Abstract
Background
Outer hair cells' (OHCs') dysfunctions as the extent of temporary and permanent threshold shifts (TTS and PTS) and cochlear damage were assessed in rabbits exposed to continuous noise
Methods
Twelve New Zealand white rabbits were studied in noise (N) (n=6; exposed to continuous noise; 95 dB SPL, 500-8000 Hz for 8 h per day during 5 consecutive days) and control (C) (n=6; not exposed to noise). OHCs' functions were assessed by distortion product otoacoustic emission (DPOAE) level (Ldp) measurements in different periods and comparing TTS and PTS. Animals were anaesthetized by CO2; cochleae were extracted, fixed in 10% formaldehyde for 48 hours, decalcified by 10% nitric acid for 24 hours, and dehydrated, embedded, sectioned 5 µm thickness and stained by Hematoxylin and Eosin for light microscopy.
Results
The most and least Ldp or TTS or PTS were related to 5888.50 Hz and 588.00 Hz respectively in noise subjected rabbits (P<0.05). TTS and PTS were decreased up to 17.79 dB and to 16.01 dB respectively. TTS were more than PTS over all test frequencies, especially at 5888.50 Hz (P<0.05). Ldp or TTS or PTS were found to be equal across ears (P>0.05). Severely vacuolated OHCs, pyknotic IHCs, swollen SC, and slightly thickened BM were found.
Conclusion
Continuous noise extensively led to OHCs' dysfunctions as decreased Ldp (both TTS and PTS) and highly damage to cochlea.
Keywords: Noise-induced Hearing Loss, Outer hair cells' function, Cochlear damage, Distortion product Otoacoustic emissions
Introduction
Noise-induced hearing loss (NIHL) is referred as the most common potentially preventable form of sensorineural hearing impairment in industries (1). Most of conducted studies regarding NIHL are mainly related to continuous noise exposure (1). It must also be emphasized that noise exposures in life, environment, and industries are mostly as continuous noise exposure (2). Continuous noise exposure can cause temporary or permanent damage to the auditory system (3). So the ears have considerable comeback power from brief exposure to intense continuous noise and ordinarily recover within 24 hours to 48 hours, called as temporary threshold shift (TTS) (3). It must be considered that repeated or prolonged exposure to intense continuous noise gradually damages the cochlear hair cells of the inner ear, resulting in a permanent threshold shift (PTS) across multiple frequencies (4,5,6). Continuous noise exposure is believed that can induce higher TTS and PTS than intermittent noise exposure in animals and humans (7). Continuous noise over-stimulation can damage to the cochlea, hair cell membranes, and changes in size and shape of hair cells through different processes (6,7). Other effects of noise indicates include interference with communication, altered performance, annoyance, distraction, and interference with work or relaxation and physiological responses such as elevated blood pressure and sleep disturbances (8).
Whether or not continuous noise would alter hearing function or damage OHCs can be investigated on different laboratory animals (9). In order to assess the alterations and damage, distortion product otoacoustic emissions (DPOAEs) are assigned as a useful clinical tool for the early and differential diagnosis of damage to the OHCs in animals and humans (10-12). DP frequency is precisely related to the stimulus frequencies f1 and f2 by the formulas f1−N(f2−f1) for the lower band and f2+N(f2−f1) for the upper side band (13-16). In normal hearing, DPOAE-grams are close to each other at high and more separated at low stimulus levels, reflecting cochlear nonlinear sound processing (17-19). In cochlear hearing loss, DPOAE-grams are more separated even at high stimulus levels, revealing loss of cochlear amplifier compression (20). There are some limitations of Ldp recordings. First, electric microphone noise, physiological noise (breathing, blood flow) and external acoustic noise do not allow Ldp measurements at very low stimulus levels (20). Especially below 0.5 KHz, reliable Ldp measurements are not possible even at high stimulus levels (21-23). Second, because of the limited frequency range of the sound probe’s electroacoustic transducers, high-frequency Ldp measurements are difficult without using specialized devices (21,22). Third, standing waves in the outer ear canal make a defined stimulus setting difficult to obtain. Fourth, besides the main DPOAE source at f2, a secondary DPOAE source is present at the 2f1−f2 place, which interacts with the main source constructively or destructively at the f2 place (19,20). Therefore, DPOAE does not exactly reflect OHCs function at f2 place. There are also several technical aspects that must be considered in correct and acceptable DPOAE-gram recording (21,22,23). The most commonly used calibration method is the in-the-ear calibration based on the measurement of the sound-pressure level at the ear probe microphone for constant voltage at the loudspeaker (21,22). To access to maximum interaction site and preserve optimum overlap of the primary-tone traveling waves, the primary-tone level difference has to be increased with decreasing stimulus level, resulting in a L1│L2 setting described by L1=0.4L2+39.(22,23) The recording of Ldp requires the use of a highly sensitive low-noise microphone; loudspeakers need to exhibit a low distortion factor to minimize technical distortion; a tight fit of the probe is essential for Ldp recording; and the ear canal has to be clean and that the ear probe ports has not to be blocked with cerumen (21-23).
For better finding of outer hair cells' dysfunctions and cochlear damage caused by continuous noise and due to limitations in human studies, the present research was conducted to assessment outer hair cells' dysfunctions as the extent of temporary and permanent threshold shifts (TTS and PTS) and cochlear damage in rabbits exposed to continuous noise simulated to industrial situations.
