Abstract
The Kaposi’s sarcoma-associated herpesvirus (KSHV) is the etiologic agent of Kaposi’s sarcoma (KS)—one of the most common tumors arising in the setting of immune suppression. Hallmarks of KS lesions include KSHV-infected cells of endothelial lineage and neoangiogenesis. Pro-migratory factors secreted in the tumor microenvironment by KSHV-infected cells promote endothelial cell (EC) migration and angiogenesis, but existing approaches targeting these pathways are not widely utilized for KS. This underscores the need for additional characterization of KSHV-host interactions relevant to EC pathogenesis to identify new therapeutic targets. We recently demonstrated that de novo infection by KSHV promotes EC invasion through upregulation of emmprin—a multifunctional glycoprotein previously shown to induce tumor cell invasion and regional angiogenesis through upregulation of signal transduction and promotion of tumor-stroma interactions. The present study was undertaken to determine whether EC invasion for KSHV-infected cells is induced through activation of specific signal transduction pathways and pro-angiogenic factors by emmprin. We found that KSHV activation of emmprin induces PI3K/Akt- and mitogen-activated protein kinase (MAPK)-dependent secretion of vascular endothelial growth factor (VEGF). Moreover, EC invasion following de novo infection is induced by emmprin-dependent PI3K/Akt and MAPK activation of VEGF. These findings support the potential utility of targeting emmprin for reducing VEGF secretion and EC migration in the KS microenvironment.
Keywords: KSHV, CD147, VEGF, Kaposi’s sarcoma, signal transduction
Introduction
Kaposi’s sarcoma-associated herpesvirus (KSHV) is the etiologic agent for Kaposi’s sarcoma (KS)1—the most common AIDS-associated tumor worldwide and an important cause of morbidity and mortality for HIV-infected patients.2 Acquisition of a migratory or invasive phenotype represents one hallmark of KSHV-infected EC, with implications for both viral dissemination and angiogenesis within KS lesions.3 KSHV-encoded proteins induce EC secretion of soluble factors that promote cell migration and angiogenesis, including matrix metalloproteinases (MMPs), interleukin-8 (IL-8), IL-6, and vascular endothelial growth factor (VEGF).3-6 Existing data support the potential utility of inhibitors targeting mechanisms of angiogenesis for the treatment of KS, although toxicities and a lack of more extensive clinical trial data have limited their widespread clinical use.7-10 A better understanding of KSHV-host interactions regulating cell migration and angiogenesis may elucidate safer and more effective therapeutic approaches for KS.
The multifunctional transmembrane protein known as the extracellular matrix metalloproteinase inducer (emmprin; CD147) initiates expression and secretion of multiple MMPs, thereby promoting tumor cell invasion.11 Several studies suggest that emmprin regulation of signal transduction initiates secretion of pro-migratory factors by tumor cells and stromal cells in the tumor microenvironment.12-15 For example, overexpression of emmprin stimulates phosphorylation of Akt and MAPK intermediates in breast cancer cells, and tumors propagated with these cell lines in vivo exhibit VEGF expression along with Akt and MAPK activation.12 MAPK signaling is also activated following upregulation of emmprin in human myelomonocytic cells,13 and emmprin stimulates activation of IL-18 via Rac 1-dependent PI3K/Akt/NF-κB and MAPK signaling pathways in murine cardiomyocytes.14 KSHV initiates constitutive activation of PI3K/Akt, MAPK and NF-κB during de novo infection of various cell types, including EC,16-22 and we recently reported that enhancement of EC invasion following de novo KSHV infection results from upregulation of emmprin by the KSHV-encoded latency-associated nuclear antigen (LANA).23 Therefore, the present study was undertaken to determine whether KSHV/emmprin-mediated invasion for EC is initiated through activation of specific signal transduction pathways and pro-angiogenic factors.
Materials and Methods
Cell culture and infection assays
BCBL-1 were maintained in RPMI 1640 media (Gibco) supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES (pH 7.5), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, 0.05 mM β-mercaptoethanol, and 0.02% (wt/vol) sodium bicarbonate. Human umbilical vein endothelial cells (HUVEC) were grown in DMEM/F-12 50/50 medium (Cellgro) supplemented with 5% FBS. To obtain KSHV for infection experiments, BCBL-1 cells were incubated with 0.6 mM valproic acid for 6 days, and the concentration of infectious viral particles within concentrated culture supernatants determined prior to infection experiments as described previously.17
qRT-PCR
Total RNA was isolated using the RNeasy Mini kit according to the manufacturer’s instructions (QIAGEN). cDNA was synthesized from equal total RNA using SuperScript III First-Strand Synthesis SuperMix Kit (Invitrogen) according to the manufacturer’s instructions. The primers for target gene amplification are provided in Supplemental Table 1. Amplification experiments were carried out using an iCycler IQ Real-Time PCR Detection System (Bio-Rad), and cycle threshold (Ct) values were tabulated in duplicate for each gene of interest for each experiment. “No template” (water) controls were used to ensure minimal background contamination. Mean Ct values were calculated following completion of three independent experiments. Using Ct values for β-actin as loading controls, fold changes for experimental groups relative to assigned controls were calculated using automated iQ5 2. 0 software (Bio-Rad).
RNA interference
For RNA silencing, HUVEC were transfected for 48 h with either emmprin- or control non-target-siRNAs (ON-TARGET plus SMART pool, Dharmacon) using a commercially available transfection reagent (Dharmacon) according to the manufacturer’s instructions. 3 independent transfections were performed for each experiment, and all samples were analyzed in triplicate for each transfection.
