Abstract
Kartagener's syndrome is a rare, autosomal recessive genetic ciliary disorder comprising the triad of situs inversus, chronic sinusitis, and bronchiectasis. The basic problem lies in the defective movement of cilia, leading to recurrent chest infections, ear/nose/throat symptoms, and infertility. We hereby report three unusual cases of this rare entity – an infertile male with azoospermia in whom Bochdalek's diaphragmatic hernia coexisted, another case of an infertile female, and a third of an infertile male with oligospermia. The need for a high index of suspicion to make an early diagnosis cannot be overemphasized in such patients so that wherever possible, options for timely treatment of infertility may be offered and unnecessary evaluation of symptoms is avoided.
KEY WORDS: Bronchiectasis, Kartagener's syndrome, sinusitis, situs inversus
INTRODUCTION
Kartagener's syndrome (KS) is a subset of a larger group of ciliary motility disorders called primary ciliary dyskinesias (PCDs). It is a genetic condition with an autosomal recessive inheritance,[1,2] comprising a triad of situs inversus, bronchiectasis and sinusitis.[1,2] Although Siewart first described this condition in 1904, it was Kartagener who recognized the etiological correlation between the elements of the triad and reported four cases in 1933.[2] The estimated prevalence of PCD is about 1 in 30,000,[3] though it may range from 1 in 12,500 to 1 in 50,000.[1] In KS, the ultrastructural genetic defect leads to impaired ciliary motility which causes recurrent chest, ear/nose/throat (ENT), and sinus infections, and infertility. A high index of suspicion is needed to make an early diagnosis so that timely treatment options may be offered for infertility in these young patients, wherever feasible. Also, although unproven, it seems likely that early diagnosis is important for the preservation of pulmonary function, quality of life, and life expectancy in this disease.[4,5] However, this has not been confirmed and further large prospective studies are needed.
CASE REPORTS
Case 1
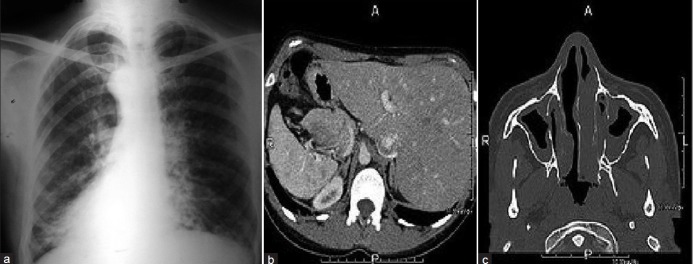
This was a 34-year-old non-smoker male, born to non- consanguineous parents. He presented to the outpatient with chief complaints of recurrent episodes of common cold, sneezing, and cough with expectoration for past 10 years, exertional shortness of breath for last 5 years, and not having children despite being married for last 14 years. The patient also revealed that he frequently developed cough, cold, rhinorrhea, nasal blockade, and ear discharge during childhood. He received anti-tubercular treatment for these complaints 5 years back with no relief. Chest X-ray at that time was done, but was not available. On examination, the vital parameters were within normal limits. Physical examination revealed grade 2 digital clubbing and apex beat on the right side in fifth intercostal space. On auscultation, bilateral wheeze and right basal crackles were audible, with heart sounds being best heard on the right side of the chest. Electrocardiogram showed evidence of dextrocardia. Chest X-ray postero-anterior (PA) view [Figure 1a] revealed cardiac apex and aortic arch on the right side, suggesting dextrocardia along with left-sided Bochdalek's hernia. Radiograph of sinuses showed mucosal thickening in maxillary sinuses. An ultrasound of the abdomen revealed a normal liver and gall bladder on the left side and a normal spleen on the right side. Contrast-enhanced computed tomography (CECT) chest [Figure 1b] revealed dextrocardia, nodular opacities in right lower lobe suggestive of bronchiectasis, and left-sided Bochdalek's hernia containing omentum and large bowel loops. A semen analysis revealed azoospermia, while the hormone profile was suggestive of hyperprolactinemia with normal luteinizing hormone and follicle stimulating hormone levels.
Figure 1.