Materials and Methods
Twelve male New Zealand white (NZW) rabbits (2000±200 g body weight) were maintained in animal house at 20-22°C temperature, 30-70 % relative humidity, and 10 times/hour air displacement. Rabbits were fed to nutritional food and soft drink water. "General principles of Helsinki law related to laboratory animal" were used absolutely. Sample size was calculated 6 for any group according to pilot study. Noise group were exposed to 95 dBA SPL continuous noise at 500-8000 Hz for 8 hours per day during 5 consecutive days. Experimental protocol was such: baseline audiometry (day 0), rest periods (3 days; day 1 to 3), exposure periods (only for N group), secondary audiometry (an hour after latest exposure on day 8); rest period (3 days; day 9 to 11), and third audiometry (72 hours after latest exposure on day 11). Situations for control group were the same as noise group except for exposing to noise. Noise exposure was occurred in a transparent poly carbonated Plexiglas chamber dimensioned 50×50×50 cm based on calculating clearances needed for 6 rabbits, ventilated air volume, and reverberation environment (that SPL was independent on distances) (Figure 1).
Figure 1. A cross-sectional view of exposure chamber (50×50×50 cm polycarbonate Plexiglas) has been shown. All rabbits were located into this chamber for exposure to noise pollutant (N group) and control group for 8 hr per day during 5 consecutive days. Exposure to noise has been carried out through a noise loudspeaker mounted on the roof of the chamber. Control group is just posed onto the chamber resembled to other groups with identical situations, but without any exposure.

Noise was delivered to animals in chamber equipped by a pair of loud speakers hanging on its roof. Noise was generated by means of Signal software manufactured by Pardisan Technology and Science Park, and delivered using Cool Edit Pro v. 2.1 manufactured by Syntrillium Software Corporation. Generated noise was amplified by an amplifier model ES-2000s manufactured by ES Audio Industrial Corporation, and propagated by a pair of loudspeakers type Micro Lab, model Subwoofer M-563 manufactured by Probit Company. SPL in chamber systematically monitored by Sound Level Meter (SLM type Precision) model CEL-490 manufactured by Cassella-CEL Company equipped to an analyzer located at animal hearing zone. Background noise in animal house and lab was below 20±2 dB.
Animals were anaesthetized by 60% Ketamine (40 mg/kg) and 40% Xylazine (10 mg/kg) mixture and examined otologically to exclude any infection or ear channel blocking wax. Middle ear health was examined by tympanometry. Ldp recording were done in left ear using DPOAE analyzer (DPOAE Model 4000 I/O, HOMOTH Company). Ldp audiograms were measured using two pure tone stimuli: f1–f2 with f2/f1 ratio of 1.25. Intensity levels of the two tones, L1 and L2 were equal to 75 and 65 dB SPL respectively. Before any Ldp recording, signal levels were calibrated in ear canal by using emission probe microphone. All data were collected into two stimuli; f1 and f2. Contents of these stimuli were summed, and summed energy in 2f1–f2 frequency buffer was served to estimate Ldp measurements at 0.5-10 KHz. Both Ldp and signal to noise ratio (SNR) were measured at 2f1–f2 and blotted respect to geometric mean of f1 and f2. Pass criterion for a valid signal evaluation procedure was typically set to SNR of 6 dB. Animals' body temperature was tried to keep constant during tests, since constant body temperature plays main role in Ldp measurement.
Animals were anaesthetized by carbon dioxide (CO2), decapitated, and their cochleae were extracted. Cochleae were fixed in 10% formaldehyde for 48 hours, decalcified by 10% nitric acid for 24 hours, dehydrated and cleared by Xylol. Specimens were embedded by paraffin in two-step; paraffin blocks were prepared and sectioned by 5 µm thickness by a calibrated precision microtome (Model Leitz). Sections were stained by Hematoxylin and Eosin (H&E). Cover slips were mounted on slides, left to dry and examined by light microscope (LM) (Zeiss model). Various segments of organ of corti in control group were histomorphologically examined under LM. Main parts involved in examination were inner hair cells (IHCs), outer hair cells (OHCs), supporting cells (SC), stria vascularis (SV), basilar membrane (BM), and tectorial membrane (TM). Noticeable parameters were cell size, relative cell count, inter- or intra-cellular distances, and cell polarity degree for each mentioned parts. It was allocated a score 0 to any parameter. Thus, control group was attributed as criteria for comparison. In the blind state, noise group were examined under LM at a magnification of 10×, 20× and 40×. Thus, any histomorphological damages of any parameter classified by scores -2, -1, 0, +1, and +2. Atrophy, edema, proliferation, and damages caused by cell injury were discriminated. Kolmogorov-Smirnov was used to determine data normality. Repeated Measures Analysis of Variance was served for comparing Ldp and Lnf among days 0, 8, and 11. One-way Analysis of Variance (ANOVA) was applied to multiple comparisons of Ldp and its Lnf at different frequencies. Tukey's Honestly Significant Difference as a Post hoc multiple comparisons were either used to determine differential Ldp and its Lnf. Paired-Sample T-test was used to compare Ldp and its Lnf between right and left ears. Significant level was considered 0.05 as judgment.