Transduction
For overexpression of emmprin, HUVEC were transduced as previously described with a recombinant adenoviral vector (MO1 ~ 10) encoding emmprin, or a control vector, for 24-48 h prior to subsequent analyses.24
Inhibition of signal transduction
Selective inhibitors targeting the mitogen-activated protein kinase kinase (MEK; U0126), Akt1/2 (A6730), PI3K (LY294002) and NF-κB (Bay11-7082) were reconstituted according to the manufacturer’s instructions (Sigma). Serial dilutions of these compounds were added to cell cultures for 2 h (U0126 and Bay11-7082) or 24 h (A6730 and LY294002) and perturbations in signal transduction confirmed using immunoblot assays (see below).
Immunoblotting
Total cell lysates (20 μg) were resolved by 10% SDS–PAGE, transferred to nitrocellulose membranes, and immunoblotted with the following antibodies: phospho-Akt (Ser473), phospho-p44/42 ERK (Thr202/Tyr204), phospho-NF-κB p65 (Ser536), t-Akt, t-p44/42 ERK, and t-NF-κB p65 (Cell Signaling Technologies), and emmprin (BD Pharmingen). For loading controls, blots were reacted with antibodies detecting β-Actin (Sigma). Immunoreactive bands were developed using an enhanced chemiluminescence reaction (Perkin-Elmer), and visualized by autoradiography.
Transwell invasion assays
Matrigel Invasion Chambers (Becton Dickinson), containing an 8μM pore size PET membrane with a thin layer of basement membrane covering the pores, were used in transwell invasion assays. The chambers were hydrated for 4 h at 37°C with culture media. After hydration, the media in the bottom of the well was replaced with fresh media, then 2 × 104 HUVEC were plated in the top of the chamber. Trypan blue staining was used to ensure that an equal number of live cells for each group was implanted into the transwell chambers. For experiments to determine the role of VEGF and IL-6 in cell invasiveness, 5 ng/mL of recombinant human VEGF (LONZA) or 10 ng/mL of IL-6 (BD Biosciences) were added to the top of chambers. After 24 h incubation, cells were fixed using 4% formaldehyde for 15 minutes at room temperature and the chambers rinsed in PBS and stained with 0.2% crystal violet for 10 minutes. After washing the chambers 5 times with dH2O, the cells at the top of the Matrigel membrane were removed with cotton applicators. The cells at the bottom of the membrane were counted using a phase contrast microscope. Relative invasion for cells in experimental groups was tabulated as follows: relative invasion = # invading cells in experimental group / # invading cells in control group.23
ELISA
Concentrations of IL-6, IL-8 and VEGF in culture supernatants were determined using human IL-6 (eBioscience), IL-8 (Becton Dickinson) and VEGF-A (Pierce Biotechnology) ELISA kits according to the manufacturers’ instructions.
Cell viability assays
Cell viability was assessed using a standard MTT assay.17 Briefly, a total of 5×103 cells were incubated in individual wells in a 96-well plate for 24 h. Serial dilutions of the signal transduction inhibitors were then added and after 24 h, cells were incubated in a 1 mg/mL MTT solution (Sigma) at 37°C for 3 h then 50% DMSO overnight prior to determination of optical density at 570 nm using a spectrophotometer (Thermo Labsystems).
Statistical Analysis
Significance for differences between experimental and control groups was determined using the two-tailed Student’s t-test (Excel 8.0).
Results
KSHV induction of emmprin promotes EC invasion through upregulation of VEGF
