Abstract
Aim:
To study the efficacy and safety of paclitaxel and platinum doublet chemotherapy in penile cancer patients with high-risk features of local failure.
Materials and Methods:
Retrospective analysis was done of patients with 19 carcinoma of the penis who were offered adjuvant chemotherapy with paclitaxel and platinum combination. The data regarding the surgical details, high-risk features for which chemotherapy was offered, chemotherapy toxicity details (in accordance with CTCAE vs 3), failure pattern, and survival data were noted. SPSS version 16 was used for statistical analysis. Descriptive and Kaplan–Meier survival analysis was performed.
Results:
Median age of patients was 48 years. Fifteen patients received paclitaxel in combination with cisplatin and four received paclitaxel with carboplatin in view of their low serum creatinine clearance. The treatment was completed by 12 patients (63.2%). Of 79 planned cycles, 50 were taken. The treatment was well tolerated with grade 3-4 gastrointestinal toxicity was seen in 1 patient, grade 3 neurological toxicity in one and grade 5 neutropenia in one patient. Treatment related death occured in one patient. The median follow-up was 15.33 months and 6 loco-regional relapsed had taken place. The estimated median DFS was 16.2 months and the estimated median OS was not reached. The estimated DFS for treatment completed patients was 23.13 months as against 2.16 months for patients not completing treatment.
Conclusion:
The platinum and taxane doublet chemotherapy was found to be safe and effective.
Keywords: Adjuvant chemotherapy, carcinoma of the penis, platinum
INTRODUCTION
Penile carcinoma is a rare malignancy, with the reported incidence in western literature of 0.84 per 100,000 population,[1] as against that in developing world where it is in the range of 2.3-8 per 100,000 population.[2] The delayed presentation of this malignancy in a developing country accounts for the fact that most of these patients present with bilateral bulky inguinal or pelvic lymph nodes.[2] In this malignancy, the involvement of lymph nodes changes the prognosis quite dramatically.[3] In men with unilateral inguinal lymph node involvement without perinodal extension, the reported 5-year survival with only surgery is in the range of 80-90%. However, in patients with bilateral lymph node metastasis with perinodal extension or pelvic lymph node involvement, the reported 5-year survival after surgery alone is only 10-20%, which warrant adjuvant treatment.[4] Due to lack of randomized studies, various approaches are being used for the treatment of such patients. Radiation, chemotherapy, or both, have been used in literature. However, the use of adjuvant radiation comes at the cost of lymphedema in such patients who have already undergone extensive groin and pelvic dissection.[5] The option of adjuvant chemotherapy has been advocated in such high-risk cases.[6] The use of adjuvant chemotherapy is less likely to contribute to lymphedema and further morbidity related to the same.
In this paper, we have retrospectively analyzed the outcomes of penile cancer patients from a tertiary cancer institute, who were selected for adjuvant chemotherapy in view of high-risk features in a multidisciplinary joint clinic.
MATERIALS AND METHODS
This is a retrospective analysis conducted for the year 2008-2009 in a tertiary cancer institute in a developing country. The case records were retrieved of patients diagnosed with carcinoma of the penis and who were offered adjuvant chemotherapy. All patients underwent a plain radiograph of the chest and contrast-enhanced computed tomography of the abdomen and pelvis prior to surgery.
Patients with high-risk features for failure such as perinodal extension, bilateral lymph node involvement, pelvic lymph node involvement, and those in whom R1 resection was performed were offered adjuvant chemotherapy. R0 surgery was said to have been performed when no tumor was present at the surgical margin. R1 surgery was done when tumor was not identified grossly at the margin, but was present in the microscopic margin.
The adjuvant chemotherapy was planned with paclitaxel and cisplatin. Paclitaxel was administered in a dose of 175 mg/m2 over 3 hour and cisplatin was administered in a dose of 75 mg/m2 over 1 hour. Four cycles were planned at an interval of 21 days. Standard premedications and antiemetics were prescribed. In patients whom serum creatinine clearance was less than 50, carboplatin was substituted for cisplatin. Toxicity data were abstracted from the charts and graded as per the Common Terminology Criteria for Adverse Events, National Cancer Institute, version 4.0 (CTCAE v 4.0) criteria.