Results
The pre- and post-exposure DPOAE levels (Ldp) analysis showed that Ldp were found to be the same across days in control rabbits (P=0.065) (Table 1). Ldp were also equal over all test frequencies on each day (P=0.071). Ldp were showed to be the same between the right and left ears (P=0.068) (Table 1).
Table 1. Comparison of mean and standard deviation of DPOAE levels (Ldp) and noise floor levels (Lnf) across times in control group.
| Frequency (Hz) | DPOAE levels (Ldp) (dB) | Noise floor levels (Lnf) (dB) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 8 | Day 11 | p | Day 0 | Day 8 | Day 11 | p | |||
| 588.00 | 5.64 (0.12) | 5.39 (0.15) | 5.36 (0.18) | 0.084 | -0.97 (0.03) | -0.47 (0.05) | -0.88 (0.07) | 0.062 | ||
| 867.00 | 9.28 (0.11) | 9.53 (0.09) | 9.06 (0.15) | 0.091 | -1.21 (0.03) | -0.76 (0.07) | -1.48 (0.06) | 0.059 | ||
| 1133.00 | 13.12 (0.08) | 13.34 (0.17) | 13.40 (0.11) | 0.318 | -1.53 (0.05) | -2.75 (0.02) | -2.11 (0.03) | 0.074 | ||
| 1677.00 | 18.56 (0.28) | 18.29 (0.21) | 18.80 (0.17) | 0.090 | -3.17 (0.09) | -3.42 (0.11) | -2.03 (0.06) | 0.053 | ||
| 1967.00 | 23.21 (0.19) | 23.45 (0.22) | 23.25 (0.31) | 0.067 | -2.21 (0.04) | -3.24 (0.9) | -2.58 (0.07) | 0.081 | ||
| 3098.50 | 27.28 (0.42) | 27.55 (0.37) | 27.42 (0.45) | 0.088 | -3.45 (0.04) | -3.28 (0.08) | -4.87 (0.10) | 0.411 | ||
| 3956.00 | 31.77 (0.31) | 31.49 (0.42) | 31.99 (0.34) | 0.129 | -3.02 (0.09) | -3.13 (0.07) | -4.16 (0.16) | 0.129 | ||
| 5888.50 | 36.11 (0.43) | 36.26 (0.32) | 36.38 (0.37) | 0.058 | -4.91 (0.014) | -4.02 (0.11) | -4.79 (0.14) | 0.056 | ||
| 8166.50 | 34.89 (0.32) | 34.98 (0.55) | 34.75 (0.43) | 0.066 | -4.83 (0.10) | -4.26 (0.15) | -5.52 (0.18) | 0.081 | ||
| 9855.00 | 33.99 (0.42) | 33.73 (0.57) | 33.84 (0.53) | 0.062 | -5.74 (0.14) | -4.09 (0.13) | -5.36 (0.08) | 0.059 | ||
The most and least post-exposure Ldp were related to 5888.50 Hz and 588 Hz respectively in noise rabbits (Table 2). Ldp were decreased on days 8 and 11, significantly on day 8, in rabbits exposed to noise compared to control rabbits (P=0.006). Decreased Ldp at 5888.50 Hz were found to be more than other test frequencies (P<0.001). Ldp were found to be the same across ears (P=0.071). (Table 2)
Table 2. Comparison of mean and standard deviation of DPOAE levels (Ldp) and noise floor levels (Lnf) across times in noise group.
| Frequency (Hz) | DPOAE levels (Ldp) (dB) | Noise floor levels (Lnf) (dB) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 8 | Day 11 | p | Day 0 | Day 8 | Day 11 | p | |||
| 588.00 | 5.16 (0.08) | 0.58 (0.02) | 1.95 (0.10) | 0.013 | -5.11 (0.04) | -6.15 (0.08) | -6.73 (0.07) | 0.091 | ||
| 867.00 | 8.87 (0.12) | 3.24 (0.26) | 3.68 (0.23) | 0.001 | -6.68 (0.08) | -6.19 (0.10) | -6.06 (0.06) | 0.077 | ||
| 1133.00 | 13.08 (0.15) | 6.39 (0.27) | 6.85 (0.21) | 0.008 | -7.23 (0.06) | -6.63 (0.12) | -6.97 (0.05) | 0.179 | ||
| 1677.00 | 18.65 (0.32) | 11.27 (0.22) | 11.91 (0.35) | 0.022 | -7.04 (0.15) | -6.49 (0.11) | -7.75 (0.13) | 0.088 | ||
| 1967.00 | 23.14 (0.26) | 12.82 (0.38) | 14.63 (0.45) | 0.016 | -7.36 (0.17) | -8.96 (0.15) | -6.28 (0.17) | 0.452 | ||
| 3098.50 | 27.82 (0.38) | 15.72 (0.43) | 16.79 (0.29) | 0.031 | -8.32 (0.13) | -7.38 (0.16) | -8.56 (0.12) | 0.089 | ||
| 3956.00 | 31.18 (0.44) | 18.01 (0.50) | 19.10 (0.31) | 0.002 | -9.44 (0.12) | -8.21 (0.15) | -9.19 (0.16) | 0.057 | ||
| 5888.50 | 36.87 (0.53) | 19.08 (0.41) | 20.86 (0.35) | 0.011 | -9.23 (0.17) | -9.55 (0.13) | -8.88 (0.19) | 0.266 | ||
| 8166.50 | 34.96 (0.47) | 17.74 (0.27) | 19.28 (0.33) | 0.009 | -11.62 (0.18) | -10.77 (0.16) | -10.09 (0.17) | 0.085 | ||
| 9855.00 | 33.25 (0.39) | 17.04 (0.49) | 18.45 (0.41) | 0.010 | -11.04 (0.13) | -11.11 (0.15) | -12.71 (0.19) | 0.151 | ||
The most and least temporary threshold shifts (TTS) or permanent threshold shifts (PTS) were related to 5888.50 Hz and 588.00 Hz respectively in noise exposed rabbits (p=0.005) (Table 3). TTS and PTS were decreased up to 17.79 dB and to 16.01 dB respectively. TTS were more than PTS over all test frequencies, especially at 5888.50 Hz in noise rabbits (P=0.015). TTS or PTS in rabbits subjected to noise were larger than those in control rabbits (P<0.05). TTS or PTS were found to be equal across ears in noise exposed rabbits (P=0.071).