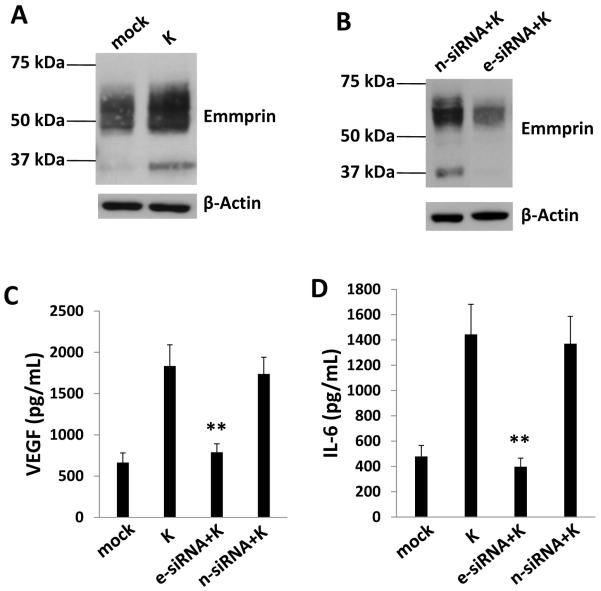
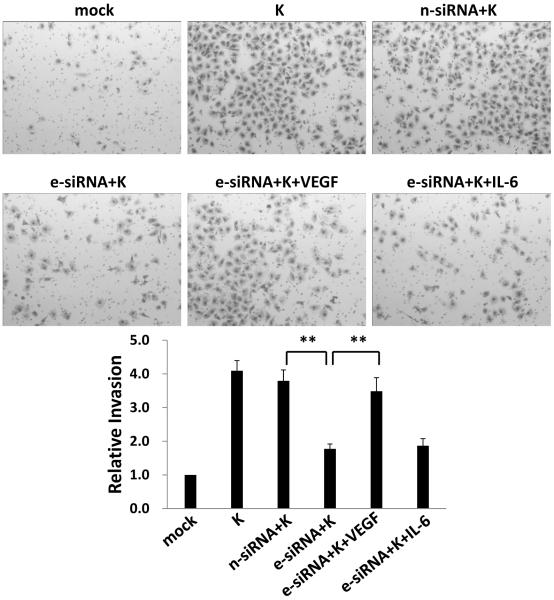
We found previously that KSHV upregulates emmprin and emmprin-associated MMP expression following de novo infection of endothelial cells.23 To determine whether KSHV upregulation of emmprin induces EC secretion of pro-migratory factors other than MMP that are associated with KS pathogenesis, we quantified VEGF-A, IL-6 and IL-8 in human EC (HUVEC) culture supernatants following EC incubation with purified KSHV in the context of emmprin knockdown using RNA interference (RNAi). High and low molecular weight glycoforms of emmprin induce MMP expression,25 and we confirmed KSHV upregulation of both high (~65 kDa) and low (~35 kDa) molecular weight glycoforms of emmprin in HUVEC during de novo infection (Fig. 1A), as well as effective suppression of KSHV-induced emmprin expression using RNAi (Fig. 1B). We found that emmprin knockdown significantly reduced KSHV-initiated secretion of VEGF and IL-6 by EC (Fig. 1C, D). In contrast, emmprin knockdown had no impact on KSHV-induced secretion of IL-8 (data not shown). Using transwell invasion assays, we also found that recombinant VEGF (rVEGF), but not recombinant IL-6 (rIL-6), restored KSHV-induced EC invasion in the context of emmprin knockdown (Fig. 2).
Figure 1. Targeting emmprin suppresses VEGF and IL-6 secretion during KSHV infection of endothelial cells.
(A, B) HUVEC were incubated with media (mock) or purified KSHV (K) for 2 h (A), in some cases following their transfection for 48 h with control siRNA (n-siRNA) or emmprin-siRNA (e-siRNA) (B). Cells were subsequently incubated in fresh media for an additional 24 h prior to performance of immunoblots to identify high (~65 kDa) and low (~35 kDa) MW glycoforms of emmprin. β-actin was identified for loading controls. Data shown represent one of three independent experiments. (C, D) Cells were treated as in (B) and concentrations of VEGF and IL-6 in culture supernatants determined using ELISAs. Error bars represent the S.E.M. for three independent experiments. ** = p<0.01.
Figure 2. Targeting emmprin suppresses KSHV-induced, VEGF-dependent invasion for endothelial cells.
HUVEC were incubated with media (mock) or purified KSHV (K) for 2 h, in some cases following their transfection for 48 h with control siRNA (n-siRNA) or emmprin-siRNA (e-siRNA). Cells were subsequently incubated in fresh media for an additional 24 h prior to completion of transwell invasion assays in the presence or absence of 5 ng/mL recombinant human VEGF or 10 ng/mL recombinant human IL-6. Relative invasiveness was calculated as described in Methods. Error bars represent the S.E.M. for three independent experiments. ** = p<0.01.
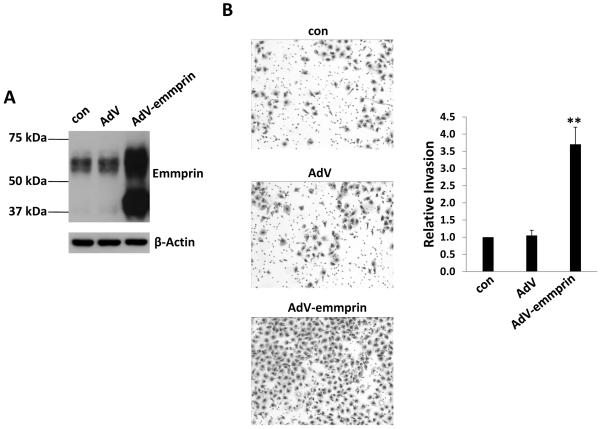
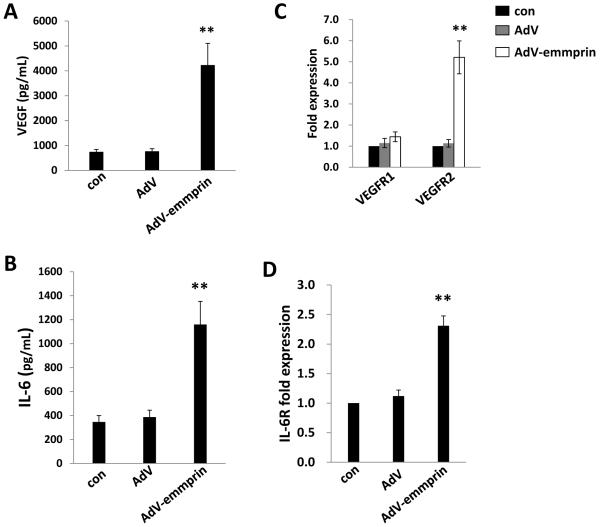
Next, we transduced HUVEC with a recombinant adenoviral vector expressing emmprin (AdV-emmprin) as described previously24 and confirmed that this approach resulted in overexpression of both glycoforms of emmprin (Fig. 3A). Subsequent transwell invasion assays revealed that overexpression of emmprin significantly enhanced EC invasion (Fig. 3B), VEGF and IL-6 secretion (Fig. 4A and B), and expression of transcripts for VEGF receptor 2 (VEGFR2) and the IL-6 receptor (IL-6R) (Fig. 4C and D). Overexpression of emmprin had no impact on EC secretion of IL-8 (data not shown), consistent with the lack of suppression of KSHV-induced IL-8 secretion with emmprin knockdown.
