antibodies (27, 28).
Mass spectrometric identification of β-glucan in oocyst walls.
Saccharomyces cerevisiae cells and Eimeria unsporulated oocysts were broken using glass beads at 4°C in a bead beater for 2 min and then washed twice in PBS. The oocyst walls were deproteinated by incubation with 1 N NaOH at 90°C for 1 h and washed three times in PBS. Prior to zymolyase treatment, oocyst walls were resuspended in α-amylase buffer (100 mM potassium phosphate [pH 6.5]) and incubated with 400 units of α-amylase at 30°C overnight with shaking to remove starch. The oocyst walls were washed three times in PBS, resuspended in zymolyase buffer (10 mM 2-mercaptoethanol, 50 mM potassium phosphate [pH 7.5]), incubated with 5 units zymolyase (Zymo Research, Irvine, CA) at 30°C overnight with shaking, and washed three times in PBS (25). Alternatively, control organisms were treated with α-amylase only. Partially digested walls were then separated from solubilized material by centrifugation at 13,000 × g for 10 min at 4°C.
The supernatants were removed, dried in a Speed-Vac, and kept at −20°C until analysis by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) or electrospray ionization-mass spectrometry (ESI-MS). Before being subjected to mass spectral analysis, dried Eimeria walls were solubilized in a glass tube with 100 µl of a suspension of dimethyl sulfoxide (DMSO) over crushed NaOH pellets, and incubated at room temperature (RT) for 30 min with vortexing to initiate permethylation. Next, 50 µl of iodomethane was added, and the tube was vortexed. After 1 h at RT, the permethylation procedure was repeated once by adding 100 µl of DMSO-NaOH plus 50 µl of iodomethane and incubating the suspension for another hour. To stop the reaction, 600 µl of water was added, and the permethylated carbohydrates were extracted with 1 ml of CHCl3. The organic phase was recovered, washed five times with 2 ml CHCl3-H2O (1:1), and dried under vacuum. The permethylated samples were dissolved as described above and spotted on a MALDI-MS target plate after being mixed 1:1 with a 10-mg/ml 2,5-dihydroxybenzoic acid (DHB) matrix that had been dissolved in the same solvent. MALDI-TOF mass spectra were obtained on a Bruker Daltonics Reflex IV (Bruker Daltonics, Billerica, MA) mass spectrometer operated in the positive-ion reflectron mode. The spectra resulting from 150 and 200 shots from a 337-nm nitrogen laser were summed. The laser pulse width was 3 ns.
For monosaccharide analysis, walls of E. tenella that had been deproteinated by treatment with NaOH were dissolved in 500 µl of 1 M methanolic-HCl at 80°C for 16 h. Sugars were trimethylsilylated in 200 µl of N,O-bis-(trimethylsilyl)-trifluoroacetamide (BSTFA) at 80°C for 45 min. GC-MS analysis was performed on a 6890 series GC-MS system instrument (Agilent, Santa Clara, CA). Sample retention times and mass spectral fragmentation patterns were then compared with those of hexose standards.
Fluorescence microscopy.
Toxoplasma and Eimeria oocysts were purified on a three-step CsCl gradient (ρ = 1.05, 1.1, and 1.15) by ultracentrifugation at 16,000 × g for 1 h, washed in PBS, and permeabilized by four cycles of freeze-thawing. Permeabilized oocysts were incubated with 2 µg/ml Alexa Fluor-labeled Dectin-1, SpGBD, or TgGBD in PBS plus 1% bovine serum albumin (BSA) for 1 h at RT and then washed three times in PBS. Alternatively, oocysts were incubated with rabbit anti-β-glucan (a gift from Gerald Fink) or anti-TgGBD antibodies, washed twice in PBS, and then incubated with Alexa Fluor-labeled anti-rabbit antibodies for another hour and washed three times. Alexa Fluor-labeled Maclura pomifera agglutinin (MPA), which binds to sporocyst walls, and cyanovirin-N, which binds to sporozoites, were used at 2 µg/ml to verify permeability (9, 33). In cases where Eimeria oocysts were deproteinated, oocysts were incubated for 1 h in 1 N NaOH at 90°C, washed three times in PBS, and labeled as described above.
Toxoplasma gondii wild-type and glucan synthase knockout (ΔTgGS) tissue cysts grown on glass coverslips were fixed in 4% paraformaldehyde for 30 min at RT, washed three times in PBS, permeabilized with 0.1% Triton X-100 for 10 min, and then washed three times in PBS. Tissue cysts were incubated with 2 µg/ml of anti-SAG2Y antibody (a gift from John Boothroyd of Stanford University) in 1% BSA in PBS for 1 h at RT, washed three times in PBS, incubated with Alexa Fluor-labeled anti-mouse antibody in 1% BSA in PBS for another hour at RT, and then washed three times in PBS (28). Alternatively, tissue cysts were fixed and permeabilized in methanol for 10 min, washed three times in PBS, incubated in 1% BSA in PBS for 1 h at RT with Alexa Fluor-labeled DBL at 2 µg/ml, and then washed three times in PBS (27). The nuclei of host and Toxoplasma cells were labeled by 2 µg/ml DAPI (4′,6′-diamidino-2-phenylindole) in PBS for the last 10 min of antibody or lectin labeling.