Table 3. Comparison of temporary threshold shifts (TTS) and permanent threshold shifts (PTS) between noise and control groups.
| Frequency (Hz) | Temporary threshold shifts (TTS) (dB) | Permanent threshold shifts (PTS) (dB) | ||||
|---|---|---|---|---|---|---|
| Control group | Noise group | p | Control group | Noise group | p | |
| 588.00 | 0.25 (0.03) | 4.58 (0.06) | 0.032 | 0.28 (0.02) | 3.21 (0.09) | 0.002 |
| 867.00 | 0.25 (0.05) | 5.63 (0.08) | 0.021 | 0.22 (0.02) | 5.19 (0.10) | 0.005 |
| 1133.00 | 0.22 (0.04) | 6.69 (0.11) | 0.017 | 0.28 (0.01) | 6.23 (0.09) | 0.023 |
| 1677.00 | 0.27 (0.01) | 7.38 (0.13) | 0.011 | 0.24 (0.02) | 6.74 (0.16) | 0.019 |
| 1967.00 | 0.24 (0.05) | 10.32 (0.14) | 0.007 | 0.04 (0.01) | 8.51 (0.12) | 0.033 |
| 3098.50 | 0.27 (0.02) | 12.10 (0.17) | 0.003 | 0.14 (0.01) | 11.03 (0.21) | 0.020 |
| 3956.00 | 0.28 (0.02) | 13.17 (0.12) | 0.029 | 0.22 (0.04) | 12.08 (0.19) | 0.017 |
| 5888.50 | 0.15 (0.01) | 17.79 (0.19) | 0.003 | 0.27 (0.02) | 16.01 (0.22) | 0.018 |
| 8166.50 | 0.09 (0.05) | 17.22 (0.13) | 0.006 | 0.14 (0.02) | 15.68 (0.17) | 0.025 |
| 9855.00 | 0.26 (0.02) | 16.21 (0.16) | 0.014 | 0.15 (0.01) | 14.80 (0.18) | 0.019 |
Control group examination showed normal cochlea (Figure 2). Therefore, there were no abnormal cases in examination of all slides of this group under microscopic observation. While severely vacuolated OHCs as well as intensively cell injury as hydropic degeneration type was obvious in noise group (Figure 3). Mild to moderately pyknotic inner hair cells (IHCs) were varied in some slides, but this state was not confirmed in all slides. SC was swollen, but not vacuolated. No status is found to be implying to injured and damaged TM, but slightly thickened has been shown.
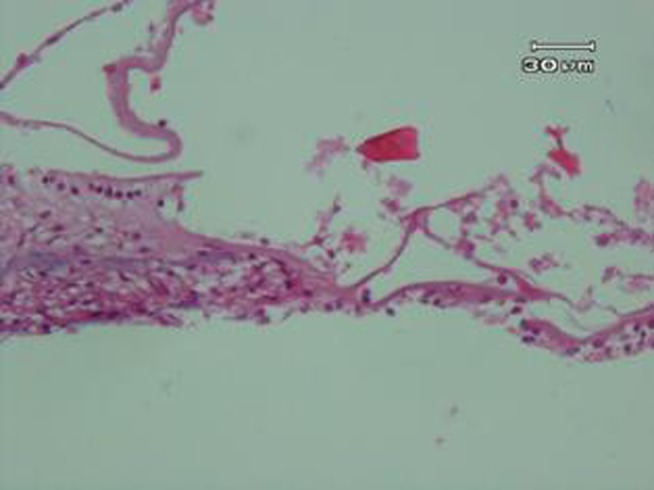
Figure 2. Control group. A photograph of the organ of corti of rabbits not exposed to any physical agents, showing healthy and normal cochlear hair cells (OHCs and IHCs), supporting cells (SC), basilar membrane (BM), and tectorial membrane (TM).