Figure 3. Ectopic expression of emmprin induces endothelial cell invasion.
(A) HUVEC were transduced using a recombinant human emmprin-encoding adenovirus (AdV-emmprin), or control adenovirus (AdV), and expression of emmprin and β-actin (for loading controls) was quantified 48 h later by immunoblotting. Data shown represent one of three independent experiments. (B) Cells were treated as (A), then transwell invasion assays performed to determine relative invasiveness as detailed in Methods. Error bars represent the S.E.M. for three independent experiments. ** = p<0.01.
Figure 4. Ectopic expression of emmprin induces expression and secretion of VEGF and IL-6 by endothelial cells.
HUVEC were transduced using AdV-emmprin or control vector for 48 h, and concentrations of VEGF (A) and IL-6 (B) in culture supernatants determined using ELISAs. In parallel, transcripts encoding VEGF receptors 1 and 2 (C) and the IL-6 receptor (D) were quantified using qRT-PCR as detailed in Methods and normalized using non-transduced control cells (con). Error bars represent the S.E.M. for three independent experiments. ** = p<0.01.
KSHV initiation of VEGF secretion and EC invasion is mediated by emmprin activation of PI3K/Akt and MAPK signal transduction
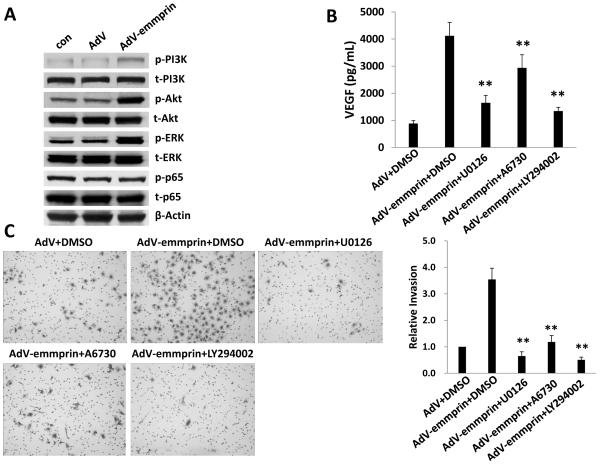
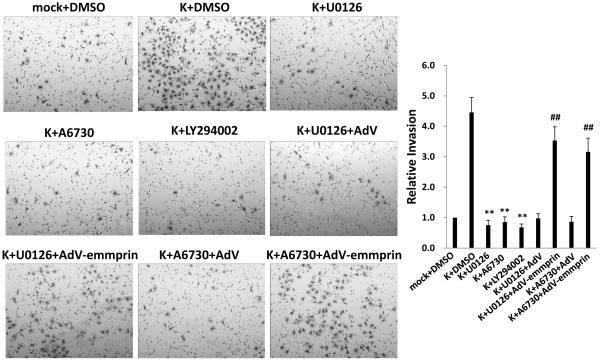
KSHV initiates constitutive activation of signal transduction involving PI3K/Akt, MAPK (including the extracellular signal-regulated kinase, or ERK), and canonical (p65-dependent) NF-κB pathways during de novo infection of EC.16-22 Moreover, existing data implicate emmprin in the activation of these pathways in some tumors.12-15 To first determine whether emmprin plays a role in activating these pathways in EC, we transduced HUVEC with AdV-emmprin and found that emmprin overexpression enhanced phosphorylation of PI3K, Akt and ERK, but not p65 (Fig. 5A). Next, to determine whether activation of one or more of these pathways was involved in emmprin-mediated initiation of VEGF secretion and EC invasion, we incubated transduced cells with concentrations of selective activation inhibitors of PI3K (2 μM LY294002), Akt (2 μM A6730), and MAPK (10 μM U0126) exhibiting no discernable cytotoxicity for HUVEC using standard MTT assays (Fig. S1). We found that inhibition of PI3K, Akt, or MAPK activation significantly suppressed emmprin-induced VEGF secretion and invasion for HUVEC (Fig. 5B and C). To extend the relevance of these findings, we used signal transduction inhibitors to selectively suppress KSHV-induced PI3K, Akt, or MAPK activation during de novo infection of HUVEC (Fig. S2). Of note, individual inhibitors had little or no impact on phosphorylation of intermediates other than their intended targets (for example, inhibition of PI3K had no impact on p65 phosphorylation), indicating that these pathways are not interdependent in HUVEC. We found that suppression of KSHV-induced PI3K, Akt, and MAPK activation also suppressed KSHV-initiated invasion for EC (Fig. 6). Furthermore, overexpression of emmprin restored invasiveness for KSHV-infected HUVEC in the presence of PI3K/Akt or MAPK inhibition (Fig. 6). These findings suggest that KSHV initiates EC invasion through upregulation of emmprin-mediated activation of PI3K/Akt and MAPK signaling.
Figure 5. Ectopic expression of emmprin increases endothelial cell invasion through activation of PI3K/Akt and ERK signaling.