Statistical analysis was done with SPSS version 16 (Statistical Package for Social Sciences, Armonk, New York, USA). Descriptive statistics are provided. The Disease-Free Survival (DFS) was calculated from the day of surgery for groin lymph nodes to the date of either of the events, local recurrence, or death related to disease or which ever occur the earliest. The Overall Survival (OS) was calculated from the day of surgery for groin to the date of death due to any cause. Kaplan-Meier survival analysis was used. The log rank test was used to make comparisons between patients who took four cycles or against those who took less than four cycles.
RESULTS
Over a period of 2 years, 19 case records could be retrieved. The details of surgery and post surgical staging are provided in Tables 1 and 2.
Table 1.
Details of surgical procedure

Table 2.
Details of pathological characteristics
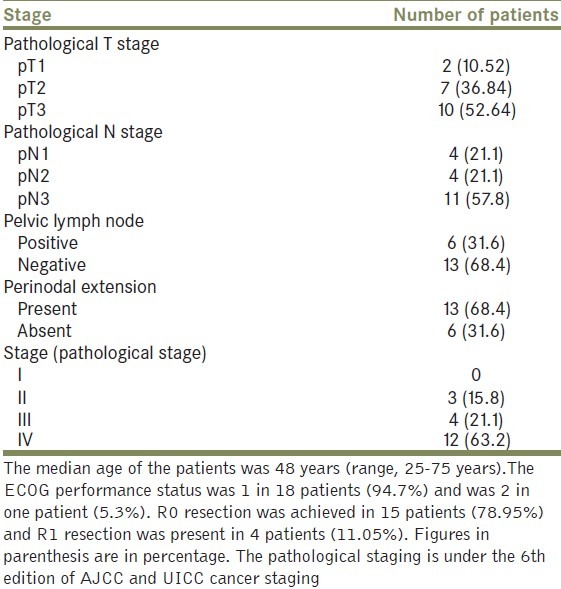
The median age of the patients was 48 years (range, 25- 75 years). The ECOG performance status was 1 in 1 8 patients (94.7%) and was 2 in one patient (5.3%). R0 resection was achieved in 15 patients (78.95%) and R1 resection was present in 4 patients (11.05%).
Of 19 patients, 15 received paclitaxel and cisplatin (88.95%), while 4 received paclitaxel and carboplatin (21.05%).The planned number of cycles was four in all patients. The planned course of adjuvant chemotherapy was completed by 12 patients (63.2%). The reason for not completing the planned course of chemotherapy was non-compliance because of logistical reasons in 6 patients (85.7%) and intolerable side effects in one patient (14.3%). The number of cycles planned were 76, and 50 of them were taken. The median number of cycles delivered was four. The median dose intensity of chemotherapy delivered was 0.89.
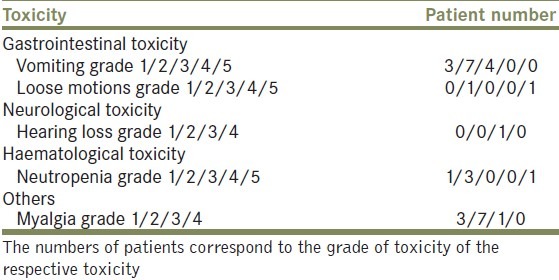
The detailed toxicity of chemotherapy is provided in Table 3. One patient had grade 5 diarrhea along with febrile neutropenia, leading to mortality on day 8 post first cycle of adjuvant chemotherapy. No dose reductions were done in patients who experienced toxicity. These patients were provided (Granulocyte stimulating factor) G-CSF support in subsequent cycles.
Table 3.
Chemotherapy toxicity
The median follow-up is 15.33 months (2-34 months). Six loco-regional relapses and three deaths occurred, one related to toxicity and the other two related to the disease. The estimated median DFS for the population was 16.2 months [Figure 1] and estimated median OS for the population was not reached [Figure 2]. The estimated median DFS for patients who received four cycles of chemotherapy was better than for those who did not complete their chemotherapy (P=0.0001) [Figure 3].
Figure 1.
