Abstract
Background:
Mexico's National Institute of Respiratory Diseases (NIRD) is a third-level national reference center. Primary adenoid cystic carcinoma (PACC) is an uncommon neoplastic disorder; hence improvements in the description of this disease are needed.
Materials and Methods:
This is a retrospective clinical study based on all consecutive patients with pathological diagnoses of PACC seen at the NIRD between January 1, 2000 and December 31, 2009.
Results:
We identified 9 cases of PACC (67% female) out of a total of 2,634 patients with lung cancer seen during the period analyzed. The mean age of those 9 patients was 41 years (IQR 36-57), and the frequency of PACC at our center was 0.3%. It is important to note that 67% of those patients had a history of smoking and that 6 of the 9 had the antecedent of previous exposure to biomass fuel smoke. Baseline arterial blood gas analyses revealed a median of 61 mmHg for pO2 and 28.5 mmHg for pCO2. Median FVC was 78%, while FEV1 was 77% with an FEV1 /FVC ratio of 78. Death occurred in 56% of cases, and the median survival time was 17 months (IQR 6-26) after the initial diagnosis.
Conclusions:
The frequency of tracheobronchial PACC among patients with lung cancer was similar to that previously reported (0.3%). According to our results, lung function has no specific phenotype in this disease; however, some abnormalities could be related to potential risk factors such as tobacco use and exposure to biomass fuel smoke.
KEY WORDS: Fatal outcomes, primary adenoid cystic carcinoma, prevalence, respiratory function tests, tracheobronchial neoplasms
INTRODUCTION
In adults, 90% of primary tracheobronchial tumors are malignant. The two pathological varieties found are squamous-cell carcinoma and primary adenoid cystic carcinoma (PACC)[1,2] though the latter accounts for less than 1% of all malignant tumors.[3] Such tumors may appear in the respiratory epithelium, the salivary glands, or in the mesenchymal tissue. Therefore, they tend to occur mainly in the trachea, carina or the main or lobar bronchus.[4]
Some studies have reported that patients with PACC complain of chronic symptoms and have divided them into two groups: First, those with airway obstruction who are treated for obstructive lung disease (i.e., asthma); and, second, patients with associated lung infections.[5,6] In most cases, reaching a definitive diagnosis requires a relatively long period of time because the symptoms tend to be non-specific and of extended duration; thus most patients present forms of the disease that are already locally advanced.[3] Despite this, prognoses are good unless metastasis is documented.
Mexico's National Institute of Respiratory Diseases (NIRD) is a third-level, national reference center where the average number of hospital admissions is approximately 4000 patients per year. Of those, only for the year 2009, malignant respiratory diseases represented the third highest cause of hospitalization, with 10.5% of all cases. This study reviewed cases from 10 years of experience in this exclusive respiratory center for the purpose of ascertaining: 1) the frequency of PACC; 2) its clinical symptoms; 3) possible risk factors; 4) a more complete description of the characteristic lung function of PACC patients; and, 5) outcomes.
MATERIALS AND METHODS
This is a retrospective analysis that included all the medical charts from patients admitted to the NIRD consecutively with pathological diagnoses of PACC between January 1, 2000 and December 31, 2009. The study was approved by the NIRD's Institutional Review Board. An informed consent was not required, but all information was kept confidential and used exclusively for research purposes.
The following variables were identified on the basis of a systematic review of the medical charts of the 9 cases found: Age, gender, history of smoking, the tobacco index (pack-years), biomass exposure (hours/day), main symptoms (cough, dyspnea, chest pain, wheezing, hemoptysis, stridor, fever and weight loss), average time from the onset of symptoms to diagnosis, results of radiographic evaluation (chest X-ray, CT scan, radionuclide bone scan, and PET/CT scan), measurements of lung function (post-bronchodilator spirometry and arterial blood gas analysis), treatment, survival time, and outcomes.