(A) HUVEC were transduced with AdV-emmprin or control vector (AdV) for 48 h, then total and phosphorylated signaling intermediates identified using immunoblots. β-actin was also identified for loading controls. (B) Cells were transduced as (A), then incubated with either drug vehicle (DMSO) or specific inhibitors of MEK (10 μM U0126) for 2 h, Akt1/2 (2 μM A6730) or PI3K (2 μM LY294002) for 24 h. Concentrations of VEGF in culture supernatants were then determined using ELISAs. (C) Cells were transduced and incubated with signal transduction inhibitors as in (B) then transwell invasion assays were performed to determine relative invasiveness as detailed in Methods. Error bars represent the S.E.M. for three independent experiments. ** = p<0.01.
Figure 6. KSHV induction of endothelial cell invasion is mediated through emmprin-dependent activation of PI3K/Akt and MAPK signaling.
HUVEC were incubated with media (mock) or purified KSHV (K) for 2 h, then incubated with either drug vehicle (DMSO) or specific inhibitors of NF-κB (10 μM Bay11-7082 [Bay11]) or MEK (10 μM U0126) for 2 h, Akt1/2 (2 μM A6730) or PI3K (2 μM LY294002) for 24 h. Some cells were transduced during drug incubation with AdV-emmprin or control vector (AdV) for 48 h as shown. Transwell invasion assays were then performed to determine relative invasiveness as detailed in Methods. Error bars represent the S.E.M. for three independent experiments. ** = p<0.01 (relative to K + DMSO group); ## = p<0.01 (relative to K + drug + AdV groups).
Discussion
Existing data indicate that emmprin-induced signal transduction facilitates cancer pathogenesis, including activation and secretion of pro-migratory factors.12-15 KSHV also induces signal transduction and EC secretion of pro-migratory factors,3-6, 16-22 and we previously described KSHV upregulation of emmprin expression and emmprin-dependent invasion for EC.23 The present study was undertaken to identify specific signal transduction pathways and pro-migratory factors through which emmprin facilitates invasion for KSHV-infected EC.
We found that KSHV upregulation of emmprin, or ectopic overexpression of emmprin, induces secretion of VEGF and IL-6 by EC. In addition, rVEGF, but not rIL-6, restores invasiveness for KSHV-infected EC in the context of emmprin knockdown. Collectively, these data suggest that VEGF is a key mediator of emmprin-induced EC invasion during KSHV infection. IL-6 is present within the KS microenvironment and the peripheral circulation of patients with KSHV-associated tumors.26-28 In addition to its role in the induction of cell migration and angiogenesis,29, 30 IL-6 may also impair dendritic cell maturation (putatively suppressing virus- or tumor-specific immune activation) and promote B cell expansion (putatively increasing availability of B cell targets for expansion of the viral reservoir) in the microenvironment of KSHV-associated tumors.31, 32 Our observation that both PI3K/Akt and IL-6 activation are emmprin-dependent in KSHV-infected EC is in agreement with existing data indicating that IL-6 activation is PI3K/Akt-dependent for some cancer cells.30 Additional studies are, therefore, warranted to determine the significance of emmprin-induced IL-6 secretion by KSHV-infected EC in the local environment. Our data also suggest that IL-8 activation following KSHV infection of EC is emmprin-independent since targeting emmprin had no impact on KSHV induction of IL-8 secretion. Moreover, we found that ectopic overexpression of emmprin did not induce canonical activation of NF-κB in HUVEC, consistent with published data indicating that KSHV activation of IL-8 is NF-κB-dependent.5, 33
We found that KSHV-induced VEGF secretion and EC invasion are mediated through KSHV upregulation of emmprin and activation of PI3K/Akt and MAPK pathways. We focused on PI3K/Akt, MAPK, and canonical NF-κB signaling since existing data support activation of these pathways by both emmprin12-14 and KSHV.16-22 Our data are in agreement with published data supporting a role for KSHV in autocrine and paracrine induction of VEGF secretion through activation of PI3K/Akt and MAPK.18, 22 We have also previously demonstrated emmprin induction of chemotherapeutic resistance for KSHV-infected lymphoma cells.34 PI3K/Akt and MAPK signaling are linked with chemotherapeutic resistance for many cancer cell types,35-40 and clinical data indicate that many KS tumors are resistant to chemotherapy.41, 42 These findings justify future studies to determine whether KSHV induction of emmprin facilitates drug resistance during de novo infection through activation of PI3K/Akt and/or MAPK signaling.
We observed no inhibition of KSHV-induced p65 phosphorylation in the presence of selective inhibition of PI3K/Akt activation. These results conflict with other studies showing that KSHV-encoded genes expressed in other cell lines induce PI3K/Akt-dependent canonical NF-κB activation,19 and that Akt phosphorylation of IKB-kinase (IKK) facilitates tumor necrosis factor (TNF)-mediated canonical activation of NF-κB.43 We hypothesize that interdependence of PI3K/Akt and NF-κB activation in KSHV-infected cells is cell type-specific. This underscores the importance of using primary cells and relevant cell lines when addressing questions related to interdependence of signal transduction pathways for KSHV-associated cancers. We also observed that ectopic overexpression of emmprin induced EC invasiveness, although it did not induce p65 phosphorylation in HUVEC. Data published by our group and others have revealed a role for NF-κB in KSHV-induced secretion of VEGF and invasiveness for KSHV-infected EC.18, 44 Therefore, it is possible that NF-κB-induced invasiveness for KSHV-infected cells is emmprin-independent. However, our studies do not address whether inhibition of specific signal transduction pathways inhibits upregulation of emmprin itself in KSHV-infected cells. In fact, existing data indicate that NF-κB plays a role in transactivation of emmprin.45 Therefore, an alternative possibility is that inhibition of signal transduction suppresses KSHV-mediated EC invasion through suppression of KSHV gene expression (if accomplished prior to viral entry and nuclear trafficking), or KSHV-induced expression of emmprin which is itself dependent upon NF-κB, MAPK, or other signaling intermediates. Additional studies are, therefore, needed to identify signaling pathways and transcription factors responsible for KSHV-induced emmprin expression23 and the role of other KSHV-encoded genes in emmprin-mediated pathogenesis.