(a) Chest X-ray PA view showing dextrocardia and left-sided Bochdalek's hernia; (b) CECT-chest showing right-sided bronchiectasis, dextrocardia, and left-sided Bochdalek's herina
Case 2
This was a 55-year-old non-smoker female. She presented to us with chief complaints of recurrent cold, cough with copious expectoration, anosmia, headache, and progressively increasing shortness of breath for last 30 years. She had been married for the last 36 years, but had no children. She received anti-tubercular treatment for these complaints 12 years back for 6 months, with no relief. Her past history was significant as she had frequent visits to the pediatrician for recurrent chest infections. Her family history revealed no parental consanguinity. On examination, the patient had a blood pressure of 110/72 mmHg, pulse rate 92/minute regular, and oxygen saturation 76% on room air. General examination revealed bilateral pedal edema, raised jugular venous pressure, facial puffiness, and grade 3 clubbing. On auscultation, diffuse ronchi and crackles (more on the left side) were heard. Rest of the systemic examination was within normal limits. Routine blood investigations were normal except hemoglobin (9.5 gm%). Electrocardiogram showed “p” pulmonale, and ultrasound of abdomen revealed situs inversus. X-ray paranasal sinuses revealed mucosal thickening with hazy sinuses [Figure 2a]. Chest X-ray [Figure 2b] and high-resolution computed tomography (HRCT) thorax revealed bronchiectasis and situs inversus.
Figure 2.

(a) X-ray PNS showing opacified maxillary sinuses; (b) chest X-ray PA view showing dextrocardia and left-sided bronchiectasis
Case 3
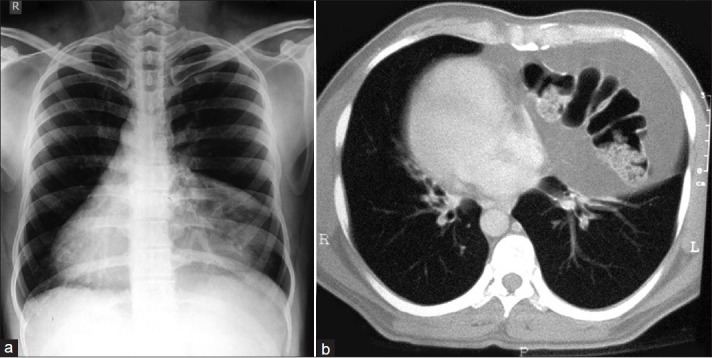
A 40-year-old male patient, non-smoker, and born to non-consanguineous parents presented with recurrent productive cough, rhinorrhea, and headache since last 20 years with episodic fever and worsening of symptoms. He had been previously treated with antibiotics, antihistamines, bronchodilators, inhaled and oral corticosteroids, and even anti-tuberculous drugs, but the response was only partial and temporary. He also had similar complaints, off and on, during childhood.
On examination, he was febrile with nasal discharge, wheezy chest, and bilateral coarse crackles. His heart sounds were heard best on the right side of the chest. There was no digital clubbing. Chest X-ray [Figure 3a] showed cystic bronchiectactic changes in the lower and mid zones with dextrocardia. Ultrasound of the abdomen showed spleen on the right side of the abdomen, while liver on the left was suggestive of complete situs inversus.
Figure 3.
(a) Cystic bronchiectactic changes in the lower and mid zones with dextrocardia; (b) axial CT image abdomen showing situs inversus with the liver and IVC on the left and the spleen and aorta on the right; (c) axial CT image paranasal sinuses showing mucosal thickening and opacified sinus cavities
Axial CT-chest showed dextrocardia with the inferior vena cava (IVC) and morphologic right ventricle on the left and the left ventricle on the right. Axial CT-abdomen showed situs inversus with the liver and IVC on the left and the spleen and aorta on the right Figure 3b. HRCT- chest showed dilated and thickened medium-sized airways with signet ring appearances. Axial CT-paranasal sinuses showed mucosal thickening and opacified sinus cavities Figure 3c. Hematological and biochemical parameters were within normal limits except semen analysis which showed oligospermia with immotile live sperms.
DISCUSSION
Disorders of ciliary motility may be congenital or acquired. Congenital disorders are labeled as PCDs. Nearly 50% of PCD patients have situs inversus. Such cases of PCD with situs inversus are known as Kartagener's syndrome.[6]
PCD is a phenotypically and genetically heterogeneous condition wherein the primary defect is in the ultrastructure or function of cilia.[7,8] Such defects are identified in approximately 90%[9] of PCD patients and involve the outer dynein arms, inner dynein arms, or both. 38%[9] of the PCD patients carry mutations of the dynein genes DNAI[10] and DNAH5.[6]
Pathophysiologically, the underlying defect which leads to accumulation of secretions and consequent recurrent sinusitis, bronchiectasis, infertility, and situs inversus is the defective ciliary motility/immotility. The severity of symptoms and the age at which the condition is diagnosed is quite variable, even though the symptoms are present from birth.[11,12] Occasionally, Kartagener's syndrome may be associated with reversible airflow obstruction.[13] Clinical progression of the disease is variable with lung transplantation required in severe cases.