All data were analyzed statistically using SPSS statistics software (version 17, SPSSinc, Chicago Ill.) Continuous data are expressed as medians with interquartile range (IQR), while categorical data are described as frequencies with percentages.
RESULTS
In this series, we described a total of 9 cases. The median age was 41 years (IQR 36-57), and 67% of patients were female. Also, 67% had a history of smoking. The average time from the onset of symptoms to the final diagnosis was 21 months (IQR 6-30). Clinical manifestations are described in Table 1. The prevalence of PACC in this study population was 9 cases out of a total of 2,634 patients with lung cancer attended during the period analyzed. This represents 0.3% of cases (1 out of every 292 lung cancer patients).
Table 1.
Characteristics of tracheobronchial adenoid cystic carcinoma patients (n = 9)
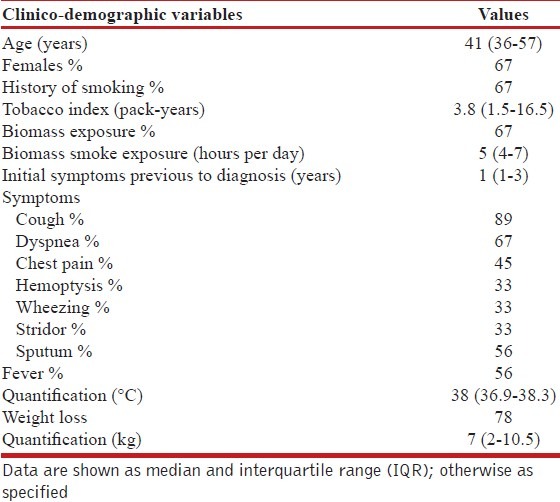
Laboratory tests performed at the time of admission highlighted the levels of hemoglobin (11.7, IQR 11-13.2 gr/dL), serum albumin (3.6, IQR 3-4.2 gr/dL), and the carcinoembryonic antigen (0.8, IQR 0-3.2 UI/L). Atelectasis was the abnormality most often reported in chest X-rays (56% of cases), while abnormal uptake on the PET/CT scan was observed in 44% of cases. Other radiological findings are described in Table 2.
Table 2.
Radiological and lung function findings in this series (n = 9)
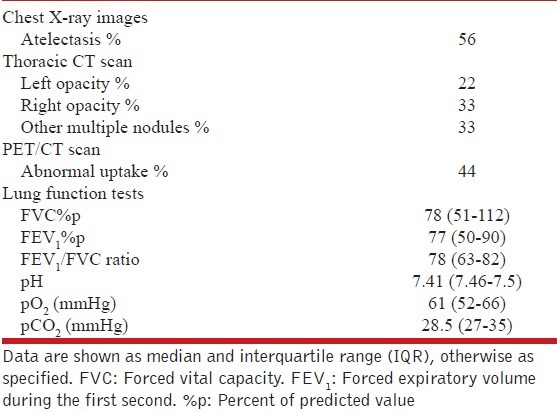
The results of lung function tests measured by spirometry and reported as the percentage of predicted values (% p) using ATS/ERS standardized reference equations[7] were as follows: FVC showed a median of 78% p (IQR, 51-112) and FEV1 a median of 77% p (IQR, 50-90) with an FEV1/FVC ratio of 78 (IQR, 63-82). Flow-volume loops were normal.