In summary, our data suggest that targeting emmprin as a therapeutic approach for KS may suppress pathogenesis for KSHV-infected EC in the tumor microenvironment through inhibition of KSHV-induced VEGF and IL-6 production and EC migration. It is possible that complimentary, emmprin-independent mechanisms induced by KSHV, including NF-κB-mediated activation of IL-8, may circumvent these effects. Additional studies, including in vivo models incorporating inhibitors of emmprin and related pathways, may clarify whether targeting emmprin is a viable strategy for the treatment and prevention of KSHV-associated pathogenesis while helping to identify other emmprin-dependent pathogenic determinants of KS progression.
Supplementary Material
Supplemental Figure 1. Determination of endothelial cell cytotoxicity during inhibition of signal transduction. HUVEC were incubated with the indicated concentrations of the MEK inhibitor U0126 (A), the Akt1/2 inhibitor A6730 (B), the PI3K inhibitor LY294002 (C) or the NF-κB inhibitor Bay11-7082 (Bay11) (D) for 24 h. Cell viability was determined using a standard MTT assay as described in Methods. Error bars represent the S.E.M. for three experiments.
Supplemental Figure 2. Selective inhibition of signal transduction in KSHV-infected endothelial cells. HUVEC were incubated with either media (mock) or purified KSHV (K) for 2 h then treated with drug vehicle (DMSO) or signal transduction inhibitors as in Suppl. Fig. 1. Immunoblots were then used to identify total and phosphorylated signaling intermediates and β-actin for loading controls. Data shown represent one of three independent experiments.
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01-CA142362 to CP and CA082867 to BPT), the South Carolina COBRE for Oral Health (P20-RR-017696; CP subproject investigator), and the MUSC Hollings Cancer Center (core grant P30-CA-138313).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Seaberg EC, Wiley D, Martinez-Maza O, Chmiel JS, Kingsley L, Tang Y, Margolick JB, Jacobson LP. Cancer incidence in the multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer. 2010;116:5507–16. doi: 10.1002/cncr.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian LW, Xie J, Ye F, Gao SJ. Kaposi’s sarcoma-associated herpesvirus infection promotes invasion of primary human umbilical vein endothelial cells by inducing matrix metalloproteinases. J Virol. 2007;81:7001–10. doi: 10.1128/JVI.00016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Q, Matta H, Lu G, Chaudhary PM. Induction of IL-8 expression by human herpesvirus 8 encoded vFLIP K13 via NF-kappaB activation. Oncogene. 2006;25:2717–26. doi: 10.1038/sj.onc.1209298. [DOI] [PubMed] [Google Scholar]
- 5.Montaner S, Sodhi A, Servitja JM, Ramsdell AK, Barac A, Sawai ET, Gutkind JS. The small GTPase Rac1 links the Kaposi sarcoma-associated herpesvirus vGPCR to cytokine secretion and paracrine neoplasia. Blood. 2004;104:2903–11. doi: 10.1182/blood-2003-12-4436. [DOI] [PubMed] [Google Scholar]
- 6.Pati S, Cavrois M, Guo HG, Foulke JS, Jr., Kim J, Feldman RA, Reitz M. Activation of NF-kappaB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi’s sarcoma pathogenesis. J Virol. 2001;75:8660–73. doi: 10.1128/JVI.75.18.8660-8673.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrario A, Gomer CJ. Avastin enhances photodynamic therapy treatment of Kaposi’s sarcoma in a mouse tumor model. J Environ Pathol Toxicol Oncol. 2006;25:251–9. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.160. [DOI] [PubMed] [Google Scholar]
- 8.Dezube BJ, Krown SE, Lee JY, Bauer KS, Aboulafia DM. Randomized phase II trial of matrix metalloproteinase inhibitor COL-3 in AIDS-related Kaposi’s sarcoma: an AIDS Malignancy Consortium Study. J Clin Oncol. 2006;24:1389–94. doi: 10.1200/JCO.2005.04.2614. [DOI] [PubMed] [Google Scholar]
- 9.Fife K, Howard MR, Gracie F, Phillips RH, Bower M. Activity of thalidomide in AIDS-related Kaposi’s sarcoma and correlation with HHV8 titre. Int J STD AIDS. 1998;9:751–5. doi: 10.1258/0956462981921512. [DOI] [PubMed] [Google Scholar]
- 10.Little RF, Wyvill KM, Pluda JM, Welles L, Marshall V, Figg WD, Newcomb FM, Tosato G, Feigal E, Steinberg SM, Whitby D, Goedert JJ, Yarchoan R. Activity of thalidomide in AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2000;18:2593–02. doi: 10.1200/JCO.2000.18.13.2593. [DOI] [PubMed] [Google Scholar]
- 11.Yan L, Zucker S, Toole BP. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost. 2005;93:199–04. doi: 10.1160/TH04-08-0536. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y, Nakada MT, Rafferty P, Laraio J, McCabe FL, Millar H, Cunningham M, Snyder LA, Bugelski P, Yan L. Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3K-Akt signaling pathway. Mol Cancer Res. 2006;4:371–7. doi: 10.1158/1541-7786.MCR-06-0042. [DOI] [PubMed] [Google Scholar]