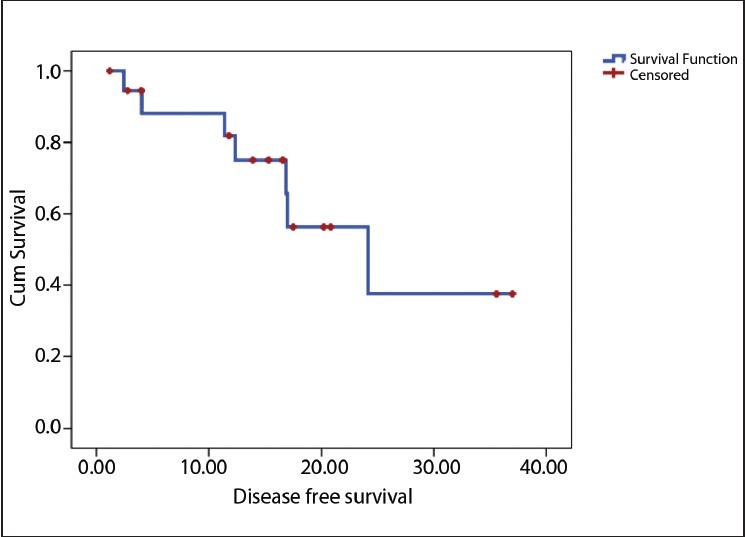
Estimated minimum disease-free survival in whole population
Figure 2.
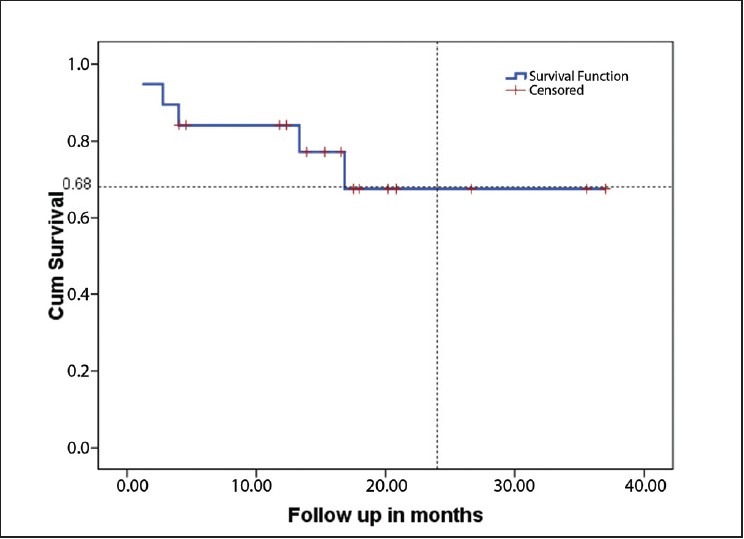
Estimated minimum overall survival in whole population
Figure 3.
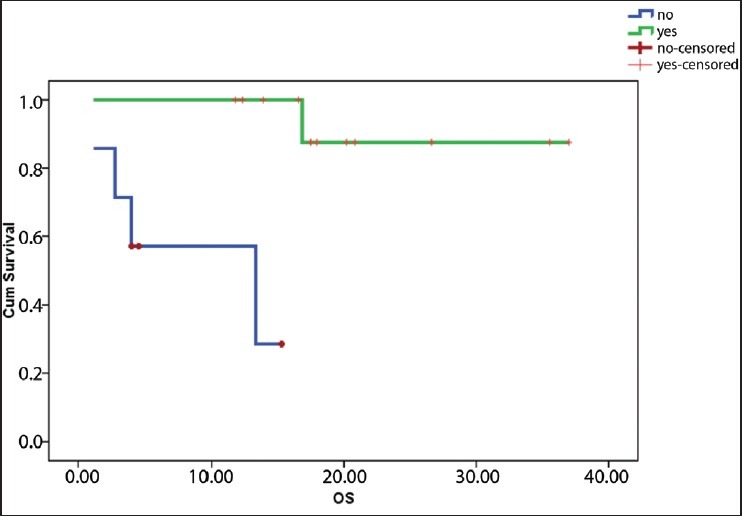
Overall survival according to number of cycles
Of the six loco-regional relapse, five patients were deemed unsuitable for any treatment and offered only palliative care. Ifosfamide-based palliative chemotherapy was offered to one patient. However, the prognosis of these patients remained dismissal with median survival post failure two months only.