Diagnostic criteria for this condition include[14] clinical picture suggestive of recurrent chest infections, bronchitis, and rhinitis since childhood, along with one or more of the following: (1) situs inversus in the patient/sibling; (2) alive but immotile spermatozoa; (3) reduced or absent transbronchial mucociliary clearance; and (4) cilia showing characteristic ultrastructural defect on electron microscopy.
Apart from fulfilling the criteria mentioned above, two types of tests are done for diagnosis of PCD – screening tests (exhaled nasal nitric oxide measurement which is usually low in PCD, and saccharin test to assess mucociliary function of nasal epithelium) and diagnostic tests (ciliary beat pattern and frequency analysis using video recording, and electron microscopic confirmation of the ultrastructural ciliary defect). The samples for these tests for examining motility and ultrastructure of cilia may be obtained by biopsy of nasal mucosa and laparoscopic biopsies of tubal mucosa in females, as was done by Halbert et al.[15] In our cases, however, we could not perform these tests and the diagnosis was essentially clinico-radiological, with variation in view of azoospermia and oligospermia. These situations have been infrequently reported previously,[16–18] and it could be possible that they are a variant associated with KS. Most infertile patients with KS have a normal spermatozoid count, but with a structural defect and a complete lack of motility.[19] The hyperprolactinemia present in our first case could possibly be coincidental, as a search of literature has reported it to be a cause of infertility but has not revealed an association between this condition and KS.
The issue of fertility was not addressed in the initial published reports of patients with KS until Arge[20] reported three male patients with this syndrome having immotile spermatozoa and sterility. Male patients with KS invariably present infertility, while women present reduced fertility.[19] Infertility in male KS patients is due to diminished sperm motility, while in females it is due to defective ovum transport because of dyskinetic motion of oviductal cilia, suggesting that the ciliated endosalpinx is essential for human reproduction.[21]
The development of assisted reproductive techniques has allowed rational treatment for these patients, and to date, there have been reported pregnancies using subzonal insemination (SUZI) and intracytoplasmic sperm injection (ICSI).[22] The case report of Kordus et al.[11] shows that even in severe cases of asthenozoospermia, ICSI[23] can overcome the inability of the spermatozoa to reach the ovum and produce healthy offspring. However, both SUZI and ICSI require expertise not available in all units and there are concerns over costs and outcomes.[22] Until more is known with regard to the genetic control of PCD, it is suggested that treatment should be individualized depending on sperm motility. In cases where there is no sperm motility, ICSI may be the most appropriate treatment. However, if sperm motility is present, a trial of in vitro fertilization (IVF) should be considered.[22] One concern regarding the fertility treatment of men with PCD is the possibility that the resultant child has the risk of being affected by the same condition. It is therefore necessary to counsel couples regarding the possibility of genetic risks and to follow-up children fathered by men affected by PCD.[22]
To conclude, KS patients are frequently troubled by repeated infection episodes for which they have to seek medical attention and this is largely the reason for their morbidity. But infertility is also one important aspect that needs to be adequately addressed in their evaluation so that they may be offered a suitable option that could help them have children.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Barthwal MS. Kartagener's syndrome in a fertile male - An uncommon variant. Lung India. 2006;23:123–5. [Google Scholar]
- 2.Dixit R, Dixit K, Jindal S, Shah KV. An unusual presentation of immotile-cilia syndrome with azoospermia: Case report and literature review. Lung India. 2009;26:142–5. doi: 10.4103/0970-2113.56352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seaton D. Bronchiectasis. In: Seaton A, Seaton D, Leitch AG, editors. Crofton and Douglas's respiratory diseases. 5th ed. Oxford: Blackwell Science; 2004. pp. 794–828. [Google Scholar]