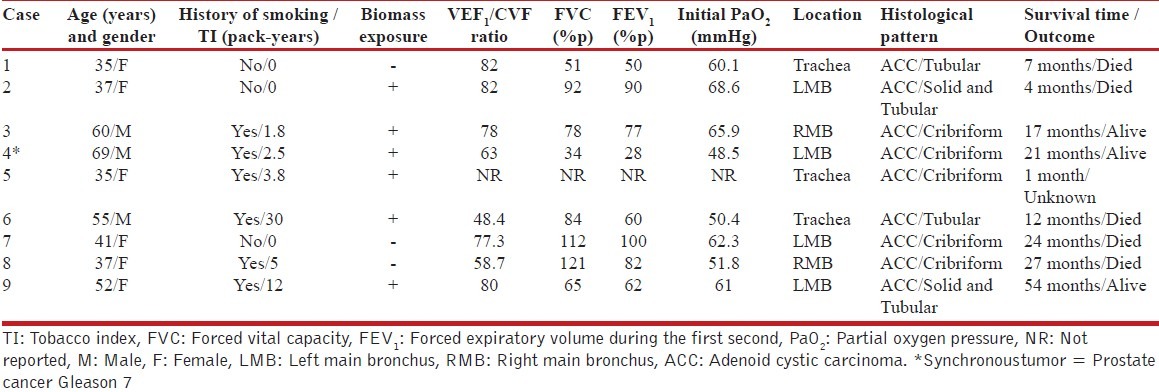
The primary location of tumors was the left main bronchus (44% of cases). A total of 56% of cases were classified as being in clinical stage IV (other scores, such as Karnofsky and ECOG, are shown in Table 3). Treatment consisted of chemotherapy (CHT), either alone or combined with surgery and/or radiation therapy (RT). The CHT drug schemes used were based on platinum, doxorubicin, and paclitaxel. Finally, 56% of patients died after a median survival time of 17 months. Of the remaining patients, 22% are considered disease-free, while another 22% are currently undergoing palliative treatment for distant metastasis. Individual case descriptions are listed in Table 4.
Table 3.
Location, staging and treatment of the group analyzed (n = 9)
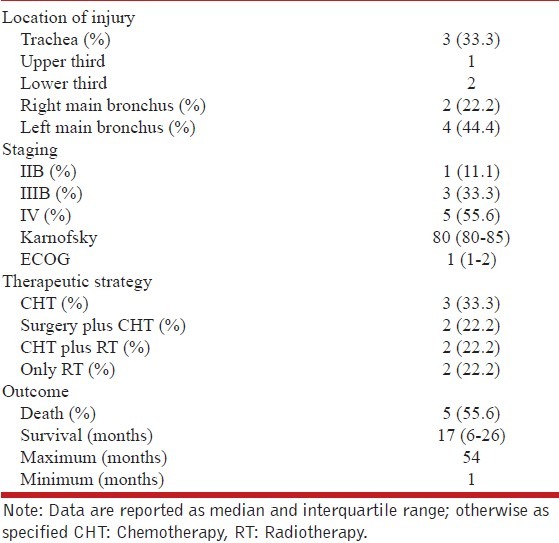
Table 4.
Characteristics of the population studied involving lung function and its association with exposure factors, anatomical location, histological type, and survival
DISCUSSION
The main findings of our study were as follows: 1) disease frequency was low and similar to that previously reported (0.3%, representing 1 out of every 292 hospital discharges due to pulmonary malignancy); 2) two-thirds of our series had previous exposure to tobacco and/or biomass fuel smoke; 3) hemoptysis, wheezing, and stridor showed a lower frequency in this study population; 4) a more complete description of the lung function of PACC patients; and, 5) mean survival time and outcomes.
The frequency of PACC has been reported previously as ranging from 0.1 to 0.5%.[6,8] Our results offer important support for this finding, especially because they come from a hospital that is devoted exclusively to treating cases of respiratory disease. The mean age at presentation was also similar to that previously reported in the literature.[8] With regards to gender prevalence, our sample had a higher number of female cases (male to female ratio 1:2). This proportion constitutes an infrequent finding because PACC has been described as having no gender predilection. However, the small number of patients included in earlier studies may account for this difference.[9] Shimizu et al., significantly, reported the results of a recent study over a 27-year period in which all patients were female (100%).[10]
A history of smoking was documented in 67% of the cases, with a median tobacco index of 3.8 pack-years. Although a relationship between tobacco use and PACC has not been described previously, it is reasonable to consider smoking as a risk factor as is the case in other strains of bronchogenic carcinoma.[1,11] In addition, it is noteworthy that two-thirds of our series had prior exposure to biomass combustion (median of 5 hours/day). To the best of our knowledge, this finding has never been described in association with this particular disease. Biomass smoke exposure seems to cause disruptions in the bronchial epithelium and can eventually cause abnormalities related to pre-malignant conditions such as those described in patients with chronic obstructive pulmonary disease (COPD).[12] For these reasons, future research should explore whether tobacco use and exposure to biomass smoke might be risk factors associated with PACC.