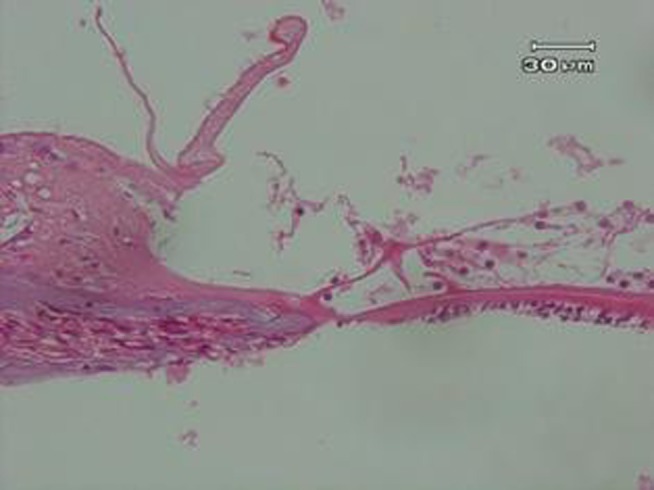
Figure 3. Noise group. A photograph of the organ of corti of rabbits exposed to noise, showing severely vacuolated outer hair cells (OHCs) with intensely cell injury as hydropic degeneration type, mild to moderately pyknotic inner hair cells (IHCs), swollen supportive cells, slightly thickened basilar membrane (BM), and not injured tectorial membrane (TM).
Discussion
Significantly decreased DPOAE levels (Ldp) caused by noise exposure reached up to 20.86 dB (for day 8) and 19.08 dB (for day 11) at 5888.50 Hz. Like this study, most studies were indicated that prolonged and repeated exposure of awake animals to continuous noise led to significantly diminished Ldp at a wide test frequencies range as a reduction in cochlear outer hair cells' function depending on exposure duration, frequency and noise intensity (13-18,24,25). The difference in affected frequencies in the present study with other similar researches can be referred to the use of broad-band noise, while most of the others used narrow-band or pure tone stimulation in their efforts (24,25).
Contrary to these findings, several factors were found to be culprit in inducing enhanced DPOAE response amplitudes such as hypoxia, low frequency electromagnetic fields, induced labyrinthitis, and some ototoxic drugs (11,26). Consistent with the findings of the study, some studies showed that the DPOAE response amplitudes were significantly depressed following a number of factors include the administration of ototoxic drugs, acoustic trauma or noise overexposure, Meniere’s disease, sudden idiopathic sensori-neural hearing loss, acoustic neuroma, presbycusis, and hereditary hearing disorders (26,27).
DPOAE levels (Ldp) were found to be significantly different on various occasions. Ldp decreased on day 8, and then increased at a level slightly higher than baseline measurements on day 11. Similar reversible and temporary differences were reported after interrupting the exposure to different noxious agents such as noise overexposure or acoustic trauma, ototoxic drugs, sudden idiopathic sensori-neural hearing loss, and thermoprobe lesioning (11,26). These decreases in DPOAE levels (Ldp) might be attributed to the temporary and reversible effect of the vibration exposure as a basal cochlear lesion progressed through the frequency region being monitored. Consistently, some confirm that the temporary reduction in DPOAE amplitudes occurring before enhancements can be interpreted as relating to an improvement of the general condition of the exposed rabbits over time (26,27).
TTS and PTS were significantly decreased up to 17.79 dB and 16.01 dB respectively in animals under exposure to continuous noise. Like the results obtained from this study, PTS may be caused by a brief exposure to extremely high-intensity sounds, but it is more commonly caused by prolonged repetitive exposure or continuous exposure to lower levels of hazardous noise (4-6,27). Susceptibility to NIHL is highly variable; while some individuals are able to tolerate high noise levels for prolonged periods of time, others who are subjected to the same environment more rapidly lose hearing (27). Risk of PTS is related to the duration and intensity of the exposure as well as to genetic susceptibility to noise trauma (4,27). Inner ear is believed that partially protected from the effects of continuous noise by the acoustic reflex which is triggered when the ear is subjected to noise louder than 90 dB, causes the middle ear muscles (the stapedius and tensor tympani) to contract and thereby stiffen the conductive system, making it more resistant to sound entry (4). Because this protective reflex is neurally mediated, it is delayed in onset for a period ranging from 25 ms to 150 ms, depending on noise intensity (4).
Very highly vacuolation and intensively cell injury with the type of hydropic degeneration in outer hair cells (OHCs), mild to moderately pyknotic inner hair cells (IHCs), swollen supportive cells (SC), slightly thickened basilar membrane (BM) were found in noise group. Reasons for reduced Ldp is believed that can be attribute to misalignment of hair bundles on adjacent hair cells, non-linearity in stiffness of stereocilia, and damage of the tectorial membrane (2-4,9,28,29). Most studies found that the noise exposure causes permanent loss of hair cell stereocilia with apparent fracture of the rootlet structures and destruction of the sensory cells, which are replaced by nonfunctioning scar tissue. NIHL results from trauma to the sensory epithelium of the cochlea (4,9,28). In TTS, several potentially reversible effects such as regional decrease in stiffness of stereocilia secondary to contraction of rootlet structures which are anchored to the cuticular plate of hair cells, intracellular changes within the hair cells including metabolic exhaustion and microvascular changes, edema of the auditory nerve endings, and degeneration of synapses within the cochlear nucleus, can be occurred (2-4,9,28). While in PTS, the changes become irreversible and include breaks in the rootlet structures, disruption of the cochlear duct and organ of corti causing mixing of endolymph and perilymph, loss of hair cells, and degeneration of cochlear nerve fibers (2-4).