Slides were examined by three-dimensional multiple-wavelength fluorescence microscopy using an Olympus IX70 microscope equipped for Deltavision deconvolution (Applied Precision, Issaquah, WA). This system employs restorative and deconvolution techniques. The images were collected at 0.2-mm optical sections for the indicated wavelengths and were subsequently deconvolved using SoftWoRx (Applied Precision). Data were examined either as optical sections or as a projection of the entire stack.
TEM.
For negatively stained transmission electron microscopy (TEM), Eimeria and Toxoplasma unsporulated oocysts were either left untreated or treated with zymolyase. For untreated samples, unsporulated oocysts were broken with glass beads in a glass bead beater for 2 min at 4°C with protease inhibitor cocktail (Roche Applied Science, Madison, WI) and then washed three times in PBS. For zymolyase treatment, unsporulated oocysts were broken and washed as described above, resuspended in α-amylase buffer incubated with 400 units of α-amylase at 30°C overnight, washed in PBS, incubated with 5 units of zymolyase at 30°C overnight, and washed in PBS.
The samples were then incubated in 0.1% ruthenium red for 5 min, loaded onto carbon-coated EM grids, washed in 20 mM Tris, stained with 1% uranyl acetate (UA) for 5 min, washed in Tris, and then stained again with UA. Alternatively, in order to visualize the outer layer alone, unsporulated oocysts were first incubated with ruthenium red, washed three times in PBS, stained with UA, washed three times in PBS, and then broken and washed as described above before they were loaded on grids. Images were recorded at 120 kV in a Philips CM12 transmission electron microscope on Kodak SO163 film and scanned on a Nikon 9000 scanner.
For thin-section TEM, Eimeria unsporulated oocysts and Saccharomyces cells were left untreated, sonicated (Eimeria), or broken with glass beads at 4°C (yeast), before washing with PBS and deproteinating with 1 N NaOH at 90°C for 1 h. Alternatively, intact yeast and sonicated oocysts were treated with 5 units of zymolyase at 30°C overnight and washed in PBS. Alternatively, sonicated oocysts were incubated with 40 µg proteinase K (Invitrogen) in 250 µl PBS at 50°C overnight and then washed in PBS.
Treated and untreated oocysts and yeast were fixed in 2.5% paraformaldehyde, 5% glutaraldehyde, and 0.06% picric acid in 200 mM cacodylate buffer for 1 h. Organisms were postfixed for >12 h at 4°C in 1% osmium tetroxide in the presence of 0.1% ruthenium red in cacodylate buffer (24). Water was removed from pellets in a graded series of solutions of ethanol and propylene oxide, and organisms were embedded in Epon at the Harvard Medical School Electron Microscopy Facility. Ultrathin sections (60 to 80 nm) were cut on a Reichert Ultracut-S microtome, picked up and placed on copper grids, and stained with 1% UA and 0.2% lead citrate, and images were recorded at 80 kV in a JEOL 1200EX transmission electron microscope using DITABIS digital imaging.
For immunoelectron microscopy, oocysts of Toxoplasma were fixed in 2% paraformaldehyde and 0.1% glutaraldehyde, dehydrated, and embedded in LR White resin. Ultrathin sections were picked up on copper grids, which were incubated with rabbit anti-TgGBD antibodies. Rabbit antibodies were localized using protein A-gold. Control incubations omitted the anti-TgGBD antibody.
SUPPLEMENTAL MATERIAL
Sequence of a novel Toxoplasma gondii glucan-binding domain (TgGBD). Toxoplasma glucan hydrolase (TgGH17) contains an N-terminal signal peptide (gray), residues conserved within the glucan-binding domain of the Eimeria tenella glucan hydrolase (EtGH17) (red), and a GH17 glycohydrolase domain (green). See Fig. 1 in the text for diagrams of glucan synthases and glucan hydrolases of Toxoplasma and Eimeria. Download Figure S1, TIF file, 2.4 MB.