As mentioned above, the main symptoms identified were chronic coughing and dyspnea. Unfortunately, both of these symptoms are non-specific. Wheezing and stridor were present in 33% of our population; however, Albers et al. have reported these clinical features in up to 50% of cases.[11] In addition, Yang et al. have reported that 71% of their cases showed hemoptysis as an initial symptom, a clue that might allow us to reach more timely diagnoses.[9] Despite the advanced clinical stages characteristic of our series, however, we observed hemoptysis in only one-third of cases.
As far as we know, the results of pulmonary function tests of patients with PACC have not been published previously. Thus, one of the significant findings of our series was the presence of hypoxemia. It is important to note that severe hypoxemia was present in those patients whose lung function showed serious abnormalities [Table 4]. Taken together, these findings may explain the mechanism of hypoxemia, which is most likely due to V/Q mismatches. In addition, we were able to identify that obstructive patterns were related to those patients who had a history of smoking. The overall FEV1/FVC ratio was normal (78% p); however, 3 patients had values indicating obstruction with different degrees of severity. No patient showed any response to bronchodilator use, and the flow-volume loops showed no abnormalities. With respect to this finding, it is important that the physicians who treat patients with this condition be particularly cautious with their lung function reports. It is imperative that spirometry findings be analyzed as a whole, using validated or local reference values and flow-volume loops. In this way, it may be possible to determine abnormalities, such as delays in gas-filling, and parameters capable of differentiating between intrathoracic and extrathoracic obstructions in patients with a fixed obstructive pattern that could be a clue that leads to more accurate diagnoses.[7] Also, the pulmonary function test technologist must be encouraged to obtain a maximum number of repeatable maneuvers of expiratory and inspiratory flow-volume loops, to allow the attending physician to make a correct interpretation. Finally, there were cases with concomitant decreases in both FVC and FEV1, which suggest the presence of lung restriction. Apparently, no specific lung function phenotype is closely related to this disease though a higher prevalence of an obstructive pattern was observed.
The main bronchus was the primary disease site. Given its level of involvement, atelectasis was the most frequent finding in X-ray imaging. We would hypothesize that these patients might have complained of wheezing in the early stages of disease development, but because of the advanced stage of the illness in our series of cases, such symptoms could have been absent or minimized by the patients’ general condition at the time of the initial evaluation.
Based on the delay in reaching diagnoses (median evolution time with symptoms, 12 months), a metastatic expression of the disease was to be expected. In this regard, 60% of our patients showed a metastatic uptake on radionuclide bone scans during their initial evaluation. It is important to mention that this same frequency has been reported in other studies.[8,9] Due to the advanced clinical stage of disease evolution, treatment was mainly palliative. Recently, a new modality of RT techniques, that includes adjuvant tomotherapy (intensity-modulated radiation therapy), has been used in palliative care and appears to be of some use.[13]
CONCLUSIONS
In this series of cases, the frequency of PACC was low (0.3%). Both, tobacco and exposure to biomass fuel smoke, appears to be an important factor. Lung function tended to show an obstructive pattern; however, additional prospective studies will have to describe lung function in greater detail including the description of the flow-volume loops, which could provide clues to the most effective diagnostic approach.