DISCUSSION
It must be admitted that in carcinoma of the penis, due to relative rarity of the disease it is difficult to carry out a high-quality randomized study and data generated from small retrospective analysis such as ours cannot be neglected. This is a fairly homogenous subgroup of population with high-risk features of local recurrence treated with only surgery followed by adjuvant chemotherapy.
The chemotherapy schedule used in this study differed from previously reported studies of adjuvant chemotherapy. The regimen used in those studies was Cisplatin, Methotrexate and Bleomycin (CMB); Vincristine, Methotrexate and Bleomycin (VMB); and cisplatin with 5-Fluorouracil (5FU). However, these regimens were associated with substantial and significant morbidity and mortality.[7,8] Probably, this was the reason that in a nationwide survey from Germany on the use of chemotherapy in carcinoma of the penis, the need for a new combination of chemotherapy was warranted.[9]
The reported single-agent response rates for platinum and paclitaxel in metastatic penile cancer were 15% and 25%, respectively.[2,10] This regimen was chosen on the basis of proven effectiveness of these drugs in squamous cell carcinoma of the head and neck region.[11] To the best of our knowledge, this is first report of adjuvant chemotherapy with paclitaxel and platinum doublet.
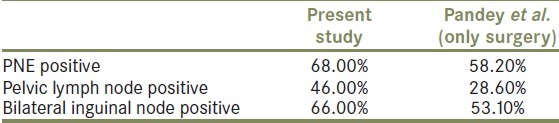
For comparison with other studies, the OS was estimated in patients with selected prognostic factors, which were shown to be independent prognostic factors on a multivariate analysis by Pandey et al. [Table 4]. The effectiveness of this regimen in reducing loco-regional relapse was proven in this analysis. In patients with high-risk features after surgery alone, 5-year failure rate is of the order of 80% and long-term survival is only 20%.[3,4,7,12,13] As compared to this historical data, it can be noted that local failure rates in the present study were comparable and probably better than results obtained by Pandey et al. [Table 4].[13] The limited follow-up in this patient subgroup may be viewed as a limitation of this study and the authors acknowledge this fact. However, it would be worthwhile to note that in the series by Pandey et al., among patients with high-risk features, a majority of patients had failed within 2 years and died. This highlights that even though the follow-up is limited, the effectiveness of this combination cannot be denied in this high-risk group as a majority of failures occur in the initial period.
Table 4.
Two-year overall survival comparison of the present study with only surgery data
Further, in this data, the comparison of patients who had completed chemotherapy showed significant difference in OS. As the intention-to-treat analysis was done, all patients offered adjuvant chemotherapy were analysed, but not those who completed the chemotherapy regimen. The group that completed the chemotherapy regimen would be the one to receive the highest possible benefit of adjuvant chemotherapy.
In the study reported by Pizzocaro, adjuvant chemotherapy was used in 12 patients over a period of 6 years from 1979 to 1985. The results of data obtained over such a prolonged period are likely to reflect changing practices in the management over the years; moreover, given the comparison of such small numbers of patients with that in the present series would not be meaningful. In their series, adjuvant chemotherapy was offered to any patients with lymph node-positive disease. With a median follow-up of 42 months, only one patient had failed.[8]
The toxicity profile of paclitaxel cisplatin/carboplatin doublet was better in comparison with previous studies of adjuvant chemotherapy. Grade 3-4 hematological toxicity was observed in more than 66% of population in whom the CMB regimen was used.[7] Though in this study, one mortality occurred related to toxicity, the patient had loose motions with febrile neutropenia on day 8 of the first cycle and was advised admission, but he did not follow the advice. The authors believe that the patient probably would have been salvaged had he followed the advice of the treating oncologist. In the VMB protocol, hematological toxicity was mild but pulmonary fibrosis was noted, resulting in treatment interruptions in two patients.[8]
The results of this study suggest that adjuvant chemotherapy regimen used in this study can reduce loco-regional relapse. Pending confirmation in a randomized trial, it seems worthwhile to consider adjuvant chemotherapy in this malignancy.