A strongly reason for cochlear OHCs' dysfunction (as decreased Ldp) and damage to organ of corti is based on oxidative stress mechanism (30-33), Metabolic damage or exhaustion is believed that occurred when toxic waste products so-called as free radicals (FRs), including reactive oxygen species (ROS) or reactive nitrogen species (RNS), are formed after cochlear cells are stressed by reductions in cochlear blood flow, excessive and toxic levels of neurotransmitters like glutamate, changes in calcium balances in the cell, and other stress-related changes that are induced by noise (30-33). These free radicals injure a wide variety of critical structures in the cochlea, causing cell damage and cell death (32,33). Noise exposure affects several structural elements in hair cells, including the cell membrane and intracellular biochemical pathways (28). These changes may evoke the formation of free radicals, resulting in sensorineural hearing loss (33-37). FRs may increase dramatically within a few minutes or hours of an intense noise exposure (30,38,39). Noise-induced cochlear FRs endanger HC’s intrinsic antioxidant system as GSH that is found to be the powerful natural antioxidant glutathione peroxidase system in cochlear hair cells. Depletion of cochlear hair cells' GSH in organ of Corti due to exposure to noise can cause more susceptibility to hearing loss (38,39).
No any significance was observed about DPOAEs levels (Ldp) between right and left ear in animals exposed to noise. Creation of reverberation field in exposure chamber seems to be the most important reason. Some studies have been reported results similar (9,25,28), but some reported different results regarding Ldp between two ears (40-42). Sato et al. (1991) showed that an efferent influence may also help to explain the systematic difference between the magnitude of left and right ear Ldp in humans and animals (40). Sininger & Cone-Wesson (2004) also indicated that tone-evoked Ldp are larger in left ear (41). van den Brink, (1970) reported pitch differences between left and right ears when presented with the same frequency stimulus (42).
Ldp measurements were examined in New Zealand white (NZW) rabbits as a species of rabbits experimented in this study, while the role of species differences must be taken as an important factor. It has been proved that there are clear species differences in the dependence of Ldp on frequency, in that Ldp tend to be largest in the regions of best hearing sensitivity in each species, and these regions vary between species (43). It has been reported that systematic variations in DPOAEs parameters such as L1=L2 and L1-L2, and f2/f1 generally produce qualitatively similar changes in emission levels in humans, monkeys, cats, rabbits, and rodents (23). They believes that these similarities occur despite the quantitative differences in particularly the f2/f1 ratio that elicits the largest DPOAEs, which is greater in rabbits and rodents (1.25) than in humans (1.22) (23).
Sex differences seem to play a key role in measuring Ldp, while only male rabbits were used in present study. Some reported Ldp are larger in human and rhesus monkey females than in males (40,44). They found that the larger Ldp may be correlated to better hearing thresholds for females of the same species (43,44). Some are believed that this difference partly referred to different hormonal exposure (40,43), while others thought it can be attributed to a sex difference in OHC electromotility and/or in the mechanism(s) responsible for stereociliary bundle motility (40,44). Both of these reasons can be the result of gender differences in membrane lipid profiles that would alter lipid–protein interactions (44). A research cited that another possibility is the shorter length of female cochleae (40), or gender differences in the size of the middle ear (40,44). Ldp is expected to be varied or larger if the studied animals were selected females or variety of both male and female rabbits. A study reported Ldp is slightly stronger in female animals as compared to males (44).
DPOAEs can be attributed as a useful screening and diagnostic clinical tool for early detecting NIHL in rabbits with normal audiograms. Outer hair cells were affected early in NIHL, and DPOAEs were detected subtle changes in OHCs' function as temporary or permanent hearing shifts and cochlear damage. Ldp temporarily and permanently diminished in rabbits that underwent exposure to noise. Therefore, DPOAEs are an attractive tool for obtaining information about small temporary or permanent threshold shifts, even when the pure tone audiogram is normal. Noise exposure led to decreased Ldp and injury to IHCs, OHCs, SC, and BM. These cochlear dysfunction and histological changes seem to be the main reason for explaining the noise-induced hearing loss in rabbits subjected to excessive continuous noise.
Acknowledgments
We would like to specially thank Professor Roger P. Hamernik and Professor Richard D. Kopke for sincerely critical comments in the present study. This research has been conducted in Tarbiat Modares University (TMU) laboratory.