Four glucan-binding reagents label the oocyst wall of Eimeria. (A) The oocyst wall of Eimeria, which has been permeabilized by freezing and thawing, is labeled with antiglucan antibodies (green) and Dectin-1 (red). (B) Glucan-binding domains of Schizosaccharomyces pombe glucan hydrolase (SpGBD) (green) and T. gondii glucan hydrolase (TgGBD) (red) bind to oocyst walls that have been deproteinated with NaOH. Sporocyst walls of Eimeria, which are present within sporulated oocysts, are not labeled with any of these reagents. See Fig. 2 in the text for binding of these reagents to sporulated oocysts of Toxoplasma. Download Figure S2, TIF file, 2.4 MB.
Electron ionization mass spectrum of the hexose peak from gas chromatography of sugars released by acid hydrolysis of deproteinated Eimeria oocyst walls. The retention time and fragments identify the peak as α-d-glucopyranoside. Download Figure S3, TIF file, 0.3 MB.
ACKNOWLEDGMENTS
We thank Rudi Beiler and Kath Hardcastle of the Boston University Lab Animals Center for help with wing vein injections. We thank Raymond Fetterer and Ruth Barfield of the USDA for their guidance in Eimeria chicken infections. We thank Maria Ericson of the Harvard Medical School for help with electron microscopy.
This work was supported in part by grants from the National Institutes of Health (NIH) (grant AI48082 to J.S., grant AI07642 [T32] to G.G.B., grants RR010888, GM104603, and RR015942 to C.E.C., grant AI081924 to M.-J.G., and grant GM31318 to P.W.R.). This work was also supported in part by the United States Department of Agriculture (USDA) (CRIS 1265-31320-075-00D to K.B.M.).
Footnotes
Citation Bushkin GG, et al. 2012. β-1,3-Glucan, which can be targeted by drugs, forms a trabecular scaffold in the oocyst walls of Toxoplasma and Eimeria. mBio 3(5):e00258-12. doi:10.1128/mBio.00258-12.
REFERENCES
- 1. Boothroyd JC. 2009. Toxoplasma gondii: 25 years and 25 major advances for the field. Int. J. Parasitol. 39:935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weiss LM, Dubey JP. 2009. Toxoplasmosis: a history of clinical observations. Int. J. Parasitol. 39:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dubey JP, Lindsay DS, Speer CA. 1998. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 11:267–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belli SI, Smith NC, Ferguson DJ. 2006. The coccidian oocyst: a tough nut to crack! Trends Parasitol. 22:416–423 [DOI] [PubMed] [Google Scholar]
- 5. Ferguson DJ, Belli SI, Smith NC, Wallach MG. 2003. The development of the macrogamete and oocyst wall in Eimeria maxima: immuno-light and electron microscopy. Int. J. Parasitol. 33:1329–1340 [DOI] [PubMed] [Google Scholar]
- 6. Chapman HD, Jeffers TK, Williams RB. 2010. Forty years of monensin for the control of coccidiosis in poultry. Poult. Sci. 89:1788–1801 [DOI] [PubMed] [Google Scholar]
- 7. Sharman PA, Smith NC, Wallach MG, Katrib M. 2010. Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunol. 32:590–598 [DOI] [PubMed] [Google Scholar]
- 8. Fritz HM, Bowyer PW, Bogyo M, Conrad PA, Boothroyd JC. 2012. Proteomic analysis of fractionated Toxoplasma oocysts reveals clues to their environmental resistance. PLoS One 7:e29955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chatterjee A, et al. 2010. Evidence for mucin-like glycoproteins that tether sporozoites of Cryptosporidium parvum to the inner surface of the oocyst wall. Eukaryot. Cell 9:84–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Possenti A, et al. 2010. Molecular characterisation of a novel family of cysteine-rich proteins of Toxoplasma gondii and ultrastructural evidence of oocyst wall localisation. Int. J. Parasitol. 40:1639–1649 [DOI] [PubMed] [Google Scholar]
- 11. Gajria B, et al. 2008. ToxoDB: an integrated Toxoplasma gondii database resource. Nucleic Acids Res. 36:D553–D556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blake DP, et al. 2012. EmaxDB: availability of a first draft genome sequence for the apicomplexan Eimeria maxima. Mol. Biochem. Parasitol. 184:48–51 [DOI] [PubMed] [Google Scholar]
- 13. Lesage G, Bussey H. 2006. Cell wall assembly in Saccharomyces cerevisiae. Mol. Biol. Rev. 70:317–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drummond RA, Brown GD. 2011. The role of Dectin-1 in the host defence against fungal infections. Curr. Opin. Microbiol. 14:392–399 [DOI] [PubMed] [Google Scholar]
- 15. Cohen NR, et al. 2011. Innate recognition of cell wall β-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe 10:437–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eschenauer G, Depestel DD, Carver PL. 2007. Comparison of echinocandin antifungals. Ther. Clin. Risk Manag. 3:71–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Käll L, Krogh A, Sonnhammer EL. 2004. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 338:1027–1036 [DOI] [PubMed] [Google Scholar]