ACKNOWLEDGEMENT
The authors would like to thank Paul Kersey for correcting errors in language usage.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Haresh KP, Prabhakar R, Rath GK, Sharma DN, Julka PK, Subramani V. Adenoid cystic carcinoma of the trachea treated with PET-CT based intensity modulated radiotherapy. J Thorac Oncol. 2008;3:793–5. doi: 10.1097/JTO.0b013e31817c9245. [DOI] [PubMed] [Google Scholar]
- 2.Aubry MC, Heinrich MC, Molina J, Lewis JE, Yang P, Cassivi SD, et al. Primary adenoid cystic carcinoma of the lung: Absence of KIT mutations. Cancer. 2007;110:2507–10. doi: 10.1002/cncr.23075. [DOI] [PubMed] [Google Scholar]
- 3.Jaso J, Malhotra R. Adenoid cystic carcinoma. Arch Pathol Lab Med. 2011;135:511–5. doi: 10.5858/2009-0527-RS.1. [DOI] [PubMed] [Google Scholar]
- 4.Park CM, Goo JM, Lee HJ, Kim MA, Lee CH, Kang MJ. Tumors in the tracheobronchial tree: CT and FDG PET features. Radiographics. 2009;29:55–71. doi: 10.1148/rg.291085126. [DOI] [PubMed] [Google Scholar]
- 5.Takeda S, Hashimoto T, Kusu T, Kawamura T, Nojiri T, Funakoshi Y, et al. Management and surgical resection for tracheobronchial tumors; institutional experience with 12 patients. Interact Cardiovasc Thorac Surg. 2007;6:484–9. doi: 10.1510/icvts.2007.152280. [DOI] [PubMed] [Google Scholar]
- 6.Gaissert HA, Mark EJ. Tracheobronchial gland tumors. Cancer Control. 2006;13:286–94. doi: 10.1177/107327480601300406. [DOI] [PubMed] [Google Scholar]
- 7.Pellegrino R, Viegi G, Brusasco V, Crapo R, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 8.Kanematsu T, Yohena T, Uehara T, Ushijima C, Asoh H, Yoshino I, et al. Treatment outcome of resected and non-resected primary adenoid cystic carcinoma of the lung. Ann Thorac Cardiovasc Surg. 2002;8:74–7. [PubMed] [Google Scholar]
- 9.Yang PY, Liu MS, Chen CH, Lin CM, Tsao TC. Adenoid cystic carcinoma of the trachea: A report of seven cases and literature review. Chang Gung Med J. 2005;28:357–63. [PubMed] [Google Scholar]
- 10.Shimizu J, Oda M, Matsumoto I, Arano Y, Ishikawa N, Minato H. Clinicopathological study of surgically treated cases of tracheobronchial adenoid cystic carcinoma. Gen Thorac Cardiovasc Surg. 2010;58:82–6. doi: 10.1007/s11748-009-0467-4. [DOI] [PubMed] [Google Scholar]
- 11.Albers E, Lawrie T, Harrell JH, Yi ES. Tracheobronchial adenoid cystic carcinoma: A clinicopathologic study of 14 cases. Chest. 2004;125:1160–5. doi: 10.1378/chest.125.3.1160. [DOI] [PubMed] [Google Scholar]
- 12.Moran-Mendoza O, Pérez-Padilla JR, Salazar-Flores M, Vazquez-Alfaro F. Wood smoke-associated lung disease: A clinical, functional, radiological and pathological description. Int J Tuberc Lung Dis. 2008;12:1092–8. [PubMed] [Google Scholar]
- 13.Alongi F, Di Muzio N, Motta M, Broggi S, De Martin E, Bolognesi A, et al. Adenoid cystic carcinoma of trachea treated with adjuvant hypofractionated tomotherapy. Case report and literature review. Tumori. 2008;94:121–5. doi: 10.1177/030089160809400122. [DOI] [PubMed] [Google Scholar]