- 13.Huang Z, Wang C, Wei L, Wang J, Fan Y, Wang L, Wang Y, Chen T. Resveratrol inhibits EMMPRIN expression via P38 and ERK1/2 pathways in PMA-induced THP-1 cells. Biochem Biophys Res Commun. 2008;374:517–21. doi: 10.1016/j.bbrc.2008.07.058. [DOI] [PubMed] [Google Scholar]
- 14.Venkatesan B, Valente AJ, Prabhu SD, Shanmugam P, Delafontaine P, Chandrasekar B. EMMPRIN activates multiple transcription factors in cardiomyocytes, and induces interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB andMKK7/JNK/AP-1 signaling. J Mol Cell Cardiol. 2010;49:655–63. doi: 10.1016/j.yjmcc.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bougatef F, Quemener C, Kellouche S, Naimi B, Podgorniak MP, Millot G, Gabison EE, Calvo F, Dosquet C, Lebbe C, Menashi S, Mourah S. EMMPRIN promotes angiogenesis through hypoxia-inducible factor-2alpha-mediated regulation of soluble VEGF isoforms and their receptor VEGFR-2. Blood. 2009;114:5547–56. doi: 10.1182/blood-2009-04-217380. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Damania B. Kaposi’s sarcoma-associated herpesvirus confers a survival advantage to endothelial cells. Cancer Res. 2008;68:4640–8. doi: 10.1158/0008-5472.CAN-07-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin Z, DeFee M, Isaacs JS, Parsons C. Extracellular Hsp90 serves as a co-factor for MAPK activation and latent viral gene expression during de novo infection by KSHV. Virology. 2010;403:92–102. doi: 10.1016/j.virol.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bais C, Van Geelen A, Eroles P, Mutlu A, Chiozzini C, Dias S, Silverstein RL, Rafii S, Mesri EA. Kaposi’s sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/ KDR. Cancer Cell. 2003;3:131–43. doi: 10.1016/s1535-6108(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 19.Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 2001;61:2641–8. [PubMed] [Google Scholar]
- 20.Sadagopan S, Sharma-Walia N, Veettil MV, Raghu H, Sivakumar R, Bottero V, Chandran B. Kaposi’s sarcoma-associated herpesvirus induces sustained NF-kappaB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J Virol. 2007;81:3949–68. doi: 10.1128/JVI.02333-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin D, Galisteo R, Ji Y, Montaner S, Gutkind JS. An NF-kappaB gene expression signature contributes to Kaposi’s sarcoma virus vGPCR-induced direct and paracrine neoplasia. Oncogene. 2008;27:1844–52. doi: 10.1038/sj.onc.1210817. [DOI] [PubMed] [Google Scholar]
- 22.Jham BC, Ma T, Hu J, Chaisuparat R, Friedman ER, Pandolfi PP, Schneider A, Sodhi A, Montaner S. Amplification of the Angiogenic Signal through the Activation of the TSC/mTOR/HIF Axis by the KSHV vGPCR in Kaposi’s Sarcoma. PLoS One. 2011;6:e19103. doi: 10.1371/journal.pone.0019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin Z, Dai L, Slomiany MG, Toole BP, Parsons C. Direct activation of emmprin and associated pathogenesis by an oncogenic herpesvirus. Cancer Res. 2010;70:3884–89. doi: 10.1158/0008-5472.CAN-09-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li R, Huang L, Guo H, Toole BP. Basigin (murine EMMPRIN) stimulates matrix metalloproteinase production by fibroblasts. J Cell Physiol. 2001;186:371–9. doi: 10.1002/1097-4652(2000)9999:999<000::AID-JCP1042>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Belton RJ, Jr., Chen L, Mesquita FS, Nowak RA. Basigin-2 is a cell surface receptor for soluble basigin ligand. J Biol Chem. 2008;283:17805–14. doi: 10.1074/jbc.M801876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oksenhendler E, Carcelain G, Aoki Y, Boulanger E, Maillard A, Clauvel JP, Agbalika F. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric castleman disease in HIV-infected patients. Blood. 2000;96:2069–73. [PubMed] [Google Scholar]
- 27.Aoki Y, Yarchoan R, Braun J, Iwamoto A, Tosato G. Viral and cellular cytokines in AIDS-related malignant lymphomatous effusions. Blood. 2000;96:1599–601. [PubMed] [Google Scholar]
- 28.Drexler HG, Meyer C, Gaidano G, Carbone A. Constitutive cytokine production by primary effusion (body cavity-based) lymphoma-derived cell lines. Leukemia. 1999;13:634–40. doi: 10.1038/sj.leu.2401371. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794–800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jee SH, Chu CY, Chiu HC, Huang YL, Tsai WL, Liao YH, Kuo ML. Interleukin-6 induced basic fibroblast growth factor-dependent angiogenesis in basal cell carcinoma cell line via JAK/STAT3 and PI3-kinase/Akt pathways. J Invest Dermatol. 2004;123:1169–75. doi: 10.1111/j.0022-202X.2004.23497.x. [DOI] [PubMed] [Google Scholar]
- 31.Jego G, Bataille R, Pellat-Deceunynck C. Interleukin-6 is a growth factor for nonmalignant human plasmablasts. Blood. 2001;97:1817–22. doi: 10.1182/blood.v97.6.1817. [DOI] [PubMed] [Google Scholar]