Footnotes
Conflict of interest: No
References
- 1.Ohinata Y, Miller JM, Schacht J. Protection from noise induced lipid peroxidation and hair cell loss in the cochlea. Brain Res. 2003;966:265–73. doi: 10.1016/s0006-8993(02)04205-1. [DOI] [PubMed] [Google Scholar]
- 2.Rabinowitz PM, Rees TS. Occupational Hearing Loss. In: Rosenstock L, Cullen MR, Brodkin CA, Redlich CA, editors. Textbook of clinical occupational and environmental medicine. 2nd ed. Philadelphia: Elsevier's Health Sciences Rights Department Publishers; 2005. pp. 423–33. [Google Scholar]
- 3.Han Y, Hong L, Zhong C, Chen Y, Wang Y, Mao X, Zhao D, Qiu J. Identification of new altered genes in rat cochleae with noise-induced hearing loss. Gene. 2012;499(2):318–22. doi: 10.1016/j.gene.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 4.Lalwani AK. Current diagnosis & treatment in otolaryngology-head & neck surgery. A LANGE medical book. 2nd ed. New York: McGraw-Hill; 2008. [Google Scholar]
- 5.Sataloff RT, Sataloff J. Occupational Hearing Loss. 3rd ed. New York: Taylor & Francis Publishers. CRC Press; 2006. [Google Scholar]
- 6.Maltby M. Occupational Audiometry: Monitoring and protecting hearing at work. 1st ed. London: Butterworth-Heinemann; 2005. [Google Scholar]
- 7.Ronen Perez, Sharon Freeman, Haim Sohmer. Effect of an initial noise induced hearing loss on subsequent noise induced hearing loss. Hear Res. 2004;192(1–2):101–106. doi: 10.1016/j.heares.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Berger EH, Royster LH, Royster JD, Driscoll DP, Layne M. The Noise Manual. 5th ed. American Industrial Hygiene Assoc: AIHA. Fairfax, VA; 2000. [Google Scholar]
- 9.Emmerich E, Richter F, Reinhold U, Linss V, Linss W. Effects of industrial noise exposure on distortion product Otoacoustic emissions (DPOAEs) and hair cell loss of the cochlea-long term experiments in awake guinea pigs. Hear Res. 2000;148:9–17. doi: 10.1016/s0378-5955(00)00101-5. [DOI] [PubMed] [Google Scholar]
- 10.Wagner W, Plinkert PK. The relationship between auditory threshold and evoked otoacoustic emissions. Eur Arch Otorhinolaryngol. 1999;256:177–88. doi: 10.1007/s004050050136. [DOI] [PubMed] [Google Scholar]
- 11.Manley GA, Fay RR, Popper AN. Active Processes and Otoacoustic Emissions in Hearing. 1st ed. New York: Springer Science & Business Media; 2008. [Google Scholar]
- 12.Brownell WE. Outer hair cell electromotility and otoacoustic emissions. Ear & Hearing. 1990;11:82–90. doi: 10.1097/00003446-199004000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankel R, Freeman S, Sohmer H. Use of ABR and OAEs in detection of noise induced hearing loss. J Basic Clin Physiol Pharmacol. 2003;14(2):95–118. doi: 10.1515/jbcpp.2003.14.2.95. [DOI] [PubMed] [Google Scholar]
- 14.Chan VS, Wong EC, McPherson B. Occupational hearing loss: screening with distortion-product otoacoustic emissions. Int J Audiol. 2004;43(6):323–9. doi: 10.1080/14992020400050041. [DOI] [PubMed] [Google Scholar]
- 15.Lucertini M, Moleti A, Sisto R. On the detection of cochlear damage by otoacoustic emission analysis. J Acoust Soc Am. 2002;111(2):972–8. doi: 10.1121/1.1432979. [DOI] [PubMed] [Google Scholar]
- 16.Nagy AL, Tóth F, Vajtai R, Gingl Z, Jóri J, Kiss JG. Effects of noise on distortion-product otoacoustic emissions. Int Tinnitus J. 2002;8(2):94–6. [PubMed] [Google Scholar]
- 17.Kiss JG, Toth F, Venczel K. Distortion-product otoacoustic emissions following pure tone and wide-band exposures. Scand Audiol Suppl. 2001;(52):138–40. doi: 10.1080/010503901300007335. [DOI] [PubMed] [Google Scholar]
- 18.Gorga MP, Neely ST, Dierking DM, Dorn PA, Hoover BM, Fitzpatrick DF. Distortion product otoacoustic emission suppression tuning curves in normal-hearing and hearing-impaired human ears. J Acoust Soc Am. 2003;114(1):263–78. doi: 10.1121/1.1575751. [DOI] [PubMed] [Google Scholar]
- 19.Kemp DT. Otoacoustic Emissions: Concepts and Origins. In: Manley GA, Fay RR, Popper AN, editors. Active Processes and Otoacoustic Emissions in Hearing. 1st ed. Springer Science & Business Media, LLC; New York, USA: 2008. pp. 1–38. [Google Scholar]
- 20.Gorga MP, Neely ST, Dorn PA, Hoover BM. Further efforts to predict pure tone thresholds from distortion product otoacoustic emission input/output functions. J Acoust Soc Am. 2003;113(6):3275–84. doi: 10.1121/1.1570433. [DOI] [PubMed] [Google Scholar]
- 21.Lonsbury-Martin BL, Martin GK. Otoacoustic Emissions: Basic Studies in Mammalian Models. In: Manley GA, Fay RR, Popper AN, editors. Active Processes and Otoacoustic Emissions in Hearing. 1st ed. New York: Springer Science & Business Media; 2008. pp. 261–303. [Google Scholar]
- 22.Janssen T, Müller J. Otoacoustic emissions as a diagnostic tool in a clinical context. In: Manley GA, Fay RR, Popper AN, editors. Active Processes and Otoacoustic Emissions in Hearing. 1st ed. New York: Springer Science & Business Media; 2008. pp. 421–60. [Google Scholar]