- 4.Barbato A, Frischer T, Kuehni CE, Snijders D, Azevedo I, Baktai G, et al. Primary ciliary dyskinesia: A consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34:1264–76. doi: 10.1183/09031936.00176608. [DOI] [PubMed] [Google Scholar]
- 5.Marthin JK, Petersen N, Skovgaard LT, Nielsen KG. Lung function in patients with primary ciliary dyskinesia: A cross-sectional and 3-decade longitudinal study. Am J Respir Crit Care Med. 2010;181:1262–8. doi: 10.1164/rccm.200811-1731OC. [DOI] [PubMed] [Google Scholar]
- 6.Olbrich H, Häffner K, Kispert A, Völkel A, Volz A, Sasmaz G, et al. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat Genet. 2002;30:143–4. doi: 10.1038/ng817. [DOI] [PubMed] [Google Scholar]
- 7.Noone PG, Bali D, Carson JL, Sannuti A, Gipson CL, Ostrowski LE, et al. Discordant organ laterality in monozygotic twins with primary ciliary dyskinesia. Am J Med Genet. 1999;82:155–60. doi: 10.1002/(sici)1096-8628(19990115)82:2<155::aid-ajmg11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 8.Chodhari R, Mitchison HM, Meeks M. Cilia, primary ciliary dyskinesia and molecular genetics. Paediatr Respir Rev. 2004;5:69–76. doi: 10.1016/j.prrv.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Zariwala MA, Knowles MR, Omran H. Genetic defects in ciliary structure and function. Annu Rev Physiol. 2007;69:423–50. doi: 10.1146/annurev.physiol.69.040705.141301. [DOI] [PubMed] [Google Scholar]
- 10.Loges NT, Olbrich H, Fenske L, Mussaffi H, Horvath J, Fliegauf M, et al. DNAI2 mutations cause primary ciliary dyskinesia with defects in the outer dynein arm. Am J Hum Genet. 2008;83:547–58. doi: 10.1016/j.ajhg.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kordus RJ, Price RL, Davis JM, Whitman-Elia GF. Successful twin birth following blastocyst culture of embryos derived from the immotile ejaculated spermatozoa from a patient with primary ciliary dyskinesia: A case report. J Assist Reprod Genet. 2008;25:437–43. doi: 10.1007/s10815-008-9254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coren ME, Meeks M, Morrison I, Buchdahl RM, Bush A. Primary ciliary dyskinesia: Age at diagnosis and symptom history. Acta Paediatr. 2002;91:667–9. doi: 10.1080/080352502760069089. [DOI] [PubMed] [Google Scholar]
- 13.Kant S, Kushwaha RAS, Verma SK, Singhal S, Mehra S, Mahajan V, et al. Kartagener syndrome associated with reversible airflow obstruction. J Intern Med India. 2007;10:63–6. [Google Scholar]
- 14.Afzelius BA, Mossberg B. Immotile cilia. Thorax. 1980;35:401–4. doi: 10.1136/thx.35.6.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halbert SA, Patton DL, Zarutskie PW, Soules MR. Function and structure of cilia in the fallopian tube of an infertile woman with Kartagener's syndrome. Hum Reprod. 1997;12:55–8. doi: 10.1093/humrep/12.1.55. [DOI] [PubMed] [Google Scholar]
- 16.Bashi S, Khan MA, Guirjis A, Joharjy IA, Abid MA. Immotile cilia syndrome with azoospermia: A case report and review of the literature. Br J Dis Chest. 1988;82:194–6. doi: 10.1016/0007-0971(88)90043-5. [DOI] [PubMed] [Google Scholar]
- 17.Gill TS, Sharma S, Mishra RR, Lahiri TK. Syndrome of primary ciliary dyskinesia: Kartagener's syndrome with empyema thoracis and azoospermia. Indian J Chest Dis Allied Sci. 1996;38:201–4. [PubMed] [Google Scholar]
- 18.Mittal V, Shah A. Situs inversus total: The association of Kartagener's syndrome with diffuse bronchiolitis and azoospermia. Arch Bronconeumol. 2012;48:179–82. doi: 10.1016/j.arbres.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Afzelius BA, Eliasson R. Male and female infertility problems in the immotile-cilia syndrome. Eur J Respir Dis Suppl. 1983;127:144–7. [PubMed] [Google Scholar]
- 20.Arge E. Transposition of the viscera and sterility in men. Lancet. 1960;1:412–4. doi: 10.1016/s0140-6736(60)90340-8. [DOI] [PubMed] [Google Scholar]
- 21.McComb P, Langley L, Villalon M, Verdugo P. The oviductal cilia and Kartagener's syndrome. Fertil Steril. 1986;46:412–6. [PubMed] [Google Scholar]
- 22.Kay VJ, Irvine DS. Successful in-vitro fertilization pregnancy with spermatozoa from a patient with Kartagener's syndrome: Case report. Hum Reprod. 2000;15:135–8. doi: 10.1093/humrep/15.1.135. [DOI] [PubMed] [Google Scholar]
- 23.Palermo G, Joris H, Devroey P, van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]