- 32.Cirone M, Lucania G, Aleandri S, Borgia G, Trivedi P, Cuomo L, Frati L, Faggioni A. Suppression of dendritic cell differentiation through cytokines released by Primary Effusion Lymphoma cells. Immunol Lett. 2008;120:37–41. doi: 10.1016/j.imlet.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Sun Q, Matta H, Lu G, Chaudhary PM. Induction of IL-8 expression by human herpesvirus 8 encoded vFLIP K13 via NF-kappaB activation. Oncogene. 2006;25:2717–26. doi: 10.1038/sj.onc.1209298. [DOI] [PubMed] [Google Scholar]
- 34.Qin Z, Dai L, Bratoeva M, Slomiany MG, Toole BP, Parsons C. Cooperative roles for emmprin and LYVE-1 in the regulation of chemoresistance for primary effusion lymphoma. Leukemia. 2011 doi: 10.1038/leu.2011.144. doi: 10.1038/leu.2011.144. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghayad SE, Vendrell JA, Larbi SB, Dumontet C, Bieche I, Cohen PA. Endocrine resistance associated with activated ErbB system in breast cancer cells is reversed by inhibiting MAPK or PI3K/Akt signaling pathways. Int J Cancer. 2010;126:545–62. doi: 10.1002/ijc.24750. [DOI] [PubMed] [Google Scholar]
- 36.Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Manzoli L, McCubrey JA. Targeting the PI3K/AKT/mTOR signaling network in acute myelogenous leukemia. Expert Opin Investig Drugs. 2009;18:1333–49. doi: 10.1517/14728220903136775. [DOI] [PubMed] [Google Scholar]
- 37.Zhang HY, Zhang PN, Sun H. Aberration of the PI3K/AKT/mTOR signaling in epithelial ovarian cancer and its implication in cisplatin-based chemotherapy. Eur J Obstet Gynecol Reprod Biol. 2009;146:81–6. doi: 10.1016/j.ejogrb.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 38.Misra S, Ghatak S, Zoltan-Jones A, Toole BP. Regulation of multidrug resistance in cancer cells by hyaluronan. J Biol Chem. 2003;278:25285–8. doi: 10.1074/jbc.C300173200. [DOI] [PubMed] [Google Scholar]
- 39.Guo X, Ma N, Wang J, Song J, Bu X, Cheng Y, Sun K, Xiong H, Jiang G, Zhang B, Wu M, Wei L. Increased p38-MAPK is responsible for chemotherapy resistance in human gastric cancer cells. BMC Cancer. 2008;8:375. doi: 10.1186/1471-2407-8-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daouti S, Wang H, Li WH, Higgins B, Kolinsky K, Packman K, Specian A, Jr., Kong N, Huby N, Wen Y, Xiang Q, Podlaski FJ, He Y, Fotouhi N, Heimbrook D, Niu H. Characterization of a novel mitogen-activated protein kinase kinase 1/2 inhibitor with a unique mechanism of action for cancer therapy. Cancer Res. 2009;69:1924–32. doi: 10.1158/0008-5472.CAN-08-2627. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen HQ, Magaret AS, Kitahata MM, Van Rompaey SE, Wald A, Casper C. Persistent Kaposi sarcoma in the era of highly active antiretroviral therapy: characterizing the predictors of clinical response. AIDS. 2008;22:937–45. doi: 10.1097/QAD.0b013e3282ff6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flaitz CM, Jin YT, Hicks MJ, Nichols CM, Wang YW, Su IJ. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences (KSHV/HHV-8) in oral AIDS-Kaposi’s sarcoma: a PCR and clinicopathologic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:259–64. doi: 10.1016/s1079-2104(97)90014-7. [DOI] [PubMed] [Google Scholar]
- 43.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 44.Defee M, Qin Z, Dai L, Toole BP, Isaacs JS, Parsons C. Extracellular Hsp90 serves as a co-factor for NF-κB activation and cellular pathogenesis induced by an oncogenic herpesvirus. Am J Cancer Res. 2011;1:686–99. [PMC free article] [PubMed] [Google Scholar]
- 45.Rucci N, Millimaggi D, Mari M, Del Fattore A, Bologna M, Teti A, Angelucci A, Dolo V. Receptor activator of NF-kappaB ligand enhances breast cancer-induced osteolytic lesions through upregulation of extracellular matrix metalloproteinase inducer/CD147. Cancer Res. 2010;70:6150–60. doi: 10.1158/0008-5472.CAN-09-2758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Determination of endothelial cell cytotoxicity during inhibition of signal transduction. HUVEC were incubated with the indicated concentrations of the MEK inhibitor U0126 (A), the Akt1/2 inhibitor A6730 (B), the PI3K inhibitor LY294002 (C) or the NF-κB inhibitor Bay11-7082 (Bay11) (D) for 24 h. Cell viability was determined using a standard MTT assay as described in Methods. Error bars represent the S.E.M. for three experiments.
Supplemental Figure 2. Selective inhibition of signal transduction in KSHV-infected endothelial cells. HUVEC were incubated with either media (mock) or purified KSHV (K) for 2 h then treated with drug vehicle (DMSO) or signal transduction inhibitors as in Suppl. Fig. 1. Immunoblots were then used to identify total and phosphorylated signaling intermediates and β-actin for loading controls. Data shown represent one of three independent experiments.