- 23.Harding GW, Bohn BA, Ahmad M. DPOAE level shifts and ABR threshold shifts compared to detailed analysis of histopathological damage from noise. Hear Res. 2002;174(1-2):158–71. doi: 10.1016/s0378-5955(02)00653-6. [DOI] [PubMed] [Google Scholar]
- 24.Vassilakis PN, Meenderink SWF, Narins PM. Distortion product otoacoustic emissions provide clues to hearing mechanisms in the frog. J Acoust Soc Am. 2004;116:3713–26. doi: 10.1121/1.1811571. [DOI] [PubMed] [Google Scholar]
- 25.Long-term sound conditioning increases distortion product otoacoustic emission amplitudes and decreases olivocochlear efferent reflex strength. NeuroReport. 2007;18:1167–70. doi: 10.1097/WNR.0b013e32820049a8. [DOI] [PubMed] [Google Scholar]
- 26.Moussavi-Najarkola SA, Khavanin A, Mirzaei R, Salehnia M, Akbari M. Effects of whole body vibration on outer hair cells' hearing response to distortion product otoacoustic emissions. In Vitro Cell Dev Biol Anim. 2012 doi: 10.1007/s11626-012-9490-3. [DOI] [PubMed] [Google Scholar]
- 27.Rosenstock L, Cullen MR, Brodkin CA, Redlich CA. Textbook of clinical occupational and environmental medicine. 2nd ed. Philadelphia: Elsevier's Health Sciences Rights Department; 2005. [Google Scholar]
- 28.Chen GD, Fechter LD. The relationship between noiseinduced hearing loss and hair cell loss in rats. Hear Res. 2003;177(1-2):81–90. doi: 10.1016/s0378-5955(02)00802-x. [DOI] [PubMed] [Google Scholar]
- 29.Kitcher ED, Ocansey G, Tumpi DA. Early occupational hearing loss of workers in a stone crushing industry: Our experience in a developing country. Noise Health. 2012;14(57):68–71. doi: 10.4103/1463-1741.95134. [DOI] [PubMed] [Google Scholar]
- 30.Chung IS, Chu IM, Cullen MR. Hearing effects from intermittent and continuous noise exposure in a study of Korean factory workers and firefighters. BMC Public Health. 2012;12:87. doi: 10.1186/1471-2458-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang T, Cheng AG, Stupak H, Liu W, Kim A, Staecker H, Lefebvre PP, Malgrange B, Kopke R, Moonen G, Van De Water TR. Oxidative stress-induced apoptosis of cochlear sensory cells: otoprotective strategies. Int J Devl Neuroscience. 2000;18:259–70. doi: 10.1016/s0736-5748(99)00094-5. [DOI] [PubMed] [Google Scholar]
- 32.Kaygusuz I, Ozturk A, Ustundag B, Yalcin S. Role of free oxygen radicals in noise-related hearing impairment. Hear Res. 2001;162:43–7. doi: 10.1016/s0378-5955(01)00365-3. [DOI] [PubMed] [Google Scholar]
- 33.Ohinata Y, Yamasoba T, Schacht J, Miller JM. Glutathione limits noise-induced hearing loss. Hear Res. 2000;146(1-2):28–34. doi: 10.1016/s0378-5955(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 34.Le Prell CG. Noise-induced hearing loss: from animal models to human trials. Adv Exp Med Biol. 2012;730:191–5. doi: 10.1007/978-1-4419-7311-5_43. [DOI] [PubMed] [Google Scholar]
- 35.Campbell KCM, Meech RP, Klemens JJ, Gerberi MT, Dyrstad SS, Larsen DL, Mitchell DL, El-Azizi M, Verhulst SJ, Hughes LF. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007;226(1-2):92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Duan M, Qiu J, Laurell G, Olofsson A, Counter SA, Borg E. Dose and time-dependent protection of the antioxidant N-Lacetylcysteine against impulse noise trauma. Hear Res. 2004;192:1–9. doi: 10.1016/j.heares.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Fechter LD. Oxidative stress: A potential basis for potentiation of noise-induced hearing loss. Environ Toxicol Pharmacol. 2005;19:543–546. doi: 10.1016/j.etap.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita D, Jlang HY, Prell GL, Schacht J, Miller M. Post-exposure treatment attenuates noise-induced hearing loss. Neuroscience. 2005;134:633–642. doi: 10.1016/j.neuroscience.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Chen GD, McWilliams M, Fechter LD. Succinate dehydrogenase (SDH) activity in hair cells: a correlate for permanent threshold elevations. Hear Res. 2000;145:101–10. doi: 10.1016/s0378-5955(00)00076-9. [DOI] [PubMed] [Google Scholar]
- 40.McFadden D, Pasanen EG, Raper J, Lange HS, Wallen K. Sex differences in Otoacoustic emissions measured in rhesus monkeys (Macaca mulatta). Horm Behav. 2006;50:274–84. doi: 10.1016/j.yhbeh.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Sininger Y, Cone-Wesson B. Asymmetric cochlear processing mimics hemispheric specialization. Science. 2004;305(5690):1581. doi: 10.1126/science.1100646. [DOI] [PubMed] [Google Scholar]
- 42.Van den Brink G. Experiments in binaural diplacusis and tonal perception. In: Plomp R, Smoorenburg GF, editors. Frequency Analysis and Periodicitiy Detection in Hearing. 1st ed. Leiden: Sijthoff AW; 1970. pp. 362–74. [Google Scholar]
- 43.Brown AM. Acoustic distortion from rodent ears: a comparison of responses from rats, guinea pigs and gerbils. Hear Res. 1987;31:25–38. doi: 10.1016/0378-5955(87)90211-5. [DOI] [PubMed] [Google Scholar]
- 44.Bilger R, Matthies ML, Hammel DR, Demorest ME. Genetic implications of gender differences in the prevalence of spontaneous otoacoustic emissions. J Speech Hear Res. 1990;33:418–32. doi: 10.1044/jshr.3303.418. [DOI] [PubMed] [Google Scholar]