- 18. Marchler-Bauer A, et al. 2011. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 39:D225–D229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aurrecoechea C, et al. 2007. ApiDB: integrated resources for the apicomplexan bioinformatics resource center. Nucleic Acids Res. 35:D427–D430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martín-Cuadrado AB, et al. 2008. The Schizosaccharomyces pombe endo-1,3-beta-glucanase Eng1 contains a novel carbohydrate binding module required for septum localization. Mol. Microbiol. 69:188–200 [DOI] [PubMed] [Google Scholar]
- 21. Austin BP, Nallamsetty S, Waugh DS. 2009. Hexahistidine-tagged maltose-binding protein as a fusion partner for the production of soluble recombinant proteins in Escherichia coli. Methods Mol. Biol. 498:157–172 [DOI] [PubMed] [Google Scholar]
- 22. Fetterer RH, Jenkins MC, Miska KB, Cain GD. 2010. Metam sodium reduces viability and infectivity of Eimeria oocysts. J. Parasitol. 96:632–637 [DOI] [PubMed] [Google Scholar]
- 23. Dubey JP. 2006. Comparative infectivity of oocysts and bradyzoites of Toxoplasma gondii for intermediate (mice) and definitive (cats) hosts. Vet. Parasitol. 140:69–75 [DOI] [PubMed] [Google Scholar]
- 24. Nanduri J, Williams S, Aji T, Flanigan TP. 1999. Characterization of an immunogenic glycocalyx on the surfaces of Cryptosporidium parvum oocysts and sporozoites. Infect. Immun. 67:2022–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magnelli PE, Cipollo JF, Robbins PW. 2005. A glucanase-driven fractionation allows redefinition of Schizosaccharomyces pombe cell wall composition and structure: assignment of diglucan. Anal. Biochem. 336:202–212 [DOI] [PubMed] [Google Scholar]
- 26. Weiss LM, et al. 1995. A cell culture system for study of the development of Toxoplasma gondii bradyzoites. J. Eukaryot. Microbiol. 42:150–157 [DOI] [PubMed] [Google Scholar]
- 27. Knoll LJ, Boothroyd JC. 1998. Molecular biology’s lessons about Toxoplasma development: stage-specific homologs. Parasitol. Today 14:490–493 [DOI] [PubMed] [Google Scholar]
- 28. Saeij JP, Arrizabalaga G, Boothroyd JC. 2008. A cluster of four surface antigen genes specifically expressed in bradyzoites, SAG2CDXY, plays an important role in Toxoplasma gondii persistence. Infect. Immun. 76:2402–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huynh MH, Carruthers VB. 2009. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot. Cell 8:530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donald RG, Roos DS. 1998. Gene knockouts and allelic replacements in Toxoplasma gondii: HXGPRT as a selectable marker for hit-and-run mutagenesis. Mol. Biochem. Parasitol. 91:295–305 [DOI] [PubMed] [Google Scholar]
- 31. Rosowski EE, et al. 2011. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J. Exp. Med. 208:195–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roos DS, Donald RG, Morrissette NS, Moulton AL. 1994. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45:27–63 [DOI] [PubMed] [Google Scholar]
- 33. Bushkin GG, et al. 2010. Suggestive evidence for Darwinian selection against asparagine-linked glycans of Plasmodium and Toxoplasma. Eukaryot. Cell 9:228–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence of a novel Toxoplasma gondii glucan-binding domain (TgGBD). Toxoplasma glucan hydrolase (TgGH17) contains an N-terminal signal peptide (gray), residues conserved within the glucan-binding domain of the Eimeria tenella glucan hydrolase (EtGH17) (red), and a GH17 glycohydrolase domain (green). See Fig. 1 in the text for diagrams of glucan synthases and glucan hydrolases of Toxoplasma and Eimeria. Download Figure S1, TIF file, 2.4 MB.
Four glucan-binding reagents label the oocyst wall of Eimeria. (A) The oocyst wall of Eimeria, which has been permeabilized by freezing and thawing, is labeled with antiglucan antibodies (green) and Dectin-1 (red). (B) Glucan-binding domains of Schizosaccharomyces pombe glucan hydrolase (SpGBD) (green) and T. gondii glucan hydrolase (TgGBD) (red) bind to oocyst walls that have been deproteinated with NaOH. Sporocyst walls of Eimeria, which are present within sporulated oocysts, are not labeled with any of these reagents. See Fig. 2 in the text for binding of these reagents to sporulated oocysts of Toxoplasma. Download Figure S2, TIF file, 2.4 MB.
Electron ionization mass spectrum of the hexose peak from gas chromatography of sugars released by acid hydrolysis of deproteinated Eimeria oocyst walls. The retention time and fragments identify the peak as α-d-glucopyranoside. Download Figure S3, TIF file, 0.3 MB.