CONCLUSION
It is feasible to administer paclitaxel with platinum as adjuvant chemotherapy in carcinoma of the penis. Adjuvant therapy with this combination appears to improve outcome in high-risk patients of carcinoma of the penis. Further prospective studies may be done to firmly establish the role of adjuvant chemotherapy in carcinoma of the penis.
Footnotes
Source of Support: Nil,
Conflict of Interest: None
REFERENCES
- 1.Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, Giuliano AR. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361–7. doi: 10.1016/j.urolonc.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 2.Misra S, Chaturvedi A, Misra NC. Penile carcinoma: A challenge for the developing world. Lancet Oncol. 2004;5:240–7. doi: 10.1016/S1470-2045(04)01427-5. [DOI] [PubMed] [Google Scholar]
- 3.Ficarra V, Akduman B, Bouchot O, Palou J, Tobias-Machado M. Prognostic Factors in Penile Cancer. Urology. 2010;76:S66–73. doi: 10.1016/j.urology.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Novara G, Galfano A, De Marco V, Artibani W, Ficarra V. Prognostic factors in squamous cell carcinoma of the penis. Nat Clin Pract Urol. 2007;4:140–6. doi: 10.1038/ncpuro0751. [DOI] [PubMed] [Google Scholar]
- 5.Ravi R. Morbidity following groin dissection for penile carcinoma. Br J Urol. 1993;72:941–5. doi: 10.1111/j.1464-410x.1993.tb16304.x. [DOI] [PubMed] [Google Scholar]
- 6.Pizzocaro G, Algaba F, Horenblas S, Solsona E, Tana S, Van Der Poel H, et al. EAU Penile Cancer Guidelines 2009. Eur Urol. 2010;57:1002–12. doi: 10.1016/j.eururo.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Hakenberg OW, Nippgen JB, Froehner M, Zastrow S, Wirth MP. Cisplatin, methotrexate and bleomycin for treating advanced penile carcinoma. BJU Int. 2006;98:1225–7. doi: 10.1111/j.1464-410X.2006.06496.x. [DOI] [PubMed] [Google Scholar]
- 8.Pizzocaro G, Piva L. Adjuvant and neoadjuvant vincristine, bleomycin, and methotrexate for inguinal metastases from squamous cell carcinoma of the penis. Acta Oncol. 1988;27:823–4. doi: 10.3109/02841868809094366. [DOI] [PubMed] [Google Scholar]
- 9.Protzel C, Ruppin S, Milerski S, Klebingat K, Hakenberg OW. The current state of the art of chemotherapy of penile cancer: results of a nationwide survey of German clinics. Urologe A. 2009;48:1495–8. doi: 10.1007/s00120-009-2108-z. [DOI] [PubMed] [Google Scholar]
- 10.Di Lorenzo G, Cartenì G, Autorino R, Gonnella A, Perdonà S, Ferro M, et al. Activity and toxicity of paclitaxel in pretreated metastatic penile cancer patients. Anticancer Drugs. 2009;20:277–80. doi: 10.1097/CAD.0b013e328329a293. [DOI] [PubMed] [Google Scholar]
- 11.Posner M. Evolving strategies for combined-modality therapy for locally advanced head and neck cancer. Oncologist. 2007;12:967–74. doi: 10.1634/theoncologist.12-8-967. [DOI] [PubMed] [Google Scholar]
- 12.Lont AP, Kroon BK, Gallee MP, van Tinteren H, Moonen LM, Horenblas S. Pelvic lymph node dissection for penile carcinoma: extent of inguinal lymph node involvement as an indicator for pelvic lymph node involvement and survival. J Urol. 2007;177:947–52. doi: 10.1016/j.juro.2006.10.060. discussion 952. [DOI] [PubMed] [Google Scholar]
- 13.Pandey D, Mahajan V, Kannan RR. Prognostic factors in node-positive carcinoma of the penis. J Surg Oncol. 2006;93:133–8. doi: 10.1002/jso.20414. [DOI] [PubMed] [Google Scholar]


